Abstract
Chronic abdominal pain (CAP) represents a common pediatric primary pain disorder that can have long-term effects on physical and mental health into adulthood. Pediatric CAP and Control cohorts recruited in childhood (~11 years old, T1) and then assessed in emerging adulthood (~20 years old, T2) were evaluated again for health outcomes in early adulthood (~30 years old, T3) for the current study. Further, the study evaluated the mental and physical health of offspring of participants who had become parents. Participants who agreed to enroll at T3 (CAP: n = 90, Control: n = 55) completed measures regarding current health, health-related quality of life (HRQoL), and their child’s health when applicable. Results indicated close to 20% of the CAP cohort reported recurrent CAP across all three timepoints. Participants with current CAP reported poorer HRQoL compared to participants with remitted CAP who reported poorer HRQoL compared to Control participants. The CAP cohort reported higher health-related anxiety compared to the Control cohort regardless of current pain status. CAP compared to Control participants reported greater emotional problems and fewer conduct problems in their children. Longitudinal studies are needed to assess the developmental course of pediatric chronic pain and intergenerational pathways of risk and resilience.
Perspective:
This article evaluates patterns of chronic abdominal pain from childhood into early adulthood. Patients with pediatric chronic abdominal pain continue to present with health-related anxiety in adulthood and report greater emotional problems in offspring.
Keywords: functional abdominal pain, irritable bowel syndrome, longitudinal, family, gastrointestinal
Introduction
Chronic abdominal pain (CAP) is a common childhood concern, affecting approximately 14% of children and adolescents.10,26,38 When extensive medical evaluations have ruled out disease-based causes (e.g., inflammatory bowel disease), medical providers have historically considered CAP a functional gastrointestinal disorder.10,12,62 Recent changes in taxonomy now consider CAP a disorder of the gut-brain interaction (DGBI, Rome IV) or chronic primary visceral pain (International Classification of Diseases 11).49,57 The percentage of children with CAP who continue to have pain 1–5 years later averages 31%, with 25–50% continuing to experience symptoms into adulthood.7,8,12,30,34
A biopsychosocial perspective is valuable in evaluating factors associated with the onset and maintenance of CAP.11,23,37 Inpatient care for abdominal pain, sleep difficulties, pain outside the abdominal region, anxiety, and depressive symptoms have all been associated with increased risk for the onset or continuation of CAP.12,24,27,37 Children with CAP that persists for up to 15 years have exhibited increased risk for non-abdominal chronic pain, missing school, job loss due to illness, and psychiatric disorders including anxiety and depression.2,25,45,58,63.
Although several studies have evaluated outcomes in late adolescence and emerging adulthood (~20-years-old24,34,58,60), few have assessed outcomes further into early adulthood around the average age of first-time parenthood (late 20’s, early 30’s7,25). A history of CAP could have intergenerational implications for an individual’s parenting style and their offspring’s physical and psychological health.21,27 Indeed, parental chronic pain, parental anxiety, and maternal neuroticism have all been associated with a higher risk of having a child with CAP.21,25,27,45,46 While parental negative affect could be a mechanism for the intergenerational transmission of risk for chronic pain development and maintenance,29,32 genetic factors,31 neurobiological effects,9 and protective parenting behaviors33 all might contribute as well. Many of these studies, however, typically are cross-sectional and evaluate parental risk factors in the context of a child presenting with CAP or chronic pain. In contrast, studying the longitudinal course of CAP from childhood into parenthood provides a broader context for evaluating health-related risk and resilience in offspring of individuals with CAP.
The present study aimed to evaluate 20-year outcomes of pediatric patients who originally presented to a pediatric gastroenterology clinic with CAP compared to a Control group of children without pain recruited from the public schools. First, the study evaluated the patterns of remission and recurrence of CAP from childhood (T1: ~11-years-old), into late adolescence/emerging adulthood (T2: ~20-years-old24), and into early adulthood (T3: ~30-years-old). Next, we tested differences between CAP and Control cohorts on measures of non-abdominal chronic pain, health-related quality of life (HRQoL), and health-related anxiety at 20-year follow-up. We hypothesized that patients with recurrent CAP would report additional non-abdominal chronic pain sites, poorer HRQoL, and greater health-related anxiety than patients with remitted CAP, and that patients with remitted CAP would report poorer HRQoL and greater health-related anxiety than the Control group.
Participants who had become parents in the years since childhood participation reported on the physical and behavioral health of one of their children. As an exploratory aim, we sought to evaluate differences between original groups (Control vs. CAP) on offspring birth outcomes, health care use, and behavioral health. Based on cross-sectional studies of parental chronic pain,19 we hypothesized the CAP group would report lower birth weight, higher health care use, and greater emotional and behavioral difficulties in their offspring compared to the Control group.
