PURPOSE
Intravenous paclitaxel (IVpac) is complicated by neuropathy and requires premedication to prevent hypersensitivity-type reactions. Paclitaxel is poorly absorbed orally; encequidar (E), a novel P-glycoprotein pump inhibitor, allows oral absorption.
METHODS
A phase III open-label study comparing oral paclitaxel plus E (oPac + E) 205 mg/m2 paclitaxel plus 15 mg E methanesulfonate monohydrate 3 consecutive days per week versus IVpac 175 mg/m2 once every 3 weeks was performed. Women with metastatic breast cancer and adequate organ function, at least 1 year from last taxane, were randomly assigned 2:1 to oPac + E versus IVpac. The primary end point was confirmed radiographic response using RECIST 1.1, assessed by blinded independent central review. Secondary end points included progression-free survival (PFS) and overall survival (OS).
RESULTS
Four hundred two patients from Latin America were enrolled (265 oPac + E, 137 IVpac); demographics and prior therapies were balanced. The confirmed response (intent-to-treat) was 36% for oPac + E versus 23% for IVpac (P = .01). The PFS was 8.4 versus 7.4 months, respectively (hazard ratio, 0.768; 95.5% CI, 0.584 to 1.01; P = .046), and the OS was 22.7 versus 16.5 months, respectively (hazard ratio, 0.794; 95.5% CI, 0.607 to 1.037; P = .08). Grade 3-4 adverse reactions were 55% with oPac + E and 53% with IVpac. oPac + E had lower incidence and severity of neuropathy (2% v 15% > grade 2) and alopecia (49% v 62% all grades) than IVpac but more nausea, vomiting, diarrhea, and neutropenic complications, particularly in patients with elevated liver enzymes. On-study deaths (8% oPac + E v 9% IVpac) were treatment-related in 3% and 0%, respectively.
CONCLUSION
oPac + E increased the confirmed tumor response versus IVpac, with trends in PFS and OS. Neuropathy was less frequent and severe with oPac + E; neutropenic serious infections were increased. Elevated liver enzymes at baseline predispose oPac + E patients to early neutropenia and serious infections (funded by Athenex, Inc; ClinicalTrials.gov identifier: NCT02594371).
INTRODUCTION
Taxane-based regimens, including paclitaxel, are among the most effective and commonly used systemic therapies for both early- and late-stage breast cancer; the role of taxanes in the metastatic setting continues to evolve as clinicians seek to optimize patient outcomes.1,2 Taxanes require intravenous (IV) administration in a setting equipped to manage potential hypersensitivity-type reactions.3 Neuropathy, which may be persistent, is a major dose-limiting side effect of IV paclitaxel (IVpac)4-8 that significantly affects the quality of life and limits treatment; hair loss is universal.9,10
CONTEXT
Key Objective
Potential benefits make oral administration of paclitaxel appealing, including home administration, lack of need for intravenous (IV) access, and as oral paclitaxel (oPac) does not contain Cremophor EL, lack of hypersensitivity reactions or need for corticosteroid and antihistamine prophylaxis.
Knowledge Generated
Overall tumor response in the intent-to-treat population and in clinically important subgroups was generally consistent and favored oPac plus encequidar over IV paclitaxel by at least 10% in most clinically important subgroups. Careful patient selection, use of growth factors, and dose reductions allow successful management of neutropenia.
Relevance
The study demonstrates that oPac can provide an alternative treatment option to every 3-week paclitaxel with lower rates and severity of neuropathy for patients with advanced or metastatic breast cancer.
Potential benefits make oral administration of paclitaxel appealing, including home administration, lack of need for IV access, and as oral paclitaxel (oPac) does not contain Cremophor EL, lack of hypersensitivity reactions or need for corticosteroid and antihistamine prophylaxis.11 Paclitaxel has low oral bioavailability because of active excretion by the P-glycoprotein pump, which is highly expressed on the luminal surface of enterocytes.12 Coadministration of oPac with encequidar (E), a minimally absorbed, highly specific, and potent P-glycoprotein pump inhibitor, facilitates absorption of orally administered paclitaxel. A randomized cross-over study demonstrated that oPac 205 mg/m2 plus 15 mg E methanesulfonate monohydrate (oPac + E) once daily for 3 days resulted in systemic exposure similar to a single dose of IVpac 80 mg/m2 with peak concentrations approximately 15% of IVpac.13 A 16-week phase II study of oPac + E once daily for 3 consecutive days per week in 28 women with previously treated metastatic breast cancer (mBC) reported a 42% best response rate.14
This phase III trial evaluated the efficacy and safety of oPac + E once daily for 3 consecutive days per week compared with IVpac at the approved dose and schedule, 175 mg/m2 once every 3 weeks in patients with mBC.
