PURPOSE
In VELIA trial, veliparib combined with carboplatin-paclitaxel, followed by maintenance (veliparib-throughout) was associated with improved progression-free survival (PFS) compared with carboplatin-paclitaxel alone in patients with high-grade ovarian carcinomas. We explored the prognostic value of the modeled cancer antigen (CA)-125 elimination rate constant K (KELIM), which is known to be an indicator of the intrinsic tumor chemosensitivity (the faster the rate of CA-125 decline, the higher the KELIM and the higher the chemosensitivity), and its association with benefit from veliparib.
PATIENTS AND METHODS
Individual KELIM values were estimated from longitudinal CA-125 kinetics. Patients were categorized as having favorable (≥ median) or unfavorable (< median) KELIM. The prognostic value of KELIM for veliparib-related PFS benefit was explored in cohorts treated with primary or interval debulking surgery, according to the surgery completeness, the disease progression risk group, and the homologous recombination (HR) status (BRCA mutation, HR deficiency [HRD], or HR proficiency [HRP]).
RESULTS
The data from 854 of 1,140 enrolled patients were analyzed (primary debulking surgery, n = 700; interval debulking surgery, n = 154). Increasing KELIM values were associated with higher benefit from veliparib in HRD cancer, as were decreasing KELIM values in HRP cancer. The highest PFS benefit from veliparib was observed in patients with both favorable KELIM and BRCA mutation (hazard ratio, 0.28; 95% CI, 0.13 to 0.61) or BRCA wild-type HRD cancer (hazard ratio, 0.43; 95% CI, 0.26 to 0.70), consistent with the association between poly (adenosine diphosphate-ribose) polymerase inhibitor efficacy and platinum sensitivity. In contrast, seventy-four percent of patients with a BRCA mutation and unfavorable KELIM progressed within 18 months while on veliparib. The patients with HRP cancer and unfavorable KELIM might have benefited from the veliparib chemosensitizing effect.
CONCLUSION
In addition to HRD/BRCA status, the tumor primary chemosensitivity observed during the first-line chemotherapy might be another complementary determinant of poly (adenosine diphosphate-ribose) polymerase inhibitor efficacy.
INTRODUCTION
Platinum-based chemotherapy followed by maintenance treatment with bevacizumab and/or poly (adenosine diphosphate-ribose) polymerase inhibitors (PARPis) is the standard first-line systemic treatment for patients with high-grade carcinomas of the ovary, fallopian tube, or peritoneum (high-grade ovarian carcinomas [HGOC]).1-6 Which patients truly benefit from PARPi maintenance remains controversial. Although BRCA mutation and, to a lesser extent, other measures of homologous recombination deficiency (HRD) are undoubtedly associated with PARPi activity,7-10 data suggest that the efficacy of these drugs cannot be unequivocally explained by these biomarkers, and there are still knowledge gaps in the understanding of determinants of PARPi efficacy. Among potential predictive biomarkers, sensitivity to platinum-based chemotherapy is very relevant. Strong relationships between platinum sensitivity and PARPi efficacy have been reported in several studies in patients treated in first-line or recurrent settings.10-12
CONTEXT
Key Objective
To understand the role of the tumor primary chemosensitivity (assessed by the modeled cancer antigen-125 kinetic parameter elimination rate constant K, KELIM) regarding the efficacy of the maintenance with the poly (adenosine diphosphate-ribose) polymerase inhibitor veliparib, with respect to the homologous recombination (HR) status, in patients with advanced ovarian carcinoma.
Knowledge Generated
The analysis of VELIA trial revealed that higher tumor chemosensitivity was associated with higher efficacy of veliparib in terms of progression-free survival in patients with HR-deficient cancer (with/without BRCA mutation). Conversely, poor chemosensitivity was associated with limited efficacy of veliparib in patients with BRCA mutation or BRCA wild-type HR deficiency disease. In patients with HR-proficient cancer characterized by poor chemosensitivity, veliparib might have induced the chemosensitizing effect.
Relevance
The tumor primary chemosensitivity, assessable by elimination rate constant K score (KELIM calculable online), may be a complementary parameter to integrate with BRCA mutation and HR status for understanding the prognosis and the benefit to expect from maintenance with the poly (adenosine diphosphate-ribose) polymerase inhibitor veliparib.
Recent publications on more than 12,000 patients enrolled in five randomized trials, along with The Netherlands Cancer Registry and the Gynecology Cancer InterGroup meta-analysis data set, have indicated that cancer antigen (CA)-125 elimination rate constant K (KELIM), a modeled kinetic parameter on the basis of CA-125 measurements during the first 100 days of adjuvant or neoadjuvant chemotherapy, represents a reproducible indicator of tumor-intrinsic chemosensitivity in the first-line setting.13,14 KELIM exhibited a strong prognostic and predictive value for response to neoadjuvant chemotherapy, completeness of interval debulking surgery (IDS), risk of subsequent platinum-resistant relapse, progression-free survival (PFS), benefit from bevacizumab in the ICON-7 trial, and long-term survival in HGOC.15-18 These data suggest that the longitudinal kinetics of CA-125 during the first three cycles of chemotherapy, as captured by KELIM, may reflect an intrinsic property of cancer cells related to their sensitivity to chemotherapy, which is independent of the regimen received. Indeed, the analyses performed on ICON-7, AGO-OVAR 9, and CHIVA trials showed that the addition of third drug (chemotherapy or antiangiogenic drugs) to carboplatin-paclitaxel did not alter CA-125 KELIM values and that KELIM was prognostic and strongly related to overall survival regardless of whether patients received doublet or triplet regimens. Of note, KELIM was shown to be more accurate than the CA-125 Gynecology Cancer InterGroup response criterion for characterizing chemotherapy efficacy in the first-line setting.15,17,19-21
Veliparib is an oral PARPi that inhibits poly (adenosine diphosphate-ribose) polymerase (PARP) activity without substantial trapping of PARP onto DNA damage repair intermediates,22,23 thereby making veliparib more suitable for administration in combination with platinum-based chemotherapy.24 The efficacy of veliparib was assessed in combination with standard frontline platinum-based chemotherapy (concomitant-veliparib arm; dose reduced during combination therapy) or in the same regimen followed by veliparib continued as a monotherapy in the maintenance phase (veliparib-throughout arm) in the VELIA phase III trial.7 Compared with the chemotherapy-only (placebo) arm, the veliparib-throughout arm was associated with significantly improved PFS in patients with BRCA mutations, in those with high genomic instability score (GIS, considered a reflection of tumor HRD), and in the intent-to-treat population.7,25 Patients enrolled in VELIA trial were not selected on the basis of their response to platinum-based chemotherapy, thereby making it possible to investigate the association between the tumor chemosensitivity and the efficacy of this PARPi beyond homologous recombination (HR) status.
