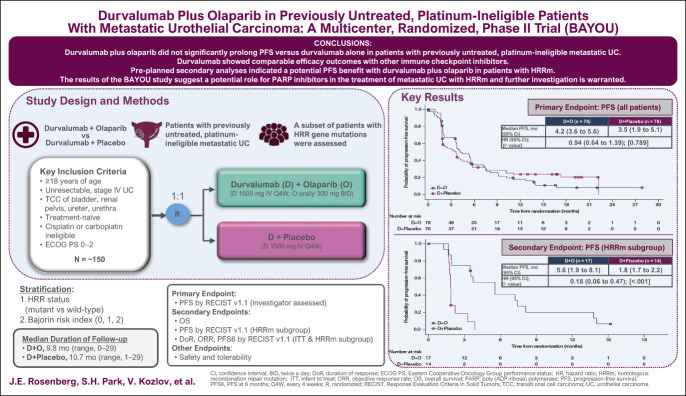
PURPOSE
Homologous recombination repair gene mutations (HRRm) are common in urothelial carcinoma (UC), rendering tumor cells sensitive to poly (ADP-ribose) polymerase (PARP) inhibition. We assessed efficacy and safety of durvalumab (anti–programmed cell death ligand-1) plus olaparib (PARP inhibitor) in patients with metastatic UC (mUC).
METHODS
This randomized, multicenter, double-blind, phase II trial enrolled untreated, platinum-ineligible patients with mUC. Patients (N = 154) were randomly assigned 1:1 to receive durvalumab (1,500 mg intravenously once every 4 weeks) plus olaparib (300 mg orally, twice daily) or durvalumab plus placebo. The primary end point was progression-free survival (PFS) assessed by investigators per RECIST version 1.1. Secondary end points included overall survival in all patients and PFS in patients with HRRm.
RESULTS
Overall, median PFS was 4.2 months (95% CI, 3.6 to 5.6) for durvalumab plus olaparib and 3.5 months (95% CI, 1.9 to 5.1) for durvalumab plus placebo (hazard ratio [HR], 0.94; 95% CI, 0.64 to 1.39; log-rank P value, .789). Median overall survival was 10.2 months (95% CI, 7.0 to 13.9) and 10.7 months (95% CI, 7.2 to 17.3), respectively (HR, 1.07; 95% CI, 0.72 to 1.61). In the 20% of patients with HRRm, median PFS was 5.6 months (95% CI, 1.9 to 8.1) and 1.8 months (95% CI, 1.7 to 2.2), respectively (HR, 0.18; 95% CI, 0.06 to 0.47). Treatment-related grade 3 or 4 adverse events occurred in 18% and 9% of patients, respectively.
CONCLUSION
Adding olaparib to durvalumab did not improve survival outcomes in an unselected mUC population. Efficacy outcomes with durvalumab were similar to those reported for other anti–programmed cell death-1/programmed cell death ligand-1 agents. However, the results of secondary analyses suggest a potential role for PARP inhibition in patients with UC harboring HRRm.
INTRODUCTION
Cisplatin-based chemotherapy remains the standard of care for the first-line treatment of patients with metastatic urothelial carcinoma (mUC), but approximately 40% of patients are unfit for these regimens because of renal impairment, poor performance status, or other comorbidities.1 Cisplatin-ineligible patients may receive carboplatin-based regimens, although median overall survival (OS) is longer with the former regimens.2 Patients ineligible for cisplatin-based chemotherapy are also frequently ineligible for carboplatin-containing regimens; treatment options for these patients include pembrolizumab (anti–programmed cell death-1 [PD-1]) and atezolizumab (anti–programmed cell death ligand-1 [PD-L1]) regardless of tumor PD-L1 status.3 However, outcomes for platinum-ineligible patients treated with anti–PD-1/PD-L1 agents are poor, with a median OS of 10.4 months,4 and there remains a high unmet need for new treatments in this population.
CONTEXT
Key Objective
We aimed to evaluate the efficacy and safety of durvalumab in combination with the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in untreated, platinum-ineligible patients with metastatic urothelial carcinoma (mUC). To our knowledge, BAYOU is the first randomized, controlled study to evaluate the combination of a PARP inhibitor and an immune checkpoint inhibitor in patients with mUC.
Knowledge Generated
The study did not meet its primary end point as there was no progression-free survival benefit with durvalumab plus olaparib versus durvalumab plus placebo in a biomarker-unselected population. However, the results of secondary analyses suggested a progression-free survival benefit with durvalumab plus olaparib in patients whose tumors harbor mutations in homologous recombination repair genes. The combination of durvalumab and olaparib had a manageable safety profile.
Relevance
The results of the BAYOU study suggest a potential role for PARP inhibitors in the treatment of patients with mUC and homologous recombination repair gene mutation, and further investigation is warranted.
The characterization of genetic abnormalities in mUC has led to the identification of new therapeutic targets.5,6 Some of the most common genetic abnormalities in mUC are alterations in DNA damage response (DDR) genes, such as mutations in homologous recombination repair (HRR) genes (eg, BRCA1 and BRCA2),6,7 with an overall mutation frequency of approximately 24%.8 These mutations render tumor cells less efficient at repairing DNA damage, and inhibition of poly (ADP-ribose) polymerase (PARP) in these sensitive tumor cells promotes the accumulation of DNA damage and cell death (known as synthetic lethality).9 The accumulation of DNA damage has the potential to modify the immunogenicity of tumors, such as promoting the formation of neoantigens and upregulation of PD-L1 expression.10 Thus, we hypothesized that PARP inhibition may enhance the effects of anti–PD-1/PD-L1 agents in tumor cells harboring HRR gene mutations.
Olaparib, an orally bioavailable inhibitor of PARP, has demonstrated activity in several platinum-sensitive, DNA repair-deficient cancers, including UC.11 The anti–PD-L1 antibody, durvalumab, has shown activity in both previously untreated and platinum-refractory mUC.2,12 We conducted a study to determine whether olaparib can enhance the activity of durvalumab in untreated, platinum-ineligible patients with mUC.
METHODS
Study Design and Participants
The BAYOU study is a randomized, multicenter, double-blind, phase II trial designed to evaluate the efficacy and safety of durvalumab plus olaparib versus durvalumab plus placebo in patients with previously untreated, unresectable, stage IV UC who were ineligible for platinum-based chemotherapy (ClinicalTrials.gov identifier: NCT03459846). Ineligibility for platinum-based chemotherapy was defined as unfit for carboplatin-based chemotherapy (as assessed by the investigators) and meeting one of the following criteria: creatinine clearance < 60 mL/min, grade ≥ 2 audiometric hearing loss/peripheral neuropathy, New York Heart Association Class III heart failure, or an Eastern Cooperative Oncology Group performance status (ECOG PS) of 2. The study was conducted at 38 sites in seven countries according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The Protocol (online only) was approved by institutional review boards at each study site. The trial was overseen by an independent data monitoring committee. All patients provided written informed consent to participate in the trial.
