Abstract
MicroRNAs (miRNA) post-transcriptionally repress gene expression by guiding Argonaute proteins to target mRNAs. While much is known about regulation of miRNA biogenesis, miRNA degradation pathways are comparatively poorly understood. Although miRNAs generally exhibit slow turnover, they can be rapidly degraded through regulated mechanisms that act in a context or sequence-specific manner. Recent work has revealed a particularly important role for specialized target interactions in controlling rates of miRNA degradation. Engagement of these targets is associated with addition and removal of nucleotides from the 3′ ends of miRNAs, a process known as tailing and trimming. Here we review these mechanisms of miRNA modification and turnover, highlighting the contexts in which they impact miRNA stability and discussing important questions that remain unanswered.
Keywords: MicroRNA, degradation, tailing and trimming, target-directed microRNA degradation, Argonaute, ZSWIM8
MicroRNAs: tightly controlled post-transcriptional regulators
MicroRNAs (miRNA) are small noncoding RNAs that play a role in virtually every aspect of animal development by broadly regulating gene expression [1]. In animals, miRNAs are initially transcribed as long primary transcripts that undergo a defined series of processing events to produce mature miRNAs that are loaded into Argonaute (AGO) proteins (see Glossary) [2]. miRNAs guide AGO to sites of imperfect complementarity in 3′ untranslated regions (UTRs) of target messenger RNAs (mRNAs), resulting in degradation and reduced translation of targets [3]. The human genome encodes hundreds of conserved miRNAs [4] and, since each can regulate dozens to hundreds of target mRNAs, the impact of miRNA activity on the transcriptome is profound. In fact, over 45,000 conserved miRNA target sites are predicted in the 3′ UTRs of human mRNAs [5]. Not surprisingly, loss of single miRNAs often yields dramatic phenotypes in model organisms such as Drosophila, C. elegans, and mice [1]. Moreover, altered miRNA expression contributes to the pathogenesis of many human diseases [6].
Due to their importance in diverse biological settings, the mechanisms that regulate miRNA biogenesis and activity have been extensively investigated [7]. Comparatively less is known about the mechanisms through which mature miRNAs are turned over. Once loaded into an AGO protein, the labile 5′ and 3′ termini of a miRNA are buried in the AGO Middle (MID) and PIWI/Argonaute/Zwille (PAZ) domains, shielding them from degradation (Fig. 1) [8–10]. As a result, miRNAs are extremely stable and generally exhibit much slower turnover than most mRNAs [11]. Nevertheless, miRNAs with short half-lives have been observed [12–20]. These findings point to the existence of mechanisms that can actively and selectively promote miRNA degradation. As reviewed elsewhere [21–23], specific ribonucleases have been implicated in the decay of selected miRNAs. Recent data, however, have revealed a particularly important role for target engagement in determining rates of miRNA turnover.
Figure 1. Structure of human AGO2 bound to a small RNA.

(A) Domain organization of human AGO2. (B) The 5′ and 3′ termini of the small RNA (in black) are buried in the AGO MID and PAZ domains (PDB ID: 4W5N) [10]. Protein structures were rendered by UCSF ChimeraX [109].
In this review, we will discuss the intimate relationship between the engagement of targets by miRNAs, modifications of miRNA 3′ ends through a process called tailing and trimming, and miRNA decay. This review will focus primarily on mechanisms of miRNA turnover, including target-directed miRNA degradation (TDMD), but related mechanisms that impact the stability of other classes of small RNAs will also be discussed (summarized in Table 1).
Table 1.
Factors involved in tailing, trimming, and turnover of small RNAs
| Factor(s) | Substrates | Activity | Organism(s) | Condition(s) | References |
|---|---|---|---|---|---|
| Unknown ubiquitin ligase | Global miRNA decay |
Ubiquitylation of AGO proteins | Mus musculus | T cell stimulation | [24] |
| Vp55 | Global miRNA decay |
3′-adenylation of miRNAs | Poxvirus | Poxvirus infection | [25] |
| Wispy | Global miRNA decay |
3′-adenylation of miRNAs | Drosophila melanogaster | Maternal-to-zygotic transition | [26] |
| HEN1 SUPRESSOR1 (HESO1) and UTP:RNA Uridylyltransferase 1 (URT1) | miR-165 miR-166 miR-167 miR-169 miR-170 miR-171 miR-173 |
3′-uridylation of miRNAs | Arabidopsis thaliana | Degradation of small RNAs lacking 2′-O-methyl group at 3′ end | [40–43] |
| SMALL RNA DEGRADING NUCLEASE (SDN1–3) | miR-156 miR-159 miR-163 miR-164 miR-165 miR-166 miR-167 miR-172 miR-173 siR1003 |
Removal of 2′-O-methylated nucleotide at miRNA 3′ end | Arabidopsis thaliana | Degradation of miRNAs loaded into AGO10 (miR-165/166) and other unknown conditions | [44–46] |
| ZSWIM8/Dora/EBAX-1 ubiquitin ligase | Numerous miRNAs |
Ubiquitylation of AGO proteins | Homo sapiens, Mus musculus, Drosophila melanogaster, Caenorhabditis elegans | Extensive complementarity between miRNA and target | [76–84, 91, 92, 96] |
| HAWAIIAN SKIRT (HWS) | miR-156 miR-159 miR-160 miR-163 miR-164 miR-167 miR-168 miR-319 miR-398 |
Ubiquitylation of AGO proteins | Arabidopsis thaliana | Association of miRNA with target mimic | [104–106] |
Global miRNA decay
It is instructive to begin by first considering examples of global miRNA turnover, since common mechanisms have been implicated in both global miRNA degradation and miRNA-specific decay pathways. The concept that particular cell types or cell states are associated with globally accelerated miRNA degradation was first suggested by Filipowicz and colleagues, who observed that many miRNAs have relatively short half-lives in neurons [20]. Globally accelerated miRNA decay was also observed upon activation of T cells [24]. While the mechanism of enhanced miRNA degradation in neurons remains unresolved, stimulation of T cells was found to trigger ubiquitylation of AGO proteins, resulting in their degradation by the proteasome and wholesale clearance of miRNAs (Fig. 2A). Although the ubiquitin ligase that mediates AGO ubiquitylation upon T cell activation has not been identified, this connection between the ubiquitin-proteasome system and miRNA turnover foreshadowed the later discovery, discussed in detail below, of a similar mechanism that can act in a miRNA-specific manner.
Figure 2. Mechanisms of global miRNA turnover.