The present exploratory post hoc study was meant to confirm the prognostic value of KELIM as an indicator of intrinsic chemosensitivity and test it as a predictive marker in relation to veliparib efficacy.
PATIENTS AND METHODS
Patients and Treatments
The VELIA study is a three-arm phase III trial in which patients with newly diagnosed HGOC were randomly assigned in a 1:1:1 ratio to the control arm (arm 1: chemotherapy plus placebo, followed by placebo maintenance), veliparib-combination-only arm (arm 2: chemotherapy plus veliparib, followed by placebo maintenance), or veliparib-throughout arm (arm 3: chemotherapy plus veliparib, followed by veliparib maintenance) in women with newly diagnosed stage III or IV HGOC.7 The trial design mandated that CA-125 values would be measured at every cycle for assessing the prognostic/predictive value of CA-125 kinetics.
To be included in the present analysis, patients had to have at least three available values of CA-125 during the first 100 days of adjuvant chemotherapy after primary debulking surgery (PDS) or before the date of IDS. The following data were collected: age, somatic or germline BRCA mutation status (BRCA mutation or BRCA wild-type), HR status as determined by the Myriad BRACAnalysis CDx or myChoice HRD CDx assay (HRD: BRCA-mutated or GIS ≥ 33; or HR-proficient (HRP): BRCA wild-type and GIS < 33), disease stage (III or IV), completeness of surgery on the basis of postoperative lesions assessed by the surgeon (complete surgery with no residual lesions or incomplete surgery with microscopic residual lesions between 0 and 1 cm or with macroscopic residual lesions > 1 cm).
Estimation of KELIM for Patients Enrolled in the VELIA Trial
In a collaboration with the Lyon University team (EA 3738, CICLY, France), the development of the KELIM model was conducted by the AbbVie team based on previous publications,15,17 with the following modifications: patients in the VELIA study were initially pooled together to model individual KELIM values, on the basis of the CA-125 kinetics during the first 100 days of chemotherapy (starting on cycle 1 day 1). The patients were then separated into two subgroups according to timing of surgery (PDS or IDS). The median values of KELIM for each subgroup were then calculated to categorize the patient as having a high tumor primary chemosensitivity (favorable KELIM value ≥ median) or an poor tumor primary chemosensitivity (unfavorable KELIM value < median).
Statistical Analysis
The prognostic value of KELIM for PFS, along with the association between KELIM and veliparib efficacy, was assessed in patients treated in the veliparib-throughout arm (arm 3) and in the placebo arm (arm 1), using the Kaplan-Meier method and univariate and multivariate Cox proportional hazards models.
Patients were analyzed all together (whole population) and then separately in the PDS and IDS cohorts as a way of accounting for their different prognosis. In addition to KELIM, the other prognostic factors assessed in univariate and multivariate analyses were as follows: treatment arm (3 v 1), disease stage (III v IV), surgery outcome on the basis of postoperative lesions (no residual lesion, v microscopic residual lesion, v any other residual lesion), and HR status (BRCA mutation, BRCA wild-type HRD, and HRP). The final Cox survival models were obtained using backward selection.
Additional analyses were performed to further assess the interactions between KELIM and treatment arms regarding veliparib-related PFS benefit, along with the association between KELIM and veliparib activity across important prognostic subgroups: disease stage (stage III or stage IV), completeness of surgery on the basis of postoperative residual lesions; disease progression risk groups (low-risk disease: stage III disease with no or microscopic residual lesions after surgery; high-risk diseases: stage III disease with postoperative macroscopic residual lesions or stage IV diseases),26 and HRD/HRP/BRCA mutation status.
All survival analyses were implemented with a landmark time point set at 100 days after the start of neoadjuvant chemotherapy. As CA-125 was modeled from days 0 to 100, the patients who progressed during the first 100 days were excluded to avoid bias related to the links between early progression and CA-125 kinetics or radiological tumor responses.27
The hazard ratios were computed using the Cox proportional hazard model and with the 95% CI.
All analyses were performed using R program (Lucent technology, Murray Hill, NJ).28
RESULTS
Patient Characteristics
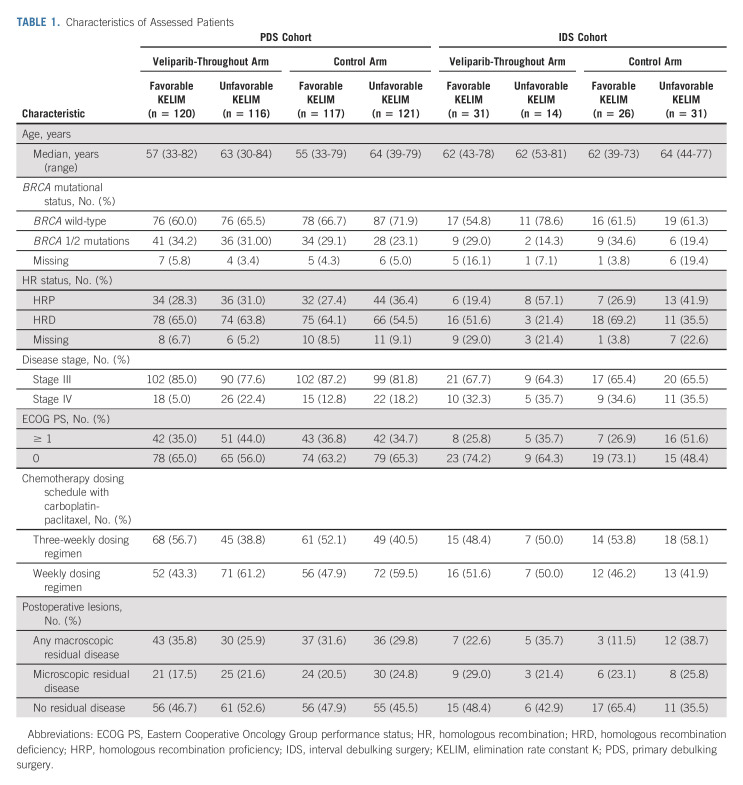
One thousand one hundred forty patients were enrolled in the VELIA study. Among 854 patients with ≥ 3 available measurements of CA-125 during the first three cycles of neoadjuvant or adjuvant chemotherapy, 700 patients (81.9%) received PDS and 154 patients (18.1%) received IDS (Data Supplement, online only and Table 1). The distribution of patients treated with the 3-weekly or the weekly carboplatin-paclitaxel regimens was well balanced between patients with favorable or unfavorable KELIM. The median number of CA-125 measurements per patient was four in the PDS cohort and three in the IDS cohort. Key demographic and clinical characteristics of the 854 patients are given in Table 1.
