Abstract
A previous study had shown that the expression of gp90, a stage-specific surface glycoprotein of Trypanosoma cruzi metacyclic trypomastigotes, is inversely correlated with the parasite's ability to invade mammalian cells. By using antisense oligonucleotides complementary to a region of the gp90 gene implicated in host cell adhesion, we investigated whether the selective inhibition of gp90 synthesis affected the capacity of metacyclic forms to enter target cells. Parasites were incubated for 24 h with 20 μM PS1, a phosphorothioate oligonucleotide based on a sequence of the gp90 coding strand; PS2, the antisense counterpart of PS1; or PO2, the unmodified version of PS2 containing phosphodiester linkages, and the expression of surface molecules was analyzed by flow cytometry and immunoblotting using specific monoclonal antibodies. PS2 but not PS1 or PO2 inhibited the expression of gp90. Inhibition by PS2 was dose dependent. Northern blot analysis revealed that steady-state gp90 mRNA levels were diminished in PS2-treated parasites compared to untreated controls. Treatment with PS2 did not affect the expression of other metacyclic stage surface glycoproteins involved in parasite-host cell interaction, such as gp82 and the mucin-like gp35/50. Expression of gp90 was also inhibited by other phosphorothioate oligonucleotides targeted to the gp90 gene (PS4, PS5, PS6, and PS7) but not by PS3, with the same base composition as PS2 but a mismatched sequence. Parasites treated with PS2, PS4, or PS5 entered HeLa cells in significantly higher numbers than untreated controls, whereas the invasive capacity of PS1- and PS3-treated parasites was unchanged, confirming the inverse association between infectivity and gp90 expression.
A critical step for the establishment of infection by Trypanosoma cruzi, the agent of Chagas' disease, is the invasion of host cells by metacyclic trypomastigotes, the developmental forms from the insect vector. We have found in a previous study that gp90, a metacyclic stage-specific surface glycoprotein, is differentially expressed in different T. cruzi strains and that the parasite's ability to invade target cells is inversely correlated with gp90 expression (17). gp90 binds to mammalian cells in a receptor-mediated manner without triggering Ca2+ signal, a requirement for T. cruzi entry into target cells (6, 12, 21, 25), in contrast to the interaction mediated by gp82, the metacyclic stage surface glycoprotein implicated in host cell invasion (15), which induces Ca2+ mobilization in both the parasite and the target cell (6, 17).
Recent studies aimed at defining the function of protozoan parasite components have used approaches such as gene disruption by homologous recombination and antisense RNA. By gene knockout, the surface molecules of malaria parasites circumsporozoite protein (CS) and thrombospondin-related anonymous protein (TRAP) have been shown to play a critical role in development in mosquitoes (11) and for infection of the liver (20). In Toxoplasma gondii, the targeted inhibition of nucleoside triphosphate hydrolase by antisense RNA blocked parasite proliferation (13). The T. cruzi gp90 gene is not amenable to gene knockout by homologous recombination because it is present in multiple copies in the genome (8), but the antisense strategy appears to be an interesting possibility. Antisense oligodeoxynucleotides designed to function as inhibitors of specific mRNAs and made resistant to nucleases by introduction of modifications such as phosphorothioate linkages have been used for gene inhibition in mammalian cells in tissue culture assays and in vivo studies (1, 23). Using such an approach, antiparasitic activities of antisense oligonucleotides towards obligatory intracellular protozoans have been reported. By treating macrophages infected with Leishmania amazonensis for 24 h with phosphorothioate oligonucleotide targeted to the miniexon sequence present at the 5′ end of every parasite mRNA, cure of about 30% of infected macrophages was observed (14). Rapaport et al. (16) demonstrated that the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum is a good target for sequence-dependent inhibition of plasmodial growth by exogenously administered phosphorothioate oligonucleotides.
In the present study, we used antisense oligonucleotides targeted to gp90 gene sequences in addition to control oligonucleotides to determine the effect of this treatment on metacyclic trypomastigote gp90 expression and on the the parasite's ability to enter host cells.
MATERIALS AND METHODS
Oligodeoxynucleotides.
The following phosphorothioate oligonucleotides were used: PS1, based on a sequence of the gp90 coding strand (5′-GTGGCGTCGGTGACC ATCGAAGAG-3′); PS2, corresponding to a sequence complementary to PS1 (5′-CTCTTC GATGGTCACCGACGCCAC-3′); PS3, with the same base composition as PS2 but with a scrambled sequence (5′-TTCCCTGTGTAGGCCAGCCCAACC-3′); and PS4 PS5, PS6, and PS7, complementary to different sequences of the g90 coding strand, with the following base compositions, respectively: 5′-TGTGAAGTTGTGGCTCAAGGATAC-3′, 5′-AGCGTCCGCACTCGGAGCCTCTTC-3′, 5′-TTGGTATTCTTTCCCCGG-3′, and 5′-GCCATCAACGTACACCGAGCTCTTGTTACC-3′. In addition, PO2, the unmodified version of PS2 containing phosphodiester linkages, was tested.
