Abstract
Objective:
With increasing experience in fenestrated endovascular aneurysm repair (FEVAR) over time, devices designed to treat juxta-/pararenal aortic aneurysms (JR/PRAA) have evolved in complexity to extend to more proximal landing zones and incorporate more target vessels. We assessed perioperative outcomes in patients who underwent juxta-/pararenal FEVAR with supraceliac versus infraceliac sealing in the Vascular Quality Initiative (VQI).
Methods:
We identified all patients who underwent elective FEVAR (commercially available-FEVAR and physician-modified endografts) for JR/PRAA in the VQI between 2014-2021. Supraceliac sealing was defined as proximal sealing in aortic zone 5, or zone 6 with a celiac scallop/fenestration/branch or celiac occlusion. Primary outcomes were perioperative and 3-year mortality. Secondary outcomes included completion-endoleaks, in-hospital complications, and factors associated with 3-year mortality. We calculated propensity scores and used inverse probability-weighted Cox-regression and logistic regression modeling to assess outcomes.
Results:
Among 1,486 patients identified, 1,246 (84%) patients underwent infraceliac sealing and 240 (16%) patients underwent supraceliac sealing. Of the supraceliac patients, 74 (31%) had a celiac scallop, 144 (60%) had a celiac fenestration/branch, and 22 (9.2%) had a celiac occlusion (intentional or unintentional). After risk-adjusted analyses, there were no differences in perioperative mortality following supraceliac sealing compared with infraceliac sealing (2.3% vs. 2.5%; Hazard Ratio [HR]: 0.67 [95%CI: 0.26-1.8], p=.42), or 3-year mortality (12% vs. 15%; HR: 0.89 [0.53-1.5]; p=.67). Compared with infraceliac sealing, supraceliac sealing was associated with lower odds of type-IA completion endoleaks (Odds Ratio [OR]: 0.24 [0.05-0.67]), but higher odds of any complication (12% vs. 6.9%; OR: 1.6 [95%CI: 1.01-2.5]) including cardiac complications (5.5% vs. 1.9%; OR: 2.6 [95%CI: 1.3-5.1]), lower-extremity ischemia (3.0% vs. 0.9%; OR: 3.2 [95%CI: 1.02-9.5]), and acute kidney injury (16% vs. 11%; OR: 1.6 [95%CI: 1.05-2.3]). Though non-significant, there was a trend towards higher risk of spinal cord ischemia following supraceliac sealing compared with infraceliac sealing (1.7% vs. 0.8%; OR: 2.2 [95%CI: 0.70-6.4]). There were no differences in bowel ischemia between groups (1.7% vs. 1.5%; OR: 0.83 [95%CI: 0.24-1.23]). A more proximal aneurysm disease extent was associated with higher 3-year mortality (HR zone-8 vs. 9: 1.7 [95%CI: 1.1-2.5]), whereas procedural characteristics had no influence.
Conclusion:
Compared with sealing at an infraceliac level, supraceliac sealing was associated with lower risk of type-IA endoleaks and similar mortality. However, clinicians should be aware that supraceliac sealing was associated with higher perioperative morbidity. Future studies with longer follow-up are needed to adequately assess durability differences in order to comprehensively weigh the risks and benefits of utilizing a higher sealing zone within the visceral aorta for juxta-/pararenal FEVAR.
Keywords: Juxtarenal AAA, Pararenal AAA, Fenestrated Endovascular Aneurysm Repair, Proximal Landing Zone, Supraceliac, Infraceliac, Visceral Aorta
Table of Contents Summary:
In juxta-/pararenal FEVAR, supraceliac sealing is associated with lower type-IA completion endoleaks and similar mortality. Nevertheless, clinicians should be aware that supraceliac sealing may be associated with higher perioperative morbidity. Future studies with longer follow-up are needed to assess durability outcomes with infraceliac versus supraceliac sealing.
INTRODUCTION
Fenestrated endovascular aneurysm repair (FEVAR) has expanded the applicability of endovascular aneurysm repair (EVAR) to abdominal aortic aneurysms (AAA) with short necks (<1-15 mm), as well as juxta-/pararenal (JR/PRAA) and suprarenal AAA. Current studies on fenestrated technology show high technical success and improved peri-operative outcomes compared with open surgical repair of JR/PRAA1,2, and has led to an increase in utility of FEVAR.3 However, just like in infrarenal EVAR4,5, this advantage seems to diminish over time.6,7
Previous studies have reported that the types of FEVAR devices to treat JR/PRAA have risen in complexity by evolving to extend into more proximal landing zones and incorporate more target vessels.8,9 However, sealing in a proximal segment of the aorta may come with added risk as sealing at a more proximal levels entails the inclusion of a higher number of target vessels which are known to further complicate the operative procedure, have a higher risk of patency loss, or endoleaks over time.9,10 Previous studies on thoracoabdominal aortic aneurysms reported that sealing in a more proximal segment of the thoracic aorta was associated with higher risk of spinal cord ischemia.11,12 Furthermore, prior studies demonstrated a trend or association of higher perioperative mortality with increased complexity.9,13,14 However, despite these findings there is a temporal rise in FEVAR complexity, highlighting the importance of more quality data regarding the safety of a landing zone in a more proximal segment of the visceral aorta.
Thus, in this study, we aimed to assess the perioperative outcomes and all-cause mortality of a proximal sealing zone at a supraceliac level compared with an infraceliac sealing zone in juxta-/pararenal FEVAR.
METHODS
Data Source
We performed a retrospective cohort study using the Society for Vascular Surgery Vascular Quality Initiative (SVS-VQI; Research Advisory Committee [RAC] approval number: 4598). The VQI is a quality improvement registry established to improve patient care through the prospective collection of clinical data. The VQI mainly constitutes of participating centers from the United States with only few participating centers from outside of the United States. More information about the VQI can be found at www.vascularqualityinitiative.org. The VQI research Advisory Committee and the Institutional Review Board at the Beth Israel Deaconess Medical Center approved this study and gave permission to use data without the need for informed consent given the retrospective, deidentified nature of the data.
Patient Cohort
All patients who underwent elective FEVAR (excluding any devices implanted under Investigational Device Exemption [IDE] studies) between January 2014 and August 2021 were included in the study (n=3,143) and were identified as previously described.3 Hybrid procedures with a chimney branch were excluded (n= 79). Furthermore, patients with non-aneurysmal disease including dissections or traumatic aortic injury were excluded (n=112), as were patients with aneurysms with a non-degenerative etiology (e.g. anastomotic; n=55), or patients with saccular aneurysms (n=303). Patients with suprarenal (n=420) and TAAAs (type IV; n=713) were also excluded. Finally, patients with missing information regarding proximal aneurysm extent were excluded (n=131).