TABLE 1.
Characteristics of Assessed Patients
Model Adjustment and Qualification
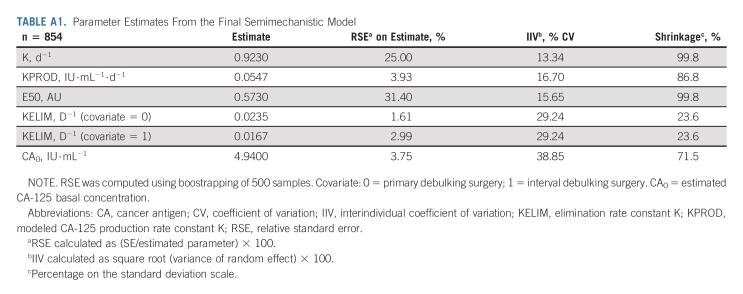
Typical parameter estimates, along with the qualification analyses from the final semimechanistic model, are presented in Appendix 1 (online only; Appendix Table A1, online only; and Data Supplement).
Characterization of KELIM in VELIA Trial
The median KELIM was 0.023 for all 854 patients, 0.017 for the IDS cohort, and 0.024 for the PDS cohort. Patients in the IDS cohort tended to have a less favorable KELIM than those in the PDS cohort, which was expected since these patients still had tumor in place. The distributions of KELIM values across treatment arms in the PDS and IDS are shown in the Data Supplement. Consistent with previous publications, KELIM did not differ significantly across treatment arms, confirming that KELIM could be assessed as an indicator of the primary tumor chemosensitivity. As expected, the percentages of BRCA mutations and HRD tumors tended to be higher in favorable KELIM groups, compared with those in unfavorable KELIM groups, regardless of the treatment arms and surgery types (Table 1 and Data Supplement).
Associations Between KELIM and PFS
Prognostic value of KELIM.
The median follow-up times were 28.6 and 28.2 months for the veliparib-throughout arm and the control arm, respectively.
In the whole population, KELIM status was a significant prognostic covariate in univariate (hazard ratio, 0.63; 95% CI, 0.51 to 0.79) and multivariate survival analyses (hazard ratio, 0.68; 95% CI, 0.56 to 0.85), together with the treatment arm and HR status (Data Supplement).
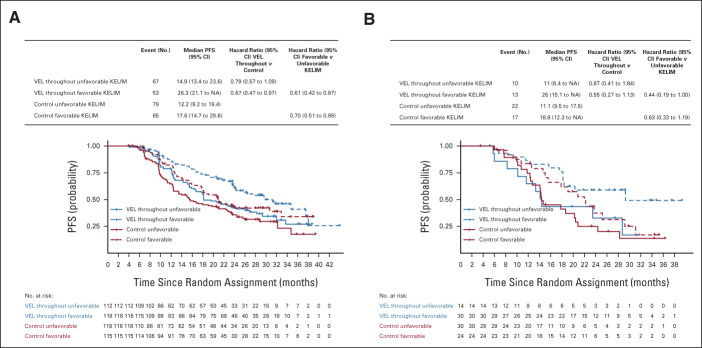
In the PDS cohort, the median PFS was longer in patients with favorable KELIM compared with those with unfavorable KELIM in both treatment arms, consistent with the previously reported prognostic value of KELIM (Fig 1). For example, the median PFS of patients treated in the veliparib-throughout arm was 29.6 months for those who had favorable KELIM and 18.2 months for those who had unfavorable KELIM (hazard ratio, 0.61; 95% CI, 0.42 to 0.87). In the control arm, the median PFS was 20.9 months for patients with favorable KELIM and 15.4 months for patients with unfavorable KELIM (hazard ratio, 0.69; 95% CI, 0.49 to 0.95; Fig 1). In multivariate analysis, KELIM status was a significant independent prognostic covariate (hazard ratio, 0.67; 95% CI, 0.52 to 0.85), along with the treatment arm, surgery outcomes on the basis of postoperative lesions, and HR status (Data Supplement).
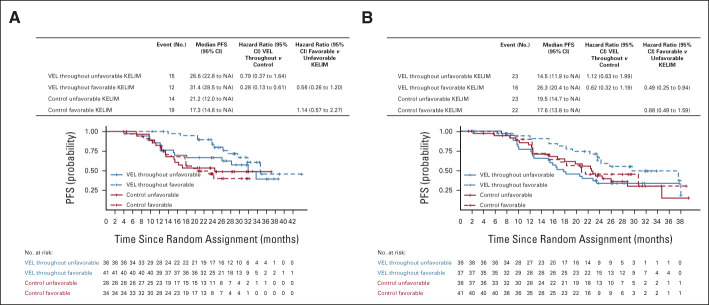
FIG 1.
PFS according to treatment arms (VEL-throughout v placebo [control]) and KELIM (favorable or unfavorable) in cohort of patients operated with (A) PDS and (B) neoadjuvant chemotherapy and IDS. IDS, interval debulking surgery; KELIM, elimination rate constant K; NA, not available; PDS, primary debulking surgery; PFS, progression-free survival; VEL, veliparib.
Similar data of the prognostic value of KELIM were observed in the IDS cohort, with larger CIs because of the lower number of patients (Fig 1 and Data Supplement).
Association between KELIM and benefit from veliparib.
