Abstract
Gasdermin B (GSDMB) belongs to a family of structurally-related proteins (i.e., gasdermins) that distinguishes itself from other members by the lack of autoinhibition, but clear bioactivity, of its full-length form, its preference to bind to phosphatidylinositol phosphates and sulfatides, and the ability to promote both lytic as well as non-lytic cellular functions. It is the only gasdermin that lacks a mouse ortholog, making in vivo mechanistic studies challenging to perform. GSDMB is abundantly expressed in epithelial cells lining organs that directly interface with the external environment, such as the gastrointestinal tract, with emerging evidence supporting its role in enteric infections, inflammatory bowel disease, and colorectal cancer. This review discusses the unique features of GSDMB among other gasdermin family members and controversies surrounding GSDMB-dependent mammalian inflammatory cell death (i.e., pyroptosis), including recent discoveries revealing both lytic and non-lytic functions of epithelial-derived GSDMB, particularly during gut health and disease.
Keywords: epithelial restitution and repair, mucosal wound healing, intestinal inflammation, non-lytic cell death, pyroptosis
GSDMB is a unique member of the gasdermin family
Gasdermins (GSDMs) comprise a family of lipid binding proteins and their related isoforms that have redefined the process of programmed cell death and secretion of IL-1 family members, including IL-1β and IL-18. In humans, six GSDM genes exist, GSDMA, GSDMB, GSDMC, GSDMD, GSDME/DFNA5, and PJVK/DFNB59, some of which are highly, but not exclusively, expressed in different parts of the digestive system[1–3]. GSDMs have emerged as key regulators of tissue inflammation and tumorigenesis[1,4–7], each sharing common characteristics, while also possessing significant differences, among other family members (Box I Table I). At present, GSDMD is the best characterized of the GSDMs, and much of what has been studied in regard to other GSDM family members is based on what is known about GSDMD. This includes canonical activation of GSDMs by proteolytic cleavage of their linker sequence, between the C- and N-terminal domains, with subsequent oligomerization of the latter within membrane bilayers and formation of transmembrane pores (Box I).
Box I Table I.
Characteristics of GSDM family members
| GSDM | Mouse Ortholog | Lipid Binding | Proteolytic Activator | Biological Pathways | Physiological tissue expression | Associated diseases | Ref. |
|---|---|---|---|---|---|---|---|
| GSDMA (GSDM1, FKSG9) | Gsdma1 Gsdma2 Gsdma3 |
cardiolipin, phosphatidic acid, phospatidylserine, phosphoinositides | SpeBj Unknown human activator? | pyroptosis, mitochondrial homeostasis | esophagus, stomach, skin, mammary gland | systemic sclerosis, alopecia (only in mice), gastric cancer | [9,28,44–49] |
| GSDMB (GSDMLa) | None | for both NTh and FLi: phosphatidic acid, phosphatidyl glycerolsulfatide, phosphoinositides | GzmAk Caspases? | pyroptosis, adjuvant in GSDMD-mediated pyroptosis (FL), cell proliferation, migration, adhesion (FL), Gram− bacterial lysis | airways, gastrointestinal tract, liver, neuroendocrine cells, immune cells | asthma, IBD, type 1 diabetes, systemic sclerosis, colorectal, liver, pancreatic, breast, cervical, lung cancers | [9,13,16–19,28,29,31,33,47] |
| GSDMC (MLZEb) | Gsdmc1 Gsdmc2 Gsdmc3 Gsdmc4 |
uncharacterized | Caspase 8 | pyroptosis | skin, gastrointestinal tract, trachea, spleen, bladder | colorectal cancer, melanoma | [9,28,47,50,51] |
| GSDMD (GSDMDC1c, DFNA5Ld, FKSG10) | Gsdmd | cardiolipin, phosphatidic acid, phosphoinositides | Caspases 1, 4, 5, 8 ELANEl Cathepsin G | pyroptosis, NETosisn, mitophagy, sublytic release of inflammatory mediators | immune cells, gastrointestinal tract, placenta | infection, sepsis, IBD, autoimmune encephalomyelitis, liver fibrosis, gastrointestinal, pancreatic, salivary gland cancers, melanoma, lymphoma | [9,28,35,47,52–61] |
| GSDME (DFNA5e, ICERE-1) | Dfna5, Gsdme | cardiolipin, phosphatidylserine, phosphoinositides | Caspase 3 GzmBm | pyroptosis, sublytic release of inflammatory mediators | cochlea, heart, brain, small intestine, colon, kidney, placenta | autosomal dominant hearing loss, acute kidney injury, IBD, gastric, colorectal, breast cancers, melanoma | [9,28,47,55,62–67] |
| PJVKf (DFNB59g, GSDMF?) | Pjvk, Dfbn59 | uncharacterized | Unknown, not required? | pexophagy | brain, eye, inner ear, heart, lung, kidney, liver, testis | autosomal recessive auditory neuropathy, recessive non-syndromic hearing impairment | [9,28,47,68] |
GSDML, gasdermin-like protein;
MLZE, melanoma-derived leucine zipper-containing extranuclear factor;
GSDMDC1, gasdermin domain-containing protein 1;
DFNA5L, DFNA5-like;
DFNA5, deafness, autosomal dominant 5;
PJVK, pejvakin;
DFNB49, deafness, autosomal recessive 59;
FL, full-length;
NT, N-terminal;
SpeB, streptococcal pyrogenic exotoxin B;
GzmA, granzyme A;
ELANE, elastase neutrophil expressed;
GzmB, granzyme B;
NET, neutrophil extracellular trap.
Box I. Canonical activation and pore formation of gasdermin family members.
Under homeostatic conditions, the majority of GSDMs reside in the cytoplasm of eukaryotic cells, locked in a folded conformation, wherein its N-terminal (NT), lipid-binding domain is autoinhibited by interaction with the C-terminal (CT) domain[14]. Canonical GSDM activation occurs through a mechanism that entails proteolytic cleavage at the linker sequence, between NT and CT domains, leading to release of cleaved, GSDM N-terminal domain fragments (GSDM-NT). Cleavage, to a certain extent, is unique to the specific proteases that cleave particular GSDMs; however, each GSDM can present with one or more cleavage sites that can serve as substrates for different proteases[10,69,70]. Released GSDM-NT translocates and binds to membrane lipids and oligomerizes within the membrane bilayer, resulting in assembly of transmembrane pores[71]. There are two major functional consequences of such GSDM-dependent pore-forming abilities: 1) non-canonical cell secretion of inflammatory cytokines, such as IL-1β and IL-18, and 2) rupture of the plasma membrane, leading to lytic cell death[1,4,6]. Functions of different GSDMs are regulated by multiple upstream stimuli that result in cell-specific activation of different proteases that are capable of GSDM cleavage (Table I), including inflammatory caspases (−1, −3, −4, −5, −8 in humans, and −1 and −11 in mice), cathepsin G, neutrophil elastase, and cytotoxic T lymphocyte-derived granzyme A and B[71]. Aside from these classical activation mechanisms and capabilities as membrane pore-forming proteins, more recent evidence suggests cleavage-independent processes of GSDM activation, and broader functions of these proteins in regulating cellular homeostasis and human disease[5,6,12].