Parasites, mammalian cells, and cell invasion assay.
T. cruzi strain G (26) was used throughout this study. Parasites were maintained alternately in mice and in liver infusion tryptose medium (LIT) containing 5% fetal calf serum. Metacyclic trypomastigotes harvested from LIT cultures at the stationary growth phase were purified by passage through a DEAE-cellulose column as described (22). HeLa cells, human carcinoma-derived epithelial cells, were grown at 37°C in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml) in a humidified 5% CO2 atmosphere. Experiments for mammalian cell invasion by T. cruzi were performed essentially as previously described (27).
MAbs.
The following monoclonal antibodies (MAbs) were used: 1G7, 3F6, and 10D8, directed to T. cruzi surface glycoproteins gp90, gp82, and the mucin-like gp35/50, respectively (15, 27, 28). Unrelated MAb 1C3, directed to Leishmania amazonensis gp63 (3), was kindly provided by Clara Lucia Barbieri, Universidade Federal de São Paulo.
Purification of native and recombinant gp90.
To purify the native gp90, parasite extracts were prepared by treating metacyclic trypomastigotes with 0.5% Nonidet P-40 in the presence of protease inhibitors. After centrifugation at 12,000 × g for 5 min, the supernatant was collected and mixed with the antibody affinity column prepared by coupling MAb 1G7 to CNBr-activated Sepharose 4B (Amersham/Pharmacia). After 2 h at room temperature under constant shaking, the resin was packed in a 10-ml plastic syringe and washed several times with phosphate-buffered saline (PBS). The bound antigen was eluted with 0.1 M glycine, pH 2.8, neutralized, dialyzed against double-distilled water, vacuum dried, and stored at −20°C until use. To purify the recombinant protein containing the gp90 C-terminal domain in fusion with glutathione S-transferase (GST) (8), suspensions of isopropyl-β-d-thiogalactopyranoside (IPTG) induced Escherichia coli were washed in PBS, sonicated for 10 min (1-min pulse at 30-s intervals), and centrifuged at 12,000 × g for 10 min at 4°C. The precipitate was resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled, and subjected to SDS-PAGE. The gels were treated with iced 250 mM KC1 to visualize the bands on a black background. By using molecular size markers ranging from 94 to 14 kDa (Amersham Pharmacia) as a reference, the band of high intensity and of the expected size for the recombinant protein was excised and electroeluted as described (24), using a buffer containing 25 mM Tris-HC1, 192 mM glycine-HCl, and 20% methanol. The electroeluted sample was dialyzed against 10 mM ammonium bicarbonate for 24 h and thereafter against distilled water for another 24 h. The purity of the isolated protein was assessed by staining the SDS-PAGE gel with Coomassie blue and by immunoblotting using MAb 1G7.
Immunoblotting.
Parasites were lysed with 1.0% nonionic detergent Nonidet P-40, the lysates were dissolved in loading buffer, and equal amounts of the parasite extract (∼10 μg) were loaded into SDS–10% PAGE gels. After electrophoresis under reducing conditions, the proteins were transferred to nitrocellulose membranes. The equal loading of parasite samples was monitored by staining the nitrocellulose membranes with 0.1% (wt/vol) Ponceau S in 10% acetic acid. Following blockage with PBS containing 7.5% defatted milk (PBS-milk), the membranes were incubated for 1 h at room temperature with anti-T. cruzi MAbs diluted in PBS-milk. After several washes in PBS containing 0.05% Tween 20, the membranes were further incubated with anti-mouse immunoglobulin G (IgG) conjugated to peroxidase. The final reaction was revealed with diaminobenzidine and H2O2 (27).
RNA purification and Northern blot analysis.
Purified metacyclic trypomastigotes were lysed with 1 ml of Trizol reagent (Gibco-BRL). Following complete dissolution and addition of 0.2 ml of chloroform, the parasite preparation was centrifuged for 15 min at 14,000 × g to recover the aqueous phase. An equal volume of isopropyl alcohol was added to the aqueous phase to precipitate the total RNA overnight at −20°C. The precipitate was washed with 70% ethanol and resuspended in RNase-free water. For Northern blot analysis, equivalent amounts of RNA purified from untreated and oligonucleotide-treated parasites were denatured with 50% formamide and 2.2 M formaldehyde and subjected to electrophroresis in a 1.0% agarose gel containing formaldehyde. After staining with ethidium bromide, the RNA was blotted onto a nylon membrane (Life Technologies) in 20× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) and immobilized on the filter by UV irradiation. The membrane was prehybridized in 50% formamide–5× SSC–5× Denhardt's solution–0.5% SDS–5 mM EDTA– 0.1 mg of tRNA per ml at 42°C for 2 h and then hybridized overnight at the same temperature with 32P-labeled probe which consisted of the insert of the gp90 cDNA clone (8 or β-tubulin. Following hybridization, the membrane was washed in 2× SSC containing 0.1% SDS at 65°C three times for 30 min each and exposed to X-ray film (Hyperfilm-MP; Amersham).