The magnitude of the PFS difference between patients treated in the veliparib-throughout arm or in the placebo arm was higher in patients who had favorable KELIM compared with those who had unfavorable KELIM, thereby suggesting a potential predictive value of KELIM regarding the benefit from veliparib. In the PDS cohort, the PFS hazard ratio was 0.67 (95% CI, 0.47 to 0.97) for patients with favorable KELIM and 0.77 (95% CI, 0.56 to 1.06) for patients with unfavorable KELIM (Fig 1). Moreover, in the IDS cohort, the veliparib-throughout arm was associated with longer median PFS for patients with favorable KELIM only (29.3 v 20.8 months; hazard ratio, 0.54; 95% CI, 0.27 to 1.07; Fig 1). These data suggest a potential association between veliparib benefit and KELIM value.
Association between veliparib activity and KELIM, according to the completeness of surgery and disease progression risk group.
The outcomes of the analyses are presented in Appendix 1. A strong association was found between KELIM estimated during neoadjuvant chemotherapy before surgery and the completeness of subsequent IDS (Data Supplement and Table 1). Higher benefit from veliparib in patients with favorable KELIM compared with those with unfavorable KELIM was observed, regardless of the completeness of surgery or disease risk group (Data Supplement).
Association between veliparib activity and KELIM according to HR status.
The BRCA mutation and tumor HR status were available in 794 (93%) and 763 (89%) patients.
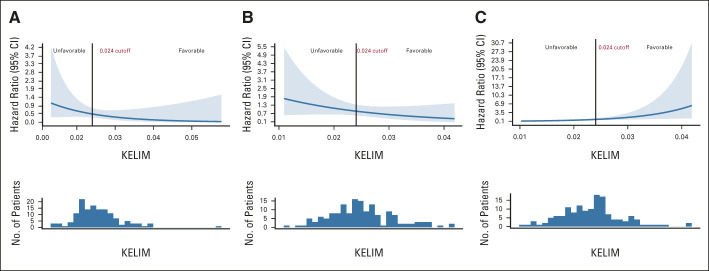
The generalized additive model with Cox proportional hazard ratios suggested different interactions between KELIM value and benefit from veliparib, depending on the HR status. In patients with BRCA mutation and BRCA wild-type HRD cancers, increasing KELIM value was associated with higher benefit from veliparib. However, in patients with HRP cancers, decreasing KELIM value seemed to be associated with higher benefit from veliparib (Fig 2).
FIG 2.
Generalized additive model with Cox proportional hazard ratios of PFS benefit in veliparib-throughout arm 3 versus placebo arm 1 according to KELIM in (A) patients with BRCA mutation, (B) BRCA wild-type HRD cancer, and (C) HRP cancer. The cutoff at 0.024 was the KELIM median value. HRD, homologous recombination deficiency; HRP, homologous recombination proficiency; KELIM, elimination rate constant K; PFS, progression-free survival.
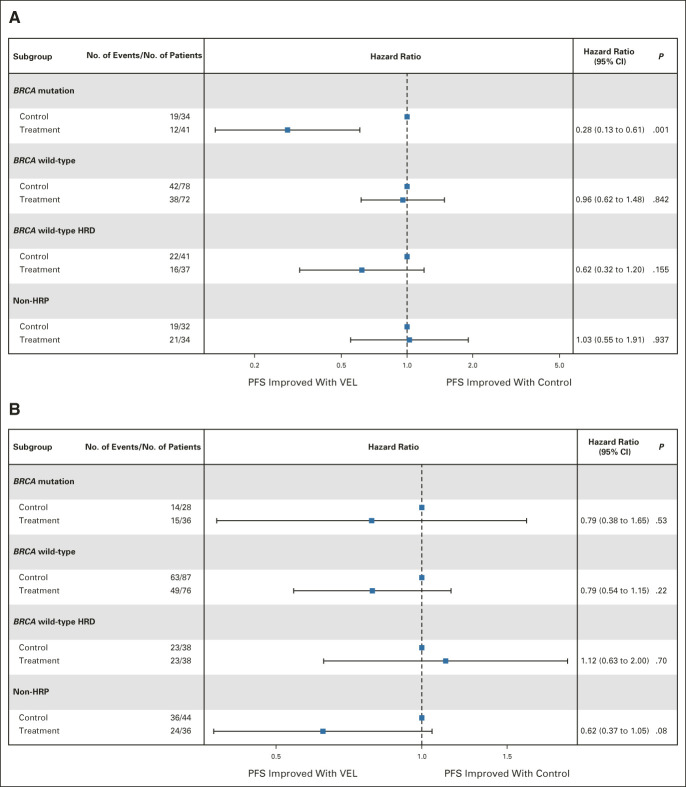
In patients treated with PDS, the subgroup of patients who derived the highest benefit from veliparib, in comparison with the placebo arm, were those with both favorable KELIM and either BRCA mutation (hazard ratio, 0.28; 95% CI, 0.13 to 0.61) or BRCA wild-type HRD cancers (hazard ratio, 0.43; 95% CI, 0.26 to 0.7), whereas those with HRP tumors had no PFS improvement (Figs 3A and 4). The interaction tests between the treatment arm and KELIM status were consistent with higher benefit from veliparib in patients with HRD cancer exhibiting favorable KELIM: BRCA mutation (n = 139 patients), hazard ratio, 0.45; 95% CI, 0.16 to 1.26; BRCA wild-type HRD cancer (n = 147 patients), hazard ratio, 0.54; 95% CI, 0.23 to 1.30; and HRD cancer (n = 286 patients), hazard ratio, 0.50; 95% CI, 0.26 to 0.96. By contrast, the patients with unfavorable KELIM derived no benefit from veliparib whether they were carrying BRCA mutation or had HRD cancer (Fig 3B). For example, 74% of patients with BRCA mutation and unfavorable KELIM treated with veliparib had short PFS < 18 months. The discriminative accuracy (receiver operating characteristic curve area under the curve) of KELIM (unfavorable v favorable) for identifying the patients likely to experience early progression < 18 months was 0.75 (95% CI, 0.63 to 0.86; sensitivity, 0.62 and specificity, 0.74 for a KELIM cutoff at 0.024; Data Supplement).
FIG 3.
Forest plots of benefit from veliparib-throughout versus placebo arms regarding PFS according to homologous recombination (HR) status in patients with (A) favorable KELIM or (B) unfavorable KELIM treated with primary debulking surgery. HRD, homologous recombination deficiency; HRP, homologous recombination proficiency; KELIM, elimination rate constant K; PFS, progression-free survival; VEL, veliparib.
FIG 4.
