Colorectal cancer (CRC) screening is recommended for adults in the U.S. aged ≥45 years.1 Screening for CRC offers the opportunity for reducing CRC incidence through detection and removal of polyps such as adenomas and sessile serrated lesions, and mortality through early detection and treatment. Post-polypectomy, evidence suggests that some individuals, such as those with advanced adenomas (defined as having an adenoma with size ≥ 1cm, villous/tubulovillous histology, or high-grade dysplasia) remain at increased risk for developing CRC.2 However, risk among these patients is not uniform nor linear. According to baseline findings and estimated risk based on the number, size, and histology of baseline polyps, the U.S. Multi-Society Task Force on Colorectal Cancer recommends repeat colonoscopy in 1 to 10 years.2 A limitation of the current approach is that risk stratification is imprecise, with current guidelines having sensitivity and specificity for predicting metachronous advanced neoplasia estimated to be 59–81%, and 43–58%, respectively.3–8 As such, novel strategies are needed to improve risk stratification and planning surveillance after polypectomy.
Genome-wide association studies (GWAS) have identified associations between common genetic variants, single nucleotide polymorphisms (SNPs) and the risk of developing CRC. Proponents argue that one powerful application of genetic markers is the use of polygenic risk scores (PRS) in improving prediction for complex disease risk. PRSs, based on panels of risk SNPs that are weighted according to their magnitude of association with disease risk, have the potential to revolutionize primary and secondary prevention of cancer through improved risk stratification and subsequent management. Indeed, PRSs have been shown in previous research to have potential for predicting risk for CRC, and to have potential for guiding the age of initiation for colorectal cancer screening.9–11 As such, PRSs might also have potential for guiding risk stratification after polypectomy, but have not been widely studied to date for this indication.
In this issue of Clinical Gastroenterology and Hepatology, Guo et al. aimed to address this evidence gap by examining whether a PRS, along with adenoma characteristics, could improve risk stratification of individuals after polyp removal.12 They conducted a population-based case-control study in Germany among 4696 CRC cases, and 3709 CRC-free controls. The study population included individuals with and without prior colonoscopy. Primary exposure of interest was a PRS which tallied the number of risk alleles a patient carried for 140 SNPs previously associated with CRC risk among populations of European ancestry. Subjects were classified as having low, medium, or high genetic risk based on the tertile distribution of the PRS, and the relationship between PRS category (the primary predictor) and CRC (primary outcome) was examined. Cumulative absolute CRC risk at 3, 5, and 10 year intervals, stratified by baseline colonoscopy status (no colonoscopy, normal colonoscopy, low risk adenoma, high risk adenoma) and PRS (low, medium, and high) was modeled. The study population was nearly 40% women, with over 60% aged 60 years or older. Cases were more likely than controls to have lower education, family history of CRC, smoking exposure, increased BMI, and lack of exposure to non-steroidal anti-inflammatory drugs (NSAIDs) or hormone replacement therapy. Additionally, colonoscopy exposure was less common among cases (69.9%) vs. controls (30.1%).
Several findings are of note. Across all individuals, increasing PRS was associated with increasing CRC risk, consistent with prior work.9–11 Also consistent with prior research, having normal colonoscopy, colonoscopy with hyperplastic polyps, or colonoscopy with low-risk adenoma (1- adenomas <10mm in size) was associated substantially reduced CRC risk compared to having no colonoscopy at up to 10 years follow up. When estimating cumulative and relative CRC risks at 3, 5, and 10 years, more variation by PRS category was seen among those with no exposure to colonoscopy than among those with exposure to colonoscopy, regardless of baseline polypectomy finding. For example, low vs. high PRS scores were associated with cumulative 10-year CRC risk of 0.8 vs. 2.1% for men and 0.5 vs. 1.3% for women after no exposure to colonoscopy, and 0.4 vs. 1.0% for men and 0.3 vs. 0.8% for women after colonoscopy with high-risk adenoma removal. The range of cumulative risk variation for low vs. high PRS for individuals with normal colonoscopy, colonoscopy with hyperplastic polyps, or colonoscopy with low-risk adenoma was smaller than observed for those with colonoscopy with high-risk adenoma or no exposure to colonoscopy, though the point estimates for cumulative risk were consistently higher for those with high PRS vs. low PRS within each baseline colonoscopy finding category. These findings show that increasing PRS is clearly associated with increased CRC risk among individuals without exposure to colonoscopy. The findings also suggest that among individuals with colonoscopy exposure, PRS may continue to contribute to risk variation, though on a smaller absolute scale.