There are two functional outcomes of GSDMD- dependent pore formation. One is membrane rupture that leads to inflammatory cell death via pyroptosis, and the other is non-lytic release of cytokines and alarmins[8–10]. The mechanism(s) responsible for these different, functional consequences of GSDMD-induced membrane pores remain poorly understood. Formation of non-lytic pores is linked to activation of the endosomal membrane repair mechanism, whereas the cell surface protein, ninjurin 1, is reported to be critical in regulating cell lysis downstream of GSDMD-dependent pore formation[10,11]. Conversely, until recently, less was known about GSDMB, which is unique among GSDMs in regard to its regulation and cellular functions[12], the latter of which are determined by GSDMB’s distinct structural features and lipid-binding properties. Crystallographic evaluation of its C-terminal domain (GSDMB-CT) reveals truncation of two alpha-helices important for the inter-domain interactions typical of other GSDM family members[13,14]. This weakens binding between GSDMB-CT and the N-terminal domain (GSDMB-NT), and relieves auto-inhibition of potential membrane binding in GSDMB’s full-length form (GSDMB-FL), while still blocking the pore-forming capacity of the N-terminal domain. Another unique feature of GSDMB involves its binding of phosphatidylinositol phosphates and sulfatides, instead of phosphoserine and cardiolipin[13] that other GSDMs commonly bind. Specifically, the ability of the N terminal domain of GSDMB isoform 1 to bind phosphoinositides, and to a lesser extent, phosphatidic acid, cardiolipin, and sulfatides, has been reported[13]; pyroptosis, however, was not evaluated in this study. Also, unlike other gasdermins, GSDMB-FL can bind phospholipids, indicating that interactions with the C-terminal domain does not inhibit the lipid binding ability of GSDMB-NT[13]. Nonetheless, it is unclear whether the C-terminal domain modulates lipid selectivity and/or GSDMB’s binding affinity by interacting with the N-terminal domain or by associating with other protein partners. These differences have given rise to speculation of alternative function(s) from other GSDMs, including those beyond pyroptosis. GSDMB, unlike other GSDMs, lacks a mouse ortholog[3,15], hampering the ability to perform more mechanistic experimentation to determine in vivo function(s), such as its role in pyroptosis, which remains controversial. In the last two years, however, critical discoveries have been made, indicating both lytic and non-lytic GSDMB-dependent cellular functions that suggest an overall protective role of GSDMB-expressing intestinal epithelial cells (IEC) during gastrointestinal inflammation, infection and cancer[16–18]. These topics will be covered, including the multiple levels of regulation that ultimately affect the biological functions of GSDMB.
Genetic regulation of GSDMB
GSDMB can be expressed as six splice variants encoding different protein isoforms, ranging in molecular weight from 18 to 50 kDa[14]. Isoforms 1–4 and 6 possess varying lengths and sequences of their interdomain linkers, whereas isoform 5 is comprised solely of GSDMB-CT[13,14]. Such differences in linker sequences could potentially modify interdomain interactions, lipid binding and/or folding/stability of GSDMB isoforms[13], although structural and functional features of the majority of GSDMB isoforms have not been fully investigated. Many of these isoforms have distinct cellular localization and functions[19,20] (Table II); however, most published studies have also not surveyed the distribution and abundance of each of the GSDMB isoforms, which are likely differentially regulated in various cell and tissue types. In addition, while GSDMB isoforms 1–4 and 6 possess caspase-1 and granzyme A (GzmA) cleavage sites, their positions in these isoforms are different, ranging from cleavage of isoform 1 at position 92–101 by caspase-1[13], position 240–246 by GzmA[17], and of isoform 4 at position 244 by GzmA[18], adding another level of complexity that may affect GSDMB’s downstream function (Figure 1).
Table II.
Differential expression and functions of GSDMB Isoforms
| GSDMB Isoform | Molecular Weight | Experimental System | Cellular Localization | Functional Effects | Ref. |
|---|---|---|---|---|---|
| Isoform 1 | 47 kDa | Overexpression in MCF-7a breast cancer cells | Cytoplasmic | Accelerates cell migration and invasion in vitro; does not affect tumor growth in vivo | [11] |
| Overexpression in bronchial epithelial cells | Nuclear | Stimulates gene expression of TGFβb, MMP9c and chemokines | [20] | ||
| Overexpression in MCF-7 breast cancer cells | Not determined | [12] | |||
| Isoform 2 | 45 kDa | Overexpression in MCF-7 breast cancer cells | Cytoplasmic | Accelerates cell migration and invasion in vitro | [11] |
| Not determined | [12] | ||||
| Isoform 3 | 45 kDa | Overexpression in MCF-7 breast cancer cells | Cytoplasmic | Not determined | [12] |
| Isoform 4 | 47 kDa | Overexpression in MCF-7 breast cancer cells | Nuclear | Not determined | [12] |
| Isoform 5 | 18 kDa | Not determined | Unknown | Speculated to repress bioactivity of the N-terminal fragment | [8] |
| Isoform 6 | 46 kDa | Not determined | Unknown | Not determined | - |
MCF-7, Michigan Cancer Foundation-7;
TGFβ, transforming growth factor-β;
MMP9, matrix metalloproteinase-9
Figure 1. GSDMB-mediated lytic activity.