Inhibition of binding of native gp90 to HeLa cells with the recombinant protein.
HeLa cells (3 × 104) placed in 96-well microtiter plates (Costar) were grown overnight at 37°C. After fixation with 4% paraformaldehyde in PBS, the cells were washed with PBS and blocked with PBS containing 10% fetal calf serum (FCS-PBS) for 1 h at room temperature. The cells were then incubated for 1 h at 37°C with the native gp90 (20 μg/ml) in FCS-PBS in the absence or presence of various concentrations of the recombinant protein fused to GST. Controls included HeLa cells incubated with gp90 in the presence of GST or J18b, the GST-fused recombinant protein containing the C-terminal domain of gp82 (19). After washes in PBS, the HeLa cells were sequentially incubated for 1 h at 37°C with MAb 1G7 diluted in FCS-PBS and anti-mouse IgG conjugated to peroxidase. Following washes in PBS, the bound enzyme was revealed using o-phenylenediamine as detailed (18).
Flow cytometry.
Live metacyclic trypomastigotes (2 × 107 cells) were incubated for 1 h on ice with MAbs directed to metacyclic stage surface molecules or with unrelated MAb 1C3. After washes in PBS, the parasites were fixed with 2% paraformaldehyde in PBS for 30 min. The fixative was washed out, and the parasites were incubated with fluorescein-labeled goat anti-mouse IgG for 1 h at room temperature. Following two more washes, the number of fluorescent parasites was estimated with a Becton Dickinson cytometer.
RESULTS
Antisense oligonucleotides based on sequences of a cDNA clone encoding the C-terminal domain of gp90 which contains the host cell binding site.
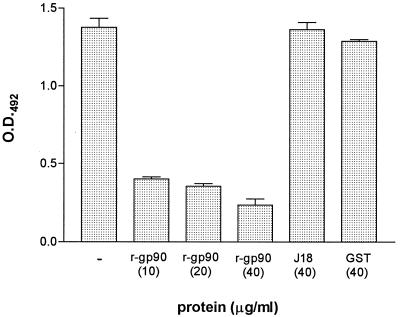
The sequence of gp90 that is currently known is that deduced from a cDNA clone corresponding to the C-terminal domain of the molecule (8). On the basis that this sequence, which is a member of a family of related sequences present in the parasite genome, encodes a recombinant protein that reacts with an MAb as well as with monospecific polyclonal antibodies directed to the native gp90 (8), we presumed that it is represented in the gp90 gene(s) actually expressed in metacyclic trypomastigotes. To determine whether the cDNA-encoded recombinant protein which binds to mammalian cells in the same manner as the native gp90 (17) was capable of inhibiting the adhesion of gp90 to host cells, assays were carried out by incubating HeLa cells for 1 h at 37°C with the native gp90 at 20 μg/ml in the absence or presence of various concentrations of gp90 recombinant protein (r-gp90) fused to GST. As controls, HeLa cells were incubated with the native gp90 plus GST or J18b at 40 μg/ml the (J18b is the GST-fused recombinant protein containing the C-teminal domain of metacyclic stage surface molecule gp82, which binds to HeLa cells through a sequence that is not represented in r-gp90) (10). Binding of gp90 to HeLa cells was revealed by MAb 1G7, which recognizes the native molecule but not r-gp90. As shown in Fig. 1, r-gp90 inhibited the binding of native gp90 to HeLa cells in a dose-dependent fashion, whereas GST and J18 did not show any inhibitory effect. Reaction of parallel wells of the enzyme-linked immunosorbent assay plate with anti-GST antibody revealed the binding of r-gp90 and J18 to HeLa cells (data not shown).
FIG. 1.
Adhesion of metacyclic trypomastigote surface glycoprotein gp90 to host cells is inhibited by the recombinant protein containing the C-terminal domain of the molecule. The assay was carried out as described in the text by incubating HeLa cells for 1 h at 37°C with native gp90 at 20 μg/ml in the absence or presence of the indicated concentrations of r-gp90. HeLa cells incubated with gp90 plus GST or J18b, the recombinant protein containing the C-teminal domain of gp82, served as controls. The binding of gp90 was revealed by MAb 1G7, which recognizes the native molecule but not the recombinant protein. Representative results of one of three experiments are shown. Values are the means ± standard deviation (SD) of triplicates.
To design the antisense oligonucleotides targeted to the gp90 gene, we selected sequences displaying low similarity with those of the gp82 gene and sequences common to both genes (Fig. 2A). The target and the various oligonucleotides containing phosphorothioate linkages used in this study are schematically represented in Fig. 2B.
FIG. 2.