Remaining patients were stratified by proximal landing zone: infraceliac or supraceliac. Supraceliac sealing was defined as proximal sealing in aortic zones 5 or zone 6, as per the reporting standards15, with integration of the celiac trunk with scallop/fenestration/branch or occlusion (Figure 1).
Figure 1.
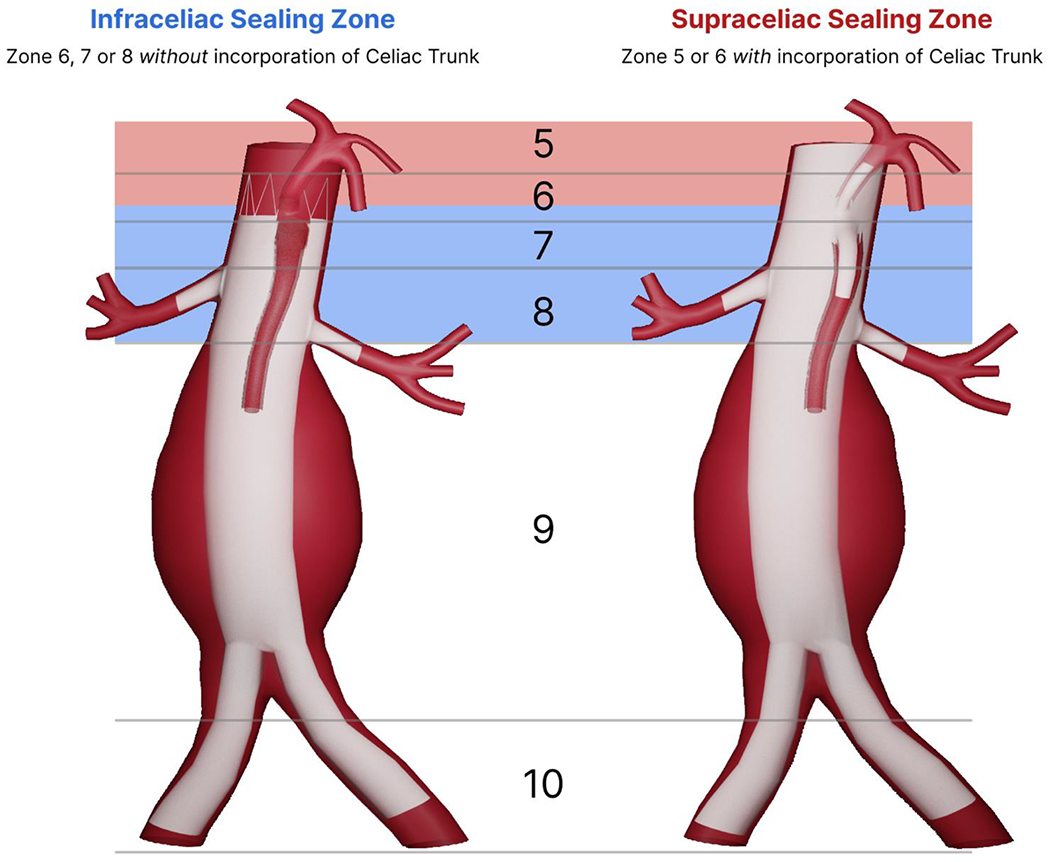
Definition of supraceliac and infraceliac sealing zones as per the Society for Vascular Surgery guidelines
Variable definitions and Outcomes
Body mass index (BMI) was calculated using weight(kg)/height(m)2 and obesity was defined as a BMI ≥ 30. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI). We further defined preoperative renal status as CKD stage 1/2 (eGFR>60 mL/min/1.73m2), CKD stage 3 (eGFR 30-60 mL/min/1.73m2), CKD stage 4/5 (eGFR<30 mL/min/1.73m2), or preoperative dialysis (regardless of eGFR).16 Preoperative chronic kidney disease (CKD) was defined as a preoperative eGFR <60 mL/min/1.73m2 or preoperative dialysis. Prior peripheral arterial disease (PAD) was defined as history of preoperative amputation, peripheral vascular intervention, or non-coronary and non-intracranial bypass. Preoperative anemia was defined as hemoglobin <10 g/dL. Proximal seal extent was defined as the difference in zones between the proximal aneurysm extent and the proximal landing zone. For example, an aneurysm with a proximal extent in zone 9 with a proximal landing zone in zone 8 would represent a difference of 1. Center and physician FEVAR procedure volumes were calculated over the 12 months preceding each operation. Suprarenal aneurysms (aneurysm extents zone 6 or 7), TAAAs (aneurysm extent more proximal to zone 6), and ruptured AAA were included in these volume calculations. Subsequently, these volumes were separated into quintiles. The lowest and highest quintiles were defined as low and high volume, respectively, and the middle three quintiles were defined as medium volume.17
The primary outcomes were perioperative and 3-year mortality. Mortality was defined as all-cause mortality, and perioperative mortality was defined as death occurring within 30 days or during index hospitalization if the primary admission exceeded 30 days. Secondary outcomes included completion endoleaks and perioperative complications during index hospitalization. Pulmonary complication was defined as pneumonia or reintubation. Cardiac complication was defined as a composite endpoint of postoperative myocardial infarction and new-onset or substantial aggravation of pre-existing congestive heart failure. Development of postoperative myocardial infarction was categorized into diagnosis based upon troponin levels, and electrocardiogram/clinical symptoms. Acute Kidney Injury (AKI) was defined according to the guidelines of the Kidney Guidelines Improving Global Outcomes (KDIGO)-criteria18, which was defined as a ≥1.5 times increase from baseline serum creatinine, or an increase of ≥26.5mmol/L from baseline. Spinal cord ischemia (SCI) was defined as the manifestation of leg weakness or paralysis which was further stratified into transient SCI or SCI present at discharge. We defined any in-hospital complication as the occurrence of a pulmonary complication, cardiac complication, stroke, bowel ischemia, leg ischemia, acute kidney injury, new-onset postoperative dialysis, SCI, or reoperation during index hospitalization. All variables had <5% missing data.
Statistical Analysis
We compared demographics, comorbidities, and procedural characteristics between the study groups. Categorical variables were presented as counts and percentages and were compared using the Pearson’s χ2-test or Fisher’s exact tests where appropriate. For continuous variables, we visually determined the normality of the data and compared between study groups using student t-tests or Wilcoxon rank-sum tests where appropriate.