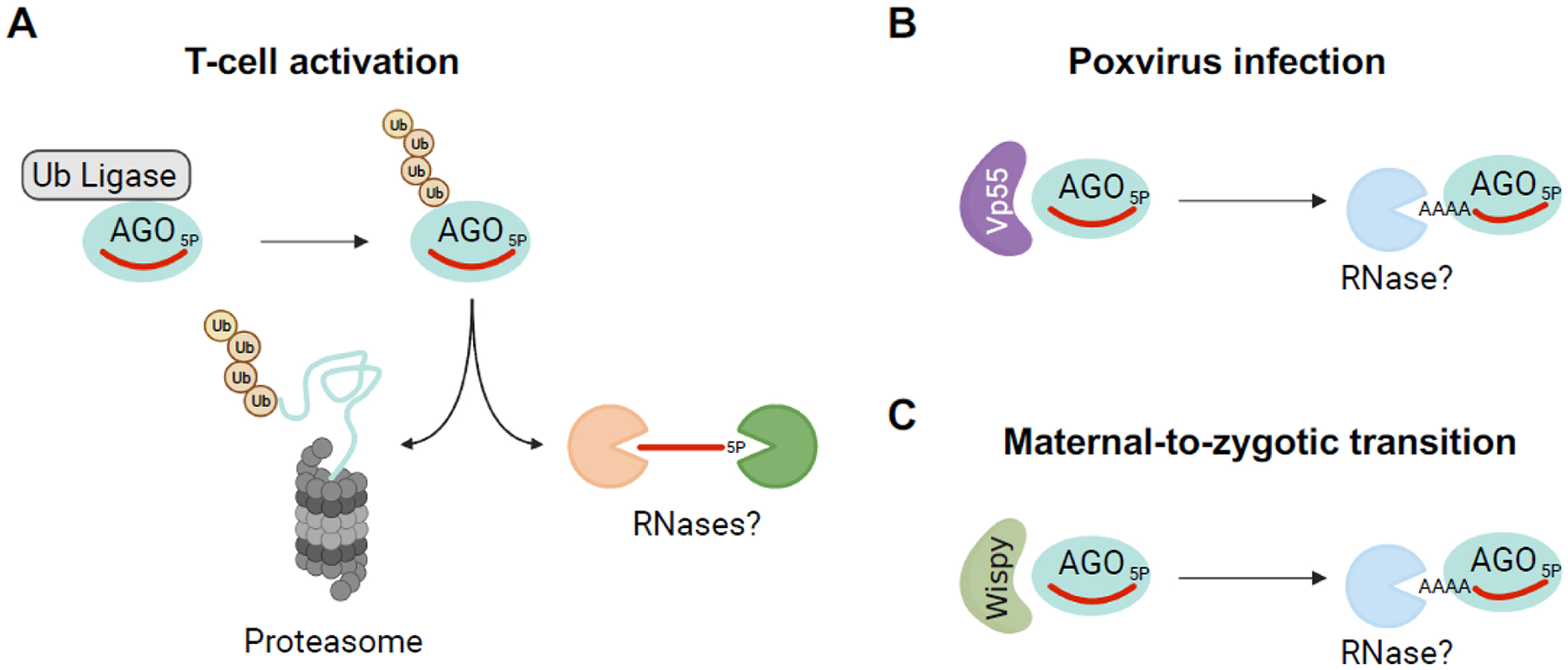
(A) During T-cell activation, AGO proteins are ubiquitylated by an unknown ubiquitin ligase and undergo proteasome-mediated degradation, resulting in global miRNA decay [24]. (B) Poxvirus Vp55 adenylates human miRNAs, leading to their degradation by an unknown nuclease(s) [25]. (C) Wispy adenylates miRNAs during the maternal-to-zygotic transition in Drosophila embryos, leading to global clearance of maternal miRNAs [26]. Figure created with Biorender.com.
Enzymes that post-transcriptionally modify miRNAs, particularly by adding non-templated nucleotides to their 3′ ends through a process called tailing, also appear to be capable of promoting global miRNA turnover. For example, poxviruses encode a poly(A) polymerase called Vp55 that efficiently polyadenylates the 3′ ends of mature miRNAs in virally-infected cells, resulting in global miRNA decay [25] (Fig. 2B). Similarly, a noncanonical poly(A) polymerase, Wispy, adenylates miRNA 3′ ends during the maternal-to-zygotic transition in Drosophila, promoting the clearance of maternally-deposited miRNAs (Fig. 2C) [26]. As discussed further below, how tailing leads to miRNA degradation, and the contexts in which this occurs, remain active areas of investigation with many unresolved questions.
Evidence for accelerated turnover of specific miRNAs
In keeping with the concept that AGO protects miRNAs from cellular nuclease activities, mature miRNAs generally exhibit very slow decay kinetics. Early studies of miRNA expression in vivo supported this notion. For example, miR-208 was found to decay with a half-life greater than 12 days in rodent heart tissue [27], while the half-life of miR-122 was estimated to be greater than 24 hours in mouse liver [28]. On the other hand, selected miRNAs with short half-lives were identified in cell lines [12, 13].
More recently, ever-improving high-throughput methods have been used to examine the stability of hundreds of miRNAs in cell culture models, providing a clearer picture of the global landscape of miRNA turnover. These methods have included blockade of transcription with actinomycin D or 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside (DRB) in mammalian cell lines, followed by microarray analysis, bead-based miRNA profiling, or high-throughput sequencing [16, 17]; acute genetic ablation of Dicer1 in mouse embryonic fibroblasts (MEFs) followed by Taqman analysis of a large panel of miRNAs [18]; and metabolic labeling of miRNAs followed by high-throughput sequencing in Drosophila S2 cells [19], 3T9 mouse fibroblasts [15], MEFs [14], and mouse embryonic stem cells (mESC) [14]. Despite using different experimental approaches, these studies all arrived at the shared conclusion that a large majority of miRNAs decay slowly, while selected miRNAs, which may differ depending on the cellular context, are rapidly turned over. These data provide strong evidence in support of the existence of active mechanisms that promote selective miRNA degradation.
Tailing, trimming, and small RNA turnover
Post-transcriptional modifications of small RNA 3′ ends have been repeatedly implicated in multiple small RNA decay pathways. The 3′ ends of small RNAs can be modified by a variety of enzymes, including terminal nucleotidyl transferases that add non-templated nucleotides, a process referred to as ‘tailing’, or exonucleases that remove nucleotides, termed ‘trimming’ [29]. The consequences of these modifications appear to depend upon multiple factors, including the type of small RNA that is modified, the identity of the nucleotides that are added to the small RNA 3′ end, and the species in which tailing and trimming occurs.
3′-end tailing by noncanonical polymerases is an established destabilizing signal for several classes of RNAs. For example, the Trf4/Air2/Mtr4 polyadenylation (TRAMP) complex adds non-templated adenosines to noncoding RNAs such as small nucleolar RNAs (snoRNAs) and ribosomal RNAs in Saccharomyces cerevisiae. The resulting poly(A) tail promotes the recruitment of 3′-to-5′ exoribonucleases including the RNA exosome for degradation or further 3′ processing of the RNA [30, 31]. In addition, 3′ terminal uridine tails provide a signal for degradation by the 3′-to-5′ exoribonuclease DIS3L2. For example, the RNA binding protein LIN28 binds let-7 miRNA precursors, leading to 3′ uridylation by terminal uridyl transferase 4 (TUT4, also known as TENT3A or ZCCHC11) and TUT7 (also known as TENT3B or ZCCHC6) [32–35], resulting in DIS3L2-mediated degradation [36, 37]. Based on these well-established noncoding RNA degradation pathways, it was logical to hypothesize that 3′ tailing could also regulate the stability of mature miRNAs.
There is strong evidence that 3′ uridylation of miRNAs in plants indeed acts as a destabilizing signal. Most plant miRNAs are post-transcriptionally modified by the addition of a 2′-O-methyl group at their 3′ ends by the methyl transferase HEN1, which prevents tailing and trimming [38, 39]. This enzyme contains double-stranded RNA (dsRNA) binding domains, enabling it to bind and methylate processed miRNA duplexes before they are loaded into an AGO protein (Fig. 3). In the absence of this protective 3′ modification, miRNAs become susceptible to uridylation by the terminal nucleotidyl transferases HEN1 SUPRESSOR1 (HESO1) and UTP:RNA Uridylyltransferase 1 (URT1), leading to tailing and reduced levels of mature miRNAs [40–43]. The precise mechanism through which 3′ uridylation of mature miRNAs in plants leads to their decay, however, remains unclear and the responsible nuclease(s) have not been identified.
Figure 3. Degradation of miRNAs in plants.