Eligible patients were ≥ 18 years old with histologically or cytologically confirmed transitional cell carcinoma (transitional cell and mixed transitional/nontransitional cell histologies) of the urothelium (including renal pelvis, ureters, urinary bladder, and urethra); no prior therapy for unresectable, stage IV disease; ineligible for cisplatin- or carboplatin-based chemotherapy; known tumor HRR mutation status (Protocol); ECOG PS of 0, 1, or 2; at least one measurable lesion at baseline, not previously irradiated, per RECIST version (v) 1.1; life expectancy ≥ 12 weeks; and adequate organ and bone marrow function. Key exclusion criteria were prior treatment with a PARP inhibitor or immune-mediated therapy (except bacillus Calmette-Guérin therapy); active or prior autoimmune or inflammatory disorders; history of active primary immunodeficiency; active infection including tuberculosis and hepatitis B, hepatitis C, or human immunodeficiency virus; active brain metastases; and uncontrolled intercurrent illness.
Study Treatment
Eligible patients were randomly assigned 1:1 to durvalumab plus olaparib or durvalumab plus olaparib-matched placebo. Random assignment was stratified by HRR status (mutant [HRRm] v wild-type [HRRwt]) and Bajorin risk index (a composite of visceral metastases [lymph node only metastasis v metastasis to any other organ system] and ECOG PS [0, 1, v 2]). Random assignment was done using an interactive voice/web response system. Durvalumab was administered intravenously at a dose of 1,500 mg once every 4 weeks. Olaparib or placebo was administered orally at 300 mg twice daily to patients with creatinine clearance ≥ 51 mL/min; patients with a creatinine clearance ≥ 31 mL/min but < 51 mL/min received olaparib 200 mg twice daily but could receive the 300 mg dose at the next treatment cycle if creatinine clearance improved to ≥ 51 mL/min. Treatment continued until confirmed disease progression, unacceptable toxicity, withdrawal of consent, or another discontinuation criterion was met.
Assessments
Tumor assessments were based on computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis, collected during screening/baseline (no more than 28 days before the start of study treatment) and at regular intervals during study treatment. Radiologic efficacy was assessed from images collected every 8 weeks for the first 48 weeks after random assignment and every 12 weeks thereafter until RECIST v1.1-defined disease progression. Assessments of tumor response were confirmed no less than 4 weeks and no more than 8 weeks after the prior assessment. Survival was assessed every 2 months after treatment discontinuation. Adverse events (AEs) were monitored throughout the treatment period and up to 90 days after the last dose of study drug. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03.
Tumor samples were assessed for HRR mutation status at a central laboratory using the FMI FoundationOne assay (Foundation Medicine, Cambridge, MA). DNA was extracted from formalin-fixed, paraffin-embedded tissue, which was obtained from either the primary tumor or metastatic biopsy. Tumor samples were tested for deleterious mutations in 15 prespecified HRR genes13: ATM, BRCA1, BRCA2, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, and RAD54L. Tumor PD-L1 expression at baseline was assessed by immunohistochemistry using the VENTANA PD-L1 (SP263) Assay (Ventana Medical Systems, Inc, a member of the Roche Group, Tucson, AZ).14 PD-L1 expression was defined as high if ≥ 25% of tumor cells exhibited membrane staining or ≥ 25% of immune cells stained for PD-L1 at any intensity if > 1% of the tumor area contained immune cells, or 100% of immune cells stained for PD-L1 at any intensity if 1% of the tumor area contained immune cells.2
End Points
The primary end point was investigator-assessed progression-free survival (PFS; according to RECIST v1.1) in the intention-to-treat (ITT) population, which included all randomly assigned patients. Secondary end points included OS in the ITT population, the proportion of patients alive at 18 months in the ITT population, and investigator-assessed PFS (according to RECIST v1.1) in the subset of patients with HRRm. In both the ITT population and in patients with HRRm, other secondary end points (as assessed by investigators according to RECIST v1.1) included objective response rate (complete or partial response), duration of response (defined as the time from the date of first documented response until the date of documented progression or death), and the proportion of patients alive and progression-free at 6 months. Safety and tolerability were assessed in all treated patients. Patient-reported outcomes were assessed as secondary and exploratory end points, including the Vulnerable Elders Survey-13, which was given at baseline to assess the frailty of patients.
Statistical Analysis
The planned sample size was 150 patients, with 75 in each treatment group. Primary analysis of PFS was performed at one time point, when approximately 118 PFS events had occurred (79% maturity) and 100 OS events had occurred (67% maturity) across both treatment groups. This provided 90% power to detect an improvement in PFS with a hazard ratio (HR) of 0.55 and a two-sided significance level of 0.05. Median PFS was calculated using the Kaplan-Meier method, with 95% CI derived from the Brookmeyer-Crowley method. PFS was compared between treatment groups using a stratified log-rank test, with HRs estimated using a stratified Cox proportional hazards model (stratified by HRR status only). Median OS was calculated using the Kaplan-Meier method, with 95% CI for median OS derived from the Brookmeyer-Crowley method; HRs were estimated using a stratified Cox proportional hazards model (stratified by HRR status only). The proportion of patients alive at 18 months, the proportion of patients alive and progression-free at 6 months, and duration of response were calculated using the Kaplan-Meier method.