We calculated propensity scores for assignment to treatment with either infraceliac or supraceliac sealing. Low outcome event rates within the independent groups precluded robust conventional multivariable adjustment, so we instead calculated inverse probability weights using the propensity scores.19 Covariates were selected a priori and introduced into the model, including age, sex, race, AAA-diameter, hypertension, diabetes, myocardial infarction, congestive heart failure (New York Heart Association [NYHA] class I or II / NYHA class III or IV), smoking status, chronic obstructive pulmonary disease, obesity, preoperative renal status, peripheral arterial disease, anemia, preoperative medication use (aspirin, p2y12-inhibitor, statin, or angiotensin-converting enzyme inhibitor [ACEi]/angiotensin receptor blockers [ARB], anticoagulation), prior abdominal aortic surgery, proximal aneurysm extent (zone 8/9), repair type (custom-made endograft/physician-modified endograft [PMEG]), distal sealing zone, center volume, and physician volume. The lowest and highest percentages of inverse probability weights were trimmed to the 5th and 95th percentile to prevent major influence of extreme inverse probability weights. We then performed inverse-probability weighted Cox regression and logistic regression to assess the primary and secondary outcomes, respectively.
To display potential outcome differences for celiac stenting patients only, separate propensity scores were created, and inverse probability weighted Kaplan-Meier methods and Cox-regression were performed to assess these outcomes in comparison with infraceliac patients. (configurations with scallops/occlusions/coverage by aortic main device for the celiac artery were excluded).
In order to examine factors associated with 3-year mortality following FEVAR for JR/PRAA, we performed a multivariable Cox regression analysis. Besides the sealing zone (supraceliac vs. infraceliac sealing zone), our cox regression model constrained the following a priori selected covariates: age, sex, race (i.e. Non-Hispanic White, Black, Asian, Hispanic, Other), prior COPD, prior CKD, and other factors that were statistically significantly different between groups at univariable analysis.
In the United States, FEVARs have a Food and Drug Administration approval for a maximum of 3-vessels which shall lead to a degree of collinearity with the repair type, as all 4-vessel FEVARs are PMEGs and almost all have a supraceliac landing zone. Although we did adjust for all available information on endograft variation, this limitation should be considered when interpreting our results. To better reflect any true differences, we also performed a sensitivity analysis comparing outcomes between the repair types (commercial FEVAR and PMEG), utilizing inverse probability weights for adjustment.
All statistical analyses were performed using R version 4.0.3 (http://www.r-proiect.org).
RESULTS
Patient characteristics
We identified 1,486 patients who underwent FEVAR for juxta-/pararenal aneurysms, of whom 1,246 (84%) had proximal sealing at an infraceliac level, and 240 (16%) had sealing at a supraceliac level. At baseline, compared with patients who had an infraceliac proximal seal, patients with a supraceliac proximal seal were more frequently on preoperative statin therapy (supraceliac vs. infraceliac: 80% vs. 74%) and ACEi/ARB therapy (63% vs. 51%). (Table I) Furthermore, patients with a supraceliac seal more frequently underwent prior aortic surgery compared with patients with an infraceliac seal (15% vs. 4.9%). Otherwise, demographics and comorbidities were similar between groups.
Table I.
Baseline Characteristics of 1,486 patients with juxta-/pararenal aortic aneurysms undergoing fenestrated endovascular aneurysm repair
| Infraceliac Sealing zone (N=1246) | Supraceliac sealing zone (N=240) | p-value | |
|---|---|---|---|
|
| |||
| Age | 74 [IQR 68, 79] | 75 [IQR 69, 80] | .048 |
| Female Sex | 274 (22%) | 45 (19%) | .48 |
| Race | .79 | ||
| Non-White Hispanic | 1092 (88%) | 207 (86%) | |
| Black | 55 (4.4%) | 7 (2.9%) | |
| Asian | 18 (1.4%) | 4 (1.7%) | |
| Hispanic | 14 (1.1%) | 5 (2.1%) | |
| Other | 8 (0.6%) | 1 (0.4%) | |
| Hypertension | 1087 (87%) | 220 (92%) | .053 |
| Diabetes | 162 (13%) | 39 (16%) | .63 |
| Prior MI | 260 (21%) | 53 (22%) | >.99 |
| Prior CHF | .21 | ||
| NYHA I/II | 135 (11%) | 36 (15%) | |
| NYHA III/IV | 21 (1.7%) | 4 (1.7%) | |
| Smoking (ever) | 1123 (90%) | 217 (90%) | .87 |
| COPD | 322 (26%) | 68 (28%) | .52 |
| Obese (BMI≥30) | 383 (31%) | 71 (30%) | .92 |
| Renal status | .24 | ||
| eGFR>60 mL/min/1.73m2 | 756 (61%) | 136 (57%) | |
| eGFR30-60mL/min/1.73m2 | 442 (36%) | 92 (38%) | |
| eGFR<30mL/min/1.73m2 | 31 (2.5%) | 11 (4.6%) | |
| Dialysis | 3 (0.3%) | 0 (0%) | |
| Prior PAD | 96 (7.7%) | 12 (9.6%) | .40 |
| Anemia (Hgb<10mg/dL) | 60 (4.8%) | 17 (7.1%) | .32 |
| Aspirin | 831 (67%) | 176 (73%) | .079 |
| P2Y12-inhibitor | 238 (19%) | 39 (16%) | .34 |
| Statin | 924 (74%) | 192 (80%) | .047 |
| Beta Blocker | 746 (60%) | 150 (63%) | 0.3 |
| ACE/ARB | 639 (51%) | 151 (63%) | .004 |
| Prior Aortic Surgery | 61 (4.9%) | 36 (15%) | <.001 |
| Prior Infrarenal Endovascular Aneurysm Repair | 2.3% | 11% | |
MI= myocardial infarction; CHF = congestive heart failure; COPD= chronic obstructive pulmonary disease; BMI = body mass index; eGFR = estimated Glomerular Filtration; PAD = peripheral arterial disease; Hgb = Hemoglobin; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blocker
Anatomical/procedural characteristics
Among all patients with a supraceliac sealing zone, 74 patients (31%) had the celiac artery incorporated as a scallop, whereas 144 patients (60%) had the celiac artery incorporated as a fenestration. (Table II) Of the remaining patients, 11 patients (4.6%) had purposeful occlusion of the celiac artery, and 11 patients (4.6%) had purposeful covering of the celiac artery. More detailed information regarding the endograft configurations is provided in Supplementary Table I. Compared with patients with an infraceliac sealing zone, patients with a supraceliac sealing zone had larger aneurysm diameter (60mm [IQR 56, 67] vs. 58mm [IQR 54, 62], p<.001) and less frequently had an aneurysm extent in zone 9 (28% vs. 41%, p<.001). Furthermore, the average proximal seal extent was significantly higher in the supraceliac sealing cohort compared with the infraceliac sealing cohort (2.89 [SD 0.63] vs. 1.18 [SD 0.79], p<.001). Again, for example, an aneurysm with a proximal extent in zone 9 with a proximal landing zone in zone 8 would represent a difference of 1.
Table II.