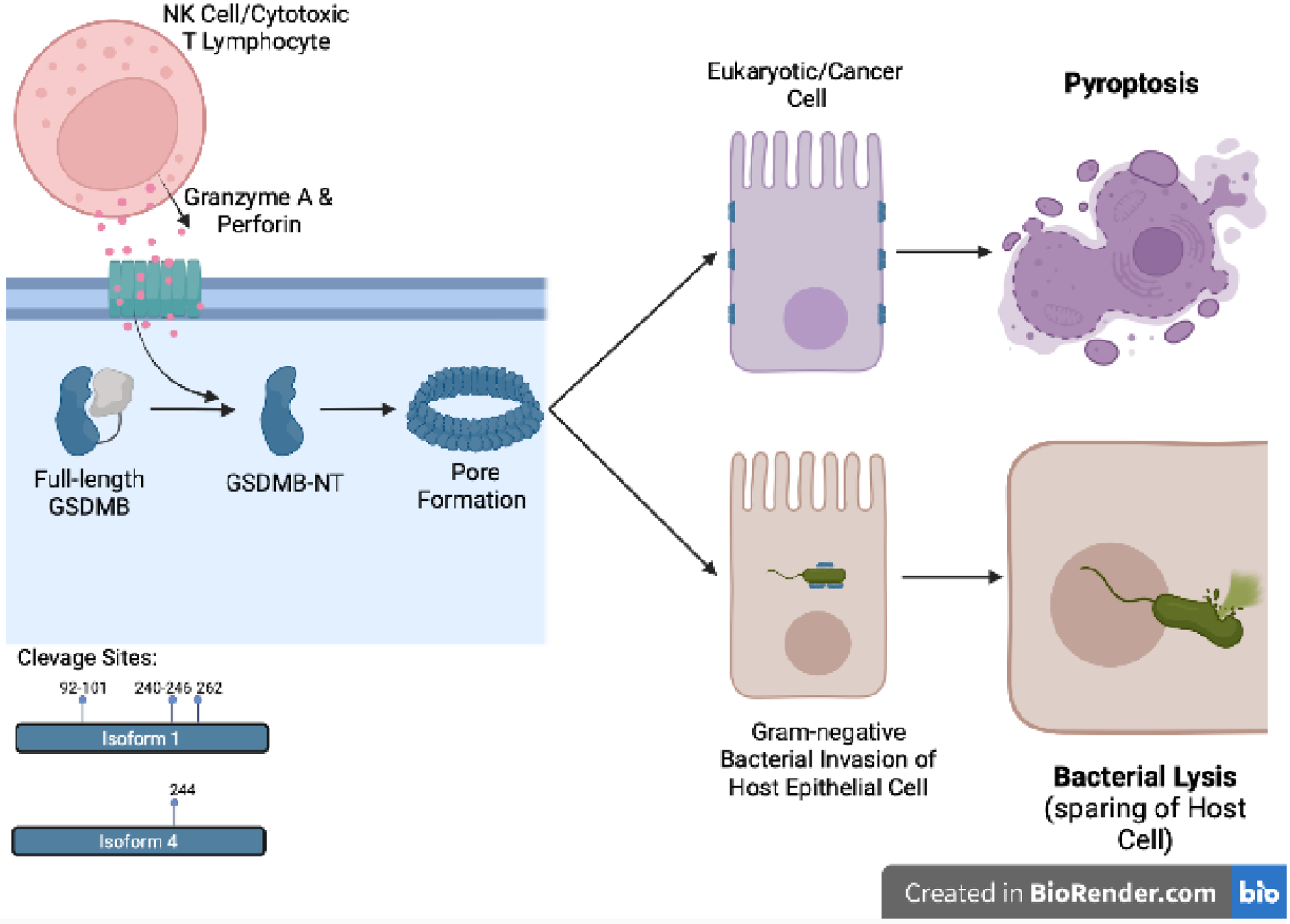
Full-length GSDMB is activated by Granzyme A, whose entry into target cells is facilitated by perforin, both of which are derived from cytotoxic T lymphocytes and NK cells. Granzyme A-mediated cleavage results in release of GSDMB-NT fragments, which translocate to lipid bilayer membranes and oligomerize into pores. GSDMB-NT has been reported to target plasma membranes of mammalian/cancer epithelial cells, leading to pyroptosis, and to membranes of Gram-negative bacteria, resulting in bacterial lysis, with sparing of host epithelial cells. GSDMB-NT, N-terminal domain of GSDMB.
A large body of evidence linking GSDMB to the pathogenesis of chronic inflammation and cancer comes from early genome-wide association studies (GWAS). Specifically, genetic polymorphisms of GSDMB, located in the 17q21 chromosomal region, are associated with susceptibility to asthma[21,22], inflammatory bowel disease (IBD)[23,24], rheumatoid arthritis[25,26] and primary biliary cholangitis[27], with single nucleotide polymorphisms (SNPs), rs2305479 (change from glycine to arginine) and rs2305480 (change from proline to serine), conferring a more rigid conformational protein structure of GSDMB, with an overall greater positive charge[13]. Such disease-related SNPs are functionally important leading to impairment of IEC proliferation, migration and adhesion[16]. Additionally, the full extent of the influence of GSDMB genetic variants on cleavage of full-length GSDMB (GSDMB-FL) has not been completely explored, but likely affects GSDMB’s overall function, similar to what is described for an asthma-associated SNP[28,29] and suggested for SNPs associated with Crohn’s disease[30], one of the eitopathogenic forms of IBD. At the protein level, GSDMB is upregulated in the bronchial and colonic epithelia of asthma[31] and IBD[16] patients, respectively; however, controversy surrounds reports of increased GSDMB in different types of cancers and consistency of its expression. In particular, elevated GSDMB is well-documented in breast, cervical and gastric cancers, with high GSDMB mRNA expression associated with a poor prognosis in breast cancer[5,12].
Nonetheless, despite accumulating evidence for disease relevance of GSDMB by both GWAS and expression profiling, its cellular and biological functions remain poorly understood. In this context, several levels of regulation likely exist, including the possibility that some GSDMB isoforms are more prone than others to proteolytic cleavage, and that specific mechanism(s) may be in place to upregulate isoform 5 that may potentially interfere with GSDMB-NT-dependent pore formation. Both scenarios can significantly affect formation of membrane pores and downstream cell lysis, and represent important issues to address in the future that require further investigation.