METHODS
Patients
Eligible patients were postmenopausal women at least age 18 years with histologically or cytologically confirmed mBC measurable radiographically using RECIST version 1.1. Initial eligibility requirements included adequate hematopoietic and kidney function, Eastern Cooperative Oncology Group performance status 0-1, total bilirubin ≤ 1.5 mg/dL (≤ 2.0 mg/dL with liver metastasis), and ALT/AST ≤ 3 times upper limit of normal (ULN) and ≤ 5 × ULN with liver metastasis. The protocol was successively amended to tighten inclusion criteria around allowable baseline liver enzyme and bilirubin elevation. Final eligibility requirements were total bilirubin within normal limits, ALT/AST < 3 times ULN, and gamma-glutamyltransferase (GGT) < five times ULN. Patients with central nervous system metastases or who received prior taxane treatment in any setting within 12 months of study entry were excluded. Final eligibility criteria are given in the Data Supplement (online only).
Study Design
Patients were randomly assigned 2:1 to oPac + E (205 mg/m2 oPac as 30 mg liquid-filled capsules plus 15 mg E methanesulfonate monohydrate equivalent to 12.9 mg free base) for 3 consecutive days per week or IVpac 175 mg/m2 once every 3 weeks, without stratification. oPac + E was administered under fasting conditions, with E 1 hour before oPac, followed by a 4-hour post-treatment fast. Prophylactic antiemetics were not allowed for oPac + E patients; however, the protocol was amended after approximately half the patients were enrolled to allow prophylactic antiemetics; steroids or H-1 receptor antagonists were not allowed. IVpac patients received premedication with corticosteroids and antihistamines per the standard of care. Patients were evaluated in the clinic every 3 weeks. Imaging assessments were obtained at baseline and weeks 10, 16, and 19. If response was observed at week 19, a confirmatory scan was obtained on week 22. Scans were evaluated by blinded independent central radiology review (BICR; Intrinsic Imaging, LLC, Bolton, MA). Each scan was evaluated by two radiologists who independently selected target lesions and evaluated tumor response according to RECIST 1.1. If the two readings were discordant (response v nonresponse), an independent adjudicator selected the reading used for analysis. Laboratory tests were obtained within 24 hours before day 1 for the first 4 weeks and 2 days before day 1 thereafter; hematology and adverse events were monitored weekly. Two dose reductions for oPac + E were allowed (165 mg/m2 and 130 mg/m2). Dose reduction criteria are given in the Data Supplement. Assigned treatment continued until radiologic or clinical disease progression, toxicity requiring more than two dose reductions, or withdrawal.
The Protocol (online only) was approved by regulatory agencies of participating countries, ethics committees, and all patients provided written informed consent. The study was designed by the sponsor (Athenex, Inc) and monitored by an independent data and safety monitoring board.
Statistical Analysis
The primary efficacy end point was the confirmed tumor response defined as response on two consecutive imaging evaluations. Secondary efficacy end points were duration of response, progression-free survival (PFS), and overall survival (OS). The study was powered to detect a 15% difference (P = .05) for the primary efficacy end point with 80% power (chi-square test) assuming a confirmed response rate of 20% with IVpac. The planned sample size was 360 evaluable patients. Two interim analyses were conducted of the primary end point after 90 and 180 evaluable patients either discontinued or completed 18-21 weeks of treatment (P value adjustment .001 and .004, respectively). At the final analysis, an approximate difference of 10% or more favoring oPac + E would achieve a P value of .045 (two-tailed).