Methods
Recruitment
Background.
For this longitudinal cohort study, participants were recruited at baseline (T1) either from a tertiary pediatric gastroenterology clinic (CAP, n = 756) or public schools in the medical center’s patient catchment area (Control, n = 343) between 1993–2007. For the CAP group, T1 data were drawn from a database of cohorts of consecutive new patients evaluated for abdominal pain in 1993–1995,61 1996–1999,59 and 2001–2007.3 Children at T1 averaged 11.47 years-old. At T2, 2009–2011, participants were older adolescents or young adults with a mean age of 20.18 years, representing approximately half of the original participants in both the CAP (n=396) and Control (n=187) groups. The sample at T2 was determined to be representative of the original sample with no significant differences in baseline pain characteristics.60 The present study (T3, 2020–2021) contacted participants who had completed T2 and at that time consented to be contacted about additional research participation in the future. As an exploratory aim, among persons who had become parents, the present study attempted to collect measures about participants’ children. All study procedures were approved by the Vanderbilt University Institutional Review Board.
Inclusion/Exclusion criteria.
At T1, CAP participants were eligible if they had abdominal pain for at least 3 months duration. CAP participants were excluded if they had a diagnosed chronic illness (e.g., diabetes) or disability (e.g., mobility impairment resulting from a genetic condition such as spina bifida or cerebral palsy or intellectual disability that would affect the participant’s ability to read and comprehend self-report measures). Control participants were eligible if they reported abdominal pain less than once a week and scored less than the median for healthy children on the Children’s Somatic Symptoms Inventory55 (formerly the Children’s Somatization Inventory10) in a school-based survey of children’s health. At T2 and T3, participants were excluded (T2: n = 3,24 T3: n=7) if they had been diagnosed with a significant illness or chronic condition such as cancer or multiple sclerosis or if they anticipated difficulty reading or completing online surveys.
Participants who had become parents indicated whether they would be willing to answer questions about one of their children. Parents were eligible to report on their child’s health if their child was under the age of 18 and lived with the parent at least 50% of the time. Study staff helped the participant select one primary child to report on for the study using a selection protocol. To maximize the number of measures valid for the age of their child, we first aimed to select an eligible child over the age of 8-years-old. If multiple children over 8-years-old were eligible, one child was selected at random. For parents of children under 8-years-old, we selected the oldest eligible child.
Recruitment procedures.
The study team previously attempted to reach all T1 participants for T2 recruitment.24 Thus, recruitment for T3 focused on contacting participants who completed T2 for whom contact information was available. Interest surveys were sent to the email addresses provided at T2 for all participants who indicated permission to be contacted for future studies and each available phone number provided at T2 was called at least once. When contact information provided at T2 was no longer accurate, we attempted to find current contact information by reaching relatives of participants and looking up numbers via the internet. Study staff made further phone attempts if they received any verifying information that the phone number was still a viable way to reach the participant (e.g., identifiable voicemail, participant answered and asked for a call back).
Study Procedures
If a participant indicated interest in the study, they completed a screening over the phone to determine their eligibility and confirm their participation in the original study. Study staff verified participant identity by their birthdate provided at T1. If they met eligibility criteria and agreed to participate, participants received an electronic informed consent form via email. These forms were generated in REDCap, a web-based, HIPAA compliant data collection system developed at Vanderbilt University.17 Study staff reviewed primary information from the consent form over the phone and answered all participants’ questions. Once enrolled, participants received the REDCap surveys appropriate for their status as a parent and the age of their child through email to complete at their convenience. Participants were compensated $20 via check if they completed 80% or more of the surveys distributed to them.
Measures
Demographic factors.
Participants reported information regarding their sex, race, status as a parent/non-parent, marital/live-in partner status, education, employment, and household income. Ages at T1, T2, and T3 were calculated using participants’ birthdays and dates of participation from each time point. In addition, because T3 data collection occurred during the COVID-19 pandemic, participants were asked: “Do you currently suffer from COVID-19 symptoms such as fever, dry cough, breathing problems, sore throat, loss of smell/taste, headaches, or diarrhea?” (yes/no).
Rome IV Diagnostic Questionnaire.
The Rome IV Diagnostic Questionnaire was used at T3 to determine whether or not participants met criteria for a disorder of the gut-brain interaction (DGBI) based on the frequency, duration and severity of self-reported symptoms.39,40 Modules administered focused on pain-related DGBIs, specifically functional dyspepsia (FD), irritable bowel syndrome (IBS), and centrally mediated pain disorders. For the purposes of this study, a positive diagnosis for any of these conditions classified the participant as having a DGBI and they were classified as having current CAP at T3.