Overall, the study was well done, with the main limitations being small sample size within colonoscopy finding sub-categories, reducing power to assess differences in CRC risk within categories across PRS risk score levels, and a focus on a PRS derived from a population of only European descent such that the PRS would likely have substantially lower performance in other ancestral groups. Strengths of the study include a population-based design, and using previously validated SNPs in their PRS, enhancing potential for generalizability. Overall, these findings are consistent with the hypothesis that heritable genetic factors contribute to CRC risk after exposure to colonoscopy and polypectomy.
Guo et al.’s findings may be considered in the larger context of attempts to optimize risk stratification and surveillance of individuals after colonoscopy. Mechanisms driving CRC risk after polypectomy may be broadly categorized into biologic factors, as well colonoscopy quality factors (Figure). Several biologic factors have been shown to be associated with risk for CRC after polypectomy, including increasing age, male sex, comorbid conditions (e.g. metabolic syndrome and diabetes), as well as exposures, such as medications (e.g., aspirin and NSAIDs), smoking, and lower physical activity.4, 13–18 Diet likely plays a role as a biologic factor given its overall proven role in driving CRC risk, though interventional and observational studies to date of the role of diet in influencing post polypectomy CRC risk have not shown a close association.19–22 Family history of CRC increases risk for metachronous advanced neoplasia after polypectomy.23 Considered together with Guo et al.’s finding that CRC-associated SNPs, as summarized by a PRS, genetic predisposition can be postulated to be a useful tool for post-polypectomy CRC risk stratification, and the clinical utility for PRS in the setting of post- polypectomy patients may be further enhanced with the additional discovery and validation of additional CRC risk SNPs.
Figure:
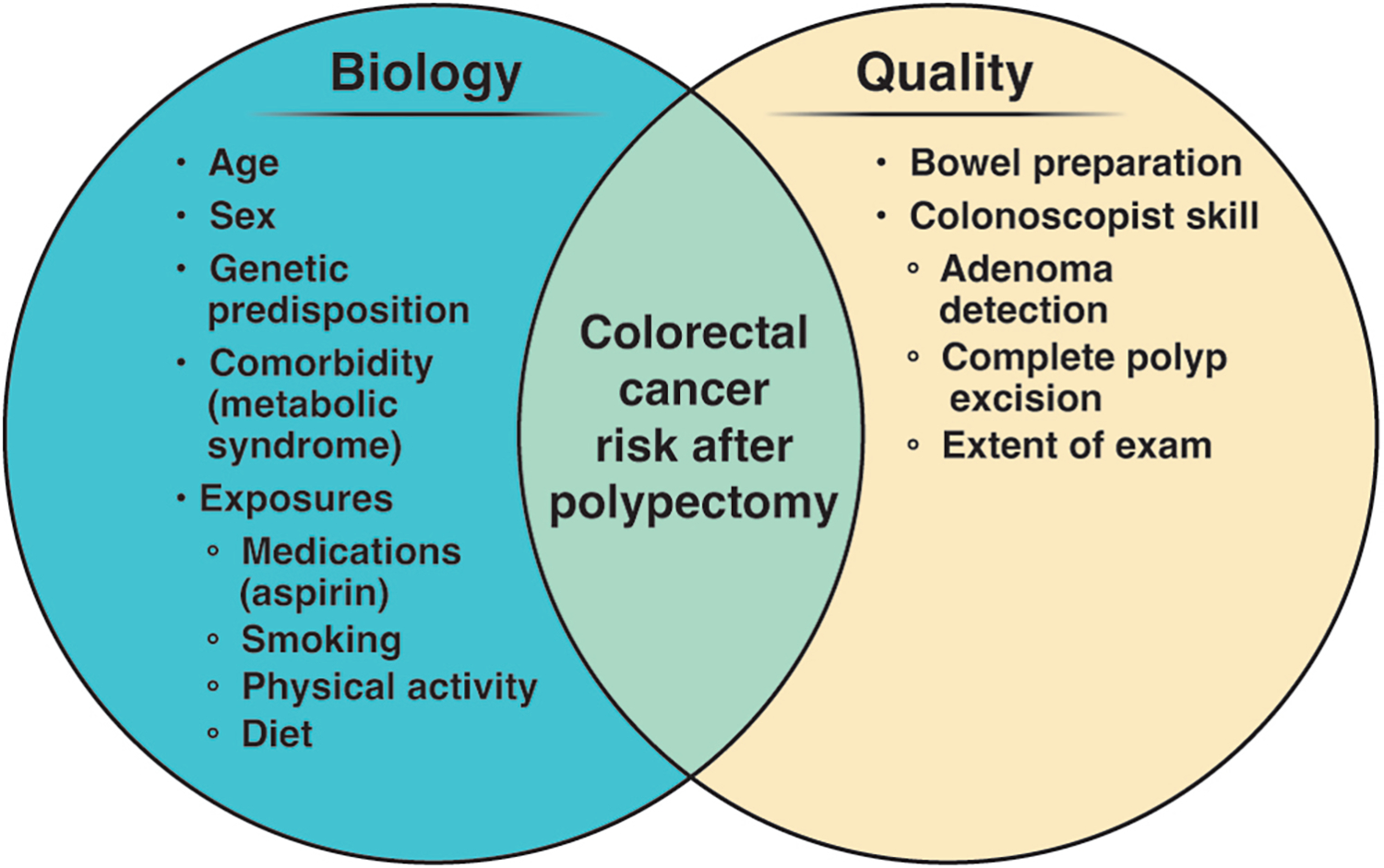
Contributors to risk for colorectal cancer and metachronous advanced neoplasia after polypectomy.