Primary analyses were performed according to intent-to-treat (ITT). Sensitivity analyses were performed on a prespecified population (modified intent-to-treat [mITT] patients with evaluable baseline target lesions by BICR who received at least seven of nine doses of oPac + E [78% of first cycle] or one dose of IVpac). Radiologic response was evaluated in clinically relevant subgroups. Kaplan-Meier estimates of time-to-event end points were calculated for PFS and OS. The Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95.5% CIs. Log-rank tests were used for descriptive comparison between the two groups. Safety was evaluated in all patients who received treatment.
RESULTS
Study Population
From December 2015 to February 2019, 402 patients were randomly assigned at 41 centers in 10 countries in Central/South America and the Caribbean (Fig 1). All patients were included in the ITT analysis of the primary efficacy end point. The safety population included 399 patients who received study treatment; 360 patients were included in the mITT population.
FIG 1.

Oraxol study CONSORT diagram. IV, intravenous; oPac + E, oral paclitaxel plus encequidar.
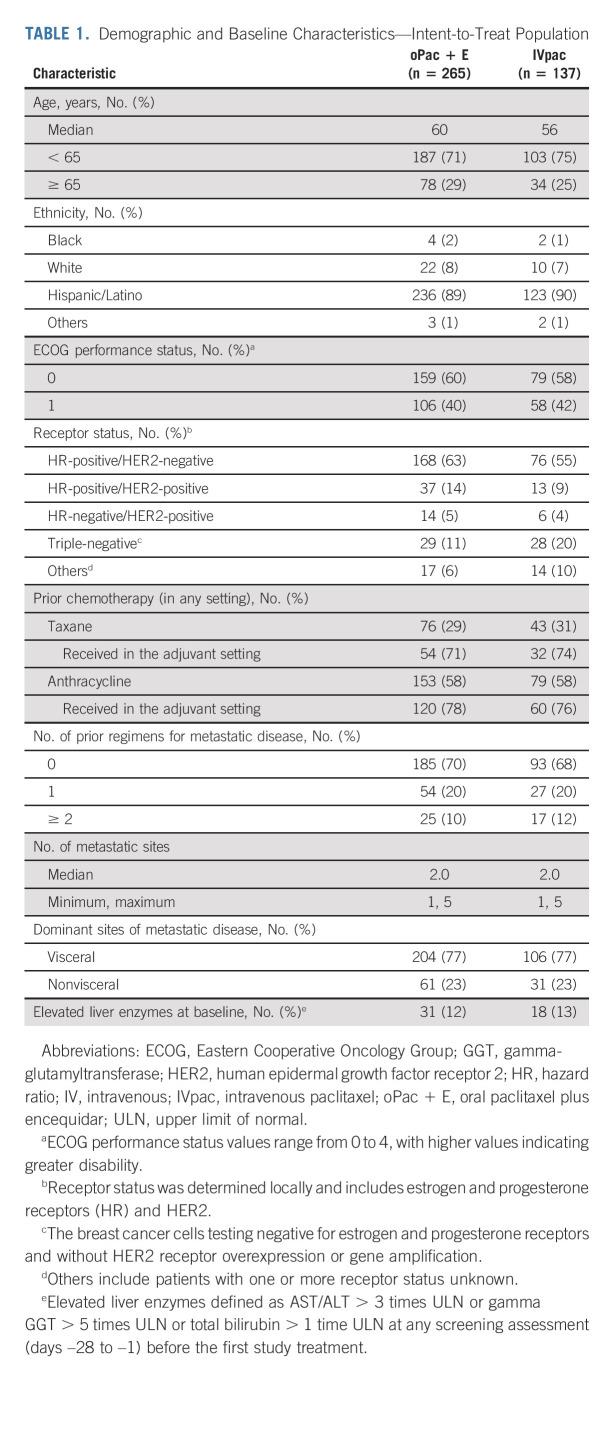
Demographic and baseline disease characteristics and prior oncology therapy were balanced (Table 1; additional details are provided in the Data Supplement).
TABLE 1.
Demographic and Baseline Characteristics—Intent-to-Treat Population
Efficacy
The database was locked (July 2019) for the primary end point after the final patients reached the week 19 evaluation. Secondary efficacy end points (PFS and OS) and safety are presented as of September 2020, approximately 14 months after the database lock for the primary efficacy end point.