Persistent Pain Questionnaire.
The Persistent Pain Questionnaire (PPQ) assessed chronic pain across multiple body regions.4,5,51,54 Participants identified where they have experienced aches or pains daily or almost daily for 3 months or more using a visual map of the body divided into many sites such as abdomen (front-left) and wrist (back-right).48 These sites were then grouped into locations based on the eight standard body locations described by the International Association for the Study of Pain36: head, neck, shoulder/arm/hand, chest, abdomen, pelvic area, upper or lower back, and legs/feet. For each location, participants were asked if their persistent pain in that location is current or was in the past. If they responded that the pain was current, they were asked to rate the current average intensity of the pain on a scale of 0–100 and indicate the frequency of the pain. Participants were categorized as having current chronic pain at a specific body location if their pain at that location was rated greater than or equal to 30/100 and they indicated pain at least weekly. Participants who met this criterion for current chronic pain at the abdominal site were classified as having current CAP regardless of whether they also met criteria for a DGBI (n = 1 participant). For the purposes of evaluating the presence of non-abdominal chronic pain, the number of non-abdominal chronic pain locations (maximum = 7) was summed to create a composite pain location score and a binary indicator (0, 1) was computed to indicate the presence or absence of a current non-abdominal chronic pain site.
If the participant indicated their persistent pain was in the past, they were asked to indicate if it started before or after the age of 18. Participants who indicated at least one past persistent pain site before the age of 18 were classified as reporting a childhood chronic pain history.
PROMIS-29 Profile.
The PROMIS-29 profile assesses both physical and emotional wellbeing and functioning.6 There are 4 questions in each of the 7 domains: Pain Interference, Physical Functioning, Anxiety, Depression, Fatigue, Sleep Disturbance, and Ability to Participate in Social Roles and Activities. If greater than 50% of the items for a domain were completed, the T-score for the domain was created using a prorated total raw score and instructions provided by the scoring manuals in the Assessment Center associated with the measure’s website. For most domains, a T-score of 50 is representative of the average for the general population of the United States. The exceptions are T-scores for Ability to Participate in Social Roles and Activities and Sleep Disturbance, which represent the average of a sample calibrated for those with chronic illness.6,28 In the current study, Cronbach’s alpha coefficient within each domain ranged from 0.83 to 0.96, indicating good to excellent internal consistency.
Health-Related Anxiety Measures
Pain Catastrophizing Scale.
The 6-item Pain Catastrophizing Scale Short Form (PCS-SF) was designed to efficiently assess the extent of pain catastrophizing.35 The short form has 2 questions from each of the three PCS subscales: rumination, magnification, and helplessness. Participants rated their agreement with statements such as “When I’m in pain, it’s awful and I feel that it overwhelms me,” on a scale of 0 (Not at all) to 4 (All the time). For the purposes of this study, the mean score of all six items was used in the analysis, with higher scores indicating greater pain catastrophizing. In the present study, Cronbach’s alpha coefficient was 0.89 indicating good internal consistency.
Body Vigilance subscale of the Short Health Anxiety Inventory.
This study utilized the 3-item subscale for body vigilance from The Short Health Anxiety Inventory.47 Body vigilance, defined as heightened attention to any changes in bodily sensations, has been shown to predict health care utilization and to play a prominent role in health-related anxiety 1. A mean score for the subscale was computed ranging from 0 to 3, with higher scores indicating greater body vigilance.
Child Measures
Child Health and Birth Outcomes.
Parents reported on their child’s current health and birth history. Specifically, parents indicated if the child was born at full term, stayed in the NICU, has ever had a chronic pain problem for at least 3 months, had surgery, had a serious illness or injury, or stayed overnight in a hospital or treatment center over the course of the child’s lifetime to date. Other items included weeks of pregnancy, birth weight, frequency of child pain over the past 3 months, and visits to a health care provider over the past 3 months.
Strengths and Difficulties Questionnaire.
The Strengths and Difficulties Questionnaire was created to assess the behavioral health of children.14,15 The measure evaluates emotional problems, conduct problems, hyperactivity/inattention problems, peer relationship problems, prosocial behavior, and total difficulties. Each subscale above has 5 items and the total difficulties score is the sum of the emotional problems, conduct problems, hyperactivity/inattention problems, and peer relationship problems subscales. This questionnaire was completed by parents of children ages 2 to 17. The SDQ has slightly different forms for the 2–4-year, 4–10 year, and 11–17-year age ranges to ensure that items are developmentally appropriate, but the number of items, response options, and scoring for each are comparable. For the present study, parents of 4-year-olds completed the 4–10-year-old version. A higher score indicates elevated levels of problems or prosocial behavior, depending on the subscale.