Gasdermin B-mediated pyroptosis is controversial
One of the most highly contested questions in the study of GSDMs is whether GSDMB can induce cellular pyroptosis. As a classical pyroptosis executioner, its related family member, GSDMD, acquires such ability after cleavage within its interdomain linker region, primarily by inflammatory caspases[11,15,32]. However, while GSDMB does not possess such inflammatory caspase cleavage sites[11,15,32], it can be cleaved at the linker region by GzmA[18] and within its N-terminal domain by apoptotic executioner caspases[13]. In fact, one of the seminal discoveries in GSDMB biology is its cleavage by GzmA, first reported to act on isoform 4 (the longest GSDMB isoform), resulting in pyroptosis of epithelial cells[18]. This mechanism requires secondary effectors cells (i.e., cytotoxic T lymphocytes, NK cells) as the source of GzmA and perforin, which is also a pore-forming protein facilitating GzmA entry into GSDMB-expressing target cells, since neither are inherently produced by the epithelium (Figure 1). In this setting, inflammatory events leading to T lymphocyte and/or NK cell expansion and recognition of MHC+ GSDMB-expressing target cells appear to be essential in order for GSDMB-dependent pyroptosis (or cell lysis in Gram-negative bacteria) to occur. It also remains unknown whether the contribution of perforin to self-destruction of targeted epithelial cells is solely dependent on GSDMB cleavage by GzmA or if there are other parallel, perforin-dependent processes occurring that lead to cell death. Indeed, the biological activity of the resulting cleaved GSDMB-NT remains controversial, with studies using almost identical experimental approaches (i.e., expression of GSDMB-NT in 293T human embryonic kidney cells), yielding conflicting results, either reporting pronounced cell death[28,29] or showing no cytotoxicity, by the expressed fragment[16,33,34]. These discrepancies are likely attributed to the evaluation of different GSDMB isoforms with various cleavage sites, some of which are unlikely to be able to form pores, or to different features of utilized 293T cells that may express different GSDMB accessory proteins required for cell lysis.
GSDMB’s pyroptotic activity is further questioned by a recent study showing the lack of interactions of either full-length GSDMB (GSDMB-FL) or GSDMB-NT isoform 1 with phospholipids typically present in eukaryotic plasma membranes, as well as the inability of GSDMB to form functional oligomeric pores in mammalian cell-derived liposomes[17]. Instead, the aforementioned study reveals specific targeting of GSDMB-NT to phospholipids of Gram-negative bacteria, and suggests a novel bactericidal function for GSDMB-NT, without causing lysis of host cells[17] (Figure 1). Results from this study also show that IpaH7.8, a bacterial ubiquitin ligase, potently suppresses NK cell-mediated killing of S. Flexneri by ubiquitinating and targeting GSDMB for proteolytic destruction, conferring an inherent self-preservation mechanism of the invading bacteria[17]. It remains unknown, however, if direct targeting of bacteria by gasdermins is a mechanism of mammalian immunity, or rather, non-specific interactions between gasdermin proteins and phospholipids found in species-specific membranes.
Another line of evidence against GSDMB’s ability to promote cell death is provided by a recent study showing marked induction of GSDMB upon treatment of IEC with methotrexate, causing translocation to the plasma membrane that creates bead-like aggregates; notably, these processes are not accompanied by either GSDMB cleavage or increased IEC death[16] (Figure 2). In addition, the reported physiological consequences of GSDMB expression in vivo are also inconsistent with its purported cell death-inducing activity. Specifically, transgenic mice expressing human GSDMB do not display overt signs of tissue injury and inflammation, but instead, demonstrate an enhanced development of peri-bronchial smooth muscles and increased deposition of extracellular matrix[31]. Together these data strongly suggest that unlike other members of the gasdermin family, the primary function(s) of GSDMB may differ from regulating cell death. Alternatively and as discussed earlier, it could be argued that GSDMB, requiring exogenous proteases (i.e., GzmA from CD8+ T cells or NK cells) for its cleavage, cannot mediate pyroptosis unless appropriate pro-inflammatory signals are activated. Finally, the possibility exists that murine proteases are unable, or ineffective, in processing an otherwise cleavable human protein.
Figure 2. Influence of genetic variants on GSDMB-mediated non-lytic activity.
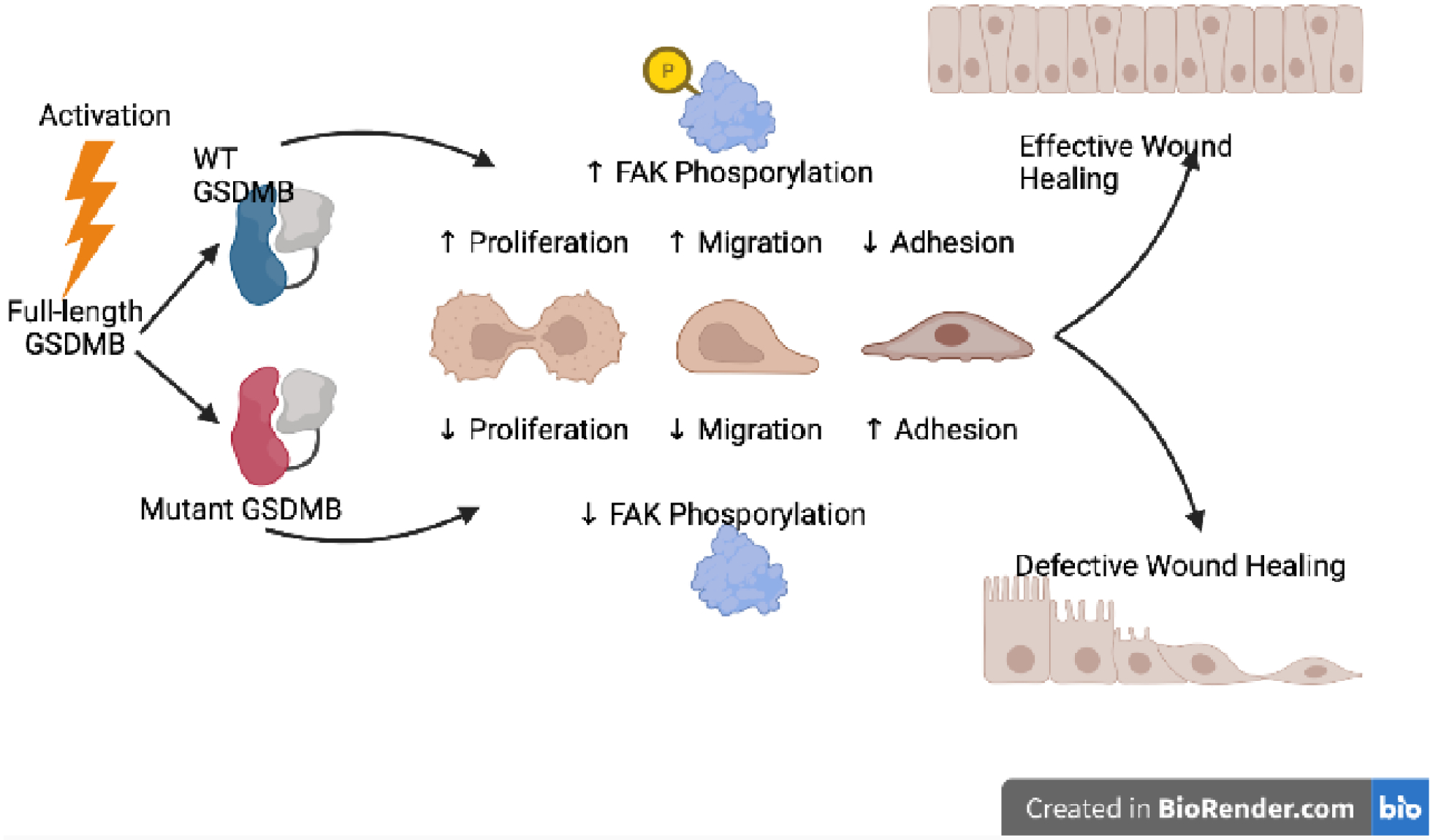
Activation of full-length GSDMB promotes translocation to the plasma membrane of IECs, and increased proliferation and migration, and decreased extracellular matrix adhesion that facilitates epithelial restitution and repair, and overall efficient wound healing. GSDMB-mediated FAK phosphorylation is thought, at least in part, to mechanistically underlie these processes. Conversely, mutant GSDMB, as a consequence of carriage of disease-associated GSDMB variants, results in decreased FAK phosphorylation, which interferes with proliferation, migration and adhesion processes, with an overall impairment of epithelial barrier function and appropriate wound healing. FAK, focal adhesion kinase; WT, wild-type.