RESULTS
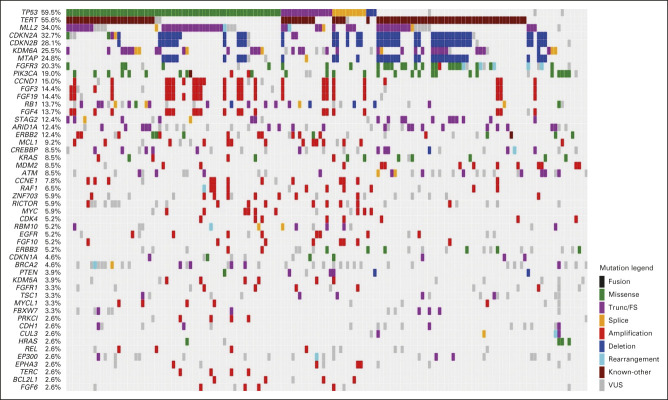
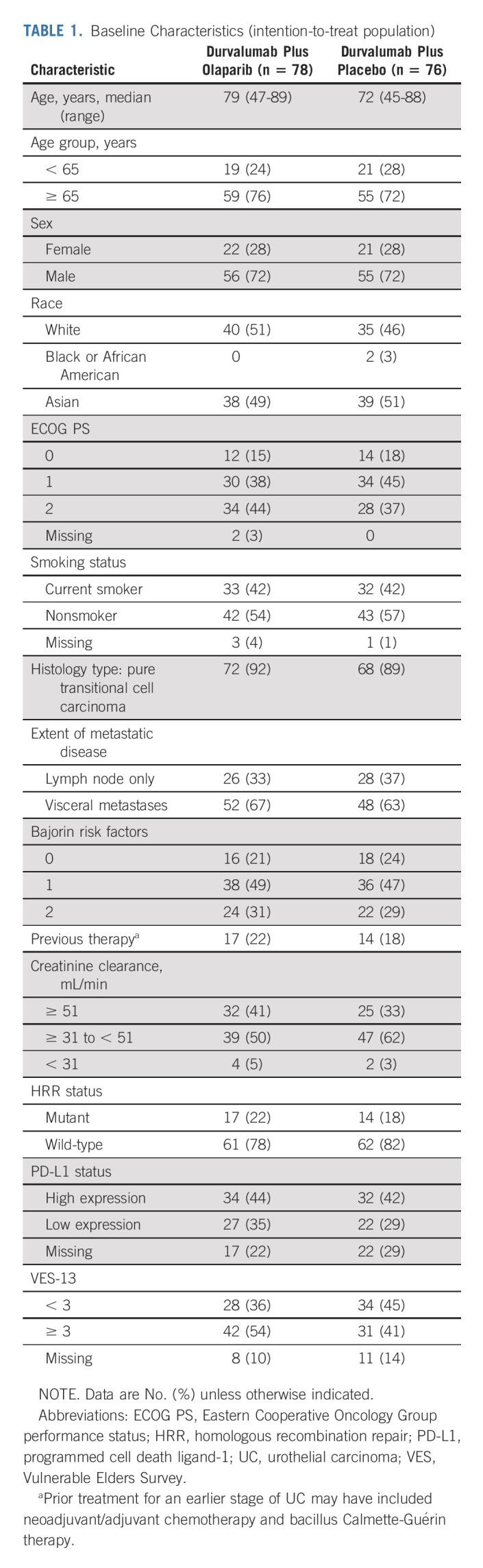
Between March 16, 2018, and September 11, 2019, 260 patients were screened and 154 were randomly assigned: 78 to durvalumab plus olaparib and 76 to durvalumab plus placebo (Fig 1). In both groups, 76 patients received study treatment. Baseline characteristics were generally well balanced between treatment groups (Table 1). Seventeen patients (21.8%) in the durvalumab plus olaparib group and 14 (18.4%) in the durvalumab plus placebo group had an HRRm. OncoPrint mapping of the 20 most common tumor gene mutations in the BAYOU population (Appendix Fig A1, online only) showed a mutational pattern consistent with that previously reported in bladder cancer.15 The two most common mutations were loss of p53 function (59.5%) and TERT promoter mutations (55.6%); mutations in DDR genes ATM and BRCA2 were present in 8.5% and 4.6% of tumors, respectively.
FIG 1.
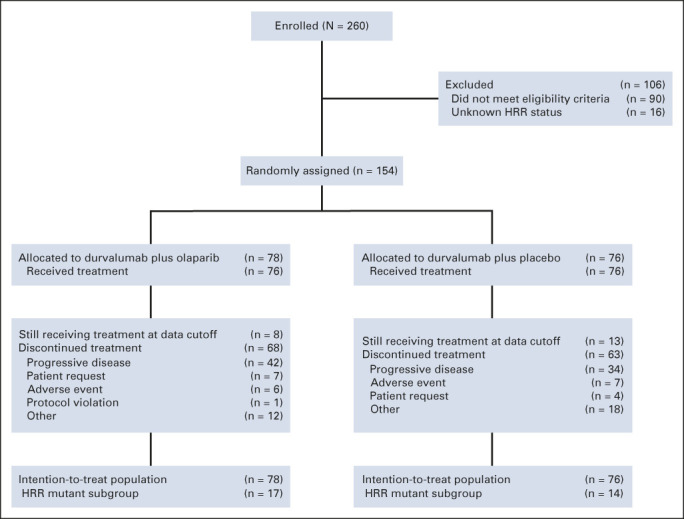
CONSORT diagram of the study. AE, adverse event; HRR, homologous recombination repair.
TABLE 1.
Baseline Characteristics (intention-to-treat population)
In the durvalumab plus olaparib group, patients received a median of 5.0 cycles (range, 1-26 cycles) of durvalumab and 4.0 cycles (range, 1-26 cycles) of olaparib, with one cycle defined as 28 days; patients in the durvalumab plus placebo group received a median of 3.5 cycles (range, 1-26 cycles) of durvalumab and 3.0 cycles (range, 1-26 cycles) of placebo. At data cutoff (October 15, 2020), median duration of follow-up was 9.8 months (range, 0.0-29.0 months) in the durvalumab plus olaparib group and 10.7 months (range, 1.0-29.0 months) in the durvalumab plus placebo group. Subsequent anticancer therapy was received by 17 patients (21.8%) in the durvalumab plus olaparib group and 14 (18.4%) in the durvalumab plus placebo group.
Efficacy
Primary PFS analysis was performed when 117 PFS events had occurred in the ITT population (62 [79.5%] in the durvalumab plus olaparib group and 55 [72.4%] in the durvalumab plus placebo group). Median PFS was 4.2 months (95% CI, 3.6 to 5.6) in the durvalumab plus olaparib group versus 3.5 months (95% CI, 1.9 to 5.1) in the durvalumab plus placebo group (HR, 0.94; 95% CI, 0.64 to 1.39; stratified log-rank P = .789; Fig 2A).
FIG 2.
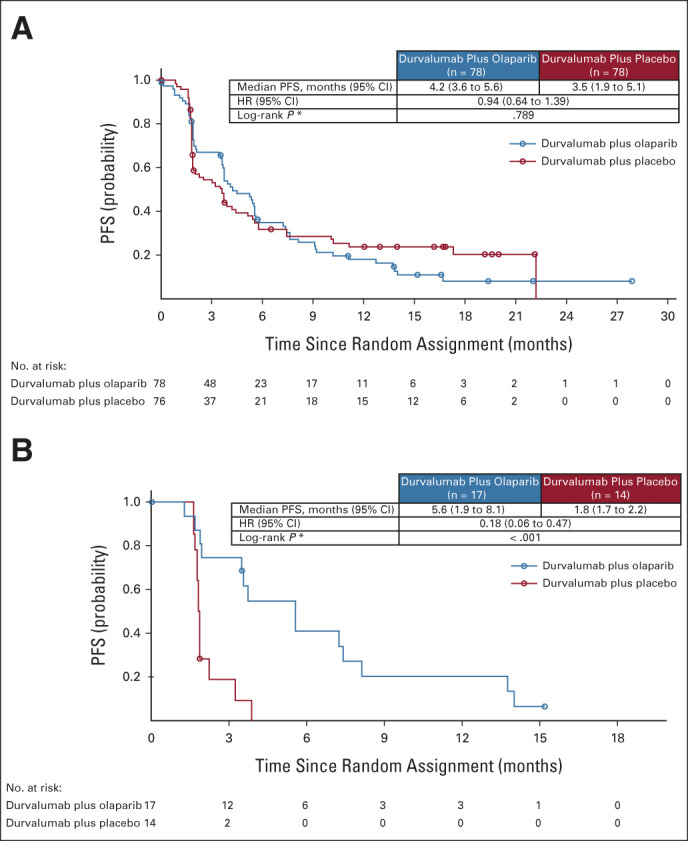
PFS in the (A) ITT population and in the (B) subset of patients with an HRRm. In the ITT population, the percentage of patients alive and progression-free at 6 months was 35.0% (95% CI, 24.1 to 46.1) in the durvalumab plus olaparib group and 31.9% (95% CI, 21.3 to 43.0) in the durvalumab plus placebo group. In the subset of patients with an HRRm, the percentage of patients alive and progression-free at 6 months was 41.3% (95% CI, 17.3 to 63.9) in the durvalumab plus olaparib group and was 0% in the durvalumab plus placebo group. HR, hazard ratio; HRRm, mutations in homologous recombination repair genes; ITT, intent-to-treat; PFS, progression-free survival.