Oligonucleotides and target gp90 gene encoding the T. cruzi metacyclic trypomastigote surface glycoprotein. (A) Nucleotide sequence of gp90 analyzed in this study, aligned with that of gp82 for comparison. The regions on which the oligonucleotides complementary to gp90 were based are indicated (PS2, PS4, PS5, PS6, and PS7). (B) Schematic representation of the currently known portion of the gp90 gene, which corresponds to that encoding the C-terminal domain of the molecule. The arrows represent the various phosphorothioate oligonucleotides (not drawn to scale): PS1, based on a sequence of the gp90 coding strand; PS2, the antisense counterpart of PS1; PS3, with the same base composition as PS2 but mismatched sequence; and PS4 to PS7, complementary to different gp90 sequences.
Expression of gp90 reduced in metacyclic trypomastigotes treated with antisense oligonucleotides targeted to the corresponding gene.
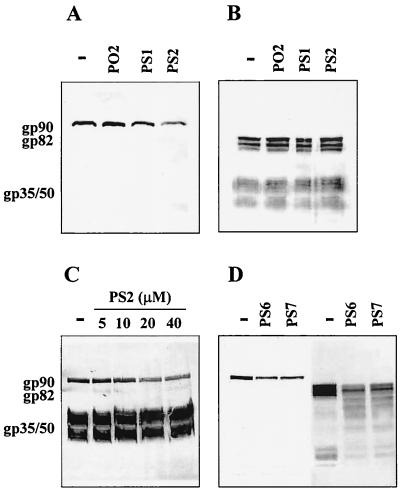
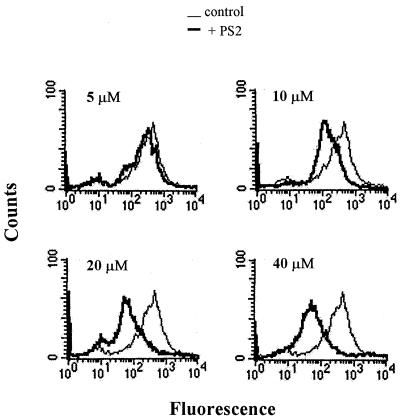
To determine whether the expression of gp90 could be blocked by the antisense approach, 4 × 107 purified metacyclic trypomastigotes were incubated for 24 h at 28°C in 0.2 ml of LIT medium in absence or presence of 20 μM PS1, a phosphorothioate oligonucleotide based on a sequence of the gp90 coding strand; PS2, the antisense counterpart of PS1; or PO2, the unmodified version of PS2 containing phosphodiester linkages. Upon treatment with the different oligonucleotides, the parasites were processed for immunoblot detection of the major metacyclic stage surface glycoproteins gp90, gp82, and gp35/50 using specific MAbs. As shown in Fig. 3A, the expression of gp90 was markedly diminished in PS2-treated metacyclic forms compared to untreated and PS1- or PO2-treated parasites. When quantified by densitometry, the intensity of the gp90 band in PS2-treated parasites was ∼50% lower than that of the controls. The expression of gp82 or gp35/50 was not significantly affected by any of these oligonucleotides (Fig. 3B). PS2 inhibited gp90 expression in a dose-dependent manner (Fig. 3C). At 5 μM, the inhibitory effect of PS2 was minimal and increased progressively at 10 and 20 μM. Further reduction in gp90 levels was not apparent in parasites treated with 40 μM PS2. This inhibitory effect of PS2 was specific inasmuch as the intensity of the gp35/50 bands in the same blot was unaltered (Fig. 3C). When metacyclic forms were treated with oligonucleotide PS6 or PS7 at 20 μM (Fig. 2), with sequences common to the gp90 and gp82 genes (matching 94 and 89%, respectively), the expression of both molecules was reduced (Fig. 3D).
FIG. 3.
Antisense oligonucleotide PS2 inhibits gp90 expression in T. cruzi metacyclic trypomastigotes. Parasites, either untreated (—) or treated for 24 h with PS1, a phosphorothioate oligonucleotide based on a sequence of the gp90 coding strand; PS2, the antisense counterpart of PS1; or PO2, the unmodified version of PS2 containing phosphodiester linkages were processed for immunoblotting. (A) MAb directed to gp90; (B) MAbs directed to gp82 and gp35/50. Note the specific inhibition of gp90 expression by PS2. (C) Parasites, untreated (—) or treated with the indicated concentrations of PS2, were analyzed by immunobloting using MAbs to gp90 and gp35/50. Note that the expression of gp90 was reduced with increasing doses of PS2, whereas that of gp35/50 remained unaltered. The difference in the intensity of the gp35/50 bands in panels B and C is due to the use of different batches of anti-gp35/50 MAb. (D) Parasites, untreated (—) or treated with antisense oligonucleotide PS6 or PS7, with sequences common to gp90 and gp82 genes, respectively, were processed for immunoblotting with MAbs to gp90 or gp82, respectively.