The 3′ ends of plant miRNAs are 2′-O-methylated by HEN1, which recognizes fully-processed miRNA duplexes through its dsRNA binding domains. This prevents HESO1/URT1-mediated 3′ uridylation and subsequent degradation of miRNAs by an unknown nuclease(s). SDN proteins can remove the 2′-O-methyl group, triggering miRNA uridylation and degradation. Figure created with Biorender.com.
Interestingly, a family of 3′-to-5′ exoribonucleases in Arabidopsis, called SMALL RNA DEGRADING NUCLEASE 1–3 (SDN1–3), are able to trim the 3′ ends of miRNAs that are loaded into AGO proteins, irrespective of the presence of 2′-O-methylation at the miRNA 3′ end [44–46]. Owing to their ability to remove the protective 2′-O-methyl group, it is believed that SDN proteins initiate miRNA decay by rendering their trimmed, AGO-associated miRNA products susceptible to tailing by HESO1/URT1 and consequent degradation (Fig. 3). Accordingly, simultaneous depletion of all SDN family members increased the abundance of a subset of miRNAs in vivo [44]. SDN1 interacts with the PAZ domain of AGO1 [46], explaining how it is recruited to the miRNA 3′ end to initiate decay. However, only selected miRNAs accumulate in SDN-depleted plants. How the specificity of SDN-initiated miRNA degradation is determined remains to be established.
Animal miRNAs lack the 2′-O-methyl modification at their 3′ ends and multiple terminal nucleotidyl transferases are capable of tailing these RNAs. For example, TUT4 and TUT7 add non-templated uridines to the 3′ ends of miRNAs [47–49], while non-templated 3′ adenylation is catalyzed by GLD2 (also known as TUT2, TENT2, or PAPD4) and GLD4 (also known as TUT3, TENT4B, or PAPD5) [50–52]. 3′ modification of miRNAs by these enzymes has been reported to modulate the stability of specific miRNAs in some settings [48, 50–53], However, a growing body of evidence suggests that miRNA tailing is not a general trigger for miRNA degradation in animals. For example, loss of 3′ miRNA adenylation by knockdown or knockout of GDL2 in a human monocytic cell line (THP-1) or mouse hippocampus did not impact miRNA stability [54, 55]. Similarly, in C. elegans, loss of CID-1 and PUP-1, which are TUT4/TUT7 family members that are necessary for 3′ uridylation of miRNAs, or loss of GLDR-2, a GLD2 homolog that adenylates miRNA 3′ ends, did not globally affect miRNA stability [56]. Consistent with these findings, global analyses of miRNA metabolism in MEFs and mESCs using metabolic labeling and high-throughput sequencing demonstrated that rates of miRNA tailing and turnover were not well correlated, suggesting that these pathways act independently of one another [14].
Unlike animal miRNAs, Piwi-interacting RNAs (piRNAs) and endogenous short interfering RNAs (siRNAs) in animals appear to share the susceptibility of plant miRNAs to tailing and trimming-mediated turnover. Also akin to plant miRNAs, these classes of small RNAs are post-transcriptionally modified by the addition of a protective 2′-O-methyl group at their 3′ ends by the methyl transferase HEN1 [57–65]. Distinct from HEN1 in plants, animal HEN1 does not contain dsRNA-binding domains and requires interaction with an AGO or PIWI protein in order to methylate the associated small RNA [58, 61]. Nonetheless, as in plants, loss of HEN1 homologs in Nematostella (sea anemone), C. elegans, Drosophila, and mice destabilizes piRNAs, strongly suggesting that 3′ modifications of these small RNAs are coupled to their turnover [58, 66–69].
The interplay between tailing and trimming and target engagement
Why do plant miRNAs and animal piRNAs and siRNAs require the addition of a 2′-O-methyl group to their 3′ ends in order to prevent tailing and trimming-mediated decay, while animal miRNAs generally appear to be insensitive to this turnover pathway? The answer, it appears, relates to the manner in which these different classes of small RNAs interact with their targets. Once loaded into an AGO protein, the 3′ end of the small RNA is buried in the PAZ domain and protected from nucleolytic attack (Fig. 1) [9, 10, 70, 71]. Nevertheless, the small RNA 3′ end can be exposed to the solvent when it base-pairs with a complementary target RNA, rendering it susceptible to tailing and trimming (Fig. 4A) [72, 73]. Notably, plant miRNAs usually exhibit extended 3′ complementarity to their target mRNAs [74]. Thus, miRNA:target interactions in plants will almost always expose the miRNA 3′ end. Similarly, piRNAs and endogenous siRNAs generally display extended complementarity to their targets and will accordingly be highly susceptible to tailing and trimming. In this context, it is logical that these small RNA classes undergo 2′-O-methylation in order to protect their 3′ ends, which are routinely exposed during target engagement.
Figure 4. AGO adopts distinct conformations when engaged with canonical or TDMD-inducing targets.
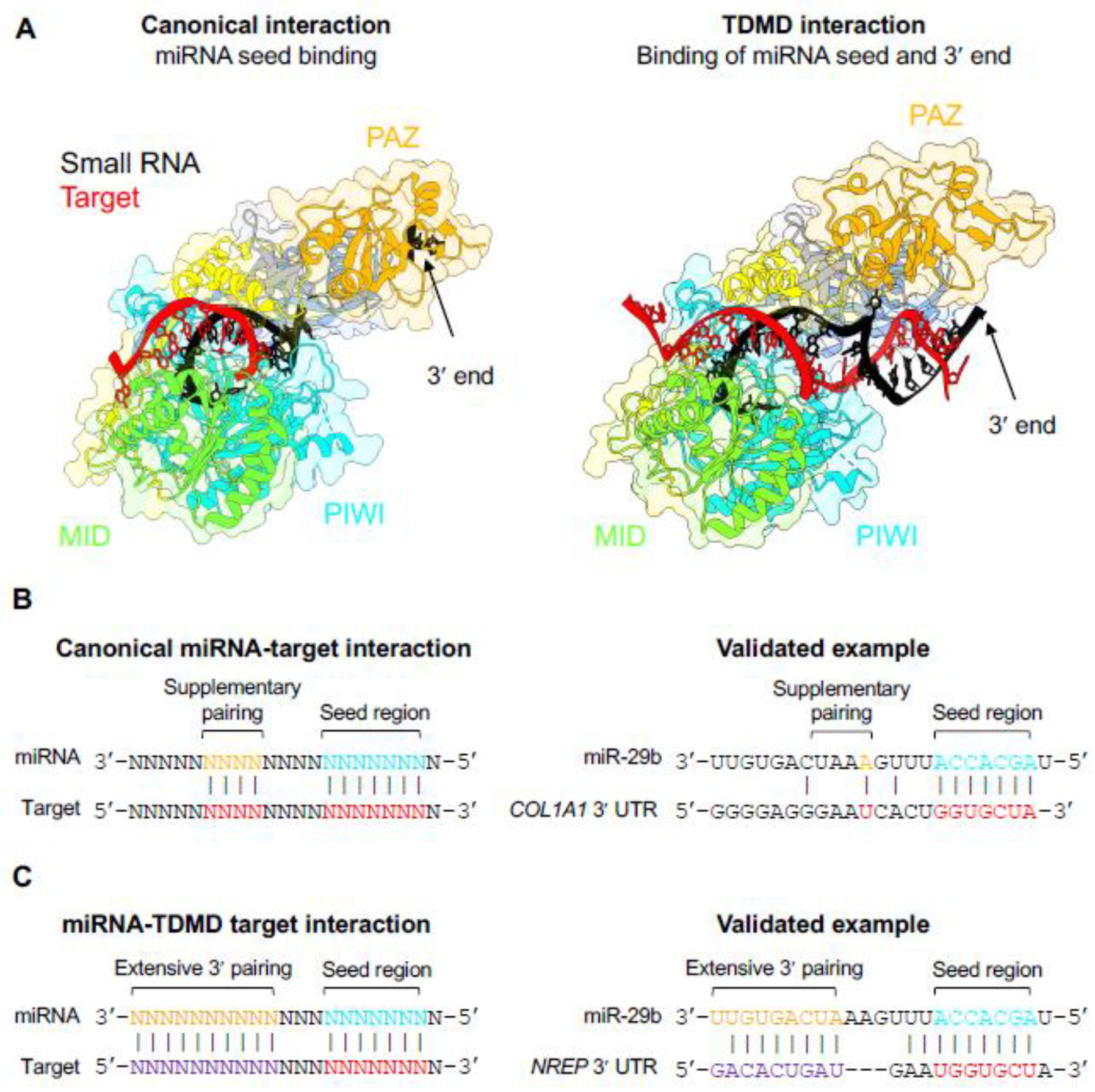
(A) When bound to a canonical target via base-pairing of the seed region, the miRNA 3′ end is buried in the PAZ domain of AGO (left panel; PDB: 4W5T) [10]. Interaction with a TDMD-inducing target with extended 3′ complementarity, however, exposes the miRNA 3′ end to solvent, which enables tailing and trimming (right panel; PDB: 6MDZ) [72]. Protein structures were rendered by UCSF ChimeraX [109]. (B) Canonical miRNA-target interactions involve base-pairing of the miRNA seed region with or without additional supplementary pairing [1]. A validated canonical binding site for miR-29b in the 3′ UTR of human COL1A1 is shown [110]. (C) Targets that induce TDMD base pair with the miRNA seed region, display extensive complementarity to the miRNA 3′ end, and have central mismatches. Interaction of miR-29b with a TDMD-inducing site in the 3′ UTR of human NREP is shown [82].