Data Analysis
Data analyses were conducted for all participants who completed the surveys required to classify current CAP status (n = 145). Current CAP status was defined as meeting criteria for a pain-related DBGI or current chronic pain at the abdominal site on the PPQ. Chi-square tests were used for categorical variables, such as demographic factors (sex, parent status, race, marital/live-in partner status, education, and household income) and non-abdominal chronic pain (yes/no). Analysis of variance (ANOVA) procedures were conducted to compare participant age across groups at each time point. Analysis of covariance (ANCOVA) procedures, controlling for T3 age and sex, were conducted for continuous variables including health-related quality of life (i.e., PROMIS-29 subscales) and health-specific anxiety. If the omnibus ANCOVA results indicated significant group differences, post hoc comparisons using the Bonferroni correction analyzed pair-wise differences in estimated marginal means. Participants who reported current COVID-19 symptoms (n = 5) were excluded from analyses of current health-related quality of life due to the potential confounding nature of the virus on these outcomes.
For analyses of offspring birth and health outcomes, chi-square tests evaluated differences in categorical variables by original CAP status (CAP vs. Control). Independent samples t-tests evaluated differences between CAP and Control offspring on parent-reported behavioral health measures. Effect sizes are reported as partial eta squared for ANCOVAs and Cohen’s d for t-tests. For partial eta squared, 0.01 indicates a small effect, 0.06 indicates a medium effect, and 0.14 indicates a large effect. For Cohen’s d, 0.2 indicates a small effect, 0.5 indicates a medium effect, and 0.8 indicates a large effect.
Results
Participants
We attempted to contact 554 potential participants (Control: n = 170, CAP: n = 384). Of those we attempted to contact, 2.7% (n= 15) refused, 61.9% (n= 343) were unable to be contacted, 1.3% (n= 7) were screened as ineligible, and 7.9% (n= 44) were reached but either did not respond to the consent form or survey after the phone call. Thus, the sample completing surveys comprised 145 participants (26.2% of possible participants; Control: n = 55, CAP: n = 90). Participants and non-participants in T3 procedures were comparable at T1 and T2, exhibiting no significant differences in age, abdominal pain severity, somatic symptoms, or depressive symptoms (all p-values > 0.05). T3 surveys were completed an average of 19.96 years (SD = 3.25, range: 15.47 – 26.8) following baseline participation in childhood. At the time of participation in the T3 evaluation, participants ranged in age from 23.20 to 42.78 years (M = 31.94, SD = 3.59).
Participants in the Control and CAP groups did not significantly differ at T3 on sex, race, marital status, educational attainment, or annual household income (Table 1). Participants in the CAP group were significantly older than the Control group, t(137.93) = −3.86, p < 0.001; this mirrors differences present at T2. In addition, CAP participants were less likely to be employed than Control participants, X2(1) = 4.94, p = 0.03.
Table 1.
Demographic factors by group
| Demographic factor | Control (n=55) | CAP (n=90) |
|---|---|---|
|
| ||
| T1 age, M (SD) | 11.85 (2.22) | 12.08 (2.58) |
|
| ||
| T2 age, M (SD) | 18.60 (2.53) | 21.01 (3.62)* |
|
| ||
| T3 age, M (SD) | 30.60 (2.72) | 32.66 (3.69)* |
|
| ||
| Sex, % (n) | ||
|
| ||
| Female | 54.5% (30) | 67.8% (61) |
|
| ||
| Male | 45.5% (25) | 32.2% (29) |
|
| ||
| Parent status, % (n) | ||
|
| ||
| Non-parent | 56.4% (31) | 54.4% (49) |
|
| ||
| Parent | 43.6% (24) | 45.6% (41) |
|
| ||
| Race | ||
|
| ||
| White | 96.4% (53) | 91.1% (82) |
|
| ||
| Black or African-American | 3.6% (2) | 4.4% (4) |
| Mixed race | 0.0% (0) | 4.4% (4) |
|
| ||
| Marital/live-in partner status, % (n) | ||
|
| ||
| Married, remarried, or live with a partner | 80.0% (44) | 71.1% (64) |
|
| ||
| Never married and not living with a partner | 18.2% (10) | 21.1% (19) |
|
| ||
| Divorced, widowed, or separated | 1.8% (1) | 7.8% (7) |
|
| ||
| Education, % (n) | ||
|
| ||
| High school or less | 7.3% (4) | 12.2% (11) |
|
| ||
| Vocational school/some college | 10.9% (6) | 16.7% (15) |
|
| ||
| Four-year college | 58.2% (32) | 55.6% (50) |
|
| ||
| Graduate/professional school | 23.6% (13) | 15.6% (14) |
|
| ||
| Employment status, % (n) | ||
| Employed | 96.4% (53) | 84.4% (76)* |
| Unemployed | 3.6% (2) | 15.6% (14)* |
|
| ||
| Household income, % (n) | ||
|
| ||
| $25,000 or less | 1.9% (1) | 7.9% (7) |
|
| ||
| $25,001–$49,999 | 7.4% (4) | 12.4% (11) |
|
| ||
| $50,000–$79,999 | 29.6% (16) | 32.6% (29) |
|
| ||
| $80,000–$119,999 | 33.3% (18) | 21.3% (19) |
|
| ||
| $120,000–$149,999 | 9.3% (5) | 15.7% (14) |
|
| ||
| $150,000 or more | 18.5% (10) | 10.1% (9) |
Groups significantly differ at p < .05 level.