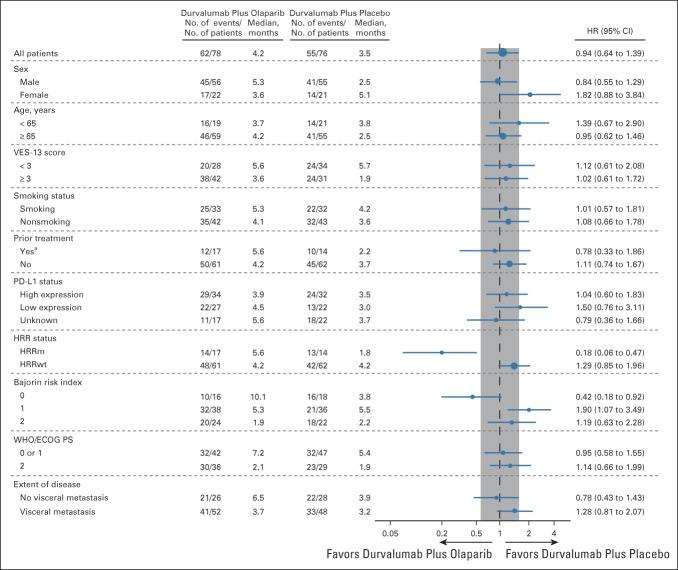
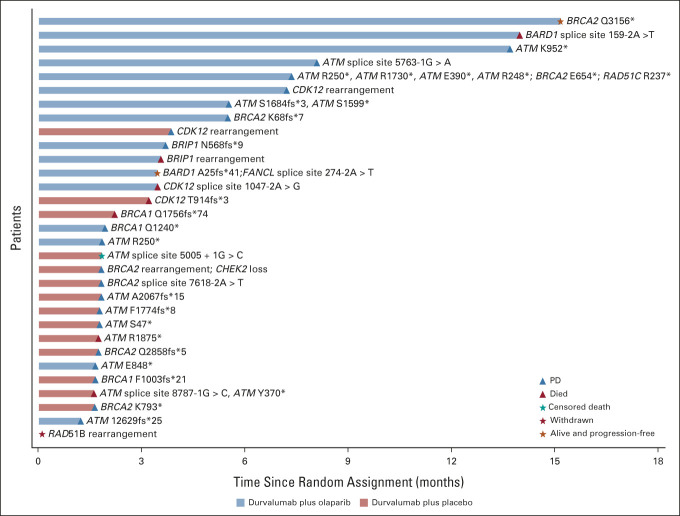
Prespecified subgroup analyses showed that the PFS HR favored durvalumab plus olaparib over durvalumab plus placebo for patients with HRRm: median PFS was 5.6 months (95% CI, 1.9 to 8.1) and 1.8 months (95% CI, 1.7 to 2.2), respectively (HR, 0.18; 95% CI, 0.06 to 0.47; Fig 2B; Appendix Fig A2, online only). Median PFS was 4.2 months in both groups for patients with HRRwt (HR, 1.29; 95% CI, 0.85 to 1.96). Subgroup analyses also showed that the PFS HR favored durvalumab plus olaparib over durvalumab plus placebo for patients with a Bajorin risk index of 0: median PFS was 10.1 months and 3.8 months, respectively (HR, 0.42; 95% CI, 0.18 to 0.92; Appendix Fig A2). A global interaction test (using the Gail and Simon approach) showed a significant qualitative interaction for both HRR status (P < .001) and Bajorin risk index (P = .017). PFS by type of HRR gene mutation is shown in Appendix Figure A3 (online only).
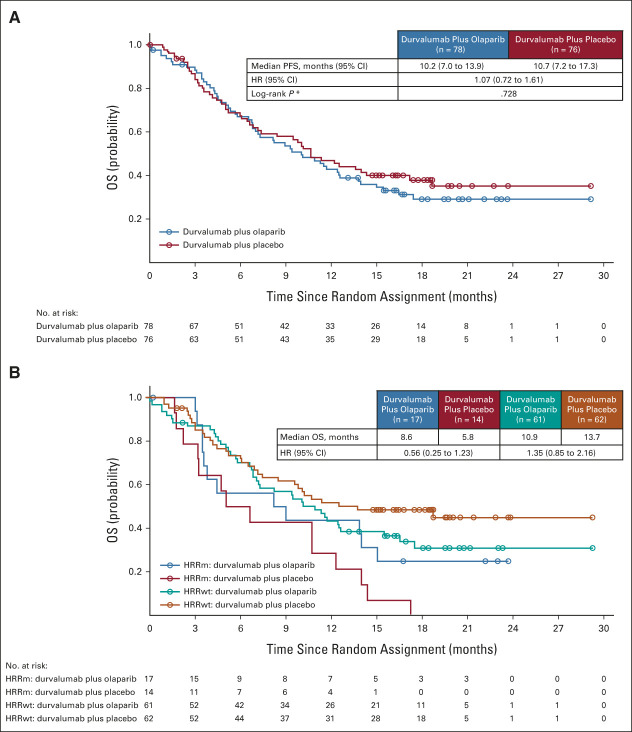
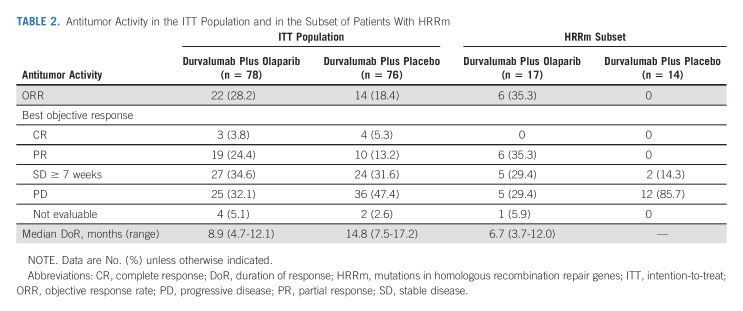
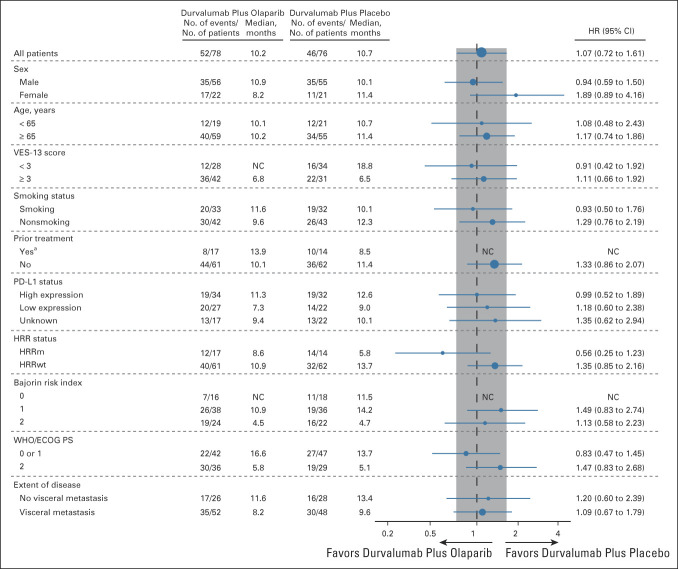
At the time of the analysis of OS, 98 OS events had occurred in the ITT population (52 [66.7%] in the durvalumab plus olaparib group and 46 [60.5%] in the durvalumab plus placebo group). Median OS was 10.2 months (95% CI, 7.0 to 13.9) in the durvalumab plus olaparib group and 10.7 months (95% CI, 7.2 to 17.3) in the durvalumab plus placebo group (Fig 3), with an HR of 1.07 (95% CI, 0.72 to 1.61). In patients with HRRm, median OS was 8.6 months in the durvalumab plus olaparib group and 5.8 months in the durvalumab plus placebo