Unlike piRNAs, siRNAs, and plant miRNAs, complementarity between animal miRNAs and their target mRNAs is usually limited to the 5′ seed region, with or without additional supplementary base-pairing of a few additional nucleotides [1] (Fig. 4B). Thus, during target engagement, miRNA 3′ ends remain protected by the AGO PAZ domain. Rare encounters with targets with more extensive 3′ complementarity could trigger limited tailing and trimming, perhaps explaining why aged miRNAs tend to have more heterogeneous 3′ ends [19]. Nevertheless, moderately tailed or trimmed miRNAs might still be accommodated by the PAZ domain and protected from degradation, thereby uncoupling tailing and trimming from global miRNA turnover. In support of this model, mutations in the PAZ domain that prevent binding to the miRNA 3′ end induce extensive tailing and trimming of miRNAs that can lead to degradation by 3′-to-5′ exoribonucleases [72, 75].
In special cases, tailing has been causally linked to the degradation of animal miRNAs. For example, as discussed above, Wispy-mediated miRNA adenylation during the maternal-to-zygotic transition in Drosophila and Vp55-mediated adenylation during poxvirus infection in mammalian cells globally destabilizes miRNAs [25, 26]. Perhaps in these cases, the addition of extremely long tails prevents accommodation of miRNA 3′ ends by the PAZ domain, triggering miRNA turnover. Nevertheless, the mechanism by which tailing of miRNAs by these enzymes ultimately results in unloading of miRNAs from AGO proteins, and the nuclease(s) responsible for their eventual degradation, remain unknown.
Target-directed microRNA degradation
What happens in animal cells when a miRNA does encounter a target with extended 3′ complementarity? These interactions, in fact, trigger perhaps the best understood mechanism of regulated miRNA degradation, further pointing to the centrality of target interactions in the turnover of small RNAs. In 2010, the Steitz laboratory reported that HSUR1, a noncoding RNA encoded by Herpesvirus simiri, could promote degradation of miR-27a in host cells by base-pairing with this miRNA through a target site with extended complementarity to both the miRNA seed region and 3′ end [76]. The Zamore laboratory contemporaneously demonstrated that synthetic targets could trigger tailing, trimming, and, ultimately, destabilization of highly complementary miRNAs in Drosophila and human cells [77]. This process, in which extensive base-pairing between a miRNA and target RNA results in miRNA decay, is referred to as target-directed miRNA degradation (TDMD).
The identification of miRNA targets that can trigger TDMD is a rapidly accelerating area of research. TDMD was initially discovered due to the ability of exogenous targets, designed in the laboratory or encoded by viruses, to direct miRNA decay. Indeed, subsequent studies found that viruses often exploit this pathway to degrade host miRNAs in order to enhance replication [78–80]. Nevertheless, TDMD has increasingly been recognized as an endogenous mechanism of miRNA regulation that can be induced by host-encoded mRNAs and long noncoding RNAs (lncRNAs). In mammals, endogenous transcripts that robustly induce TDMD include CYRANO (also known as OIP5-AS1), NREP, Serpine, and BCL2L11, which promote decay of miR-7, miR-29b, miR-30b/c, and miR-221/222, respectively [81–84]. These well-conserved TDMD targets are characterized by base-pairing with both the 5′ seed regions as well as the 3′ ends of their corresponding miRNAs (Fig. 4C). In addition, these sites contain central mismatches that prevent AGO-mediated cleavage of the target. The importance of this target site architecture for inducing TDMD is supported by studies of synthetic RNA transcripts known as ‘Tough Decoys (TuDs)’ which function as potent miRNA inhibitors [85–88]. These RNAs, which can be stably expressed from RNA polymerase II or III promoters, can strongly reduce activity of complementary miRNAs in cell culture models and in mouse tissues, at least in part by inducing miRNA degradation. Optimization of these sequences demonstrated that 5′ and 3′ base-pairing of the miRNA with central mismatches resulted in the strongest miRNA inhibition, consistent with a requirement for these features in triggering robust TDMD [86, 87].
Recent progress has been made in the identification of TDMD-inducing transcripts using both experimental and computational approaches. For example, a high throughput sequencing method originally designed to identify miRNA:target pairs, called AGO-crosslinking, ligation, and sequencing of hybrids (AGO-CLASH) was successfully adapted to identify new TDMD triggers [84]. AGO-CLASH is a modified crosslinking-immunoprecipitation (CLIP) method in which intermolecular ligation is used to physically link a miRNA to a base-paired target RNA [89]. Subsequent immunoprecipitation of AGO, followed by sequencing of the associated miRNA:target chimeras, allows the identification of miRNA binding sites throughout the transcriptome. Because the 3′ of the miRNA is ligated to the target, this protocol also allows identification of targets that induce tailing, since this will introduce non-templated nucleotides at the junction between the miRNA and target in the sequenced chimera. Given that TDMD-inducing targets often stimulate tailing due to the extended 3′ pairing of the miRNA, this class of targets can potentially be distinguished from canonical miRNA targets using AGO-CLASH. Indeed, re-analysis of existing AGO-CLASH datasets from human and mouse cells and tissues led to the identification of eight new potential TDMD triggers which, when overexpressed, led to degradation of their cognate miRNAs [84]. Among these, a conserved site in endogenous BCL2L11 was demonstrated to induce TDMD of miR-221/222 in human cell lines.