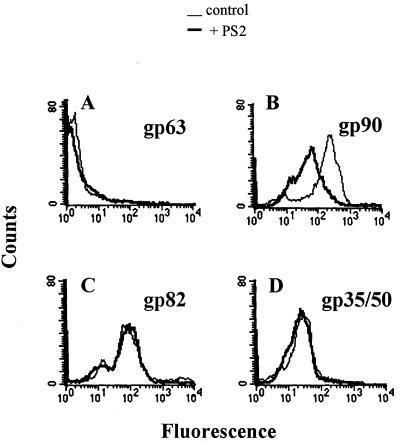
Next, we determined the amounts of gp90, gp82, and gp35/50 on the parasite surface. Live metacyclic forms, untreated or treated with oligonucleotide, were incubated with unrelated or specific MAb. After washes, fixation with paraformaldehyde, and reaction with fluorescein-conjugated anti-mouse IgG, the parasites were analyzed by flow cytometry. The levels of gp90 but not of gp82 or gp35/50 on the parasite surface were reduced in PS2-treated metacyclic forms (Fig. 4). Inhibition by PS2 was dose dependent (Fig. 5). In PS1-treated parasites, the expression of the three surface glycoproteins was unaltered (data not shown).
FIG. 4.
gp90 levels on the surface of T. cruzi metacyclic trypomastigotes are selectively reduced by the antisense oligonucleotide PS2. Parasites, untreated or treated with PS2, a phosphorothioate oligonucleotide complementary to a sequence of the gp90 coding strand, were incubated with (A) unrelated MAb 1C3, directed to Leishmania gp63, (B) anti-gp90 MAb 1G7, (C) anti-gp82 MAb 3F6, or (D) anti-gp35/50 MAb 10D8. After paraformaldehyde fixation and reaction with fluorescein-labeled anti-mouse IgG, the parasites were analyzed by flow cytometry.
FIG. 5.
Dose-dependent inhibition of gp90 expression by treatment of T. cruzi metacyclic trypomastigotes with antisense oligonucleotide PS2. Parasites were treated with the indicated concentrations of PS2, and the levels of gp90 on their surface were compared with those of untreated controls by flow cytometry using anti-gp90 MAb 1G7.
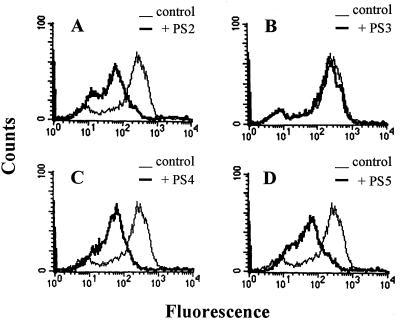
To further confirm the sequence-specific gp90 gene inhibition, the following additional phosphorothioate oligonucleotides were tested: PS3, with the same base composition as PS2 but mismatched sequence, and PS4 and PS5, which are complementary to sequences located upstream and downstream of PS1, respectively (Fig. 2). Metacyclic trypomastigotes were incubated for 24 h with 20 μM PS2, PS3, PS4, or PS5, and gp90 expression was analyzed by flow cytometry using MAb 1G7. As shown in Fig. 6, gp90 levels were significantly decreased in PS4- and PS5-treated metacyclic forms, in a manner similar to the effect obtained with PS2, whereas gp90 expression in PS3-treated parasites remained unchanged.
FIG. 6.
gp90 sequence-specific antisense oligonucleotides PS4 and PS5 affect T. cruzi metacyclic trypomastigote gp90 expression in the same manner as PS2. Parasites, untreated or treated with (A) PS2, (B) PS3, with a mismatched PS2 sequence, (C) PS4, or (D) PS5, which are complementary to gp90 sequences flanking PS1 (Fig. 2), were incubated with the anti-gp90 MAb 1G7. After paraformaldehyde fixation and reaction with fluorescein-labeled anti-mouse IgG, the parasites were analyzed by flow cytometry.
Incubation of metacyclic trypomastigotes with any of these oligonucleotides for 5 to 6 h did not result in significant inhibition of gp90 levels. A longer incubation time (48 h) with the antisense oligonucleotide was not more effective than 24 h of incubation in reducing the expression of gp90. This may be associated with the slow turnover of gp90. We treated the parasites for 30 min at 37°C with proteinase K, which greatly reduces the expression of gp90. After washes in PBS, the parasites were maintained in culture medium, and at various times samples were taken to determine the recovery of gp90 expression. Even at 20 h posttreatment, gp90 expression did not reach pretreatment levels (data not shown), indicating that its turnover rate is low. Attempts to improve the delivery of oligonucleotide by using cationic lipids, which reportedly enhance cellular uptake of phosphorothioate oligonucleotides (4), failed to augment the efficiency in inhibiting gp90 expression (data not shown).
Antisense oligonucleotide targeted to gp90 is taken up by metacyclic trypomastigotes and decreases the steady-state levels of gp90 mRNA.