group (HR, 0.56; 95% CI, 0.25 to 1.23); in patients with HRRwt, median OS was 10.9 months and 13.7 months, respectively (HR, 1.35; 95% CI, 0.85 to 2.16; Fig 3 and Appendix Fig A4, online only). Consistent with prior studies,2 higher median OS was observed in the PD-L1 high versus PD-L1 low subgroups for durvalumab plus olaparib (11.3 v 7.3 months) and durvalumab plus placebo (12.6 v 9.0 months; Appendix Fig A4).
FIG 3.
OS in the (A) ITT population and in the (B) subset of patients with an HRRm. In the ITT population, the OS rates at 18 months were 29.9% (95% CI, 19.6 to 40.8) in the durvalumab plus olaparib group and 38.7% (95% CI, 27.5 to 49.7) in the durvalumab plus placebo group. HR, hazard ratio; HRRm, mutations in homologous recombination repair genes; HRRwt, wild-type homologous recombination repair genes; ITT, intent-to-treat; OS, overall survival; PFS, progression-free survival.
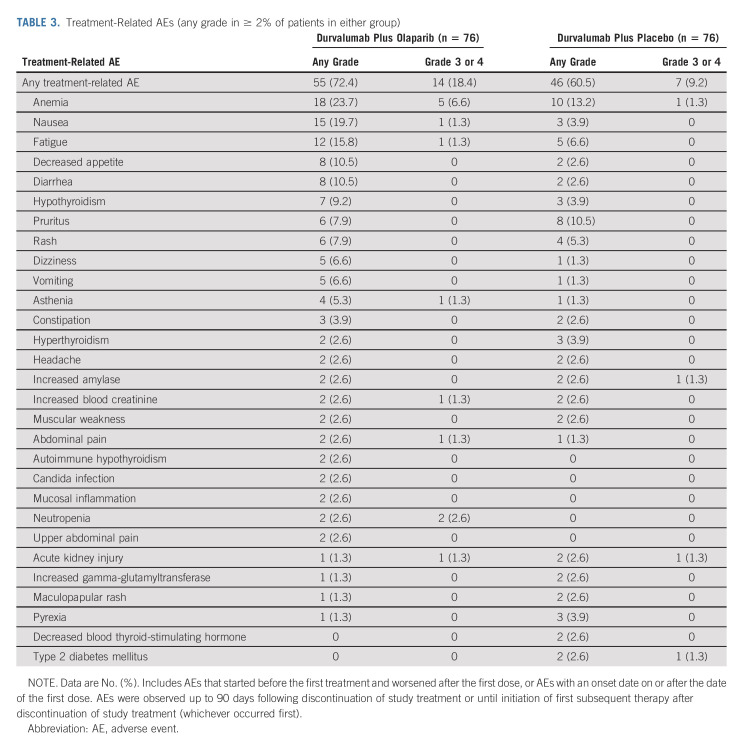
In the ITT population, objective responses occurred in 22 patients (28.2%) in the durvalumab plus olaparib group and in 14 patients (18.4%) in the durvalumab plus placebo group (odds ratio, 1.76; 95% CI, 0.82 to 3.78; Table 2). Median duration of response was shorter in the durvalumab plus olaparib group compared with the durvalumab plus placebo group (Table 2), with 32% and 64% of patients remaining in response at 12 months, respectively. In the subset of patients with HRRm, 6 of 17 patients (35.3%) in the durvalumab plus olaparib group achieved an objective response; median duration of response was 6.7 months (Table 2). No patient in the durvalumab plus placebo group achieved an objective response.
TABLE 2.