The analysis of the confirmed tumor response rate by BICR showed a statistically significant difference favoring oPac + E (36%) versus IVpac (23%; P = .011) in the ITT population (Table 2). In the mITT population, confirmed response rates also favored oPac + E (40%) versus IVpac (26%; P = .005). Tumor response in clinically important subgroups in the ITT population was generally consistent and favored oPac + E over IVpac by at least 10% in most subgroups (Fig 2).
TABLE 2.
Confirmed Tumor Response Determined by Blinded Central Radiology Review
FIG 2.
Confirmed tumor response in clinically relevant subgroups. Others category includes patients with receptor status unknown for one or more receptors.
At the data cutoff of September 2020, seven oPac + E patients and one IVpac patient remained on treatment. The median duration of response was 36 (range, 6-111+) weeks and 33 (range, 4-84+) weeks for oPac + E and IVpac, respectively.
The median PFS favored oPac + E (8.4 months v 7.4 months; HR, 0.768; 95.5% CI, 0.584 to 1.01; P = .046) in the ITT population (Fig 3) and in the mITT population (8.4 months v 7.4 months; HR, 0.739; 95.5% CI, 0.561 to 0.974; P = .023).
FIG 3.
Kaplan-Meier curve for (A) PFS and (B) OS. (A) oPac + E: median estimate 8.4, range 0-35.7, censored summary 125 of 265 (47%); IVpac: median estimate 7.4, range 0-33.1, censored summary 49 of 137 (36%); HR (95.5% CI) 0.768 (0.584 to 1.01), P = .046. (B) oPac + E: median estimate 22.7, range 0.3-49.4, event summary 154 of 265 (58%), censored summary 111 of 265 (42%), alive 107 of 265 (40%), and discontinued 4 of 265 (2%); IVpac: median estimate 16.5, range 0.3-45.3, event summary 89 of 137 (65%), censored summary 48 of 137 (35%), alive 46 of 137 (34%), and discontinued 2 of 137 (1%); HR (95.5% CI) 0.794 (0.607 to 1.037). + = censored; HR estimated with the Cox proportional hazards model at a significance level of 0.045. HR, hazard ratio; IV, intravenous; IVpac, intravenous paclitaxel; oPac + E, oral paclitaxel plus encequidar; OS, overall survival; PFS, progression-free survival.
The median OS in the ITT population was 22.7 months with oPac + E versus 16.5 months with IVpac (HR, 0.794; 95.5% CI, 0.607 to 1.037; P = .082), with 58% (154 of 265) and 65% (89 of 137) of survival events, respectively. In the mITT population, median OS favored oPac + E versus IVpac (23.3 months v 16.3 months; HR, 0.735; 95.5% CI, 0.556 to 0.972; P = .026). PFS and OS at the time of the primary efficacy analysis are presented in the Data Supplement.
Safety
Although the safety profile of oPac + E was generally consistent with the known toxicity profile of IVpac, important differences were seen. The incidence of grade 3 and 4 adverse reactions was similar with both treatments (55% oPac + E v 53% IVpac; Table 3). The mean dose intensity was 84% with oPac + E and 95% with IVpac. Dose reductions, primarily because of neutropenic events and elevated GGT for oPac + E and neutropenia and peripheral neuropathy for IVpac, were required for 41% of oPac + E and 26% of IVpac patients. Discontinuations during the first 10 weeks of treatment occurred in 26% of oPac + E and 17% of IVpac patients primarily for adverse events and disease progression, respectively. The incidence of grade ≥ 2 neuropathy was approximately four-fold higher with IVpac (31%) than oPac + E (8%), with grade 3 neuropathy in 15% (IVpac) versus 2% (oPac + E). The higher incidence and severity of neuropathy with IVpac were reflected in higher rates of pregabalin and gabapentin use (30% and 10%, respectively) versus oPac + E (10% and 2%, respectively). Neuropathy also developed more rapidly with IVpac, with cumulative risk > 50% by week 8, reaching 75% versus 30% at 1 year with IVpac and oPac + E, respectively. This difference was also observed for risk of grade ≥ 2 neuropathy with IVpac where events continued to rise with the increasing duration of therapy, reaching 45% at 1 year. By contrast, oPac + E patients had a slow rise in risk, plateauing at approximately 12% at 1 year. Overall, 8% of IVpac patients and no oPac + E patients discontinued treatment because of neuropathy (Data Supplement). Complete alopecia was less frequent with oPac + E compared with IVpac (grade 2: 29% v 48%, respectively).