Abdominal Pain Patterns
We evaluated the patterns of remission or recurrence among CAP participants at T1 to T2 to T3 (see Figure 1). A small proportion of participants (21.1%) had recurrent CAP at all three time points. The largest proportion of the original CAP participants (55.6%) had CAP in childhood that remitted by late adolescence/emerging adulthood and remained remitted into early adulthood. Some participants (15.6%) had CAP at the late adolescence/emerging adulthood evaluation (T2), but were remitted at the early adulthood assessment (T3). Only 7.8% of participants had remitted CAP in late adolescence/emerging adulthood (T2) that recurred in adulthood (T3).
Figure 1.
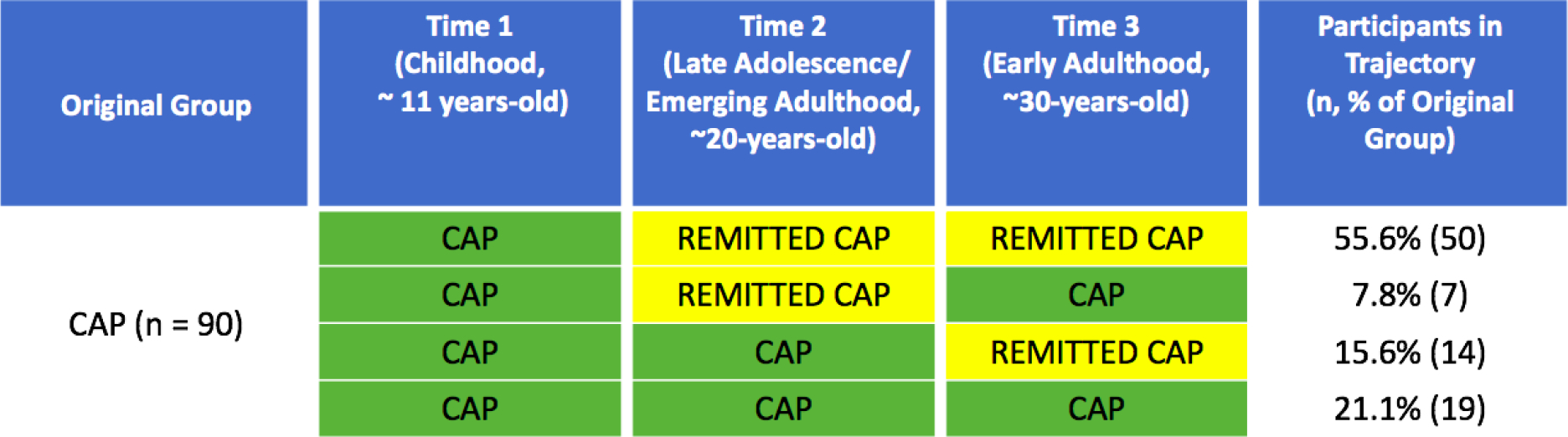
Four distinct patterns from T1 to T3 for CAP patients who participated at 20-year (T3) follow-up
Note. Remitted CAP describes patients who were not actively experiencing CAP at that time point.
At T3, participants in the original CAP group were significantly more likely to meet criteria for current CAP as compared to those in the Control group, X2(1) = 10.49, p = 0.001. Among those within the original CAP group, 28.89% (n = 26) met criteria for current CAP (FD only: n = 7, IBS only: n = 10, both FD and IBS: n = 9, CAP without FD or IBS: n = 1) as compared to 7.3% (n = 4) of the original Control group (FD only: n = 1, IBS only: n = 2, FD and IBS: n = 1). Due to the small number of Control participants who developed CAP (n = 4), we excluded these four participants from analyses of current health outcomes. Thus, the present study compared current physical and mental health among three groups: (0) Control (n = 51), (1) originally CAP without current chronic abdominal pain (CAP−; n = 64), and (2) originally CAP with current abdominal pain (CAP+; n = 26).