Evidence that colonoscopy quality drives CRC risk is growing. Inadequate bowel preparation has been associated with increased risk for incident CRC after polypectomy.24 Colonoscopist skill is also a factor, as incomplete examination has been associated with increased risk for incident CRC,24 and achieving complete polyp excision has been shown to be challenging, particularly for larger adenomas and sessile serrated lesions.25 Colonoscopist skill, as measured by the adenoma detection rate (ADR), has previously been well established to be associated with CRC risk after normal colonoscopy,26, 27 and recent evidence shows increasing ADR is associated with decreasing risk for incident CRC28 and advanced metachronous neoplasia after adenoma removal.29
Our growing understanding of the potential biologic and quality mechanisms that contribute to CRC risk after polypectomy may help to improve risk stratification of individuals after polypectomy, and ultimately, better guide surveillance intervals. As mentioned previously, current guideline recommendations for follow-up after colonoscopy and polypectomy have suboptimal sensitivity and specificity for identifying individuals with metachronous advanced neoplasia. Risk stratification models to date have mainly considered demographic and polyp characteristics, and have not achieved excellent performance.3, 28, 30, 31 A novel model considering demographic characteristics, polyp features, presence of diabetes, exposure to smoking, and colonoscopist ADR was recently shown to achieve improved sensitivity and specificity for identifying individuals with metachronous advanced neoplasia compared to 2020 USMSTF recommendations, but still did not reach excellent discriminatory performance.29
In summary, work to date on risk stratification suggests that multiple factors associated with metachronous CRC risk can be identified, and that models incorporating these factors can improve risk stratification. Work by Guo et al. and others also underscore that single factors, such as a PRS alone, are unlikely to be adequate to achieve optimal risk stratification. However, work by Guo et al. and others also suggests that we have an opportunity to leverage well established biologic and quality factors, such as age, sex, and extent of examination, as well as emerging factors such as information on genetic predisposition and colonoscopist ADR to develop even better, more comprehensive models for risk stratification. Although questionnaire-based, clinical and PRS-based tools appear to be clinically useful, incorporating additional blood-based markers, for example, information from the blood epigenome,32, 33 may provide a novel path toward improving further CRC risk prediction. Innovations in strategies for risk stratification may enable a new era of precision surveillance that might ultimately allow some low-risk patients to be offered long intervals between colonoscopy or even the option of non-invasive surveillance such as with a fecal immunochemical test, and high-risk patients to receive tailored recommendations for close interval colonoscopy surveillance. Ultimately, continued work in this area has great potential to improve the effectiveness and efficiency of post-polypectomy surveillance.
Grant Support:
Research reported in this publication was supported by VA Health Services Research & Department award I01HX001574 (Gupta, PI), and by the National Cancer Institute of the National Institutes of Health under award number R37CA222866 (Gupta, PI).
Footnotes
Conflicts of Interest:
The authors have no relevant conflicts of interest to disclose.