PFS according to KELIM (favorable or unfavorable) and treatment arms (VEL-throughout arm three v placebo [control] arms) in patients operated with PDS carrying tumors associated with (A) BRCA mutation or (B) BRCA wild-type HRD. HRD, homologous recombination deficiency; KELIM, elimination rate constant K; NA, not available; PDS, primary debulking surgery; PFS, progression-free survival; VEL, veliparib.
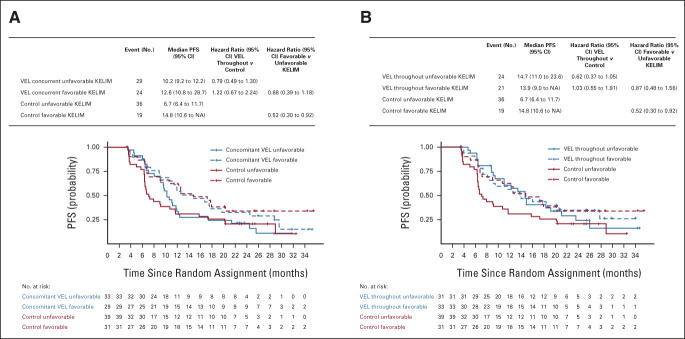
Consistent with the generalized additive model curve analysis (Fig 2), a nonsignificant PFS benefit with veliparib was observed in patients with HRP cancer characterized by unfavorable KELIM. Compared with patients treated with placebo arm 1, this PFS gain was temporary in patients treated with the concomitant-veliparib arm 2 (median PFS, 10.2 v 6.7 months; hazard ratio, 0.79; 95% CI, 0.49 to 1.30; Fig 5A) and maintained in patients treated with the veliparib-throughout arm (arm 3; median PFS, 14.7 v 6.7 months; hazard ratio, 0.62; 95% CI, 0.37 to 1.05; Figs 3B and 5B).
FIG 5.
PFS according to KELIM (favorable or unfavorable) and treatment arms ([A] VEL-concurrent arm 2 v placebo [control] arm 1 and [B] VEL-throughout arm 3 v placebo [control] arm 1) in patients operated with PDS carrying tumors associated with HRP. HRP, homologous recombination proficiency; KELIM, elimination rate constant K; NA, not available; PDS, primary debulking surgery; PFS, progression-free survival; VEL, veliparib.
In the IDS cohort, the low numbers of patients in the BRCA-mutated and HRD cancer subgroups did not allow us to perform meaningful analyses.
DISCUSSION
To our knowledge, this is the first study to explore the role of the tumor primary chemosensitivity, assessed by the modeled CA-125 kinetic parameter KELIM, in PARPi efficacy in newly diagnosed advanced HGOC.
The data confirmed our previous findings about the prognostic role of KELIM in both PDS and IDS cohorts, regardless of treatment arms. Consistent with the role of KELIM as an indicator of chemosensitivity, we found that favorable KELIM was associated with higher likelihood of complete surgery in the IDS cohort, suggesting that it could be factored into algorithms designed to optimize the timing of IDS.
We also found that favorable KELIM was associated with veliparib benefit in patients with BRCA mutation or BRCA wild-type HRD cancer. These outcomes suggesting a higher benefit from veliparib in patients with platinum-sensitive diseases are consistent with the literature, as shown with other PARPis.9-12 In contrast to SOLO-1, PAOLA-1, ATHENA, and PRIMA trials, where patients were selected on the basis of their platinum sensitivity, VELIA enrolled patients without prior assessment of their sensitivity to chemotherapy. The present study suggests that the PFS benefit from veliparib might have been higher if patients with HRD cancer had been selected for their platinum sensitivity. For example, the PFS relative benefit in the veliparib-throughout arm compared with the placebo arm in patients carrying BRCA mutation and favorable KELIM (hazard ratio, 0.28; 95% CI, 0.13 to 0.61) appears to be higher than those reported in patients who were not selected on KELIM in VELIA trial (hazard ratio, 0.44; 95% CI, 0.28 to 0.68). Conversely, lower benefit from veliparib was found in patients carrying BRCA mutation or with HRD tumor characterized by unfavorable KELIM. The large majority of patients with BRCA mutation and unfavorable KELIM experienced short PFS < 18 months, confirming that HR status is not the only determinant of veliparib efficacy. It indirectly suggests the complementary predictive role of the tumor chemosensitivity in addition to HR status and justifies the selection of patients on the basis of their response to platinum-based chemotherapy in the other phase III trials.
In patients with HRP cancer, a favorable KELIM was not associated with a higher benefit from veliparib. However, some patients carrying HRP cancer characterized by poor chemosensitivity (unfavorable KELIM) might have derived a temporary nonsignificant PFS benefit from the addition of veliparib to chemotherapy (arm 2) and a more sustained PFS benefit from veliparib-throughout (arm 3), potentially as a result of a chemosensitizing effect of veliparib. This hypothesis needs to be explored.
There are several limitations in the present study that should be considered. As all analyses were performed retrospectively, these findings could conceivably be confounded by unaccounted biases. Moreover, subgroup analyses were hindered by the low number of patients in each subgroup. For example, there were only 45 patients in the veliparib-throughout arm in the IDS cohort. Among them, a disproportionate of 31 (69%) patients were classified as having a favorable KELIM, whereas only 14 patients (31%) as unfavorable KELIM. If the BRCA mutation status of patients at the time of data collection had to be documented (mutated or wild-type), the differential BRCA1 or BRCA2 mutation status was not used for random assignment, thereby limiting the possibility of additional analyses.
Despite these limitations, the present exploratory study supports the concept that intrinsic chemosensitivity, assessed by KELIM, is a relevant complementary parameter to integrate with BRCA mutation status and HRD status for understanding the patient prognosis in the first-line setting and for identifying the patients who would receive maximum benefit from veliparib. For example, KELIM could be assessed in patients with the CA-125 values observed during the first three to four cycles of adjuvant/neoadjuvant chemotherapy, and veliparib would be associated with chemotherapy and then given as a maintenance treatment in patients with favorable KELIM and BRCA mutation or HRD cancer. Of note, KELIM of patients is easily calculable online (for both neoadjuvant chemotherapy29 and adjuvant chemotherapy30). In addition, KELIM might be of interest for identifying the patients who are more likely to benefit from other PARPis. KELIM is being prospectively assessed in the phase III trial NIVARNA-1 comparing the efficacy of niraparib with/without bevacizumab in patients operated with complete PDS (ClinicalTrials.gov identifier: NCT05183984).