TABLE 3.
Adverse Reactions (≥ 10% all grades or ≥ 2% grades 3 or 4)—Safety Population

The overall incidence of GI toxicity was higher with oPac + E compared with IVpac, including grade ≥ 3 nausea (3% v < 1%), vomiting (4% v < 1%), and diarrhea (5% v < 1%). The initial protocol did not allow prophylactic antiemetic therapy for oPac + E patients. After approximately 50% of patients were enrolled, prophylactic antiemetics (primarily 5-hydroxytryptamine-3 antagonists) and early antidiarrheal therapy were encouraged for oPac + E patients, reducing grade ≥ 3 GI toxicity by approximately half (Data Supplement).
The incidence of grade ≥ 3 neutropenia was similar with both treatments (36% oPac + E v 32% IVpac); however, grade 4 neutropenia was more common with oPac + E (15%) than with IVpac (9%). Growth factors were used as prophylaxis or management of neutropenia for 40% and 29% of oPac + E and IVpac patients, respectively. Treatment discontinuation because of neutropenia was 7% for oPac + E and 1% for IVpac.
The incidence of on-treatment deaths was similar with both treatments (8% oPac + E v 9% IVpac) although the incidence of deaths because of infectious complications and considered related to treatment (9 [3%] oPac + E v 0 [0%] IVpac) was higher for oPac + E. The primary cause of treatment-related death was septic shock/sepsis in five cases, and one case each of multisystem organ failure, anemia, febrile neutropenia, and soft tissue infection. Clinical data review demonstrated that events in six of the nine patients occurred within the first 10 weeks of treatment and six of nine had elevated hepatic enzymes or serum bilirubin at baseline. In response to this observation, inclusion criteria for liver function tests and bilirubin were progressively tightened.
Post hoc, logistic regression analyses were conducted on oPac + E patients to examine the influence of demographics, prior treatment, and liver function on the risk of grade 4 or 5 toxicities during the first 10 weeks of therapy (data not shown). Multivariate analyses showed that increasing bilirubin and GGT and decreases in albumin were associated with increased risk for these early serious toxicities.
DISCUSSION
oPac + E is a novel combination of oPac with the P-glycoprotein inhibitor E, which is highly selective for P-glycoprotein and does not inhibit drug-metabolizing enzymes. It has low bioavailability, and thus, its pharmacology is limited to the GI tract where it increases the bioavailability of P-glycoprotein substrates, such as orally administered paclitaxel. This study was a phase III trial comparing the efficacy and safety of oPac + E in mBC versus IV paclitaxel at the labeled dose and regimen.
Radiologic end points were assessed by BICR located in the United States to minimize site bias and intersite variability in scan interpretation. Treatment with oPac + E administered 3 consecutive days weekly demonstrated a superior confirmed tumor response compared with every 3-week IVpac, with supporting trends in PFS and OS.
The toxicity of IV paclitaxel is well characterized. Hematologic toxicity, neuropathy, and alopecia are common, and risk of hypersensitivity-type reactions because of the solubilizing agent Cremophor and peripheral neuropathy are treatment-limiting toxicities.2 IV nab-paclitaxel is formulated without Cremophor; treatment-limiting toxicities include neutropenia and peripheral neuropathy.15
This study was conducted to support oPac + E registration. The comparator chosen was the dose and schedule of IV paclitaxel approved for treatment of mBC by US Food and Drug Administration and health authorities in the European Union and rest of the world, and every 3-week dosing is more feasible in many parts of the world. Although paclitaxel 80 mg/m2 once weekly is frequently used to treat breast cancer, this regimen has not been approved by health authorities. In addition, the study was conducted outside the United States where the care of patients with mBC may be different, particularly for those who had actionable markers and did not necessarily receive targeted therapy before or during the trial.
Weekly IV paclitaxel demonstrated improved breast cancer outcomes in clinical trials compared with every 3-week dosing; however, the incidence of severe peripheral neuropathy was higher.4,5 In addition, there is variation in the dosing schedule, with 80 mg/m2 given once weekly or 3 of 4 weeks to manage the high rate of peripheral neuropathy.