An algorithm for prediction of TDMD-inducing targets was recently developed based on (i) the presence of 3′ pairing between the miRNA and target site; (ii) the presence of a central bulge between 2–8 nt in length; and (iii) a favorable miRNA:target hybridization energy [90]. 17 out of 37 tested predicted sites were capable of triggering TDMD of their cognate miRNAs when overexpressed. This database, which is available online (TDMDfinder), therefore provides a useful resource for future candidate-based screens for TDMD triggers, although mutation or downregulation of the endogenous TDMD-inducing transcripts will ultimately be needed to validate these predictions.
Despite significant progress, the targets that trigger TDMD of many miRNAs in Drosophila, C. elegans, and mammals remain to be identified [91, 92]. This suggests that generalizable rules for accurately predicting TDMD-inducing targets have not yet been realized. For example, sequences outside the region that base pairs with the miRNA could be important for licensing TDMD. Indeed, the existence of TDMD-promoting cis-elements in selected target transcripts has been suggested [79, 80]. An improved understanding of the characteristics of TDMD-inducing targets, both within and beyond the miRNA binding sites, promises to accelerate the identification of targets that trigger TDMD.
As described above, complementarity between the 3′ end of a miRNA and a TDMD-inducing target leads to disengagement of the miRNA 3′ end from the AGO PAZ domain, exposing it to the solvent (Fig. 4A) [72]. This configuration renders the miRNA susceptible to tailing and trimming [77, 83, 93]. Based on these observations, and the known destabilizing effect of tailing and trimming on plant miRNAs and other small RNAs that are protected by 2′-O-methyl modification, it was logical to hypothesize that tailing and trimming is integral to the TDMD mechanism. However, inhibition of terminal nucleotidyl transferases that add 3′ uridine or adenosine tails to miRNAs undergoing TDMD did not prevent miRNA decay [75, 83, 94], nor did blocking tailing and trimming during TDMD by adding a 2′-O-methyl group to the 3′ end of the miRNA [91, 92]. These experiments indicated that tailing and trimming likely occurs as a consequence of the distinct conformation of AGO:miRNA complexes engaged with TDMD targets, but is not an essential step in the miRNA decay mechanism. It nevertheless remains possible that tailing and trimming may influence the efficiency of TDMD, for example by positively or negatively impacting the stability of specific miRNA:target interactions [95].
Two independent studies recently provided important mechanistic insight into this miRNA decay pathway by identifying trans-factors that are essential for TDMD in mammalian cells [91, 92]. Through the application of genome-wide CRISPR screens, a novel cullin-RING E3 ubiquitin ligase complex containing the poorly characterized substrate adapter ZSWIM8 was found to be required for TDMD. Together with evidence that this complex interacts with AGO proteins and activity of the proteasome is necessary for TDMD, these findings led to a model wherein the ZSWIM8 ubiquitin ligase recognizes AGO:miRNA complexes engaged with TDMD targets (Fig. 5). Subsequent ubiquitylation and degradation by the proteasome would release the miRNA for decay by cytoplasmic ribonucleases. Consistent with this model, conserved, surface exposed lysine residues in human AGO2, that may represent ubiquitin acceptor sites, are necessary for TDMD. Moreover, miRNAs that undergo TDMD are enriched upon pull-down of polyubiquitin chains [91, 92]. Analysis of this pathway in Drosophila cells demonstrated that the ZSWIM8 homolog Dora is specifically able to promote the decay of Ago1-loaded, but not Ago2-loaded, small RNAs [96]. Since Drosophila Ago2 is largely loaded with siRNAs that interact with highly complementary targets, while Ago1 is predominantly loaded with miRNAs, the specificity of Dora for Ago1 ensures that TDMD acts on miRNAs while avoiding inappropriate destabilization of other classes of small RNAs.
Figure 5. Current model of TDMD.
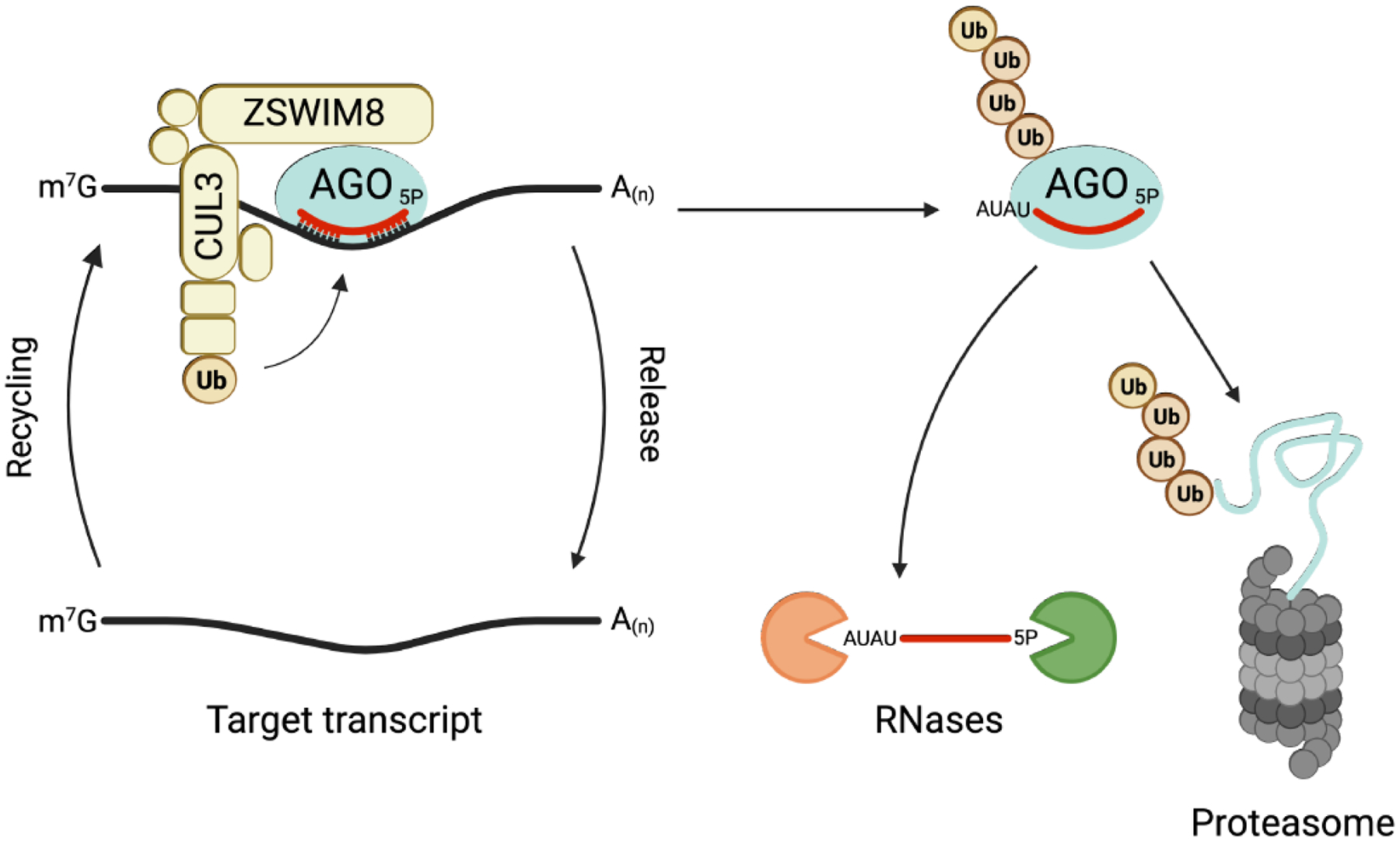
The ZSWIM8 E3 ubiquitin ligase recognizes and ubiquitylates AGO engaged with a TDMD target. Extensive 3′-pairing of the miRNA to the TDMD target exposes the 3′ end of the miRNA, enabling tailing by terminal nucleotidyl transferases. Ubiquitylated AGO is degraded by the proteasome and subsequent release of the miRNA leads to its degradation by unknown cytoplasmic RNases. The target transcript is recycled for another round of TDMD. CUL3: Cullin 3; Ub: Ubiquitin. Figure created with Biorender.com.