Anatomic/Procedural Characteristics of the included patients with juxta-/pararenal aortic aneurysms undergoing fenestrated EVAR
| Infraceliac Sealing zone (N=1246) | Supraceliac sealing zone (N=240) | p-value | |
|---|---|---|---|
|
| |||
| Large AAA-diameter (≥65mm) | 212 (17%) | 69 (29%) | <.001 |
| Preoperative AAA-Diameter (mm) | 58 [IQR 54, 62] | 60 [IQR 56, 67] | <.001 |
| Proximal Aneurysm Extent | <.001 | ||
| Zone 8 (Pararenal) | 737 (59%) | 174 (73%) | |
| Zone 9 (Juxtarenal) | 509 (41%) | 66 (28%) | |
| Proximal Sealing Zone | <.001 | ||
| Zone 5 | 0 (0%) | 147 (61%) | |
| Zone 6 | 90 (7.2%) | 93 (39%) | |
| Zone 7 | 786 (63%) | 0 (0%) | |
| Zone 8 | 370 (30%) | 0 (0%) | |
| Proximal Seal Extent (mean [zones; sd]) | 1.18 (0.79) | 2.89 (0.63) | <.001 |
| Repair type | <.001 | ||
| Commercial FEVAR | 1142 (92%) | 44 (18%) | |
| PMEG | 104 (8.3%) | 196 (82%) | |
| No. of Target vessels (mean[sd]) | 2.39 (0.71) | 3.69 (0.62) | <.001 |
| Celiac Trunk | 0 (0%) | 218 (91%) | |
| Superior Mesenteric artery | 772 (62%) | 234 (98%) | |
| Right renal artery | 1105 (89%) | 219 (91%) | |
| Left renal artery | 1098 (88%) | 215 (90%) | |
| Distal Sealing zone | .006 | ||
| Aortic | 63 (5.1%) | 24 (10%) | |
| Common iliac | 1051 (84%) | 186 (78%) | |
| External iliac or lower | 132 (11%) | 30 (13%) | |
| Celiac branch treatment | |||
| Fenestration | NA | 144 (60%) | |
| Scallop | NA | 74 (31%) | |
| Occluded | NA | 11 (4.6%) | |
| Purposely Covered* | NA | 11 (4.6%) | |
| Procedure time (min) | 186 [IQR 140, 250] | 238 [IQR 184, 307] | <.001 |
| Fluoro time (min) | 51 [IQR 37, 72] | 68 [IQR 51, 92] | <.001 |
| Blood Loss (cc) | 200 [IQR 100, 350] | 250 [IQR 100, 500] | <.001 |
| Contrast use (mL) | 105 [IQR 75, 148] | 100 [IQR 60, 160] | .42 |
| Center Volume Category | <.001 | ||
| Low volume (≤2) | 400 (32%) | 24 (10%) | |
| Medium volume (3-17) | 714 (57%) | 117 (49%) | |
| High volume (≥18) | 132 (11%) | 99 (41%) | |
| Physician Volume Category | <.001 | ||
| Low volume (<1) | 420 (33.7%) | 30 (12.5%) | |
| Medium volume (2-9) | 664 (53.3%) | 98 (40.8%) | |
| High volume (≥10) | 162 (13.0%) | 112 (46.7%) | |
FEVAR = fenestrated endovascular aneurysm repair; PMEG = physician modified endograft; mL = milliliter
Patients with a supraceliac sealing zone were more frequently treated with PMEGs (80% vs. 8.5%, p<.001) and had a higher average number of target vessels (3.7 [±0.64] vs. 2.4 [±0.71], p<.001). Also, patients with supraceliac sealing less frequently had distal sealing zones in the common iliac or lower (91% vs. 95%, p=.006). In comparison with patients who underwent juxta-/pararenal FEVAR with an infraceliac sealing zone, patients with a supraceliac sealing zone had longer procedure times (238 [IQR 184, 307] vs. 186 [IQR 140, 250], p<.001), but similar contrast use (100 [IQR 60, 160] vs. 105 [75, 148], p=.42). Finally, juxta-/pararenal FEVARs with a supraceliac sealing zone were less likely to be treated in low volume centers (7.9% vs. 24%, p<.001) and by low volume physician’s (13% vs. 34%, p<.001).
Completion Endoleaks
Patients with supraceliac sealing zones had lower rates of type-IA endoleaks (1.4% vs. 4.5%, p=.043), but similar rates of type-IB endoleaks (0.5% vs. 1.6%, p=.30), and type-III endoleaks (7.2% vs. 6.0%, p=.62). (Table III) Furthermore, patients with supraceliac sealing zones had lower rates of type-II endoleaks at completion (14% vs. 21%, p=.01), but had higher rates of type-IV endoleaks (3.2% vs. 0.8%, p=.011).
Table III.
Perioperative mortality and in-hospital complications following the use of an infraceliac versus a supraceliac proximal landing zone in fenestrated endovascular aneurysm repair of juxta-/pararenal abdominal aortic aneurysms
| Supraceliac Sealing zone (N=240) | Infraceliac Sealing zone (N=1246) | Supraceliac Sealing vs. Infraceliac Sealing Risk-adjusted Outcomes |
|||
|---|---|---|---|---|---|
|
| |||||
| Adjusted Kaplan-Meier Estimates | Hazards ratio | 95%-Confidence interval | p-value | ||
| Perioperative Mortality | 2.3% | 2.5% | 0.67 | 0.26-1.77 | .42 |
| 3-year Mortality | 11% | 12% | 0.89 | 0.53-1.50 | .67 |
| Completion Endoleaks | Unadjusted event rates | Unadjusted Odds Ratio | 95%- Confidence Interval | p-value | |
|
| |||||
| Type IA-Endoleak | 4.5% | 1.2% | .028 | ||
| Type IB-Endoleak | 1.6% | 0.5% | .30 | ||
| Type III Endoleak | 6.0% | 7.2% | .62 | ||
| Type II-Endoleak | 21% | 14% | .009 | ||
| Type IV-Endoleak | 0.8% | 3.2% | .001 | ||
| Undetermined Endoleak | 4.2% | 5.9% | .38 | ||
|
| |||||
| Graft-related Reinterventions | 1.4% | 3.1% | .81 | ||