To ascertain the incorporation of the oligonucleotide, the parasites were incubated for 20 h with various concentrations of the fluorescein-labeled version of PS2, fixed with paraformaldehyde, washed in PBS, and analyzed by flow cytometry. As shown in Fig. 7A, the intensity of fluorescence was higher in PS2-treated parasites than in untreated controls and increased with increasing PS2 concentration. Assays performed with fluorescein-labeled PS6 showed essentially the same results (data not shown). Staining was more intense in the parasite nucleus (data not shown).
FIG. 7.
Incorporation of antisense oligonucleotide targeted to gp90 gene by metacyclic trypomastigotes results in diminished steady-state levels of gp90 mRNA. Parasites were incubated with the indicated concentrations of fluorescein-labeled antisense oligonucleotide PS2, fixed in paraformaldehyde, and analyzed by flow cytometry (A). Total RNA extracted from parasites either untreated (—) or treated with oligonucleotide PS2 was fractionated on formaldehyde-containing 1% agarose gels, transferred to a nylon membrane, and probed successively with the 32P-labeled insert of the gp90 cDNA clone and the β-tubulin coding sequence (B).
In experiments to determine the steady-state levels of the transcripts corresponding to gp90, total RNA was extracted from PS2-treated and untreated parasites for Northern blot analysis. We found that gp90 mRNA was diminished in PS2-treated parasites compared to untreated controls, whereas β-tubulin mRNA was unaffected (Fig. 7B).
Infectivity of metacyclic trypomastigotes increases upon treatment with antisense oligonucleotides targeted to the gp90 gene.
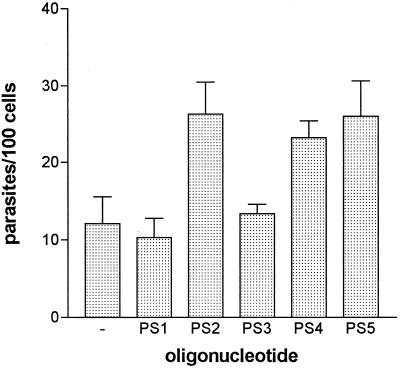
To investigate the effect of decrease in gp90 expression on parasite infectivity, cell invasion assays were performed with metacyclic trypomastigotes subjected to 24 h of treatment with the various oligonucleotides. Parasites treated with PS2, PS4, or PS5 invaded HeLa cells in significantly higher numbers than untreated controls (Fig. 8). By contrast, the infectivity of metacyclic forms treated with PS1 or PS3 remained unchanged (Fig. 8).
FIG. 8.
Metacyclic trypomastigotes with reduced gp90 expression have an increased ability to invade host cells. Parasites, untreated (—) or treated for 24 h with the indicated phosphorothioate oligonucleotide, were incubated with HeLa cells for 3 h at 37°C. After washes in PBS and staining with Giemsa, the number of intracellular parasites was counted in a total of 500 Giemsa-stained cells. Values are means ± SD of four experiments performed in duplicate. The parasites treated with PS2, PS4, or PS5 entered HeLa cells in significantly higher numbers (P < 0.01) compared to the untreated control by Student's t test.
DISCUSSION
Our results suggest that the infectivity of T. cruzi metacyclic trypomastigotes is downregulated by the surface glycoprotein gp90. By treating metacyclic forms with antisense phosphorothioate oligonucleotides complementary to sequences of the gp90 gene, the gp90 levels on the parasite surface were reduced (Fig. 3 to 6) and the parasite ability to invade host cells was significantly increased (Fig. 8). Such effects were not observed when the parasites were treated with sense or mismatched oligonucleotides, indicating that the inhibition by antisense oligonucleotides is sequence specific. This finding is compatible with our previous observation that T. cruzi strains expressing high gp90 levels are poor invaders of mammalian cells, whereas gp90 is barely detectable in highly invasive strains (17).
It should be noted that the expression of gp35/50, also present on the T. cruzi surface, was not affected by the antisense oligonucleotides targeted to the gp90 gene. This lack of inhibition on the genes encoding gp35/50 molecules was expected, for these belong to the mucin gene family (5, 9), quite distinct from that comprising the gp90 gene. However, the possibility existed that the expression of gp82, a metacyclic trypomastigote stage-specific glycoprotein with adhesive properties (15), was altered, provided that the genes coding for gp82 and gp90 are members of the same multigene family (2). When that undesired inhibitory effect was precluded by designing antisense oligonucleotides based on sequences of the gp90 gene with a minimal homology with the gp82 gene (Fig. 2), gp90 expression was specifically diminished (Fig. 3A and 4). On the other hand, treatment of metacyclic trypomastigotes with antisense oligonucleotides with sequences common to the gp90 and gp82 genes affected the expression of both molecules (Fig. 3D).
We have found that oligonucleotides targeted to the gp90 gene are taken up by metacyclic trypomastigotes in a dose-dependent manner (Fig. 7A) and appear to concentrate in the parasite nucleus (data not shown). This results in reduced levels of steady-state gp90 mRNA in oligonucleotide-treated parasites (Fig. 7B).