GSDMB’s ability to bind to lipid bilayer membranes
As touched upon earlier, GSDMB differs from other GSDM family members in its ability to bind lipid membranes both as a full-length protein and as cleaved NT fragments. However, little is known about the specific mechanisms underlying the localization of GSDMB to lipid bilayer membranes. Similar to other GSDMs, the CT domain exerts some auto-inhibitory functions[28]; however, such a model is not sufficient to reconcile the findings from different studies in IECs, reporting that: 1) GSDMB-NT causes pyroptosis in epithelial cells[18], 2) GSDMB-NT selectively targets bacterial walls and spares host cells[17], and 3) GSDMB-FL binds the plasma membrane to induce epithelial restitution[16]. Conceptually, to explain such unique, and somewhat conflicting, observations, one, or rather a combination, of the following scenarios should be considered. First, differences in lipid composition of target membranes might explain the specificity of GSDMB binding; this would account for the preferential targeting displayed by GSDMB-NT, of Gram-negative bacteria and potentially, of neoplastic cells, which may present with mis-localized or altered lipids within their membranes. However, membrane composition cannot be the only relevant factor in determining whether GSDMB binds the plasma membrane, since this would not be sufficient to reconcile the observation that in HT-29 epithelial cells, both IFNγ and methotrexate increase GSDMB-FL, but only methotrexate activates its translocation to the membrane[16]. In fact, unless we assume that methotrexate alters membrane composition, other factors need to be in play to account for the specificity of GSDMB’s membrane targeting. It would appear conceivable that, for both GSDMB-FL and GSDMB-NT to bind membranes, additional events need to occur, such as post-translational modifications other than cleavage, or the co-operation with partner proteins, and that these events are indispensable for GSDMB to undertake a specific biological function (either lytic or non-lytic). Interactions between GSDMB and the chaperone, Hsp90, have been described[19], and chaperones and supramolecular complexes have been reported to be involved in the intracellular trafficking of non-pyroptotic GSDMD-FL[35]. Finally, different isoforms could preferentially bind membranes in different forms (i.e., FL vs. cleaved) and/or with differential lipid composition, which poses an intriguing hypothesis that IFNγ and methotrexate can induce the expression of different isoforms in IECs. In this context, while IFNγ upregulates GSDMB-FL, its localization is almost exclusively cytoplasmic, and Gzma (originating from other cells) needs to enter IECs to generate GSDMB-NT that binds to the plasma membrane, thereby causing pyroptosis[18]. This would suggest that methotrexate specifically upregulates an isoform capable of binding to the plasma membrane in its FL form, while IFNγ induces expression of a different isoform that needs proteolytic activation to bind lipid membranes. Taken together, the target specificity and regulatory mechanisms of GSDMB’s binding to lipid bilayer membranes, both in its full-length form and as NT fragments, represent a major conundrum with a wide range of biological implications that needs to be addressed in the future.
GSDMB as a driver of epithelial cell proliferation and motility
A well-documented increase of GSDMB expression in different cancers[5,12] prompted investigation into GSDMB’s potential role(s) in tumor development. These studies reveal surprisingly diverse functions that appear to control fundamental cellular processes, such as proliferation and cell motility[16,19,36,37]. Specifically, overexpression of GSDMB accelerates breast cancer cell xenograft growth in mice, whereas knockdown of this protein inhibits breast tumor development in vivo[19,37]. Furthermore, a recently developed mouse model knocking in GSDMB isoform 2 shows accelerated development of breast cancer driven by the HER2/NEu oncogene[38]. Other work shows that GSDMB depletion significantly inhibits in vitro proliferation of IECs[16] and bladder cancer cells[36]. Despite these findings, the mechanisms involved in GSDMB-dependent regulation of epithelial cell proliferation are largely unknown. In bladder cancer cells, GSDMB interacts with STAT3 and regulates STAT3 activation[36], which causes upregulation in the expression of several metabolic enzymes and stimulation of glycolysis. It remains to be determined, however, whether the described mechanism is limited to bladder cancer, or could underlie GSDMB activity in other epithelial and cancer cells.
Another important functional activity of epithelial-derived GSDMB involves regulation of cell migration. Overexpression of GSDMB in breast carcinoma cells stimulates cell motility and subsequent invasive capacity, specifically demonstrated for GSDMB isoform 2 in in vivo mouse xenograft models[19], whereas its depletion in transformed IEC and primary colonic organoids attenuates both collective and individual cell migration[16]. Since GSDMB is markedly upregulated in the colonic epithelium of IBD patients[16], and chronic inflammation during IBD is known to trigger mucosal damage and ulceration[39,40], it is likely that induction of full-length GSDMB in the inflamed intestinal epithelium plays a protective role by stimulating wound healing and mucosal repair. IBD-associated GSDMB SNPs impair the pro-migratory activity of this protein[16], providing novel functional insights into understanding the association of GSDMB genetic polymorphisms with susceptibility to IBD.