APPENDIX 1.
Structure of the Semimechanistic Model of Elimination Rate Constant K
Treatment kinetics were described by a two-compartment model: central compartment (C1) receiving chemotherapy dosing (doses set to 1) and a transit compartment (C2) to describe the treatment lag-time effect. The cancer antigen (CA)-125 production inhibition induced by the treatment is expressed by an indirect effect model using an Emax (E50) relationship,
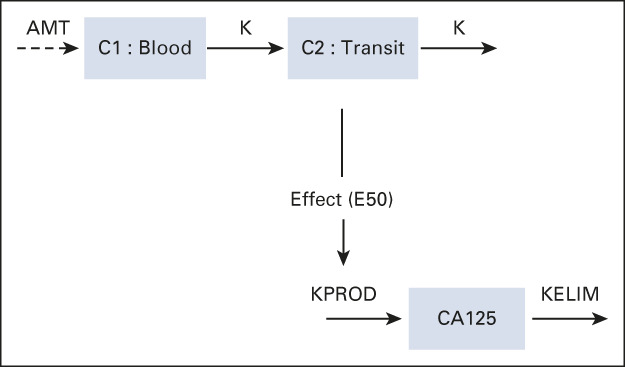
where K is the treatment kinetic rate constant (days−1), KPROD is the CA-125 tumor production rate (days−1), E50 is the concentration producing 50% of the maximum effect (AU), and elimination rate constant K (KELIM) is the CA-125 elimination rate (days−1).
Association Between Veliparib Activity and KELIM, According to the Completeness of Surgery and Disease Progression Risk Group
In the primary debulking surgery cohort, as expected, complete surgery was associated with better progression-free survival outcomes compared with incomplete surgery. The positive discriminatory impact of a favorable KELIM regarding the benefit from veliparib was especially marked in patients operated with complete primary debulking surgery (favorable KELIM: 34.7 in the veliparib-throughout arm v 32.9 months in the standard arm; hazard ratio, 0.83; 95% CI, 0.46 to 1.46; unfavorable KELIM: 19.5 months in the veliparib-throughout arm v 23.3 months in the standard arm; hazard ratio, 1.23; 95% CI, 0.76 to 2.00; Data Supplement). The same analyses were performed in the interval debulking surgery cohort, but the low number of patients reduced the power of the analyses. Nevertheless, a trend for higher benefit with veliparib was observed only among patients with favorable KELIM (Data Supplement).
Similar outcomes were found when patients were categorized according to the disease risk groups, with a trend for higher benefit from veliparib among patients with favorable KELIM compared with those with unfavorable KELIM (Data Supplement).
TABLE A1.
Parameter Estimates From the Final Semimechanistic Model
Benoit You
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Novartis, LEK, TESARO, Bayer, Amgen, Clovis Oncology, GlaxoSmithKline, ECS PROGASTRIN, Immunomedics, Daiichi Sankyo Europe GmbH, Myriad Genetics, MSD Oncology, Seattle Genetics
Research Funding: Merck Serono (Inst), Roche/Genentech (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, AstraZeneca, BMS, MSD Oncology, Bayer
Balakrishna Hosmane
Employment: AbbVie
Consulting or Advisory Role: AbbVie
Xin Huang
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Peter J. Ansell
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Minh H. Dinh
Employment: AbbVie
Leadership: AbbVie
Stock and Other Ownership Interests: AbbVie
Travel, Accommodations, Expenses: AbbVie
Katherine Bell-McGuinn
Employment: AbbVie
Stock and Other Ownership Interests: Lilly, AbbVie
Patents, Royalties, Other Intellectual Property: Author on patent for CD200R agent developed by Lilly
Xizhi Luo
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Gini F. Fleming
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Curio Science, Physicians' Education Resource
Consulting or Advisory Role: GlaxoSmithKline
Research Funding: Corcept Therapeutics (Inst), AbbVie (Inst), Iovance Biotherapeutics (Inst), Syros Pharmaceuticals (Inst), Sermonix Pharmaceuticals (Inst), Compugen (Inst), Plexxikon (Inst), Roche (Inst), GlaxoSmithKline (Inst), Celldex (Inst), AstraZeneca (Inst), Molecular Templates (Inst), CytomX Therapeutics (Inst), Astellas Pharma (Inst), K-Group Beta (Inst)
Other Relationship: DSI (Inst), Merck (Inst), Caris Life Sciences (Inst), Eisai (Inst), AstraZeneca (Inst)
Uncompensated Relationships: TTC Oncology, AbbVie
Michael Friedlander
Honoraria: AstraZeneca, MSD, Lilly, Takeda, Novartis, GlaxoSmithKline
Consulting or Advisory Role: AstraZeneca, MSD, AbbVie, Lilly, Takeda, Novartis, GlaxoSmithKline, Eisai, Incyclix
Speakers' Bureau: AstraZeneca, ACT Genomics, GlaxoSmithKline
Research Funding: BeiGene (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Michael A. Bookman
Employment: The Permanente Medical Group
Consulting or Advisory Role: AstraZeneca, AbbVie, Immunogen, Merck Sharp & Dohme, Genentech/Roche, Seattle Genetics, Aravive
Kathleen N. Moore
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicians' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, TESARO (Inst), VBL Therapeutics, Merck, Aravive, Eisai, Vavotar Life Sciences, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Pharmaceuticals (Inst), GlaxoSmithKline/Tesaro (Inst), I-Mab (Inst), InxMed (Inst), Mereo BioPharma (Inst), OncXerna Therapeutics, Onconova Therapeutics, Mereo BioPharma, Novartis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Forty Seven (Inst), Stemcentrx (Inst), Immunogen (Inst), Bayer (Inst), Novogen (Inst), AbbVie/Stemcentrx (Inst), Artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), Cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: GOG Partners (Inst)
Karina D. Steffensen
Consulting or Advisory Role: AbbVie
Research Funding: AstraZeneca (Inst)
Robert L. Coleman
Employment: US Oncology
Leadership: Onxeo
Stock and Other Ownership Interests: McKesson
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, AstraZeneca/MedImmune, Genmab, Tesaro, OncoMed, Sotio, Oncolytics, AbbVie/Stemcentrx, Immunogen, AbbVie, Agenus, Novocure, Merck, OncXerna Therapeutics, Alkermes, Gradalis, Regeneron
Research Funding: AstraZeneca/MedImmune, Esperance Pharmaceuticals, Array BioPharma, Clovis Oncology, Johnson & Johnson, Merck, Roche/Genentech, Abbott/AbbVie, Immunogen (Inst), Mirati Therapeutics (Inst), Amgen (Inst), Pfizer (Inst), Lilly (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca/MedImmune, Array BioPharma, Clovis Oncology, Roche/Genentech, Research to Practice, GOG Foundation, Sotio, Vaniam Group
Elizabeth M. Swisher
Leadership: IDEAYA Biosciences
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the International Gynecologic Cancer Society Annual Global Virtual Meeting, September 10-13, 2020.