| In-hospital Complications | Adjusted event rates | Adjusted Odds Ratio | 95%-Confidence Interval | p-value | |
|
| |||||
| Any Complication | 12% | 6.9% | 1.6 | 1.01-2.49 | .041 |
| Pulmonary Complications | 3.5% | 2.7% | 1.27 | 0.57-2.59 | .54 |
| Cardiac Complication | 5.5% | 1.9% | 2.61 | 1.29-5.13 | .006 |
| Myocardial Infarction | 4.4% | 1.8% | 2.33 | 1.12-4.76 | .023 |
| New-onset CHF | 1.1% | 0.4% | 2.69 | 0.57-12.1 | .18 |
| Bowel Ischemia | 1.7% | 1.5% | 0.83 | 0.24-2.27 | .75 |
| Stroke | 0.3% | 0.8% | 0.47 | 0.02-2.65 | .48 |
| Leg Ischemia | 3.0% | 0.9% | 3.15 | 1.02-9.47 | .039 |
| AKI | 16% | 11% | 1.55 | 1.05-2.26 | .024 |
| Postoperative Dialysis | 1.7% | 0.9% | 1.09 | 0.23-3.70 | .90 |
| Spinal Cord Ischemia | 1.7% | 0.8% | 2.21 | 0.70-6.38 | .15 |
| Reintervention/Reoperation | 6.0% | 4.2% | 1.49 | 0.80-2.64 | .19 |
This model was corrected for Age, Gender, Race, Diameter, Hypertension, Diabetes, Myocardial Infarction, Congestive Heart Failure (NYHAI/II / NYHAIII/IV), Smoking Status, Chronic Obstructive Pulmonary Disease, Obesity, Renal Dysfunction, Anemia, Prior Medication use (Aspirin, P2Y12-inhibitor, Statin, ACE/ARB), Prior Aortic Surgery, Aneurysm Extent (Zone 8/9), Repair type, Distal Sealing Zone, Low Center volume, Low Physician volume
CHF = Congestive Heart Failure; AKI = Acute Kidney Injury
Perioperative Outcomes
Following inverse probability weighted risk adjustment, we found that supraceliac sealing was associated with similar risk of perioperative mortality compared with infraceliac sealing (2.3% vs. 2.5%; Hazard Ratio [HR]: 0.67 [95%CI: 0.26-1.8], p=.42). (Table III) Supraceliac sealing was associated with higher odds of leg ischemia (3.0% vs. 0.9%; OR: 3.2 [95%CI 1.02-9.5]), acute kidney injury (16% vs. 11%; OR: 1.6 [95%CI 1.1-2.3]), and cardiac complications (5.5% vs. 1.9%; OR: 2.6 [95%CI 1.3-5.1]). Regarding cardiac complications, supraceliac sealing was specifically associated with higher risk of postoperative myocardial infarction (4.4% vs. 1.8%; OR: 2.3 [95%CI: 1.1-4.8]), but similar risk of new-onset congestive heart failure (1.1% vs. 0.4%; OR: 2.7 [95%CI: 0.57-12]). Furthermore, compared with infraceliac sealing, supraceliac sealing trended towards a higher risk of spinal cord ischemia (1.7% vs. 0.8%; OR: 2.2 [95%CI 0.70-6.4]).
Otherwise, compared with infraceliac sealing, supraceliac sealing was associated with similar risk of bowel ischemia (1.7% vs. 1.5%; OR: 0.83 [95%CI 0.24-2.3], and in-hospital reinterventions (2.3% vs. 0.9%; OR: 2.2 [95%CI 0.70-6.4]). Also, supraceliac sealing was associated with similar risk of pulmonary complications (3.5% vs. 2.7%), stroke (0.3% vs. 0.8%), and postoperative dialysis (1.7% vs. 0.9%).
3-year Mortality
The inverse-probability weighted Kaplan-Meier survival curve following juxta-pararenal FEVAR is displayed in Figure 2A. Following risk-adjustment, supraceliac sealing was associated with similar risk of 3-year mortality compared with infraceliac sealing (11% vs. 12%; HR: 0.89 [95%CI 0.53-1.5], p=.67). Furthermore, the subgroup of patients with supraceliac stenting had a similar rate of 3-year mortality compared with infraceliac sealing (14% vs. 12%; HR: 1.2 [95%CI: 0.63-2.2], p=.62). (Figure 2B)
Figure 2.
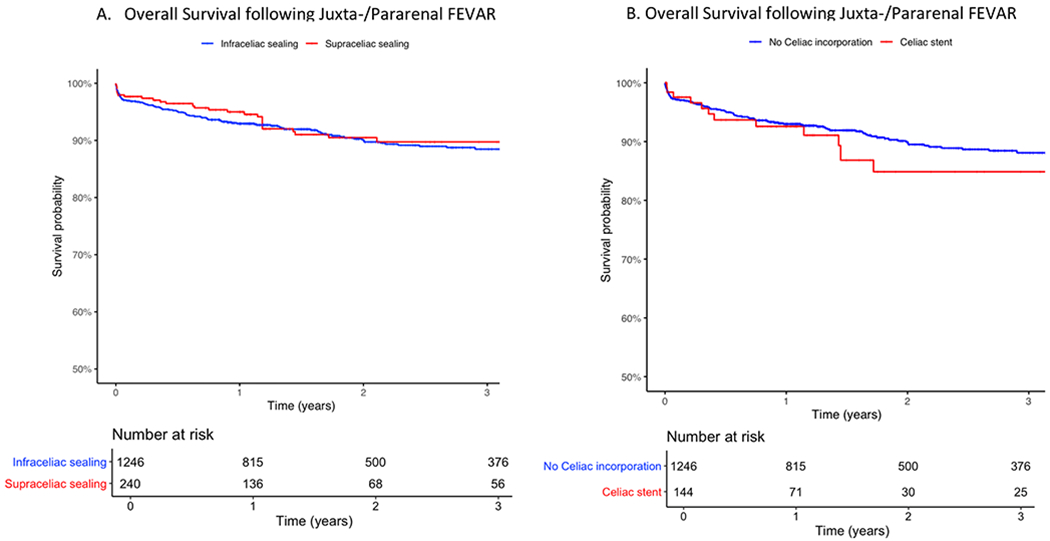
Overall Survival for Endovascular Repair of Juxtarenal Abdominal Aortic Aneurysms A) Infraceliac vs. Supraceliac sealing B) Infraceliac sealing (No Celiac Incorporation) vs. Celiac stenting; Standard Error<0.10
Comparison of Outcomes between Repair Types
Following inverse-probability weighted risk adjustment, compared with commercial FEVAR, PMEG was associated with higher rates of pulmonary complications, AKI, and a higher reintervention rate during index hospitalization. (Supplementary Table II) Between groups, there were similar risk of type-IA endoleaks at completion angiography, cardiac complications, and leg ischemia.
Factors associated with 3-year survival
Following multivariable cox-regression, factors that were independently associated with higher 3-year mortality included age (HR/decade: [95%CI: 1.1-1.9], p=.007), female sex (HR: 1.7 [95%CI: 1.1-2.5], p=.012), and more proximal disease extent (HR [Zone 8 vs. Zone 9]: 1.7 [95%CI: 1.1-2.5], p=.015). (Table IV) Supraceliac sealing was not associated with a higher risk of 3-year mortality (HR [ref infraceliac sealing]: 0.89 [95%CI: 0.46-1.7]). Other factors that were not associated with high risk of mortality included FEVAR repair type (HR [PMEG vs. Commercial FEVAR]: 1.2 [95%CI: 0.65-2.3]) and more distal sealing zone (HR [Common Iliac vs. Aortic]: 0.84 [95%CI: 0.39-1.8]).