Nonetheless, little is known regarding the mechanism(s) of GSDMB-dependent regulation of epithelial cell migration, but recent work has linked this function to the control of cell-extracellular matrix (ECM) adhesion and assembly of adhesive multiprotein complexes at the base of the cell, referred to as ‘focal adhesions’[16]. Indeed, loss of GSDMB in IECs increases focal adhesion assembly and enhances cell attachment to the ECM, resulting in hyper-adhesiveness that impairs the efficiency IEC movement. Furthermore, attenuated motility of GSDMB-deficient IECs is associated with inactivation (dephosphorylation) of a key regulator of ECM adhesion, focal adhesion kinase (FAK), with pharmacologic activation of FAK showing consistent rescue of wound healing defects in GSDMB-depleted cell monolayers[16]. This GSDMB-dependent process involves transcriptional regulation of platelet derived growth factor (PDGF)-A[16], previously reported to induce FAK phosphorylation[41,42]. Nonetheless, the described modulation of PDGF-A/FAK signaling may not be the only mechanism responsible for GSDMB-dependent regulation of epithelial cell migration. In fact, overexpression of GSDMB in breast cancer cells is reported to activate members of the Rho small GTPase family, Rac1 and Cdc-42, which are critical regulators of cytoskeletal remodeling in motile cells[19]. As such, evidence points to GSDMB promoting epithelial cell motility by controlling at least two different molecular events: the dynamics of ECM adhesions and actin filament remodeling at the leading edge of migrating epithelial sheets.
Taken together, these findings suggest overall protective functions of epithelial-derived GSDMB as opposed to those promoting autolytic events, which could prove to be catastrophic for maintaining epithelial barrier integrity and in certain settings, lead to a state of uncontrolled, chronic inflammation. However, it should also be taken into account that, during certain conditions, such as the presence neoplasia or bacterial infection, epithelial-derived GSDMB can also confer protection via lytic events, although not without some damage to epithelial barrier integrity.
Downstream effector molecules regulated by Gasdermin B
While the downstream events of GSDMB-dependent lytic functions have been more clearly delineated, the identification and contribution of effector molecules during the process of GSDMB-dependent proliferation and cell motility in epithelial cells are less defined. Indeed, the decreased PDGF-A observed in GSDMB-deficient IEC described earlier is not an isolated event, but rather, part of a global transcriptional reprogramming of these cells. In fact, RNA sequencing analysis reveals that GSDMB knockout in IECs causes global changes in gene expression that include downregulation of genes involved in cell proliferation and motility, and upregulation of ECM-adhesion-associated genes[16]. Such transcriptional changes correlate well with the observed functional effects of IEC deplete of GSDMB, and are consistent with recent findings in bladder cancer cells, wherein transient knockdown of GSDMB causes substantial changes in gene expression[36]. Furthermore, GSDMB overexpression in bronchial epithelial cells upregulate several genes that are relevant to cell migration, including transforming growth factor-β, matrix metalloproteinase 9 and different chemokines[31]. However, how GSDMB regulates downstream gene expression remains unknown and represents an understudied area of investigation. In bronchial epithelial cells, GSDMB localizes to the nucleus and deletion of its nuclear localization signal reverses the increased gene transcription caused by GSDMB expression[31]. In contrast, GSDMB activation in IECs does not result in its nuclear translocation[16]. The mechanism(s) underlying these conflicting results are unclear. One possible explanation may be the isoform-specific behavior of GSDMB. Specifically, GSDMB isoforms 1 and 4 are reported to be localized to the nucleus, where they may regulate gene expression by serving as transcription co-activators or enhancers, whereas isoforms 2 and 3 do not show nuclear localization[20,31]. However, isoform specificity of GSDMB induced in IEC has not been determined, and remains an important issue to resolve. Alternatively, transcriptional reprogramming can represent an indirect effect of GSDMB activity, involving secretion of different regulatory molecules, such as growth factors, cytokines and chemokines. These secreted mediators may signal to epithelial cells in an autocrine/paracrine fashion, thereby regulating the transcriptional program of these cells.
Clinical implications of GSDMB in GI health and disease
In regard to the clinical implications of GSDMB expression, specifically in the GI tract, the evidence presented thus far supports an overall protective role for IEC-derived GSDMB in IBD[9], CRC[[17,18], and enteric infections[[17,18], although it is worth noting that opposing functions (i.e., proliferation in the former vs. pyroptosis in the latter two) are proposed to mediate both of these processes (reviewed in[43]). A balance needs to be met in order to maintain homeostatic conditions since dysregulation can lead to exacerbation of disease. For example, increased GSDMB-dependent pyroptosis in the gut epithelium of patients with IBD would compromise barrier integrity, facilitate bacterial translocation into the underlying mucosa, and could further aggravate chronic inflammation mediated by uncontrolled mucosal immune responses. In fact, IBD patients harboring disease-associated GSDMB variants are thought to have less effective epithelial restitution and wound healing processes than those carrying WT GSDMB[9]. Conversely, if unchecked, GSDMB-dependent regulation of cell proliferation and adhesion/migration, specifically in its full-length form, may also promote tumorigenesis in susceptible hosts and play a critical role in cancer progression. On the other hand, GSDMB’s protective effects of the gut mucosa can also be conferred by pro-pyroptotic and -lytic events. For example, targeting neoplastic or malignant epithelium for pyroptotic clearance, as well as specific lysis of GSDMB-expressing bacterially-infected epithelial cells would certainly provide overall protection for affected individuals and can preserve normal gut function.
It is also possible to devise future prognostic or therapeutic tools targeting GSDMB for clinical use. For example, GSDMB protein levels in gut mucosal biopsies can be potentially used as a biomarker for monitoring disease progression of IBD and/or colon cancer. In this context, and considering the apparent, opposing functions of GSDMB in IEC, it is likely that the ratio between full-length and cleaved forms, rather than the overall protein levels, may represent a more meaningful clinical biometric to assess whether GSDMB is primarily functioning as an inducer of epithelial restitution or rather, as a pyroptotic effector. Finally, and as discussed earlier, the therapeutic potential of regulating epithelial GSDMB must take into consideration an optimal approach as to how to best bias GSDMB towards the desired function; namely, pyroptosis of tumor cells in colorectal cancer to arrest carcinogenesis, bactericidal effects during enteric infections of intracellular pathogens, or epithelial restitution in IBD to promote wound healing. Therefore, future therapies targeting GSDMB would likely require specific regulation of expression and/or activation of full-length vs. cleaved GSDMB in order to effectively leverage its therapeutic potential.