The identification of the essential role of the ZSWIM8 ubiquitin ligase in TDMD allowed, for the first time, analysis of the broad landscape of miRNAs that are regulated by this pathway in diverse contexts. Small RNA sequencing of a panel of ZSWIM8-deficient mammalian cell lines revealed more than 30 miRNAs that are upregulated upon impairment of TDMD without an increase in the abundance of their corresponding passenger strands [91, 92]. Because passenger strands are byproducts of miRNA biogenesis that are discarded when the mature miRNA is loaded into AGO [2], these results strongly implied that these miRNAs are regulated at the level of stability and are therefore bona fide TDMD substrates. Similar studies of Drosophila S2 cells depleted of the ZSWIM8 homolog Dora, or adult C. elegans depleted of the ZSWIM8 homolog EBAX-1, identified numerous additional TDMD-regulated miRNAs in these species [92]. In mammalian and Drosophila cell lines where the decay rates of miRNAs have been globally measured, miRNA half-lives were inversely correlated with sensitivity to ZSWIM8-depletion [92]. These findings indicate that susceptibility of a miRNA to TDMD is a major determinant, if not the major determinant, of its half-life. The identification of this large set of new TDMD substrates emphasizes the need for improved computational and experimental methods for accurate identification of targets that trigger this mechanism of miRNA decay. These efforts, coupled with the analysis of ZSWIM8 loss-of-function phenotypes in model organisms, promise to illuminate the role of TDMD in animal development, physiology, and, potentially, disease.
Despite the recent advances in our mechanistic understanding of TDMD, many critical questions remain unresolved. For example, how does ZSWIM8 specifically recognize the AGO:miRNA complex engaged with a TDMD target? Elegant structural studies revealed that base-pairing of a miRNA with a TDMD-inducing transcript promotes broad conformational rearrangements of human AGO2, including opening of the central cleft (Fig. 4A) [72]. Perhaps this configuration reveals a binding site that enables ZSWIM8 association or, alternatively, uniquely stimulates ubiquitylation by the ZSWIM8 complex. Interestingly, members of the C. elegans mir-35 family, whose abundance is regulated by the ZSWIM8 homolog EBAX-1 [92], are degraded in a manner dependent upon their seed sequences, but independent of sequences at their 3′ ends [97]. This suggests that there may be alternative mechanisms for ZSWIM8 recruitment beyond extended 3′ miRNA:target base-pairing, such as target-associated RNA binding proteins that interact with ZSWIM8.
Another unresolved question relates to the surprising observation that TDMD-inducing targets appear to be largely resistant to miRNA-mediated silencing. For instance, ZSWIM8 loss of function did not result in repression of the TDMD-inducing target CYRANO, despite a strong increase in abundance of the cognate miRNA miR-7 [91, 92]. Moreover, synthetic targets that induced robust TDMD in rodent primary neuronal cultures were resistant to miRNA-mediated decay [93]. This was not simply due to the architecture of the miRNA binding sites, since targets with extensive 5′ and 3′ complementarity and a central bulge can be effectively repressed by miRNAs [98, 99]. This effect allows TDMD to function as a multi-turnover pathway, with several rounds of miRNA degradation per target, but the underlying mechanism is unclear. Perhaps co-factors that are essential for target silencing, such as TNRC6/GW182, are excluded from the AGO:miRNA complex when it engages a TDMD target. For example, TDMD targets could be segregated in condensates or other subcellular compartments that exclude silencing co-factors.
The demonstration that TDMD occurs independently of tailing and trimming raises the possibility that a related mechanism could regulate the stability of other classes of small RNAs that are protected from 3′ modification by 2′-O-methylation. For example, although a ZSWIM8 homolog has not been identified in plants, specific miRNA targets, called target mimics, can strongly reduce the abundance of cognate AGO-associated miRNAs [100–103]. Like animal targets that induce TDMD, plant target mimics exhibit extended complementarity to miRNAs but have central mismatches that prevent AGO-mediated cleavage. Interestingly, the F-box protein HAWAIIAN SKIRT (HWS), which functions as a cullin-RING ubiquitin ligase adapter protein, has been shown to be required for target mimic-induced miRNA degradation in Arabidopsis [104–106]. Thus, degradation of specific AGO:miRNA complexes by the ubiquitin-proteasome system may be a common mechanism of regulated turnover of diverse classes of small RNAs across kingdoms.
Concluding remarks
Since the discovery of miRNAs almost three decades ago [107, 108], elaborate mechanisms that regulate the biogenesis and activity of these important post-transcriptional regulators have been revealed [1]. Yet we still have much to learn about how regulated degradation of miRNAs impacts their activity in different biological contexts. Recent discoveries are beginning to unveil how engagement of targets by miRNAs and other classes of small RNAs renders them susceptible to decay pathways that are associated with 3′ modifications, such as tailing and trimming, and turnover by the ubiquitin-proteasome system. Further investigation of where, when, and how these mechanisms are deployed (see Outstanding Questions) promises to advance our understanding of the roles of miRNA-mediated regulation in health and disease.
Outstanding Questions.
How does tailing and trimming lead to unloading of small RNAs from AGO proteins and what are the nucleases that function in tailing and trimming-mediated decay?
How does tailing lead to the degradation of animal miRNAs in specialized settings, such as poxvirus infection in mammalian cells and the maternal-to-zygotic transition in Drosophila embryos?
Does tailing and trimming impact the efficiency of TDMD in some cases?
How can we accurately predict miRNA target sites that trigger TDMD?
How does ZSWIM8 specifically recognize and ubiquitylate AGO:miRNA complexes engaged with TDMD targets?
Can ZSWIM8-mediated miRNA turnover be directed by specialized targets that lack extensive 3′ complementarity to the miRNA?
To what extent are miRNAs regulated by TDMD in vivo and what are the roles of TDMD in animal development, physiology, and disease?
How are transcripts that trigger TDMD protected from AGO-mediated repression?
Do target mimics in plants trigger turnover of AGO:miRNA complexes by the ubiquitin-proteasome system in a manner similar to the TDMD pathway in animals?
Highlights.
Degradation of Argonaute (AGO) proteins by the ubiquitin-proteasome system and modification of miRNA 3′ ends by tailing and trimming are mechanisms that can promote global or miRNA-specific turnover.
The consequences of tailing and trimming of small RNAs vary depending upon the class of small RNA, the identity of the nucleotides that are added to the small RNA 3′ end, and the species in which the modifications occur.
Tailing and trimming appears to regulate the stability of plant miRNAs, animal siRNAs, and animal piRNAs, but usually not animal miRNAs.
Target-directed microRNA degradation (TDMD), which is triggered by extensive base-pairing of both the miRNA 5′ and 3′ ends with a target, appears to be the major mechanism of regulated turnover of animal miRNAs.
The ZSWIM8 ubiquitin ligase mediates TDMD by promoting destruction of AGO:miRNA complexes engaged with highly complementary targets.
Acknowledgements
We thank Collette LaVigne, Frederick Rehfeld, and Kathryn O’Donnell for thoughtful suggestions on the manuscript. J.T.M. is an Investigator of the Howard Hughes Medical Institute and is supported by grants from NIH (R35CA197311), CPRIT (RP220309), and the Welch Foundation (I-1961-20210327).