Antitumor Activity in the ITT Population and in the Subset of Patients With HRRm
Safety
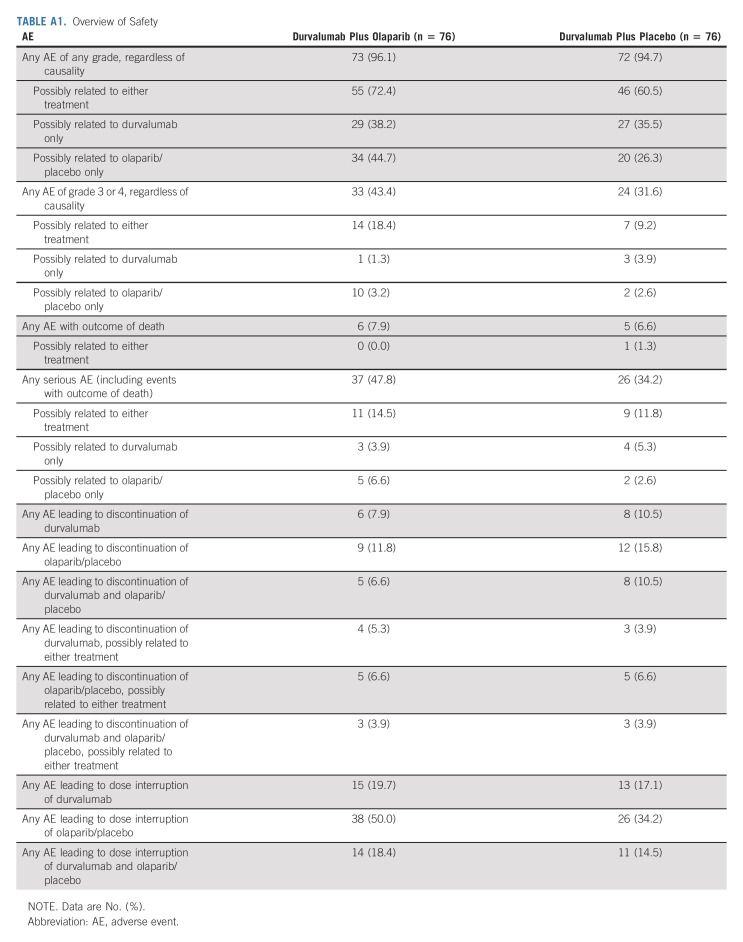
Treatment-related AEs of any grade were reported in 55 patients (72.4%) in the durvalumab plus olaparib group and in 46 patients (60.5%) in the durvalumab plus placebo group, with treatment-related grade 3 or 4 AEs in 18.4% and 9.2% of patients, respectively (Table 3). The most common treatment-related AE in both groups was anemia (Table 3). An overview of safety data is provided in Appendix Table A1 (online only). Treatment-related serious AEs occurred in 11 patients (14.5%) in the durvalumab plus olaparib group and in nine patients (11.8%) in the durvalumab plus placebo group. There were fewer AEs, regardless of causality, that led to discontinuation of durvalumab, olaparib/placebo, or both drugs in the durvalumab plus olaparib group. Incidence of AEs with an outcome of death was similar in both groups (7.9% in the durvalumab plus olaparib group and 6.6% in the durvalumab plus placebo group). One death in the durvalumab plus placebo group, because of anemia, was considered to be possibly related to both study drugs.
TABLE 3.
Treatment-Related AEs (any grade in ≥ 2% of patients in either group)
DISCUSSION
To our knowledge, BAYOU is the first randomized, controlled study to evaluate a PARP inhibitor in combination with an immune checkpoint inhibitor in patients with mUC. The primary end point of the study was not met as there was no PFS benefit with durvalumab plus olaparib versus durvalumab plus placebo in the ITT population. Although limited by small numbers, secondary analyses involving patients with HRRm showed a nearly 4-month increase in median PFS with durvalumab plus olaparib versus durvalumab alone. Median OS was nearly 3 months longer with durvalumab plus olaparib versus durvalumab alone in the subset of patients with HRRm. Notably, median OS was shorter for patients with HRRm than for patients with HRRwt, consistent with data showing that mutations in DDR genes are associated with poorer outcomes in mUC.16-18 Overall, safety data for durvalumab plus olaparib were consistent with the known safety profiles of the individual drugs. The combination was well tolerated with a manageable safety profile.
Somatic alterations in DDR genes are associated with improved response to cisplatin-based chemotherapy in muscle-invasive bladder cancer19 and to platinum-based chemotherapy in mUC.20 In patients with mUC and DDR gene mutations who had received platinum-based chemotherapy, the results of a randomized, phase II trial (ATLANTIS) showed that median PFS was significantly extended with the PARP inhibitor rucaparib as maintenance therapy versus placebo (8.1 v 3.5 months).21 However, the results of the randomized, phase II Meet-URO12 trial in platinum-treated patients with mUC and HRRm did not show a difference in median PFS with the PARP inhibitor niraparib plus best supportive care as maintenance therapy versus best supportive care (2.1 v 2.4 months).22 In previously treated patients, the results of the single-arm, phase II ATLAS trial also did not show a difference in median PFS between the HRRm and HRRwt subgroups (1.4 v 1.8 months).23
Olaparib has demonstrated activity in other tumor types known to harbor HRRm, including as maintenance therapy after response to platinum-based chemotherapy in advanced ovarian cancer24 and in metastatic castration-resistant prostate cancer after progression on a new hormonal agent.25 Preliminary data from the phase II NEODURVARIB study of neoadjuvant durvalumab plus olaparib in muscle-invasive bladder cancer showed a pathologic complete response rate of 44.5%.26 Anecdotal case reports have provided evidence for olaparib activity in previously treated patients with mUC and BRCA1/2 mutations,11 and the results from the phase Ib BISCAY biomarker study showed an objective response rate of 35.7% with durvalumab plus olaparib in platinum-refractory mUC harboring HRRm.27 The results of the BAYOU study extend these findings to previously untreated patients with mUC. However, larger, prospective studies are needed to determine the role of anti–PD-1/PD-L1 agents alone or in combination with PARP inhibitors as treatments for patients with mUC and HRRm.
Several retrospective and observational studies have shown that approximately 50% of patients with mUC do not receive chemotherapy in clinical practice.28 In addition to not being eligible for chemotherapy, many patients are unwilling to be treated with chemotherapy because of concerns over toxicities.29 Among cisplatin-ineligible patients enrolled in the phase II IMvigor210 study of atezolizumab and the phase II KEYNOTE-052 study of pembrolizumab, 70% and 49% had renal impairment and 20% and 32% had an ECOG PS of 2, respectively.30,31 In the BAYOU study, more than half of the patients had creatinine clearance < 51 mL/min and approximately 40% had an ECOG PS of 2. Despite the population differences, survival outcomes with durvalumab alone in BAYOU were comparable with those in the KEYNOTE-052 study, in which median PFS was 2.2 months and median OS was 11.3 months with pembrolizumab.31,32
In conclusion, durvalumab plus olaparib did not significantly prolong PFS versus durvalumab alone in patients with previously untreated, platinum-ineligible mUC. Durvalumab showed comparable efficacy outcomes with other immune checkpoint inhibitors. Preplanned secondary analyses indicated a potential PFS benefit with durvalumab plus olaparib in patients with HRRm. No new safety signals were observed with durvalumab plus olaparib compared with durvalumab alone. The results of the BAYOU study suggest a potential role for PARP inhibitors in the treatment of mUC with HRRm, and further investigation is warranted in both platinum-eligible and platinum-ineligible patients.
ACKNOWLEDGMENT
The authors would like to thank the patients, their families, and caregivers for their participation in the BAYOU study, the study investigators and staff at participating sites (Appendix 1, online only), and members of the independent data monitoring committee. The authors also thank Mary Li (formerly of AstraZeneca), Celina M. D'Cruz of AstraZeneca, and Erik T. Goluboff of AstraZeneca for contributions to study conception and design, data analysis, and data interpretation, and Zhongwu Lai of AstraZeneca for contributions to the biometric/molecular analyses. Medical writing and editorial assistance, in accordance with Good Publication Practice (GPP3) guidelines, were provided by Ward A. Pedersen, PhD, of Parexel and were funded by AstraZeneca.