Non-Abdominal Chronic Pain Outcomes
CAP outcome groups at T3 were significantly different regarding presence of current non-abdominal chronic pain, X2(2), = 10.75, p = 0.005. Specifically, close to half of the CAP+ group reported at least one current non-abdominal chronic pain site (42.3%, n = 11/26), which was significantly higher than the Control group (9.8%, n = 5/51), but did not significantly differ from the CAP− group (23.4%, n = 15/64).Within the CAP+ group, most commonly reported current non-abdominal pain locations included: back (30.8%, n = 8/26), shoulder, arm, or hand (19.2%, n = 5/26), pelvic (11.5%, n = 3/26), and hips or buttocks (11.5%, n = 3/26).
CAP outcome groups at T3 also significantly differed on recall of childhood chronic pain experience, X2(2) = 31.49, p < 0.001, and history of ever receiving a chronic pain diagnosis from a medical provider, X2(2) = 25.83, p < 0.001. The CAP+ group was significantly more likely to recall having experienced chronic pain in childhood (69.2%, n = 18/26) compared to the CAP− group (29.7%, n = 19/64) and the Control group, (7.8%, n = 4/47). Similarly, the CAP+ group was significantly more likely to report having ever received a chronic pain diagnosis from a medical provider (84.6%, n = 22/26) as compared to the CAP− group (51.6%, n = 33/64), who in turn reported a higher prevalence of historical chronic pain diagnoses than did the Control group (24.0%, n = 12/50). Half of the CAP+ group reported at least two overlapping chronic pain diagnoses (50.0%, n = 13/26), compared to 26.6% (n = 17.64) of the CAP− group and 2.0% (n = 1/50) of the Control group, X2(2) = 24.20, p < 0.001. See Supplemental Table 1 for the prevalence of diagnoses by group.
Health Related Quality of Life
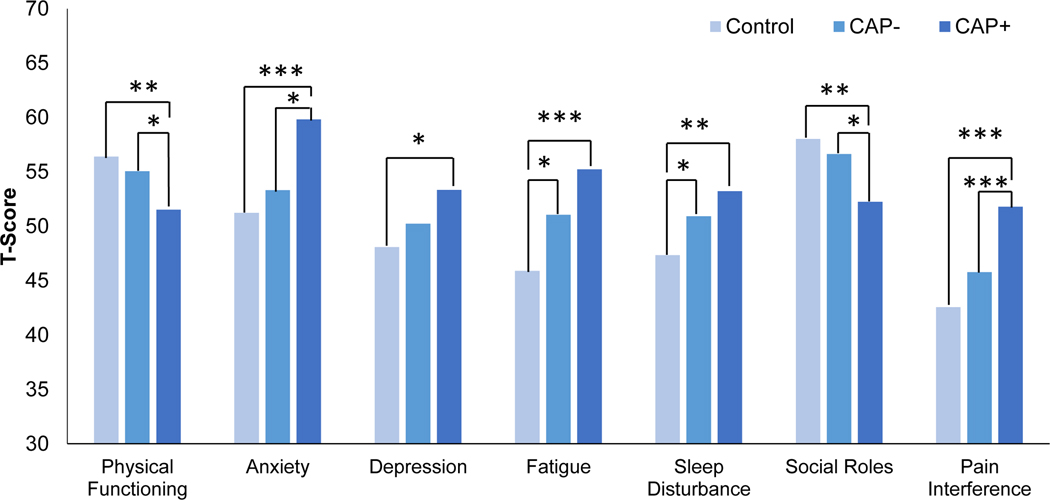
ANCOVAs, controlling for sex and age at follow-up, evaluated between-group differences on the PROMIS-29 Profile domains indexing current symptoms and functioning (see Table 2). Participants indicating current COVID-19 symptoms (n = 5) were excluded from these analyses due to the potential impact on current symptoms and functioning. Results indicated significant differences between groups on all PROMIS-29 Profile domains: physical functioning, anxiety, depression, fatigue, sleep disturbance, ability to function in social roles, and pain interference (summarized in Figure 2).
Table 2.