Glossary
- 3′ untranslated region (3′ UTR)
The region of a messenger RNA downstream of the translation termination codon where cis-acting regulatory elements, such as microRNA binding sites, are often located.
- Argonaute (AGO) proteins
A family of proteins that associate with various classes of small RNAs, including microRNAs (miRNAs) and short-interfering RNAs (siRNAs). Small RNAs guide AGO proteins to target transcripts for gene silencing.
- HEN1
An enzyme that adds a 2′-O-methyl group at the 3′ end of specific classes of small RNAs, such as plant miRNAs and animal siRNAs and piRNAs. This modification prevents tailing and trimming when these classes of small RNAs engage highly complementary targets.
- PIWI proteins
A family of proteins related to AGO proteins that are loaded with a specialized class of small RNAs known as Piwi-interacting RNAs (piRNAs). In many species, PIWI proteins and their associated piRNAs play an important role in transposon suppression in the germline.
- Tailing
Addition of non-templated nucleotides to the 3′-end of a small RNA.
- Target-directed microRNA degradation (TDMD)
A microRNA degradation pathway that is triggered by the interaction of microRNAs with specialized target transcripts that exhibit extensive 5′ and 3′ complementarity to the microRNA.
- Trimming
Removal of nucleotides from the 3′-end of a small RNA.
- ZSWIM8
A substrate adapter for a cullin-RING E3 ubiquitin ligase complex that mediates TDMD by recognizing AGO:miRNA complexes engaged with highly complementary targets and triggering their degradation by the proteasome. Known as Dora in Drosophila melanogaster and EBAX-1 in Caenorhabditis elegans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
J.T.M is a scientific advisor for Ribometrix, Inc. and Circ Bio, Inc.
References
- 1.Bartel DP (2018) Metazoan microRNAs. Cell 173, 20–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ha M and Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15, 509–524 [DOI] [PubMed] [Google Scholar]
- 3.Jonas S and Izaurralde E (2015) Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16, 421–433 [DOI] [PubMed] [Google Scholar]
- 4.Kozomara A and Griffiths-Jones S (2014) miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42, D68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman RC, et al. (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendell JT and Olson EN (2012) MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treiber T, et al. (2019) Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol 20, 5–20 [DOI] [PubMed] [Google Scholar]
- 8.Winter J and Diederichs S (2011) Argonaute proteins regulate microRNA stability: Increased microRNA abundance by Argonaute proteins is due to microRNA stabilization. RNA Biol 8, 1149–1157 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. (2008) Structure of the guide-strand-containing argonaute silencing complex. Nature 456, 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schirle NT, et al. (2014) Structural basis for microRNA targeting. Science 346, 608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen TJ, et al. (2020) The dynamics of cytoplasmic mRNA metabolism. Mol Cell 77, 786–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rissland OS, et al. (2011) MicroRNA destabilization enables dynamic regulation of the miR-16 family in response to cell-cycle changes. Mol Cell 43, 993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang HW, et al. (2007) A hexanucleotide element directs microRNA nuclear import. Science 315, 97–100 [DOI] [PubMed] [Google Scholar]
- 14.Kingston ER and Bartel DP (2019) Global analyses of the dynamics of mammalian microRNA metabolism. Genome Res 29, 1777–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzi MJ, et al. (2016) Degradation dynamics of microRNAs revealed by a novel pulse-chase approach. Genome Res 26, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, et al. (2015) Characterization of the mammalian miRNA turnover landscape. Nucleic Acids Res 43, 2326–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bail S, et al. (2010) Differential regulation of microRNA stability. RNA 16, 1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gantier MP, et al. (2011) Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res 39, 5692–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichholf B, et al. (2019) Time-resolved small RNA sequencing unravels the molecular principles of microRNA homeostasis. Mol Cell 75, 756–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krol J, et al. (2010) Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141, 618–631 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, et al. (2012) MicroRNA degradation and turnover: regulating the regulators. Wiley Interdiscip Rev RNA 3, 593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kai ZS and Pasquinelli AE (2010) MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat Struct Mol Biol 17, 5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruegger S and Grosshans H (2012) MicroRNA turnover: when, how, and why. Trends Biochem Sci 37, 436–446 [DOI] [PubMed] [Google Scholar]
- 24.Bronevetsky Y, et al. (2013) T cell activation induces proteasomal degradation of Argonaute and rapid remodeling of the microRNA repertoire. J Exp Med 210, 417–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backes S, et al. (2012) Degradation of host microRNAs by poxvirus poly(A) polymerase reveals terminal RNA methylation as a protective antiviral mechanism. Cell Host Microbe 12, 200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M, et al. (2014) Adenylation of maternally inherited microRNAs by Wispy. Mol Cell 56, 696–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rooij E, et al. (2007) Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316, 575–579 [DOI] [PubMed] [Google Scholar]
- 28.Gatfield D, et al. (2009) Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev 23, 1313–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ameres SL and Zamore PD (2013) Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 14, 475–488 [DOI] [PubMed] [Google Scholar]
- 30.Anderson JT and Wang X (2009) Nuclear RNA surveillance: no sign of substrates tailing off. Crit Rev Biochem Mol Biol 44, 16–24 [DOI] [PubMed] [Google Scholar]
- 31.Houseley J and Tollervey D (2009) The many pathways of RNA degradation. Cell 136, 763–776 [DOI] [PubMed] [Google Scholar]
- 32.Heo I, et al. (2009) TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 [DOI] [PubMed] [Google Scholar]
- 33.Lehrbach NJ, et al. (2009) LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat Struct Mol Biol 16, 1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagan JP, et al. (2009) Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol 16, 1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thornton JE, et al. (2012) Lin28-mediated control of let-7 microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA 18, 1875–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang HM, et al. (2013) A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature 497, 244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ustianenko D, et al. (2013) Mammalian DIS3L2 exoribonuclease targets the uridylated precursors of let-7 miRNAs. RNA 19, 1632–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu B, et al. (2005) Methylation as a crucial step in plant microRNA biogenesis. Science 307, 932–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, et al. (2006) HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 2’ OH of the 3’ terminal nucleotide. Nucleic Acids Res 34, 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren G, et al. (2012) Uridylation of miRNAs by hen1 suppressor1 in Arabidopsis. Curr Biol 22, 695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, et al. (2012) The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Curr Biol 22, 689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park W, et al. (2002) CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12, 1484–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu B, et al. (2015) Distinct and cooperative activities of HESO1 and URT1 nucleotidyl transferases in microRNA turnover in Arabidopsis. PLoS Genet 11, e1005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandran V and Chen X (2008) Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science 321, 1490–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Y, et al. (2017) ARGONAUTE10 promotes the degradation of miR165/6 through the SDN1 and SDN2 exonucleases in Arabidopsis. PLoS Biol 15, e2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, et al. (2018) Structural and biochemical insights into small RNA 3’ end trimming by Arabidopsis SDN1. Nat Commun 9, 3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones MR, et al. (2009) Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat Cell Biol 11, 1157–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutierrez-Vazquez C, et al. (2017) 3’ Uridylation controls mature microRNA turnover during CD4 T-cell activation. RNA 23, 882–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thornton JE, et al. (2014) Selective microRNA uridylation by Zcchc6 (TUT7) and Zcchc11 (TUT4). Nucleic Acids Res 42, 11777–11791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh T, et al. (2009) Selective stabilization of mammalian microRNAs by 3’ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev 23, 433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Ambrogio A, et al. (2012) Specific miRNA stabilization by Gld2-catalyzed monoadenylation. Cell Rep 2, 1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boele J, et al. (2014) PAPD5-mediated 3’ adenylation and subsequent degradation of miR-21 is disrupted in proliferative disease. Proc Natl Acad Sci U S A 111, 11467–11472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shukla S, et al. (2019) The RNase PARN controls the levels of specific miRNAs that contribute to p53 regulation. Mol Cell 73, 1204–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burroughs AM, et al. (2010) A comprehensive survey of 3’ animal miRNA modification events and a possible role for 3’ adenylation in modulating miRNA targeting effectiveness. Genome Res 20, 1398–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansur F, et al. (2016) Gld2-catalyzed 3’ monoadenylation of miRNAs in the hippocampus has no detectable effect on their stability or on animal behavior. RNA 22, 1492–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vieux KF, et al. (2021) Screening by deep sequencing reveals mediators of microRNA tailing in C. elegans. Nucleic Acids Res 49, 11167–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forstemann K, et al. (2007) Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell 130, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horwich MD, et al. (2007) The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol 17, 1265–1272 [DOI] [PubMed] [Google Scholar]