APPENDIX 1. Participating Investigators
Cristiano Ferrario, Brindusa Mocanu, Som D. Mukherjee, Srikala Sridhar (Canada); Yoon Ji Choi, Young Saing Kim, Jae Lyun Lee, Se Hoon Park, Sang Joon Shin (Korea); Alexander Arkhipov, Vladimir Kheifets, Boris Komyakov, Evgeny Kopyltsov, Vadim Kozlov, Sergey Mishugin (Russian Federation); Urbano Anido Herranz, Daniel Castellano, Begoña Mellado González, Rafael Morales Barrera (Spain); Yi-Hsiu Huang, Jian-Ri Li, See-Tong Pang, Wen-Pin Su, Ying-Wen Su, Yu-Li Su, Yu-Chieh Tsai, Wen-Jeng Wu (Taiwan); Thomas Bradley, Nancy Davis, Sunil Gandhi, Benjamin Gartrell, Arash Rezazadeh Kalebasty, Jonathan E. Rosenberg, Ashish Sangal, Jingsong Zhang (United States); Tu V. Dao, Hung Quach, Sam Thai (Vietnam).
TABLE A1.
Overview of Safety
FIG A1.
OncoPrint of tumor gene mutations in the BAYOU study population. The OncoPrint illustrates the 20 most common gene mutations identified in tumors of patients enrolled in the BAYOU study. The two most common mutations were loss of p53 function (59.5%) and TERT promoter mutations (55.6%). A large number of tumors presented with defects in one or more genes involved chromatin remodeling: MLL2 (34%), KDM6A (25.5%), and ARID1A (12.4%), whereas mutations in the DNA damage repair genes ATM and BRCA2 were present in 8.5% and 4.6% of tumors, respectively. Loss of the cell cycle control genes CDKN2A and CDKN2B were identified in 32.7% and 28.1%, respectively; the adjacent MTAP gene was codeleted alongside both genes in 24.8% of tumors, suggesting loss of a significant region of chromosome 9. Common mutated driver genes were FGFR3 (20.3%), PIK3CA (19%), ERBB2 (12.4%), and KRAS/HRAS (11.1%), as previously described FGFR3 mutations were mutually exclusive with KRAS/HRAS mutations and with RB1, present in 13.7% of tumors. Trunc/FS, truncation/frameshift; VUS, variant of unknown significance.
FIG A2.
Subgroup analyses of PFS in the ITT population. aPrior treatment for an earlier stage of UC may have included neoadjuvant/adjuvant chemotherapy and bacillus Calmette-Guérin therapy. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; HRR, homologous recombination repair; HRRm, homologous recombination repair gene mutation; HRRwt, wild-type homologous recombination repair genes; ITT, intention-to-treat; PD-L1, programmed cell death ligand-1; PFS, progression-free survival; UC, urothelial carcinoma; VES, Vulnerable Elders Survey.
FIG A3.
PFS by gene mutation type in the HRRm subgroup. HRRm, homologous recombination repair gene mutation; PD, progressive disease; PFS, progression-free survival.
FIG A4.
Subgroup analyses of OS in the ITT population. aPrior treatment for an earlier stage of UC may have included neoadjuvant/adjuvant chemotherapy and bacillus Calmette-Guérin therapy. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; HRR, homologous recombination repair; HRRm, homologous recombination repair gene mutation; HRRwt, wild-type homologous recombination repair genes; ITT, intention-to-treat; NC, not calculable; OS, overall survival; PD-L1, programmed cell death ligand-1; VES, Vulnerable Elders Survey; UC, urothelial carcinoma.
FIG A5.
Graphical Summary of the BAYOU Study.
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Intellisphere, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seattle Genetics, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceutical, Alligator Bioscience
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
Se Hoon Park
Honoraria: Merck, Pfizer, Ono Pharmaceutical
Consulting or Advisory Role: Janssen Oncology
Research Funding: Ono Pharmaceutical, Sanofi
Tu V. Dao
Honoraria: AstraZeneca, Roche, Novartis, MSD, Pierre Fabre, Merck Serono
Consulting or Advisory Role: MSD, AstraZeneca, Novartis
Daniel Castellano
Consulting or Advisory Role: Janssen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Pfizer, Novartis, Ipsen, Bristol Myers Squibb, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre, Boehringer Ingelheim
Research Funding: Janssen Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb, AstraZeneca Spain
Som D. Mukherjee
Consulting or Advisory Role: Novartis/Pfizer, AstraZeneca Canada, EMD Serono/Merck
Travel, Accommodations, Expenses: Amgen
Kathryn Howells
Employment: Phastar
Stock and Other Ownership Interests: GlaxoSmithKline UK Ltd
Consulting or Advisory Role: AstraZeneca
Hannah Dry
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Mark C. Lanasa
Employment: AstraZeneca, BeiGene
Stock and Other Ownership Interests: AstraZeneca, BeiGene
Ross Stewart
Employment: AstraZeneca, Pfizer
Stock and Other Ownership Interests: AstraZeneca, Pfizer
Travel, Accommodations, Expenses: AstraZeneca
Dean F. Bajorin
Consulting or Advisory Role: Merck, Dragonfly Therapeutics, Fidia Farmaceutici SpA, Bristol Myers Squibb Foundation
Research Funding: Novartis (Inst), Merck (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst)
Travel, Accommodations, Expenses: Merck
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part during The American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO-GU), San Francisco, CA, + virtual, February 17-19, 2022.