SUPPORT
VELIA trial and KELIM assessment in this study were supported by AbbVie.
AUTHOR CONTRIBUTIONS
Conception and design: Benoit You, Peter J. Ansell, Minh H. Dinh
Provision of study materials or patients: Balakrishna Hosmane, Michael Friedlander, Michael A. Bookman, Robert L. Coleman, Elizabeth M. Swisher
Collection and assembly of data: Peter J. Ansell, Minh H. Dinh, Michael Friedlander, Robert L. Coleman, Elizabeth M. Swisher
Data analysis and interpretation: Vasudha Sehgal, Balakrishna Hosmane, Xin Huang, Peter J. Ansell, Minh H. Dinh, Katherine Bell-McGuinn, Xizhi Luo, Gini F. Fleming, Michael A. Bookman, Kathleen N. Moore, Karina D. Steffensen, Robert L. Coleman, Elizabeth M. Swisher
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
CA-125 KELIM as a Potential Complementary Tool for Predicting Veliparib Benefit: An Exploratory Analysis From the VELIA/GOG-3005 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benoit You
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Novartis, LEK, TESARO, Bayer, Amgen, Clovis Oncology, GlaxoSmithKline, ECS PROGASTRIN, Immunomedics, Daiichi Sankyo Europe GmbH, Myriad Genetics, MSD Oncology, Seattle Genetics
Research Funding: Merck Serono (Inst), Roche/Genentech (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, AstraZeneca, BMS, MSD Oncology, Bayer
Balakrishna Hosmane
Employment: AbbVie
Consulting or Advisory Role: AbbVie
Xin Huang
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Peter J. Ansell
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Minh H. Dinh
Employment: AbbVie
Leadership: AbbVie
Stock and Other Ownership Interests: AbbVie
Travel, Accommodations, Expenses: AbbVie
Katherine Bell-McGuinn
Employment: AbbVie
Stock and Other Ownership Interests: Lilly, AbbVie
Patents, Royalties, Other Intellectual Property: Author on patent for CD200R agent developed by Lilly
Xizhi Luo
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Gini F. Fleming
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Curio Science, Physicians' Education Resource
Consulting or Advisory Role: GlaxoSmithKline
Research Funding: Corcept Therapeutics (Inst), AbbVie (Inst), Iovance Biotherapeutics (Inst), Syros Pharmaceuticals (Inst), Sermonix Pharmaceuticals (Inst), Compugen (Inst), Plexxikon (Inst), Roche (Inst), GlaxoSmithKline (Inst), Celldex (Inst), AstraZeneca (Inst), Molecular Templates (Inst), CytomX Therapeutics (Inst), Astellas Pharma (Inst), K-Group Beta (Inst)
Other Relationship: DSI (Inst), Merck (Inst), Caris Life Sciences (Inst), Eisai (Inst), AstraZeneca (Inst)
Uncompensated Relationships: TTC Oncology, AbbVie
Michael Friedlander
Honoraria: AstraZeneca, MSD, Lilly, Takeda, Novartis, GlaxoSmithKline
Consulting or Advisory Role: AstraZeneca, MSD, AbbVie, Lilly, Takeda, Novartis, GlaxoSmithKline, Eisai, Incyclix
Speakers' Bureau: AstraZeneca, ACT Genomics, GlaxoSmithKline
Research Funding: BeiGene (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Michael A. Bookman
Employment: The Permanente Medical Group
Consulting or Advisory Role: AstraZeneca, AbbVie, Immunogen, Merck Sharp & Dohme, Genentech/Roche, Seattle Genetics, Aravive
Kathleen N. Moore
Leadership: GOG Partners, NRG Oncology (Inst)
Honoraria: Research To Practice, Prime Oncology, Physicians' Education Resource, Great Debates and Updates
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, TESARO (Inst), VBL Therapeutics, Merck, Aravive, Eisai, Vavotar Life Sciences, Mersana (Inst), Myriad Genetics, Alkermes (Inst), Blueprint Pharmaceuticals (Inst), GlaxoSmithKline/Tesaro (Inst), I-Mab (Inst), InxMed (Inst), Mereo BioPharma (Inst), OncXerna Therapeutics, Onconova Therapeutics, Mereo BioPharma, Novartis
Research Funding: PTC Therapeutics (Inst), Lilly (Inst), Merck (Inst), Tesaro (Inst), Genentech (Inst), Clovis Oncology (Inst), Lilly Foundation (Inst), Regeneron (Inst), Advaxis (Inst), Bristol Myers Squibb (Inst), Verastem (Inst), Novartis Pharmaceuticals UK Ltd (Inst), AstraZeneca (Inst), Agenus (Inst), Takeda (Inst), Forty Seven (Inst), Stemcentrx (Inst), Immunogen (Inst), Bayer (Inst), Novogen (Inst), AbbVie/Stemcentrx (Inst), Artios (Inst), Bolt Biotherapeutics (Inst), Amgen (Inst), Daiichi Sankyo/Lilly (Inst), Cyteir (Inst), Immunocore (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: GOG Partners (Inst)
Karina D. Steffensen
Consulting or Advisory Role: AbbVie
Research Funding: AstraZeneca (Inst)
Robert L. Coleman
Employment: US Oncology
Leadership: Onxeo
Stock and Other Ownership Interests: McKesson
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, AstraZeneca/MedImmune, Genmab, Tesaro, OncoMed, Sotio, Oncolytics, AbbVie/Stemcentrx, Immunogen, AbbVie, Agenus, Novocure, Merck, OncXerna Therapeutics, Alkermes, Gradalis, Regeneron
Research Funding: AstraZeneca/MedImmune, Esperance Pharmaceuticals, Array BioPharma, Clovis Oncology, Johnson & Johnson, Merck, Roche/Genentech, Abbott/AbbVie, Immunogen (Inst), Mirati Therapeutics (Inst), Amgen (Inst), Pfizer (Inst), Lilly (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Merck, AstraZeneca/MedImmune, Array BioPharma, Clovis Oncology, Roche/Genentech, Research to Practice, GOG Foundation, Sotio, Vaniam Group
Elizabeth M. Swisher
Leadership: IDEAYA Biosciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Colombo N, Sessa C, du Bois A, et al. : ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol 30:672-705, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Karam A, Ledermann JA, Kim JW, et al. : Fifth ovarian cancer consensus conference of the Gynecologic Cancer InterGroup: First-line interventions. Ann Oncol 28:711-717, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Wright AA, Bohlke K, Armstrong DK, et al. : Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice guideline. J Clin Oncol 34:3460-3473, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA approves bevacizumab in combination with chemotherapy for ovarian cancer. Silver Spring, MD, US Food and Drug Administration, 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-bevacizumab-combination-chemotherapy-ovarian-cancer