If the expression of gp90 is specifically inhibited in metacyclic trypomastigotes treated with antisense oligonucleotides and this leads to an increase in parasite infectivity, it is reasonable to assume that gp90 is playing a modulatory role in the process of host cell invasion. How could gp90 negatively affect the infectivity of metacyclic trypomastigotes? We had previously postulated that gp82 plays a key role in host cell invasion by inducing a Ca2+ response both in the target cell and in the parasite (17), an event that is required for parasite internalization (6, 12, 21). As gp90 is also a cell adhesion molecule, the presence of high levels of this molecule on the parasite surface could impair the binding of gp82 to target cells. In that case, the reduction in gp90 levels could increase the gp82-mediated interaction of metacyclic trypomastigotes with host cells, leading to increased internalization. Our present resuls are in accord with this proposition.
We have recently mapped the host cell binding site of gp82 (10). It is located in the C-terminal domain of the molecule, in a region not equivalent to the gp90 C-terminal domain, which contains the host cell binding site. Consistent with this, r-gp90 but not r-gp82, each containing the C-terminal region of the molecule, was capable of inhibiting the adhesion of native gp90 to HeLa cells (Fig. 1). Taken together, the present data and our previous findings suggest that gp90 and gp82 interact with distinct host cell receptors. Binding of gp82 to its receptor induces an increase in the internal Ca2+ concentration in host cells. On the other hand, in metacyclic trypomastigotes, the gp82-parasite interaction triggers a signaling cascade that involves activation of a protein tyrosine kinase and culminates in Ca2+ mobilization (7, 29). By contrast, binding of gp90 to its receptor is unable to induce the Ca2+ response either in the host cell or in the parasite (17), so that this interaction is unproductive as far as cell invasion is concerned.
ACKNOWLEDGMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
We thank José Franco da Silveira for critical reading of the manuscript and Patricio M. Manque for help in flow cytometry experiments.
REFERENCES
- 1.Agrawal S. Importance of nucleotide sequence and chemical modifications of antisense oligonucleotides. Biochim Biophys Acta. 1999;1489:53–68. doi: 10.1016/s0167-4781(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 2.Araya J E, Cano M I, Yoshida N, Franco da Silveira J. Cloning and characterization of a gene for the stage-specific 82 kDa surface antigen of metacyclic trypomastigotes of Trypanoma cruzi. Mol Biochem Parasitol. 1994;65:161–169. doi: 10.1016/0166-6851(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 3.Barbiéri C L, Giorgio S, Merjan A J C, Figueiredo E N. Glycosphingolipid antigens of Leishmania (Leishmania) amazonensis amastigotes idientified by use of a monoclonal antibody. Infect Immun. 1993;61:2131–2137. doi: 10.1128/iai.61.5.2131-2137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett C F, Chiang M, Chan H, Shoemaker J E E, Mirabelli C K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1992;41:1023–1033. [PubMed] [Google Scholar]
- 5.Di Noia J M, Pollevick G D, Xavier M T, Previato J O, Mendonça-Previato L, Sanchez D O, Frasch A C C. High diversitty of mucin genes and mucin molecules in Trypanoma cruzi. J Biol Chem. 1996;271:32078–32083. doi: 10.1074/jbc.271.50.32078. [DOI] [PubMed] [Google Scholar]
- 6.Dorta M L, Ferreira A T, Oshiro M E M, Yoshida N. Ca2+ signal induced by Trypanoma cruzi metacyclic trypomastigote surface molecules implicated in mammalian cell invasion. Mol Biochem Parasitol. 1995;73:285–289. doi: 10.1016/0166-6851(94)00123-5. [DOI] [PubMed] [Google Scholar]
- 7.Favoreto S, Dorta M L, Yoshida N. Trypanoma cruzi 175-kDa protein tyrosine phosphorylation is associated with host cell invasion. Exp Parasitol. 1998;89:188–194. doi: 10.1006/expr.1998.4285. [DOI] [PubMed] [Google Scholar]
- 8.Franco F R S, Paranhos-Bacalla G, Yamauchi L M, Yoshida N, Franco da Silveira J. Characterization of a cDNA clone encoding the carboxy-terminal domain of a 90-kilodalton surface antigen of Trypanoma cruzi metacyclic trypomastigotes. Infect Immun. 1993;61:4196–4201. doi: 10.1128/iai.61.10.4196-4201.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freitas-Junior L H, Briones M R, Schenkman S. Two distinct groups of mucin-like genes are differentially expressed in the developmental stages of Trypanoma cruzi. Mol Biochem Parasitol. 1998;93:101–114. doi: 10.1016/s0166-6851(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 10.Manque P M, Eichinger D, Juliano M A A, Juliano L, Araya J E, Yoshida N. Characterization of the cell adhesion site of Trypanoma cruzi metacyclic stage surface glycoprotein gp82. Infect Immun. 2000;68:478–484. doi: 10.1128/iai.68.2.478-484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ménard R, Sultan A A, Cortes C, Autszuler R, Dijk M R, Janse C, Waters A P, Nussenzweig R S, Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- 12.Moreno S N J, Silva J, Vercesi A E, Docampo R. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med. 1994;180:1535–1540. doi: 10.1084/jem.180.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakaar V, Samuel B U, Ngo E O, Joiner K A. Targeted reduction of nucleotide triphosphate hydrolase by antisense RNA inhibits Toxoplasma gondii proliferation. J Biol Chem. 1999;274:5083–5087. doi: 10.1074/jbc.274.8.5083. [DOI] [PubMed] [Google Scholar]