Table IV.
Factors associated with 3-year mortality following fenestrated endovascular aneurysm re pair for juxta-/pararenal abdominal aortic aneurysms
| Hazard Ratio | 95%CI | p-value | |
|---|---|---|---|
|
| |||
| Sealing Zone | |||
| Ref: Infraceliac | - | - | |
| Supraceliac | 0.89 | 0.46, 1.73 | NS |
| Age by Decade | 1.43 | 1.10, 1.85 | .007 |
| Female Sex | 1.67 | 1.12, 2.49 | .012 |
| Race | |||
| Non-Hispanic White | — | — | |
| Black | 1.14 | 0.55, 2.38 | NS |
| Asian | 0.73 | 0.10, 5.33 | NS |
| Hispanic | 1.72 | 0.61, 4.86 | NS |
| Other | 2.33 | 0.32, 17.2 | NS |
| Large Preoperative AAA-diameter (≥65mm) | 1.19 | 0.76, 1.86 | NS |
| Preoperative Statin | 0.73 | 0.49, 1.08 | NS |
| Preoperative ACE/ARB | 1.12 | 0.78, 1.62 | NS |
| Prior COPD | 2.17 | 1.51, 3.11 | <.001 |
| Prior CKD | 1.4 | 0.97, 2.03 | NS |
| Anemia (Hgb < 10 mg/dL) | 2.02 | 1.10, 3.72 | .023 |
| Prior Aortic Surgery | 0.96 | 0.47, 1.99 | NS |
| Proximal Disease Extent | .015 | ||
| Ref: Zone 9 (Juxtarenal) | — | — | |
| Zone 8 (Pararenal) | 1.67 | 1.10, 2.52 | .015 |
| Graft type | |||
| Ref: Commercial FEVAR | — | — | |
| PMEG | 1.22 | 0.65, 2.28 | NS |
| Distal Landing Zone | |||
| Ref: Aortic | — | — | |
| Common Iliac Artery | 0.84 | 0.39, 1.83 | NS |
| External Iliac or lower | 2.14 | 0.90, 5.04 | NS |
| Low Center volume | 0.81 | 0.47, 1.41 | NS |
| Low Physician volume | 0.81 | 0.50, 1.32 | NS |
NS = Non-significant; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blocker; Hgb = Hemoglobin; FEVAR = fenestrated endovascular aneurysm repair; PMEG = physician modified endograft;
DISCUSSION
Previous studies have shown that the types of devices that are being used to treat similar anatomy have increased in complexity8,9, though the current literature comparing FEVARs of varying complexities remains scarce, especially within the same disease extent. In this large observational study, we included all patients who underwent elective juxta-pararenal FEVAR and compared perioperative outcomes between repairs with different levels of proximal sealing within the visceral aorta: a supraceliac versus an infraceliac sealing zone. We found that although a supraceliac proximal landing zone utilized a higher number of target vessels, it was associated with similar risk of perioperative and three-year mortality. Also, compared with infraceliac sealing, supraceliac sealing was associated with lower rates of type-IA endoleaks on completion angiogram, and despite more proximal aortic coverage, with similar risk of spinal cord and bowel ischemia. Nevertheless, supraceliac sealing was associated with higher rates of myocardial infarction, leg ischemia, and acute kidney injury.
Despite increased complexity of supraceliac repairs with longer operative time and a higher number of target vessels, our study demonstrated that a supraceliac landing zone was associated with similar risk of perioperative and 3-year mortality compared with infraceliac sealing in juxta-/pararenal FEVAR. Our findings with regard to perioperative mortality are in contrast to prior studies.9,13,14 This included a prior report by Roy et al. who previously reported that FEVARs with 2 target vessels had a seemingly lower perioperative mortality compared with 3-vessel and 4-vessel FEVARs (2% vs. 8%, p=.059).9 However, this study was limited in sample size (n=173), and outcomes were not adjusted for factors such as disease extent, as was the case with the other studies. Furthermore, in contrast to our findings, Mastracci et al. reported a trend toward a lower overall survival in patients who underwent FEVAR with a supraceliac landing zone, when comparing various endograft configurations with lower sealing levels within the aorta.8 However, this study also did not stratify for disease extent as FEVARs with supraceliac sealing were almost exclusively used for type IV TAAA, whereas infraceliac sealing FEVAR were mostly performed in juxtarenal aortic aneurysms. As such, it is possible that the lower overall survival following a supraceliac sealing technique was a result of the greater disease extent in the supraceliac group, rather than the level of proximal sealing or endograft configuration. Our finding that a more proximal disease extent rather than increased procedural complexity is associated with higher mortality, supports this argument. We also found similar overall survival in patients who underwent supraceliac sealing with celiac stenting compared with infraceliac sealing. These findings might suggest that, although previous studies demonstrated higher rates of celiac occlusion or reintervention with celiac stenting,8,9 these complications did not impact survival.
Though our results displayed similar mortality after FEVAR with supraceliac versus infraceliac sealing, we found a higher likelihood of developing perioperative complications following supraceliac sealing. Prior studies have demonstrated that higher contrast loads and longer procedure times were related to higher risk of acute kidney injury.20,21 Although there were no differences in contrast use between groups, the supraceliac sealing cohort did have longer procedure times. It has previously been suggested that longer operative times are associated with higher risk of AKI as this leads to a higher exposure of intraoperative hemodynamic variability. Additionally, supraceliac sealing could be chosen due to aortic wall thrombus in the visceral segment, which is known to lead to a higher rate of AKI too.22 With regard to leg ischemia, it could be hypothesized that the higher risk of leg ischemia in this group is in part due to the longer operation time too. Besides higher risk of acute kidney injury and leg ischemia, the rate of myocardial infarction was significantly higher following supraceliac sealing compared with infraceliac sealing, even after adjusting for prior cardiac events. However, as the VQI only includes endovascular repairs that were performed without an IDE, only the early experience of 4-vessel PMEG’s (having a supraceliac sealing zone) were included. Nevertheless, it may be that physicians doing PMEG’s have higher volume than ZFEN physician.23
We did not find statistically significant differences in risk of bowel and spinal cord ischemia, despite increased aortic coverage in the supraceliac sealing cohort. These findings in juxta-/pararenal FEVAR are in contrast to prior studies in context of fenestrated/branched EVAR for treatment of exclusively TAAA’s: Diamond et al. and Kitpanit et al. recently reported increased risk of paraparesis or spinal cord injury in more extensive disease, suggesting that this is most likely due to increased/more extensive aortic coverage within the thoracic aorta.11,12 We speculate that this has to do with the increased variability in aortic coverage in context of endovascular TAAA repair. Also, the proximal disease extent was not accounted for in these studies, which might also in part explain the difference in outcomes. However, it is also possible that our findings with respect to bowel and spinal cord ischemia might be subject to type-II errors, and would become significant in a larger sample size.