- 5.FDA approves olaparib plus bevacizumab as maintenance treatment for ovarian, fallopian tube, or primary peritoneal cancers. Silver Spring, MD, US Food and Drug Administration, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-plus-bevacizumab-maintenance-treatment-ovarian-fallopian-tube-or-primary
- 6.FDA approves niraparib for first-line maintenance of advanced ovarian cancer. Silver Spring, MD, US Food and Drug Administration, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer
- 7.Coleman RL, Fleming GF, Brady MF, et al. : Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381:2403-2415, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Martin A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Ray-Coquard I, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Monk BJ, Parkinson C, Lim MC, et al. : A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA–MONO/GOG-3020/ENGOT-ov45). J Clin Oncol 40:3952-3964, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong PC, Yap TA, Boss DS, et al. : Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 28:2512-2519, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Labidi-Galy SI, de La Motte Rouge T, Derbel O, et al. : Clinical factors associated with prolonged response and survival under olaparib as maintenance therapy in BRCA mutated ovarian cancers. Gynecol Oncol 155:262-269, 2019 [DOI] [PubMed] [Google Scholar]
- 13.You B, Freyer G, Gonzalez-Martin A, et al. : The role of the tumor primary chemosensitivity relative to the success of the medical-surgical management in patients with advanced ovarian carcinomas. Cancer Treat Rev 100:102294, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Lauby A, Colomban O, Corbaux P, et al. : The increasing prognostic and predictive roles of the tumor primary chemosensitivity assessed by CA-125 elimination rate constant K (KELIM) in ovarian cancer: A narrative review. Cancers (Basel) Dec 25; 14:98, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colomban O, Tod M, Leary A, et al. : Early modeled longitudinal CA-125 kinetics and survival of ovarian cancer patients: A GINECO AGO MRC CTU study. Clin Cancer Res 25:5342-5350, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Colomban O, Tod M, Peron J, et al. : Bevacizumab for newly diagnosed ovarian cancers: Best candidates among high-risk disease patients (ICON-7). JNCI Cancer Spectr 4:pkaa026, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You B, Robelin P, Tod M, et al. : CA-125 ELIMination rate constant K (KELIM) is a marker of chemosensitivity in patients with ovarian cancer: Results from the phase II CHIVA trial. Clin Cancer Res 26:4625-4632, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Van Wagensveld L, Colomban O, Van der AAM, et al. : The prognostic value of chemosensitivity, estimated by the modeled CA-125 KELIM, in ovarian cancer patients treated with neo-adjuvant chemotherapy in the Netherlands. Presented at ESMO 2020 virtual meeting, 2020 (abstr 847P)
- 19.You B, Clamp A, Cook A, et al. : Differential benefit from fractionated dose-dense first-line chemotherapy for epithelial ovarian cancer (EOC) according to KELIM-evaluated tumor primary chemosensitivity: Exploratory analyses of ICON-8 trial. J Clin Oncol 39, 2021. (15 suppl; abstr 5530) [Google Scholar]
- 20.Corbaux P, You B, Glasspool R, et al. : Survival prognostic and surrogate values of the early modeled CA-125 KELIM in newly diagnosed advanced ovarian cancer: Data from the GCIG meta-analysis group. Ann Oncol 32, 2021. (suppl 5; abstr S744) [Google Scholar]
- 21.You B, Van Wagensveld L, Tod M, et al. : The impact of chemosensitivity assessed by modeled CA-125 KELIM on the likelihood of long progression-free survivorship (PS) after 1st line treatment in ovarian cancer: An analysis of 4,450 patients. Presented at ESMO 2020 virtual meeting, 2020 (abstr 815MO)
- 22.Murai J, Huang SY, Das BB, et al. : Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 72:5588-5599, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pommier Y, O'Connor MJ, de Bono J: Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med 8:362ps17, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Hopkins TA, Ainsworth WB, Ellis PA, et al. : PARP1 trapping by PARP inhibitors drives cytotoxicity in both cancer cells and healthy bone marrow. Mol Cancer Res 17:409-419, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Aghajanian C, Bookman MA, Fleming GF, et al. : PFS by blinded independent central review (BICR) in the VELIA trial of veliparib (V) plus carboplatin/paclitaxel (CP) and as monotherapy in newly diagnosed patients (pts) with high-grade serous ovarian cancer (HGSC). J Clin Oncol 38, 2020. (15 suppl; abstr 6077) [Google Scholar]
- 26.Oza AM, Cook AD, Pfisterer J, et al. : Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol 16:928-936, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller G, McCormack R, Kheoh T, et al. : Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: A comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol 36:572-580, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team : R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing V, 2021 [Google Scholar]
- 29.Biomarker Kinetics: Modeled CA-125 KELIM™ in patients with stage III-IV high grade serous ovarian carcinomas treated with first line neo-adjuvant chemotherapy. https://www.biomarker-kinetics.org/CA-125-neo
- 30.Biomarker Kinetics: Modeled CA-125 KELIM™ for prognostic classification of ovarian cancer patients treated with first line chemotherapy. https://www.biomarker-kinetics.org/CA-125