Means and standard deviations for PROMIS measure T scores by pain group
| PROMIS measure | Control (n = 51) | CAP− (n=60) | CAP+ (n = 25) | F | ηp2 |
|---|---|---|---|---|---|
| Physical Functioning | 56.40 (2.47)a | 55.06 (4.45)a | 51.53 (8.00)b | 6.91* | .10 |
| Anxiety | 51.23 (9.93)a | 53.31 (9.02)a | 59.82 (8.18)b | 6.98** | .10 |
| Depression | 48.09 (8.31)a | 50.24 (8.40)a,b | 53.34 (8.62)b | 3.83* | .06 |
| Fatigue | 45.90 (7.64)a | 51.05 (9.71)b | 55.24 (10.39)b | 9.14*** | .12 |
| Sleep Disturbance | 47.34 (5.94)a | 50.92 (7.66)b | 53.22 (7.24)b | 6.51** | .09 |
| Social Roles | 58.03 (6.98)a | 56.64 (8.85)a | 52.25 (9.95)b | 5.43** | .08 |
| Pain Interference | 42.57 (3.02)a | 45.78 (6.69)a | 51.80 (9.49)b | 14.23*** | .18 |
p<.001
p < .01
p < .05
Note. Within rows, means with different superscripts differ significantly at p < .01. ANCOVA analyses included age and sex as covariates.
Figure 2.
PROMIS measure T-scores of Control, CAP−, and CAP+ participants at 20-year follow-up
Note. ***p<.001; **p < .01; *p < .05.
Post-hoc analyses using a Bonferroni correction tested for pair-wise differences between groups. The CAP+ group reported poorer physical and social functioning, greater anxiety, and greater pain interference than both the CAP− and Control groups. Both CAP+ and CAP− groups reported significantly greater sleep disturbance and fatigue than the Control group. The CAP+ group reported significantly greater depressive symptoms and poorer physical functioning than the Control group, but did not differ significantly from the CAP− group. Effect sizes (see Table 2) for between-group differences generally ranged from medium to large.
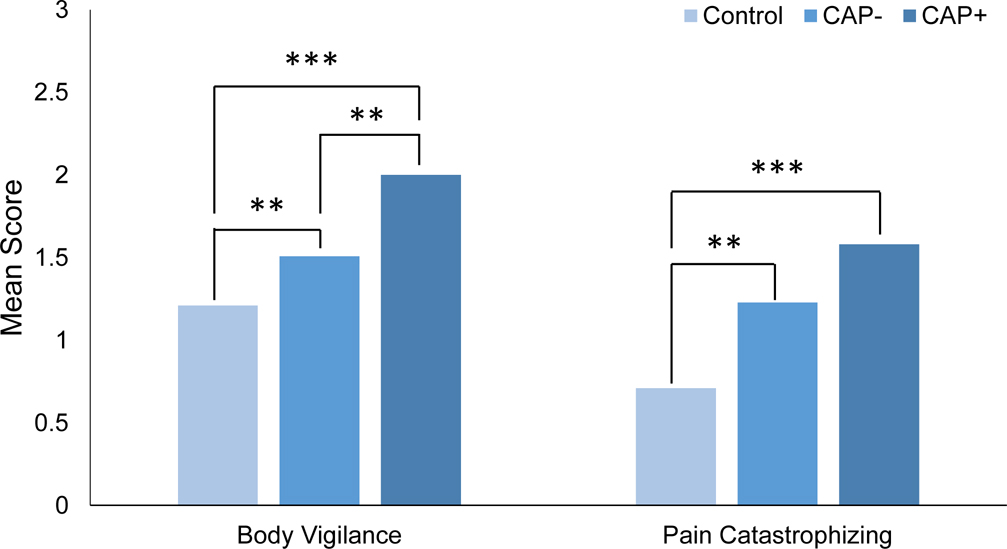
Health-Specific Anxiety: Body Vigilance and Pain Catastrophizing
Controlling for sex and age at follow-up, ANCOVAs evaluated between-group differences on measures of health-specific anxiety including body vigilance and pain catastrophizing. Results indicated significant between-group differences for both body vigilance, F(2, 135) = 16.14, p < 0.001, ηp2 = 0.19, and pain catastrophizing F(2, 135) = 8.50, p < 0.001, ηp2 = 0.11. For body vigilance, groups differed in a stair-wise manner in the expected direction (Figure 3). The CAP+ group reported significantly higher body vigilance (M = 2.00, SD = 0.47) than the CAP− group (M = 1.51, SD = 0.58) which, in turn, reported significantly higher body vigilance than the Control group (M = 1.21, SD = 0.60). Both the CAP+ and CAP− groups reported significantly higher pain catastrophizing than the Control group (CAP+ M = 1.55, SD = 0.94; CAP− M = 1.23, SD = 0.91; Control M = 0.71, SD = 0.51).
Figure 3.