SUPPORT
Supported by AstraZeneca. Supported in part by National Cancer Institute Cancer Center Support grant P30 CA008748.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data reported in this article may be obtained in accordance with AstraZeneca's data sharing policy available at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan E. Rosenberg, Kathryn Howells, Mark C. Lanasa, Dean F. Bajorin
Administrative support: Vadim Kozlov, Tu V. Dao
Provision of study materials or patients: Se Hoon Park, Tu V. Dao, Daniel Castellano, Jian-Ri Li, Som D. Mukherjee
Collection and assembly of data: Jonathan E. Rosenberg, Se Hoon Park, Vadim Kozlov, Tu V. Dao, Jian-Ri Li, Hannah Dry, Ross Stewart
Data analysis and interpretation: Jonathan E. Rosenberg, Se Hoon Park, Tu V. Dao, Daniel Castellano, Som D. Mukherjee, Kathryn Howells, Hannah Dry, Ross Stewart, Dean F. Bajorin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Durvalumab Plus Olaparib in Previously Untreated, Platinum-Ineligible Patients With Metastatic Urothelial Carcinoma: A Multicenter, Randomized, Phase II Trial (BAYOU)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Intellisphere, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seattle Genetics, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceutical, Alligator Bioscience
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
Se Hoon Park
Honoraria: Merck, Pfizer, Ono Pharmaceutical
Consulting or Advisory Role: Janssen Oncology
Research Funding: Ono Pharmaceutical, Sanofi
Tu V. Dao
Honoraria: AstraZeneca, Roche, Novartis, MSD, Pierre Fabre, Merck Serono
Consulting or Advisory Role: MSD, AstraZeneca, Novartis
Daniel Castellano
Consulting or Advisory Role: Janssen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Pfizer, Novartis, Ipsen, Bristol Myers Squibb, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre, Boehringer Ingelheim
Research Funding: Janssen Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb, AstraZeneca Spain
Som D. Mukherjee
Consulting or Advisory Role: Novartis/Pfizer, AstraZeneca Canada, EMD Serono/Merck
Travel, Accommodations, Expenses: Amgen
Kathryn Howells
Employment: Phastar
Stock and Other Ownership Interests: GlaxoSmithKline UK Ltd
Consulting or Advisory Role: AstraZeneca
Hannah Dry
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Mark C. Lanasa
Employment: AstraZeneca, BeiGene
Stock and Other Ownership Interests: AstraZeneca, BeiGene
Ross Stewart
Employment: AstraZeneca, Pfizer
Stock and Other Ownership Interests: AstraZeneca, Pfizer
Travel, Accommodations, Expenses: AstraZeneca
Dean F. Bajorin
Consulting or Advisory Role: Merck, Dragonfly Therapeutics, Fidia Farmaceutici SpA, Bristol Myers Squibb Foundation
Research Funding: Novartis (Inst), Merck (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst)
Travel, Accommodations, Expenses: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Koufopoulou M, Miranda PAP, Kazmierska P, et al. : Clinical evidence for the first-line treatment of advanced urothelial carcinoma: Current paradigms and emerging treatment options. Cancer Treat Rev 89:102072, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Powles T, van der Heijden MS, Castellano D, et al. : Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 21:1574-1588, 2020 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network : Clinical Practice Guidelines in Oncology: Bladder Cancer, Version 5.2021. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf [Google Scholar]
- 4.Pond GR, Agarwal A, Ornstein M, et al. : Clinical outcomes of platinum-ineligible patients with advanced urothelial carcinoma treated with first-line PD1/L1 inhibitors. Clin Genitourin Cancer 19:425-433, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Loriot Y, Necchi A, Park SH, et al. : Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 381:338-348, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Tran L, Xiao JF, Agarwal N, et al. : Advances in bladder cancer biology and therapy. Nat Rev Cancer 21:104-121, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Börcsök J, Diossy M, Sztupinszki Z, et al. : Detection of molecular signatures of homologous recombination deficiency in bladder cancer. Clin Cancer Res 27:3734-3743, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heeke AL, Pishvaian MJ, Lynce F, et al. : Prevalence of homologous recombination-related gene mutations across multiple cancer types. JCO Precis Oncol 2018:1-13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimar KJ, Tran PT, Matulewicz RS, et al. : The emerging role of homologous recombination repair and PARP inhibitors in genitourinary malignancies. Cancer 123:1912-1924, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peyraud F, Italiano A: Combined PARP inhibition and immune checkpoint therapy in solid tumors. Cancers (Basel) 12:1502, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweis RF, Heiss B, Segal J, et al. : Clinical activity of olaparib in urothelial bladder cancer with DNA damage response gene mutations. JCO Precis Oncol 2:1-7, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Powles T, O’Donnell PH, Massard C, et al. : Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 3:e172411, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai Z, Brosnan M, Sokol ES, et al. : Landscape of homologous recombination deficiencies in solid tumours: Analyses of two independent genomic datasets. BMC Cancer 22:13, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ventana Medical Systems : VENTANA PD-L1 (SP263) Assay, 2017. https://www.accessdata.fda.gov/cdrh_docs/pdf16/p160046c.pdf [Google Scholar]
- 15.Hayashi T, Fujita K, Hayashi Y, et al. : Mutational landscape and environmental effects in bladder cancer. Int J Mol Sci 21:6072, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellmunt J, Paz-Ares L, Cuello M, et al. : Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol 18:522-528, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kim KH, Do I-G, Kim HS, et al. : Excision repair cross-complementation group 1 (ERCC1) expression in advanced urothelial carcinoma patients receiving cisplatin-based chemotherapy. APMIS 118:941-948, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Mullane SA, Werner L, Guancial EA, et al. : Expression levels of DNA damage repair proteins are associated with overall survival in platinum-treated advanced urothelial carcinoma. Clin Genitourin Cancer 14:352-359, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouw KW: DNA repair pathway alterations in bladder cancer. Cancers (Basel) 9:28, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teo MY, Bambury RM, Zabor EC, et al. : DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res 23:3610-3618, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crabb SJ, Hussain SA, Soulis E, et al. : A randomized, double blind, biomarker selected, phase II clinical trial of maintenance PARP inhibition following chemotherapy for metastatic urothelial carcinoma (mUC): Final analysis of the ATLANTIS rucaparib arm. J Clin Oncol 40, 2022. (suppl 6; abstr 436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vignani F, Tambaro R, De Giorgi U, et al. : Randomized phase II study of niraparib plus best supportive care (BSC) versus BSC alone as maintenance treatment in patients with advanced urothelial carcinoma (UC) whose disease did not progress after first-line platinum-based chemotherapy (PBCT): The Meet-URO12 trial. J Clin Oncol 40, 2022. (suppl 6; abstr 442) [DOI] [PubMed] [Google Scholar]
- 23.Grivas P, Loriot Y, Morales-Barrera R, et al. : Efficacy and safety of rucaparib in previously treated, locally advanced or metastatic urothelial carcinoma from a phase 2, open-label trial (ATLAS). BMC Cancer 21:593, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 25.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Moreno JF, de Velasco G, Bravo Fernandez I, et al. : Impact of the combination of durvalumab (MEDI4736) plus olaparib (AZD2281) administered prior to surgery in the molecular profile of resectable urothelial bladder cancer: NEODURVARIB trial. J Clin Oncol 38, 2020. (suppl 6; abstr 542) [Google Scholar]
- 27.Powles T, Carroll D, Chowdhury S, et al. : An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat Med 27:793-801, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Rose TL, Deal AM, Nielsen ME, et al. : Sex disparities in use of chemotherapy and survival in patients with advanced bladder cancer. Cancer 122:2012-2020, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonpavde G, Watson D, Tourtellott M, et al. : Administration of cisplatin-based chemotherapy for advanced urothelial carcinoma in the community. Clin Genitourin Cancer 10:1-5, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Balar AV, Galsky MD, Rosenberg JE, et al. : Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 389:67-76, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balar AV, Castellano D, O’Donnell PH, et al. : First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 18:1483-1492, 2017 [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell PH, Balar AV, Vuky J, et al. : First-line pembrolizumab in cisplatin-ineligible patients with advanced urothelial cancer: Response and survival results up to 5 years from the KEYNOTE-052 phase 2 study. J Clin Oncol 39, 2021. (suppl 15; abstr 4508) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data reported in this article may be obtained in accordance with AstraZeneca's data sharing policy available at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.