Concluding remarks
In conclusion, recent studies reveal important and versatile functional roles of GSDMB in regulating epithelial responses to inflammation, infection and tumorigenesis, and provide a better understanding regarding the mechanism(s) by which GSDMB-dependent functions are regulated, specifically in gut epithelial cells. These studies have illustrated that the field of GSDMB biology remains at infancy with many compelling questions remaining to be addressed for future investigation (see Outstanding Questions). For example, we know very little about tissue- and disease-specific mechanisms that drive the different cellular activities of GSDMB, including formation of either lytic or non-lytic membrane pores, or pore-independent modulation of cell signaling. A crucial step in solving this conundrum could be provided by elucidating the structure of GSDMB complexes assembled at membranes using super-resolution microscopy or cryo-electron tomography. It is likely that the diverse cellular functions of GSDMB are regulated by yet to be identified accessory proteins or posttranslational modifications. Applying unbiased approaches, such as Bio-ID and mass spectroscopy, have great potential for discovery of novel molecular states and interactors for GSDMB. Another unsolved question relates to the unique biological functions and regulation of different GSDMB variants. A combination of spatial transcriptomics, particularly in the intestines, combined with single cell RNA sequencing analysis and interrogation of cellular functions of specific GSDMB isoforms holds the key to understanding the diverse biological roles of this protein. Last, but not least, the reported transcriptional activity of GSDMB remains completely enigmatic, and whether regulation is dictated by nuclear translocation of this protein or if it indirectly affects its pore-forming activity and secretion of autocrine regulators. Studies addressing these important questions have the potential to make crucial discoveries regarding alternative functions of GSDMB, as well as reveal novel regulatory mechanisms that will have important implications in designing strategies to modulate this interesting protein during disease states.
Outstanding Questions.
What are the mechanisms and structural determinants that mediate GSDMB’s interactions with eukaryotic cell membranes and lipid membranes of pathogenic bacteria?
Specifically, how does cleavage of GSDMB influence its affinity to different phospholipids? And how do GSDMB polymorphisms and different splice variants affect the lipid binding ability of this protein?
What are the roles of different GSDMB isoforms in regulating epithelial cell functions under homeostatic conditions and during disease states?
Are GSDMB isoforms tissue- and/or cell-specific? Which isoforms are induced during inflammation and tumorigenesis and what are the mechanisms of preferential induction of certain GSDMB isoforms?
What regulatory mechanisms are in place that determine the cellular distribution and function(s) of GSDMB?
For example, both Mtx and IFNγ upregulate GSDMB-FL expression in the intestinal epithelium, but only Mtx triggers formation of GSDMB aggregates at the plasma membrane. Is GSDMB-FL capable of self-assembly within membranes or does it require partner proteins? Does this process involve specific post-translational protein modification(s) (e.g. phosphorylation)?
What are the triggers and mechanisms governing lytic versus non-lytic GSDMB-dependent functions?
Under what conditions are these functions implemented, and what are the implications if these GSDMB-dependent functions are dysregulated?
What are the downstream molecular/signaling effectors of non-pyroptotic GSDMB?
A crucial unanswered question relates to the mechanisms by which gasdermin regulates transcriptional reprogramming of epithelial and cancer cells. Does GSDMB play a direct role by regulating gene transcription in the nucleus or does it orchestrate autocrine signaling feedback loops by controlling processing and secretion of different cytokines and growth factors?
Highlights.
The role of gasdermin B (GSDMB) as an executioner of pyroptosis in intestinal epithelial cells is controversial, with emerging evidence supporting its role in alternative activities that go beyond pyroptotic cell death.
Recent findings regarding GSDMB’s function during gastrointestinal (GI) disorders suggest an overall protective role of epithelial-derived GSDMB, promoting pyroptosis of cancer cells, bactericidal processes, and epithelial restitution and wound repair.
Several mechanisms for controlling GSDMB function exists, and at different levels (e.g., as a consequence of GSDMB polymorphisms, by alternative splicing, and through regulation of its cleavage, among others). We are at the advent of discovery regarding GSDMB’s biology, with recent studies providing important insights into the mechanism(s) of such regulation and its specific roles during health and disease states.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institute of Allergy and Infectious Diseases (NIAID) to T.T.P. (P01, Project 3 DK091222, R01 DK042191, R01 DK125293, P01, Project 4 AI141350), and to A.I.I. (R01 DK126702). Figures 1 and 2 were created by N.R. using BioRender.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
No conflict of interests declared.
References
- 1.Feng S et al. (2018) Mechanisms of gasdermin family members in inflammasome signaling and cell death. J. Mol. Biol 430, 3068–3080 [DOI] [PubMed] [Google Scholar]
- 2.Saeki N et al. (2009) Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer 48, 261–271 [DOI] [PubMed] [Google Scholar]
- 3.Tamura M et al. (2007) Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics 89, 618–629 [DOI] [PubMed] [Google Scholar]
- 4.Hou J et al. (2021) Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol. Cell 81, 4579–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarrio D et al. (2021) The multifaceted roles of gasdermins in cancer biology and oncologic therapies. Biochim. Biophys. Acta Rev. Cancer 1876, 188635. [DOI] [PubMed] [Google Scholar]
- 6.Zou J et al. (2021) The versatile gasdermin family: their function and roles in diseases. Front. Immunol 12, 751533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Privitera G et al. (2022) Novel insights into the interactions between the gut microbiome, inflammasomes, and gasdermins during colorectal cancer. Front. Cell. Infect. Microbiol 11, 806680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broz P et al. (2020) The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol 20, 143–157 [DOI] [PubMed] [Google Scholar]
- 9.Liu X et al. (2021) Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov 20, 384–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruhl S and Broz P (2022) Regulation of lytic and non-lytic functions of gasdermin pores. J. Mol. Biol 434, 167246. [DOI] [PubMed] [Google Scholar]
- 11.Kayagaki N et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 [DOI] [PubMed] [Google Scholar]
- 12.Li L et al. (2020) Role of GSDMB in pyroptosis and cancer. Cancer Manag. Res 12, 3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao KL et al. (2017) Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc. Natl. Acad. Sci. U S A 114, E1128–E1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan J (2019) Structural insight of gasdermin family driving pyroptotic cell death. Adv. Exp. Med. Biol 1172, 189–205 [DOI] [PubMed] [Google Scholar]
- 15.Shi J et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 [DOI] [PubMed] [Google Scholar]
- 16.Rana N et al. (2022) GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell 185, 283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen JM et al. (2021) Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell 184, 3178–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z et al. (2020) Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 [DOI] [PubMed] [Google Scholar]