- 14.Ramazeilles C, Mishra R K, Moreau S, Pascolo S E, Toulmé J J. Antisense phosphorothioate oligonucleotides: selective killing of the intracellular parasite Leishmania amazonensis. Proc Natl Acad Sci USA. 1994;91:7859–7863. doi: 10.1073/pnas.91.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez M I, Ruiz R C, Araya J E, Franco da Silveira J, Yoshida N. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanoma cruzi metacyclic trypomastigotes in host cell invasion. Infect Immun. 1993;61:3636–3641. doi: 10.1128/iai.61.9.3636-3641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapaport E, Misiura K, Agrawal S, Zamecnik P. Antimalarial activities of oligodeoxynucleotide phosphorothioates in chloroquine-resistant Plasmodium falciparum. Proc Natl Acad Sci USA. 1992;89:8577–8580. doi: 10.1073/pnas.89.18.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz R C, Favoreto S, Jr, Dorta M L, Oshiro M E M, Ferreira A T, Manque P M, Yoshida N. Infectivity of Trypanoma cruzi strains is associated with differential expression of surface glycoproteins with differential Ca2+ signaling activity. Biochem J. 1998;330:505–511. doi: 10.1042/bj3300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santori F R, Dorta M L, Juliano L, Juliano M A, Franco da Silveira J, Ruiz R C, Yoshida N. Identification of a domain of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp62 required for attachment and invasion of mammalian cells. Mol Biochem Parasitol. 1996;78:209–216. doi: 10.1016/s0166-6851(96)02626-6. [DOI] [PubMed] [Google Scholar]
- 19.Santori F R, Paranhos-Bacalla G S, Franco da Silveira J, Yamauchi J L M, Araya J E, Yoshida N. A recombinant protein based on the Trypanosoma cruzi metacyclic trypomastigotes 82-kilodalton antigen that induces an effective immune response to acute infection. Infect Immun. 1996;64:1093–1099. doi: 10.1128/iai.64.4.1093-1099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sultan A A, Thathy V, Frevert U, Robson K J H, Crisanti A, Nussenzweig V, Nussenzweig R S, Ménard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 21.Tardieux I, Nathanson M H, Andrews N W. Role in host cell invasion of Trypanoma cruzi-induced cytosolic free Ca2+ transients. J Exp Med. 1994;179:1017–1022. doi: 10.1084/jem.179.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira M M G, Yoshida N. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanoma cruzi identified by monoclonal antibodies. Mol Biochem Parasitol. 1986;18:271–282. doi: 10.1016/0166-6851(86)90085-x. [DOI] [PubMed] [Google Scholar]
- 23.Wagner R W. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994;372:333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- 24.Wheatley M. Peptide mapping and the generation and isolation of sequencable peptides from receptors. In: Hulme E C, editor. Receptor biochemistry: a practical approach. New York, N.Y: Oxford University Press; 1993. pp. 213–216. [Google Scholar]
- 25.Yakubu M A, Majumder S, Kierszenbaum F. Changes in Trypanoma cruzi infectivity by treatments that affect calcium ion levels. Mol Biochem Parasitol. 1994;66:119–125. doi: 10.1016/0166-6851(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida N. Surface antigens of metacyclic trypomastigotes of Trypanoma cruzi. Infect Immun. 1983;40:836–839. doi: 10.1128/iai.40.2.836-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida N, Mortara R A, Araguth M F, Gonzalez J C, Russo M. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35- and 50-kilodalton surface glycoconjugates of Trypanoma cruzi Infect. Immun. 1989;57:1663–1667. doi: 10.1128/iai.57.6.1663-1667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida N, Blanco S A, Araguth M F, Russo M, Gonzalez J. The stage-specific 90-kilodalton surface antigen of metacyclic trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1990;39:39–46. doi: 10.1016/0166-6851(90)90006-8. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida N, Favoreto Jr S, Ferreira A T, Manque P M. Signal transduction induced in Trypanoma cruzi metacyclic trypomastigotes during the invasion of mammalian cells. Braz J Med Biol Res. 2000;33:269–278. doi: 10.1590/s0100-879x2000000300003. [DOI] [PubMed] [Google Scholar]