Importantly, we found that supraceliac sealing leads to lower rates of type-IA endoleaks at completion compared with infraceliac sealing, with a high rate of 4.5% following infraceliac sealing. In the case of infrarenal EVAR, an important cause of technical failure is dilatation of the proximal sealing zone, resulting in type-IA endoleaks, endograft migration, and AAA rupture.5,24,25 One of the presumed advantages of FEVAR over infrarenal EVAR is the use of a proximal landing zone in a healthier segment of the aorta (visceral segment), which is possibly less prone to proximal seal dilatation over time. Though recent FEVAR studies demonstrated a low clinical risk of proximal graft failure in the mid-term, these studies also reported that seal dilatation still occurs after sealing in the visceral aorta26–28, leaving us to speculate that the risk of proximal graft failure for these grafts is not eliminated in the future. Especially as infrarenal EVAR studies have taught us that proximal graft failure is a complication that mainly presents in the long-term.29 A couple of these studies concurrently reported lower dilatation rates in more proximal segments within the visceral aorta, demonstrating potential advantages of sealing at a supraceliac level compared with sealing at an infraceliac level.26,28 Although our finding of a lower risk of type IA completion endoleaks is reassuring, future studies with long-term outcomes are needed to assess whether supraceliac sealing leads to improved endograft durability of compared with infraceliac sealing in juxta-/pararenal FEVAR.
Our study should be interpreted within the context of its retrospective design. Although mortality is well captured within the VQI, long-term complications and reinterventions are not. We were unable to investigate secondary interventions following supraceliac versus infraceliac sealing in the current study, although these data should be available in the future. Furthermore, the VQI does not capture the cause of death, precluding any analysis of aneurysm-related mortality. Also, radiation dose is not captured by the VQI, and remains an interesting characteristic to examine between the aortic visceral sealing levels. Considering that commercial FEVARs have a Food and Drug Administration approval for a maximum of 3-vessels, this shall lead to a degree of collinearity with the repair type, as all 4-vessel FEVARs are PMEGs and almost all have a supraceliac landing zone. Although we adjusted outcomes for endograft configuration, and despite a sensitivity analysis comparing the repair types demonstrated variability in outcomes, this limitation should be considered when interpreting our results. And, therefore it will remain important to compare these results with the commercially available custom-made 4-vessel grafts. Furthermore, as mentioned before, repairs performed under an IDE were not included, potentially biasing against a supraceliac sealing device as we were primarily capturing early experience of this configuration. This was not the case for 2/3-vessel commercial FEVARs, potentially biasing against the supraceliac sealing group. Also, no sealing lengths or other seal characteristics (angulation, geometry, or diameter) are described in the module in which FEVARs are included, precluding any understanding of how these factors may impact clinical outcomes.
CONCLUSION
Compared with sealing at an infraceliac level, supraceliac sealing was associated with lower risk of type-IA endoleaks and similar mortality. However, clinicians should be aware that supraceliac sealing may be associated with higher perioperative morbidity. Future studies with longer follow-up are needed to adequately assess durability differences in order to comprehensively weigh the risks and benefits of utilizing a higher sealing zone within the visceral aorta/for juxta-/pararenal FEVAR.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research:
Retrospective cohort study of prospectively collected data from the Vascular Quality Initiative registry
Key Findings:
Among 1,402 patients who underwent FEVAR for juxta-/pararenal aneurysms, compared with sealing at an infraceliac level, supraceliac sealing was associated with higher risk of myocardial infarction, lower extremity ischemia, and kidney injury, but lower risk of type-IA completion endoleaks and similar risk of mortality, bowel ischemia, and spinal cord ischemia.
Take home Message:
With the rise in FEVAR complexity, clinicians should be aware that supraceliac sealing may be associated with higher perioperative morbidity compared with infraceliac sealing, though there is no difference in mortality. Future studies with longer follow-up are required to assess durability outcomes with infraceliac versus supraceliac sealing.
ACKNOWLEDGEMENTS
Figure 1 was a 3D graphic design by Arjen van Gaal (email at arjenvangaal@gmail.com).
CM is supported by grant number F32HS027285 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
PP is supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: HV is a consultant of Medtronic, WL Gore, Terumo, Endologix, Philips.
Presenter Information
This study was presented at the 2022 Annual Symposium of the Society for Clinical Vascular Surgery, Las Vegas, Nevada, March 19-23, 2022.
REFERENCES
- 1.Doonan RJ, Girsowicz E, Dubois L, Gill HL. A systematic review and meta-analysis of endovascular juxtarenal aortic aneurysm repair demonstrates lower perioperative mortality compared with open repair. J Vasc Surg [Internet]. 2019;70(6):2054–2064.e3. Available from: 10.1016/jjvs.2019.04.464 [DOI] [PubMed] [Google Scholar]
- 2.Gupta PK, Brahmbhatt R, Kempe K, Stickley SM, Rohrer MJ. Thirty-day outcomes after fenestrated endovascular repair are superior to open repair of abdominal aortic aneurysms involving visceral vessels. J Vasc Surg [Internet]. 2017;66(6):1653–1658.e1. Available from: 10.1016/jjvs.2017.04.057 [DOI] [PubMed] [Google Scholar]
- 3.Donnell TFXO, Patel VI, Deery SE, Li C, Swerdlow NJ, Liang P, et al. The State of Complex Endovascular Abdominal Aortic Aneurysm Repairs in the Vascular Quality Initiative. J Vasc Surg. 2019;70(2):617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet [Internet]. 2016;388(10058):2366–74. Available from: 10.1016/S0140-6736(16)31135-7 [DOI] [PubMed] [Google Scholar]