- 19.Hergueta-Redondo M et al. (2014) Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS One 9, e90099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Q et al. (2008) Expression of GSDML associates with tumor progression in uterine cervix cancer. Transl. Oncol 1, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X et al. (2021) Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J. Allergy Clin. Immunol 147, 894–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H et al. (2009) Genetic variation in ORM1-like 3 (ORMDL3) and gasdermin-like (GSDML) and childhood asthma. Allergy 64, 629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christodoulou K et al. (2013) Next generation exome sequencing of paediatric inflammatory bowel disease patients identifies rare and novel variants in candidate genes. Gut 62, 977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soderman J et al. (2015) Gene expression-genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility. Biomed. Res. Int 2015, 834805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal LR and Moult J (2015) Genetic basis of common human disease: insight into the role of missense SNPs from genome-wide association studies. J. Mol. Biol 427, 2271–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl EA et al. (2010) Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet 42, 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hitomi Y et al. (2017) Identification of the functional variant driving ORMDL3 and GSDMB expression in human chromosome 17q12–21 in primary biliary cholangitis. Sci. Rep 7, 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding J et al. (2016) Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 [DOI] [PubMed] [Google Scholar]
- 29.Panganiban RA et al. (2018) A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J. Allergy Clin. Immunol 142, 1469–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong W et al. (2021) P010 GSDMB-mediated pyroptosis exacerbates intestinal inflammation by destroying intestinal epithelium in Crohn’s disease. J. Crohn’s Colitis 15, S129–S131 [Google Scholar]
- 31.Das S et al. (2016) GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc. Natl. Acad. Sci. U S A 113, 13132–13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He WT et al. (2015) Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 25, 1285–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q et al. (2019) GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J. Mol. Cell. Biol 11, 496–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi P et al. (2015) Loss of conserved Gsdma3 self-regulation causes autophagy and cell death. Biochem. J 468, 325–336 [DOI] [PubMed] [Google Scholar]
- 35.Bulek K et al. (2020) Epithelial-derived gasdermin D mediates nonlytic IL-1beta release during experimental colitis. J. Clin. Invest 130, 4218–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He H et al. (2021) USP24-GSDMB complex promotes bladder cancer proliferation via activation of the STAT3 pathway. Int. J. Biol. Sci 17, 2417–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molina-Crespo A et al. (2019) Intracellular delivery of an antibody targeting gasdermin-B reduces HER2 breast cancer aggressiveness. Clin. Cancer Res 25, 4846–4858 [DOI] [PubMed] [Google Scholar]
- 38.Sarrio D et al. (2022) Gasdermin-B pro-tumor function in novel knock-in mouse models depends on the in vivo biological context. Front. Cell Dev. Biol 10, 813929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho GT et al. (2020) Resolution of inflammation and gut repair in IBD: translational steps towards complete mucosal healing. Inflamm. Bowel Dis 26, 1131–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neurath MF and Leppkes M (2019) Resolution of ulcerative colitis. Semin. Immunopathol 41, 747–756 [DOI] [PubMed] [Google Scholar]
- 41.Hunger-Glaser I et al. (2004) PDGF and FGF induce focal adhesion kinase (FAK) phosphorylation at Ser-910: dissociation from Tyr-397 phosphorylation and requirement for ERK activation. J. Cell Physiol 200, 213–222 [DOI] [PubMed] [Google Scholar]
- 42.Singh J et al. (2019) Role of PDGF-A-activated ERK signaling mediated FAK-paxillin interaction in oligodendrocyte progenitor cell migration. J. Mol. Neurosci 67, 564–573 [DOI] [PubMed] [Google Scholar]
- 43.Privitera G and Pizarro TT (2022) Live or let die: translational insights and clinical perspectives of GSDMB-dependent intestinal epithelial cell fate. Clin. Transl. Med 12, e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin PH et al. (2015) N-terminal functional domain of Gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J. Biomed. Sci 22, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng W et al. (2022) Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602, 496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi P et al. (2015) Loss of conserved Gsdma3 self-regulation causes autophagy and cell death. Biochem. J 468, 325–336 [DOI] [PubMed] [Google Scholar]
- 47.De Schutter E et al. (2021) Punching holes in cellular membranes: biology and evolution of gasdermins. Trends Cell. Biol 31, 500–513 [DOI] [PubMed] [Google Scholar]
- 48.Saeki N et al. (2000) Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm. Genome 11, 718–724 [DOI] [PubMed] [Google Scholar]
- 49.Terao C et al. (2017) Transethnic meta-analysis identifies GSDMA and PRDM1 as susceptibility genes to systemic sclerosis. Ann. Rheum. Dis 76, 1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou J et al. (2020) PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell. Biol 22, 1264–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miguchi M et al. (2016) Gasdermin C is upregulated by inactivation of transforming growth factor beta receptor type II in the presence of mutated Apc, promoting colorectal cancer proliferation. PLoS One 11, e0166422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sollberger G et al. (2018) Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol 3, eaar6689 [DOI] [PubMed] [Google Scholar]
- 53.Karmakar M et al. (2020) N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun 11, 2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X et al. (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers C et al. (2019) Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun 10, 1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platnich JM et al. (2018) Shiga toxin/lipopolysaccharide activates caspase-4 and gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep 25, 1525–1536 [DOI] [PubMed] [Google Scholar]
- 57.Kayagaki N et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 [DOI] [PubMed] [Google Scholar]
- 58.Sarhan J et al. (2018) Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. of the Natl. Acad. Sci. USA 115, E10888–E10897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgener SS et al. (2019) Cathepsin G inhibition by Serpinb1 and Serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-driven inflammation. Cell Rep 27, 3646–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evavold CL et al. (2018) The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaul S et al. (2021) Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol 74, 156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y et al. (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z et al. (2020) Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan G et al. (2021) Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation. Cell Rep 35, 109265. [DOI] [PubMed] [Google Scholar]
- 65.Tan G et al. (2020) HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol 13, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Laer L et al. (1998) Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat. Genet 20, 194–197 [DOI] [PubMed] [Google Scholar]
- 67.Xia W et al. (2021) Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis 12, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Defourny J et al. (2019) Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc. Natl. Acad. Sci. USA 116, 8010–8017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer FA et al. (2021) Posttranslational and therapeutic control of gasdermin-mediated pyroptosis and inflammation. Front. Immunol 12, 661162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zahid A et al. (2021) Molecular and structural aspects of gasdermin family pores and insights into gasdermin-elicited programmed cell death. Biochem. Soc. Trans 49, 2697–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C and Ruan J (2021) Mechanistic insights into gasdermin pore formation and regulation in pyroptosis. J. Mol. Biol 434, 167297. [DOI] [PMC free article] [PubMed] [Google Scholar]