Results with weekly oPac + E were consistent with studies of weekly IV paclitaxel in mBC with respect to increased tumor response; however, oPac + E was associated with a lower incidence and severity of neuropathy. In this trial, the incidence of grade 3 neuropathy with every 3-week IVpac was consistent with previous reports using this dose and schedule.2,4
Compared with oPac + E, the incidence and severity of neuropathy were greater with IVpac, and onset occurred rapidly. We hypothesize that this is due to the higher peak paclitaxel plasma concentrations after IV administration. Treatment with oPac + E resulted in meaningfully lower rates and severity of peripheral neuropathy and other musculoskeletal pain–related symptoms compared with IVpac. Importantly, among those treated with oPac + E, only 2% had grade 3 peripheral neuropathy compared with 15% with IVpac. In total, 8% of IVpac patients discontinued therapy because of peripheral neuropathy; no oPac + E patients discontinued treatment because of peripheral neuropathy. Interestingly, the rate of myalgia and arthralgia was lower in patients treated with oPac + E, as was the incidence of grade 2 alopecia.
GI toxicity, primarily nausea, vomiting, or diarrhea, was more frequent with oPac + E. Antiemetic prophylaxis was not allowed for oPac + E patients because of a protocol amendment instituted after approximately 50% of patients were enrolled. Prophylactic use of antiemetics and early management of diarrhea decreased both the overall incidence and severity of nausea, vomiting, and diarrhea observed with oPac + E. Premedication with antihistamines or steroids is not required with oPac + E, and hypersensitivity-type reactions were not observed. All IVpac patients received premedication with high-dose dexamethasone and antihistamines, both of which have antiemetic activity; these drugs were not administered in the oPac + E arm. Although the incidence of grade > 3 neutropenia was similar between the two arms, oPac + E was associated with a greater incidence of grade 4 neutropenia, resulting in more infections, use of growth factors, and treatment-related deaths primarily in the first 10 weeks of treatment.
Subgroup analyses conducted to assess safety profiles demonstrated that patients with mild elevations of hepatic enzymes in both treatment arms had a higher frequency of early, serious adverse events, particularly hematologic toxicity and serious infections. In response to the emerging adverse events, a series of amendments were used to progressively tighten inclusion criteria for enzyme elevation. As paclitaxel is metabolized by cytochrome P450 2C8/9 and CYP3A4 in the liver, it is probable that abnormal hepatic function has a greater influence on orally administered paclitaxel, where first-pass metabolism by the liver may play a role. Dedicated studies to define the pharmacokinetics of orally administered paclitaxel in patients with varying degrees of hepatic dysfunction have not been conducted, and the regimen for patients with elevation of hepatic enzymes or bilirubin remains to be refined. Careful patient selection and close monitoring for early toxicity are warranted in patients with elevated hepatic enzymes or hepatic dysfunction. Treatment compliance was > 95%; dose reductions and treatment interruptions were successful in managing most other oPac + E toxicities. Per protocol, the management of unacceptable toxicities (primarily treatment-related hematologic toxicity) required dose interruption until resolution of the event, followed by restarting oPac + E at a lower paclitaxel dose, with the option for granulocyte colony-stimulating factor support for hematologic toxicity. Adverse events leading to dose interruption or reduction with both treatments were mainly due to neutropenia.
At the time of the trial initiation, the effect of food on the bioavailability of oPac was not known, and oPac + E was administered under fasting conditions. The requirement for fasting is being evaluated in an ongoing study of the effect of food, and current clinical trials use a 2-hour fast after oPac + E administration after a light breakfast.
In conclusion, oral administration of oPac + E achieved higher confirmed tumor response rates in patients with mBC compared with 3 weekly IVpac, supported by trends in PFS and OS. GI toxicity was more frequent with oPac + E and should be managed by the use of prophylactic and rescue antiemetics and antidiarrheal therapy. Neurotoxicity, a major treatment-limiting toxicity, of IV paclitaxel was less frequent and less severe with oPac + E compared with IVpac, as was alopecia. Hypersensitivity-type reactions were not observed, and prophylaxis for these events is not required.