References
- 1.U. S. Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965–1977. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158:1131–1153 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Messer K, Baron JA, et al. A prognostic model for advanced colorectal neoplasia recurrence. Cancer Causes Control. 2016;27:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148:419–426. [DOI] [PubMed] [Google Scholar]
- 6.Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol. 2009;7:86–92. [DOI] [PubMed] [Google Scholar]
- 7.Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60:1537–1543. [DOI] [PubMed] [Google Scholar]
- 8.Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Colorectal cancer risk factors in the detection of advanced adenoma and colorectal cancer. Cancer Epidemiol. 2013;37:278–283. [DOI] [PubMed] [Google Scholar]
- 9.Thomas M, Sakoda LC, Hoffmeister M, et al. Genome-wide Modeling of Polygenic Risk Score in Colorectal Cancer Risk. Am J Hum Genet. 2020;107:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archambault AN, Su YR, Jeon J, et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology. 2020;158:1274–1286 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archambault AN, Jeon J, Lin Y, et al. Risk Stratification for Early-Onset Colorectal Cancer Using a Combination of Genetic and Environmental Risk Scores: An International Multi-Center Study. J Natl Cancer Inst. 2022;114:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F, Edelmann D, Cardoso R, et al. Polygenic Risk Score for Defining Personalized Surveillance Intervals After Adenoma Detection and Removal at Colonoscopy. Clin Gastroenterol Hepatol. 2022;S1542–3565:288–289. [DOI] [PubMed] [Google Scholar]
- 13.Kim TJ, Kim JE, Choi YH, et al. Obesity-related parameters and colorectal adenoma development. Gastroenterology. 2017;52:1221–1229. [DOI] [PubMed] [Google Scholar]
- 14.Kim MC, Jung SW, Kim CS, et al. Metabolic syndrome is associated with increased risk of recurrent colorectal adenomas in Korean men. Int J Obes (Lond). 2012;36:1007–1011. [DOI] [PubMed] [Google Scholar]
- 15.Kim NH, Park JH, Park DI, et al. Metabolic syndrome is a risk factor for adenoma occurrence at surveillance colonoscopy: A single-center experience in Korea. Medicine (Baltimore). 2016;95:e4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiredo JC, Crockett SD, Snover DC, et al. Smoking-associated risks of conventional adenomas and serrated polyps in the colorectum. Cancer Causes Control. 2015;26:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molmenti CL, Hibler EA, Ashbeck EL, et al. Sedentary behavior is associated with colorectal adenoma recurrence in men. Cancer Causes Control. 2014;25:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulai PS, Singh S, Marquez E, et al. Chemoprevention of colorectal cancer in individuals with previous colorectal neoplasia: systematic review and network meta-analysis. BMJ. 2016;355:i6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunzmann AT, Coleman HG, Huang WY, et al. Fruit and vegetable intakes and risk of colorectal cancer and incident and recurrent adenomas in the PLCO cancer screening trial. Int J Cancer. 2016;138:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sardo Molmenti CL, Steck SE, Thomson CA, et al. Dietary Inflammatory Index and Risk of Colorectal Adenoma Recurrence: A Pooled Analysis. Nutr Cancer. 2017;69:238–247. [DOI] [PubMed] [Google Scholar]
- 21.Lanza E, Yu B, Murphy G, et al. The polyp prevention trial continued follow-up study: no effect of a low-fat, high-fiber, high-fruit, and -vegetable diet on adenoma recurrence eight years after randomization. Cancer Epidemiol Biomarkers Prev. 2007;16:1745–1752. [DOI] [PubMed] [Google Scholar]
- 22.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342:1149–1155. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs ET, Gupta S, Baron JA, et al. Family history of colorectal cancer in first-degree relatives and metachronous colorectal adenoma. Am J Gastroenterol. 2018;113:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80 e1. [DOI] [PubMed] [Google Scholar]
- 26.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminski MF, Wieszczy P, Rupinski M, et al. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98–105. [DOI] [PubMed] [Google Scholar]
- 28.Wieszczy P, Kaminski MF, Franczyk R, et al. Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology. 2020;158:875–883 e5. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Bustamante R, Earles A, et al. Impact of polyp features and colonoscopist adenoma detection rate on post-polypectomy risk for metachronous advanced neoplasia. Gastroenterology. 2020; 158(6):S-641–S-642. [Google Scholar]
- 30.van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013;144:1410–1418. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Park HW, Kim MJ, et al. Prediction of the risk of a metachronous advanced colorectal neoplasm using a novel scoring system. Dig Dis Sci. 2016;61:3016–3025. [DOI] [PubMed] [Google Scholar]
- 32.Gebhard C, Mulet-Lazaro R, Glatz D, et al. Aberrant DNA methylation patterns in microsatellite stable human colorectal cancers define a new marker panel for the CpG island methylator phenotype. Int J Cancer. 2022;150:617–625. [DOI] [PubMed] [Google Scholar]
- 33.Nassar FJ, Msheik ZS, Nasr RR, et al. Methylated circulating tumor DNA as a biomarker for colorectal cancer diagnosis, prognosis, and prediction. Clin Epigenetics. 2021;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]