- 59.Czech B, et al. (2008) An endogenous small interfering RNA pathway in Drosophila. Nature 453, 798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawamura Y, et al. (2008) Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature 453, 793–797 [DOI] [PubMed] [Google Scholar]
- 61.Saito K, et al. (2007) Pimet, the Drosophila homolog of HEN1, mediates 2’-O-methylation of Piwi- interacting RNAs at their 3’ ends. Genes Dev 21, 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirino Y and Mourelatos Z (2007) Mouse Piwi-interacting RNAs are 2’-O-methylated at their 3’ termini. Nat Struct Mol Biol 14, 347–348 [DOI] [PubMed] [Google Scholar]
- 63.Ohara T, et al. (2007) The 3’ termini of mouse Piwi-interacting RNAs are 2’-O-methylated. Nat Struct Mol Biol 14, 349–350 [DOI] [PubMed] [Google Scholar]
- 64.Kamminga LM, et al. (2010) Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J 29, 3688–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurth HM and Mochizuki K (2009) 2’-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA 15, 675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Modepalli V, et al. (2018) The methyltransferase HEN1 is required in Nematostella vectensis for microRNA and piRNA stability as well as larval metamorphosis. PLoS Genet 14, e1007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pastore B, et al. (2021) pre-piRNA trimming and 2’-O-methylation protect piRNAs from 3’ tailing and degradation in C. elegans. Cell Rep 36, 109640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gainetdinov I, et al. (2021) Terminal modification, sequence, length, and PIWI-protein identity determine piRNA stability. Mol Cell 81, 4826–4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim SL, et al. (2015) HENMT1 and piRNA stability are required for adult male germ cell transposon repression and to define the spermatogenic program in the mouse. PLoS Genet 11, e1005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elkayam E, et al. (2012) The structure of human argonaute-2 in complex with miR-20a. Cell 150, 100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schirle NT and MacRae IJ (2012) The crystal structure of human Argonaute2. Science 336, 1037–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheu-Gruttadauria J, et al. (2019) Structural basis for target-directed microRNA degradation. Mol Cell 75, 1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, et al. (2008) Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones-Rhoades MW, et al. (2006) MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57, 19–53 [DOI] [PubMed] [Google Scholar]
- 75.Yang A, et al. (2020) AGO-bound mature miRNAs are oligouridylated by TUTs and subsequently degraded by DIS3L2. Nat Commun 11, 2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cazalla D, et al. (2010) Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ameres SL, et al. (2010) Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Libri V, et al. (2012) Murine cytomegalovirus encodes a miR-27 inhibitor disguised as a target. Proc Natl Acad Sci U S A 109, 279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marcinowski L, et al. (2012) Degradation of cellular mir-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog 8, e1002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee S, et al. (2013) Selective degradation of host MicroRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell Host Microbe 13, 678–690 [DOI] [PubMed] [Google Scholar]
- 81.Ghini F, et al. (2018) Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nat Commun 9, 3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bitetti A, et al. (2018) MicroRNA degradation by a conserved target RNA regulates animal behavior. Nat Struct Mol Biol 25, 244–251 [DOI] [PubMed] [Google Scholar]
- 83.Kleaveland B, et al. (2018) A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 174, 350–362 e317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, et al. (2021) Widespread microRNA degradation elements in target mRNAs can assist the encoded proteins. Genes Dev 35, 1595–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haraguchi T, et al. (2012) A potent 2’-O-methylated RNA-based microRNA inhibitor with unique secondary structures. Nucleic Acids Res 40, e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haraguchi T, et al. (2009) Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res 37, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie J, et al. (2012) Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat Methods 9, 403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bak RO, et al. (2013) Potent microRNA suppression by RNA Pol II-transcribed ‘Tough Decoy’ inhibitors. RNA 19, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Helwak A, et al. (2013) Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simeone I, et al. (2022) Prediction and pan-cancer analysis of mammalian transcripts involved in target directed miRNA degradation. Nucleic Acids Res 50, 2019–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han J, et al. (2020) A ubiquitin ligase mediates target-directed microRNA decay independently of tailing and trimming. Science 370, eabc9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi CY, et al. (2020) The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science 370, eabc9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de la Mata M, et al. (2015) Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO Rep 16, 500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pawlica P, et al. (2019) How complementary targets expose the microRNA 3’ end for tailing and trimming during target-directed microRNA degradation. Cold Spring Harb Symp Quant Biol 84, 179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang A, et al. (2019) 3’ uridylation confers miRNAs with non-canonical target repertoires. Mol Cell 75, 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kingston ER and Bartel DP (2021) Ago2 protects Drosophila siRNAs and microRNAs from target-directed degradation, even in the absence of 2’-O-methylation. RNA 27, 710–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Donnelly B, et al. (2021) The developmentally-timed decay of an essential microRNA family is seed sequence-dependent. bioRxiv 10.1101/2021.11.19.469346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmitter D, et al. (2006) Effects of Dicer and Argonaute down-regulation on mRNA levels in human HEK293 cells. Nucleic Acids Res 34, 4801–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Golden RJ, et al. (2017) An Argonaute phosphorylation cycle promotes microRNA-mediated silencing. Nature 542, 197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu HJ, et al. (2013) Widespread long noncoding RNAs as endogenous target mimics for microRNAs in plants. Plant Physiol 161, 1875–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Todesco M, et al. (2010) A collection of target mimics for comprehensive analysis of microRNA function in Arabidopsis thaliana. PLoS Genet 6, e1001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ivashuta S, et al. (2011) Regulation of gene expression in plants through miRNA inactivation. PLoS One 6, e21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan J, et al. (2012) Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell 24, 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, et al. (2017) The Arabidopsis thaliana F-box gene HAWAIIAN SKIRT is a new player in the microRNA pathway. PLoS One 12, e0189788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mei J, et al. (2019) The F-box protein HAWAIIAN SKIRT is required for mimicry target-induced microRNA degradation in Arabidopsis. J Integr Plant Biol 61, 1121–1127 [DOI] [PubMed] [Google Scholar]
- 106.Lang PLM, et al. (2018) A role for the F-Box protein HAWAIIAN SKIRT in plant microRNA function. Plant Physiol 176, 730–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee RC, et al. (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 [DOI] [PubMed] [Google Scholar]
- 108.Wightman B, et al. (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 [DOI] [PubMed] [Google Scholar]
- 109.Pettersen EF, et al. (2021) UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci 30, 70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Z, et al. (2009) Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284, 15676–15684 [DOI] [PMC free article] [PubMed] [Google Scholar]