Patients with elevated liver enzymes, serum bilirubin, or low serum albumin at study entry were at increased risk of early high-grade neutropenia and infectious complications, which were in some cases fatal. Careful patient selection, use of growth factors, and dose reductions along with close monitoring of patients at increased risk is warranted. Further studies to optimize dosing in patients with hepatic dysfunction are warranted. An ongoing study is comparing oPac + E with weekly paclitaxel as neoadjuvant therapy (ClinicalTrials.gov identifier: NCT01042379).
ACKNOWLEDGMENT
Athenex, Inc would like to thank the following individuals for assistance: Johnson Y.N. Lau, MBBS, MD, FRCP for trial inception and support; John Goldfinch, Taryn Moore, Dr Carlos Caparros, Rosario Briones, and Sandra Moro for the conduct and monitoring of the trial; and Dr Harold Amkraut for contributions to the trial design and for statistical guidance. Medical writing assistance, under the direction of the authors, was provided by Dr Frances Martino of FPM Communications and was funded by Athenex, Inc.
Hope S. Rugo
Honoraria: Puma Biotechnology, Mylan, Samsung Bioepis, Chugai Pharma, Blueprint Medicines
Consulting or Advisory Role: Napo Pharmaceuticals
Research Funding: OBI Pharma (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Genentech (Inst), Merck (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Sermonix Pharmaceuticals (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Ayala Pharmaceuticals (Inst), Astellas Pharma (Inst), Seattle Genetics (Inst), MacroGenics (Inst), Boehringer Ingelheim (Inst), Polyphor (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/183398
Rosa H. Vasallo
Research Funding: Roche
Suyapa Bejarano
Research Funding: MSD Oncology, Kinex
Travel, Accommodations, Expenses: Pfizer, Asofarma
Julio R. Ramírez
Research Funding: MSD Oncology, Athenex
Luis Fein
Consulting or Advisory Role: Novartis, Pfizer, AstraZeneca/Merck
Research Funding: AstraZeneca (Inst), MSD Oncology (Inst), Novartis (Inst)
Ruben D. Kowalyszyn
Consulting or Advisory Role: BMS Argentina, MSD Oncology, Astellas Pharma, Merck Serono
Speakers' Bureau: BMS Argentina, Novartis
Research Funding: BMS, MSD Oncology, Novartis, Roche, Astellas Pharma, Lilly, Gemabiotech, Nektar, Poliphor, AstraZeneca
E. Douglas Kramer
Employment: Athenex
Stock and Other Ownership Interests: Athenex, DemeRx
Patents, Royalties, Other Intellectual Property: Athenex
Hui Wang
Employment: Athenex
Leadership: Athenex
Stock and Other Ownership Interests: Athenex
Min-Fun R. Kwan
Employment: Athenex
Leadership: Athenex
Stock and Other Ownership Interests: Athenex, Merck/Schering Plow, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Multiple patents held as an employee of Athenex Inc, no royalties from any
Travel, Accommodations, Expenses: Athenex
David L. Cutler
Employment: Athenex
Stock and Other Ownership Interests: Merck Sharp & Dohme, Athenex
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at San Antonio Breast Cancer Symposium, December 10-14, 2019, San Antonio, TX and San Antonio Breast Cancer Symposium, December 8-11, 2020, San Antonio, TX.