- 5.Schermerhom ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373(4):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones AD, Waduud MA, Walker P, Stocken D, Bailey MA, Scott DJA. Meta-analysis of fenestrated endovascular aneurysm repair versus open surgical repair of juxtarenal abdominal aortic aneurysms over the last 10 years. BJS open. 2019;3(5):572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinsakchai K, Prapassaro T, Salisatkorn W, Hongku K, Moll FL, Ruangsetakit C, et al. Outcomes of Open Repair, Fenestrated Stent Grafting, and Chimney Grafting in Juxtarenal Abdominal Aortic Aneurysm: Is It Time for a Randomized Trial? Ann Vasc Surg. 2019;56(November 2018): 114–23. [DOI] [PubMed] [Google Scholar]
- 8.Mastracci TM, Eagleton MJ, Kuramochi Y, Bathurst S, Wolski K. Twelve-year results of fenestrated endografts for juxtarenal and group IV thoracoabdominal aneurysms. J Vasc Surg [Internet]. 2015;61 (2):355–64. Available from: 10.1016/jjvs.2014.09.068 [DOI] [PubMed] [Google Scholar]
- 9.Roy IN, Millen AM, Jones SM, Vallabhaneni SR, Scurr JRH, McWilliams RG, et al. Long-term follow-up of fenestrated endovascular repair for juxtarenal aortic aneurysm. Br J Surg. 2017;104(8): 1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinelli G, Crea MA, de Waure C, Di Tanna GL, Becquemin JP, Sobocinski J, et al. A propensity-matched comparison of fenestrated endovascular aneurysm repair and open surgical repair of pararenal and paravisceral aortic aneurysms. J Vasc Surg [Internet]. 2018;68(3):659–68. Available from: 10.1016/jjvs.2017.12.060 [DOI] [PubMed] [Google Scholar]
- 11.Kitpanit N, Ellozy SH, Connolly PH, Agrusa CJ, Lichtman AD, Schneider DB. Risk factors for spinal cord injury and complications of cerebrospinal fluid drainage in patients undergoing fenestrated and branched endovascular aneurysm repair. J Vasc Surg [Internet]. 2021;73(2):399–409.e1. Available from: 10.1016/jjvs.2020.05.070 [DOI] [PubMed] [Google Scholar]
- 12.Diamond KR, Simons JP, Crawford AS, Arous EJ, Judelson DR, Aiello F, et al. Effect of thoracoabdominal aortic aneurysm extent on outcomes in patients undergoing fenestrated/branched endovascular aneurysm repair. J Vasc Surg [Internet]. 2021;74(3):833–842.e2. Available from: 10.1016/jjvs.2021.01.062 [DOI] [PubMed] [Google Scholar]
- 13.Vallabhaneni SR. Early results of fenestrated endovascular repair of juxtarenal aortic aneurysms in the United Kingdom. Circulation. 2012;125(22):2707–15. [DOI] [PubMed] [Google Scholar]
- 14.Patel SD, Constantinou J, Simring D, Ramirez M, Agu O, Hamilton H, et al. Results of complex aortic stent grafting of abdominal aortic aneurysms stratified according to the proximal landing zone using the Society for Vascular Surgery classification. J Vasc Surg [Internet]. 2015;62(2):319–325.e2. Available from: 10.1016/jjvs.2015.03.035 [DOI] [PubMed] [Google Scholar]
- 15.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg [Internet]. 2010;52(4): 1022–1033.e5. Available from: 10.1016/jjvs.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Balk E, Kausz A, Levin A, Steffes MW, et al. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann Intern Med [Internet]. 2003;139:137–47. Available from: papers2://publication/uuid/72AAA803-D341-4D04-A582-59C8EAB5C6FE [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell TFX, Boitano LT, Deery SE, Lancaster RT, Siracuse JJ, Schermerhom ML, et al. Hospital Volume Matters: The Volume-Outcome Relationship in Open Juxtarenal AAA Repair. Ann Surg. 2020;271(1):184–90. [DOI] [PubMed] [Google Scholar]
- 18.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. [Google Scholar]
- 19.Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: A primer for practitioners. BMJ. 2019;367:1–10. [DOI] [PubMed] [Google Scholar]
- 20.Sailer AM, Nelemans PJ, van Berio C, Yazar O, de Haan MW, Fleischmann D, et al. Endovascular treatment of complex aortic aneurysms: prevalence of acute kidney injury and effect on long-term renal function. Eur Radiol [Internet]. 2016;26(6):1613–9. Available from: 10.1007/s00330-015-3993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cucuruz B, Kasprzak PM, Gallis K, Schierling W, Pfister K, Kopp R. Midterm outcome of renal function after branched thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2020;71(4):1119–27. [DOI] [PubMed] [Google Scholar]
- 22.Sandri G de A, Oderich GS, Tenorio ER, Ribeiro MS, Reis de Souza L, Cha SS, et al. Impact of aortic wall thrombus on late changes in renal function among patients treated by fenestrated-branched endografts. J Vasc Surg [Internet]. 2019;69(3):651–660.e4. Available from: 10.1016/jjvs.2018.05.243 [DOI] [PubMed] [Google Scholar]
- 23.Simons JP, Shue B, Flahive JM, Aiello FA, Steppacher RC, Eaton EA, et al. Trends in use of the only Food and Drug Administration-approved commercially available fenestrated endovascular aneurysm repair device in the United States. J Vasc Surg [Internet]. 2017;65(5):1260–9. Available from: 10.1016/j.jvs.2016.10.101 [DOI] [PubMed] [Google Scholar]
- 24.De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven ELG, Cuypers PWM, et al. Longterm outcome of open or endovascular repair of abdominal aortic aneurysm. Vasc Med. 2010;15(6):515–6. [Google Scholar]
- 25.Kouvelos GN, Oikonomou K, Antoniou GA, Verhoeven ELG, Katsargyris A. A Systematic Review of Proximal Neck Dilatation after Endovascular Repair for Abdominal Aortic Aneurysm. J Endovasc Ther. 2017;24(l):59–67. [DOI] [PubMed] [Google Scholar]
- 26.Zettervall SL, Dansey K, Kline B, Singh N, Starnes BW. Significant aortic neck dilation occurs after repair of juxtarenal aneurysms with fenestrated endovascular aneurysm repair. J Vasc Surg [Internet]. 2021; Available from: 10.1016/j.jvs.2021.03.060 [DOI] [PubMed] [Google Scholar]
- 27.Tran K, Deslarzes-Dubuis C, Lee JT. Quantification of suprarenal aortic neck dilation after fenestrated endovascular aneurysm repair. J Vasc Surg [Internet]. 2021;73(1):31–8. Available from: 10.1016/j.jvs.2020.04.522 [DOI] [PubMed] [Google Scholar]
- 28.Rastogi V, de Bruin JL, Varkevisser RRB, Oliveira NFG, Bouwens E, Hoeks SE, et al. Proximal Seal Dilatation following Fenestrated Endovascular Repair for Complex Abdominal Aortic Aneurysms. J Vasc Surg [Internet]. 2022; Available from: 10.1016/j.jvs.2021.12.061 [DOI] [PubMed] [Google Scholar]
- 29.Oliveira NFG, Oliveira-Pinto J, van Rijn MJ, Baart S, Raa S Ten, Hoeks SE, et al. Risk Factors, Dynamics, and Clinical Consequences of Aortic Neck Dilatation after Standard Endovascular Aneurysm Repair. Eur J Vasc Endovasc Surg [Internet]. 2021; Available from: 10.1016/j.ejvs.2021.03.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