SUPPORT
Supported by Athenex, Inc.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Hope S. Rugo, Gerardo A. Umanzor, E. Douglas Kramer, Min-Fun R. Kwan, David L. Cutler
Provision of study materials or patients: Gerardo A. Umanzor, Francisco J. Barrios, Rosa H. Vasallo, Marco A. Chivalan, Suyapa Bejarano, Luis Fein, Ruben D. Kowalyszyn
Collection and assembly of data: Gerardo A. Umanzor, Francisco J. Barrios, Rosa H. Vasallo, Marco A. Chivalan, Suyapa Bejarano, Julio R. Ramírez, Luis Fein, Ruben D. Kowalyszyn, Hui Wang
Data analysis and interpretation: Hope S. Rugo, E. Douglas Kramer, Hui Wang, Min-Fun R. Kwan, David L. Cutler
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Open-Label, Randomized, Multicenter, Phase III Study Comparing Oral Paclitaxel Plus Encequidar Versus Intravenous Paclitaxel in Patients With Metastatic Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Hope S. Rugo
Honoraria: Puma Biotechnology, Mylan, Samsung Bioepis, Chugai Pharma, Blueprint Medicines
Consulting or Advisory Role: Napo Pharmaceuticals
Research Funding: OBI Pharma (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Genentech (Inst), Merck (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Sermonix Pharmaceuticals (Inst), AstraZeneca (Inst), Gilead Sciences (Inst), Ayala Pharmaceuticals (Inst), Astellas Pharma (Inst), Seattle Genetics (Inst), MacroGenics (Inst), Boehringer Ingelheim (Inst), Polyphor (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/183398
Rosa H. Vasallo
Research Funding: Roche
Suyapa Bejarano
Research Funding: MSD Oncology, Kinex
Travel, Accommodations, Expenses: Pfizer, Asofarma
Julio R. Ramírez
Research Funding: MSD Oncology, Athenex
Luis Fein
Consulting or Advisory Role: Novartis, Pfizer, AstraZeneca/Merck
Research Funding: AstraZeneca (Inst), MSD Oncology (Inst), Novartis (Inst)
Ruben D. Kowalyszyn
Consulting or Advisory Role: BMS Argentina, MSD Oncology, Astellas Pharma, Merck Serono
Speakers' Bureau: BMS Argentina, Novartis
Research Funding: BMS, MSD Oncology, Novartis, Roche, Astellas Pharma, Lilly, Gemabiotech, Nektar, Poliphor, AstraZeneca
E. Douglas Kramer
Employment: Athenex
Stock and Other Ownership Interests: Athenex, DemeRx
Patents, Royalties, Other Intellectual Property: Athenex
Hui Wang
Employment: Athenex
Leadership: Athenex
Stock and Other Ownership Interests: Athenex
Min-Fun R. Kwan
Employment: Athenex
Leadership: Athenex
Stock and Other Ownership Interests: Athenex, Merck/Schering Plow, GlaxoSmithKline
Patents, Royalties, Other Intellectual Property: Multiple patents held as an employee of Athenex Inc, no royalties from any
Travel, Accommodations, Expenses: Athenex
David L. Cutler
Employment: Athenex
Stock and Other Ownership Interests: Merck Sharp & Dohme, Athenex
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gradishar WJ: Taxanes for the treatment of metastatic breast cancer. Breast Cancer (Auckl) 6:159-171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera E, Cianfrocca M: Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol 75:659-670, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panday VRN, Huizing MT, Ten Bokkel Huinink WW, et al. : Hypersensitivity reactions to the taxanes paclitaxel and docetaxel. Clin Drug Invest 14:418-427, 1997 [Google Scholar]
- 4.Seidman AD, Berry D, Cirrincione C, et al. : Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642-1649, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Sparano JA, Wang M, Martino S, et al. : Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663-1671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loprinzi CL, Reeves BN, Dakhil SR, et al. : Natural history of paclitaxel-associated acute pain syndrome: Prospective cohort study NCCTG N08C1. J Clin Oncol 29:1472-1478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandos H, Melnikow J, Rivera DR, et al. : Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst 110:djx162, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara Y, Mukai H, Saeki T, et al. : A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer 120:475-480, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodd MJ, Miaskowski C, Paul SM: Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 28:465-470, 2001 [PubMed] [Google Scholar]
- 10.Pachman DR, Barton DL, Swetz KM, et al. : Troublesome symptoms in cancer survivors: Fatigue, insomnia, neuropathy, and pain. J Clin Oncol 30:3687-3696, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Eek D, Krohe M, Mazar I, et al. : Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: A review of the literature. Patient Prefer Adherence 10:1609-1621, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparreboom A, van Asperen J, van Asperen J, et al. : Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci USA 94:2031-2035, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson C, Deva S, Bayston K, et al. : An international randomized cross-over bio-equivalence study of oral paclitaxel + HM30181 compared with weekly intravenous (IV) paclitaxel in patients with advanced solid tumours. Ann Oncol 30:v180-v181, 2019 [Google Scholar]
- 14.Dai M-S, Chao T-C, Chiu C-F, et al. : Oral paclitaxel in the treatment of metastatic breast cancer (MBC) patients. J Clin Oncol 37:1084-1084, 2019 [Google Scholar]
- 15.Gradishar WJ, Tjulandin S, Davidson N, et al. : Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23:7794-7803, 2005 [DOI] [PubMed] [Google Scholar]