Body vigilance and pain catasrophizing reported by Control, CAP−, and CAP+ participants at 20-year follow-up
Note. ***p<.001; **p < .01; *p < .05. Body Vigilance mean scores range from 0 to 3. Pain Catastrophizing mean scores range from 0 to 4.
Child Birth and Health Outcomes
Due to the small sample size of participants who had become parents since T2, analyses on child outcomes compared the original T1 CAP group, regardless of current CAP status, to the original Control group (n = 60, T1 CAP: n = 39, T1 Control: n = 21). In this subset of participants, the majority of parents had either 1 (n = 25, 41.7%) or 2 (n = 26, 43.3%) biological children with a small number reporting 3 or more children (n = 9, 15.0%). Parents completed measures on one of their children (oldest when possible). The average child age was 5.55 years old (SD = 4.66). Out of those who completed measures regarding offspring, proportion of offspring ages (by category) were as follows: 21.7% (n = 13) 0–1 years of age, 11.7% (n = 7) 2-years-old, 11.7% (n = 7) 3-years-old, 3.3% (n = 2) 4-years-old, 31.7% (n = 19) 5–10 years-old, and 20.0% (n = 12) 11–17 years-old. The mean age of CAP and Control offspring did not differ significantly, t(58) = −1.50, p = 0.14. There were no significant between-group differences on child health or birth outcomes (Table 3). Types of surgeries reported by parents were consistent with the age of the child sample and primarily included tonsillectomy and/or adenoidectomy and ear tubes. Overall prevalence of any chronic pain history in offspring in the sample was low (CAP: 5.1%, n = 2/39, Control: 4.8%, n = 1/21).
Table 3.
Offspring health and birth outcomes by group
| Health or birth outcome | Well (n=21) | CAP (n=39) |
|---|---|---|
| Child born at full term, % (n) | 90.5% (19) | 84.6% (33) |
| Weeks of pregnancy, M (SD) | 39.57 (1.75) | 38.77 (2.18) |
| Birth weight in lbs., M (SD) | 7.54 (1.05) | 7.07 (1.28) |
| Child stayed in NICU, % (n) | 4.8% (1) | 10.3% (4) |
| Child had surgery, % (n) | 28.6% (6) | 20.5% (8) |
| Child with pain at least weekly over past 3 months, % (n) | 9.5% (2) | 2.6% (1) |
| Number of visits to a health care provider over past 3 months, Median (Range) | 1 (0, 4) | 0 (0, 6) |
Note. Groups did not differ significantly on statistical tests for any outcomes.
Regarding children’s behavioral health, parents in the CAP group reported significantly higher emotional problems and fewer conduct problems in their offspring compared to the Control group (Table 4). Children of CAP and Control groups did not differ on parents’ reports of children’s hyperactivity, peer problems, prosocial tendencies, or total behavioral problems.
Table 4.
Comparisons of offspring behavioral health by group on subscales of the Strengths and Difficulties Questionnaire
| Subscale | Well (n = 11) | CAP (n=30) | t(39) | Cohen’s d | 95%CI for Cohen’s d |
|---|---|---|---|---|---|
| Emotion problems | 2.36 (1.57) | 3.77 (1.85) | −2.23* | −0.79 | −1.50, −0.07 |
| Conduct problems | 4.36 (1.03) | 3.10 (1.86) | 2.75* | 0.75 | 0.03, 1.45 |
| Hyperactivity | 4.45 (3.08) | 4.17 (2.34) | 0.32 | 0.11 | −0.58, 0.80 |
| Peer problems | 3.27 (1.68) | 2.80 (1.83) | 0.75 | 0.26 | −0.43, 0.96 |
| Prosocial tendencies | 8.73 (1.27) | 8.97 (1.35) | −0.51 | −0.18 | −0.87, 0.51 |
| Total behavioral problems | 8.09 (4.91) | 8.50 (4.83) | −0.24 | −0.08 | −0.78, 0.61 |
p<.05
Discussion
In this twenty-year longitudinal cohort study, approximately one in five children presenting to a tertiary care pediatric gastroenterology clinic for CAP at initial enrollment also reported clinically significant CAP in both late adolescence/emerging adulthood (at about 20-years-old) and early adulthood (at about 30-years-old). As expected for a recurrent pain condition, a small proportion of youth showed a pattern in which they met criteria for CAP at one follow-up time point, but not both. As reported previously,24 the majority of youth with CAP experienced pain resolution between childhood and late adolescence/emerging adulthood, indicating natural resolution or responsiveness to standard pediatric or general medical care. At the 20-year follow-up (i.e., in early to mid-adulthood), the subset of participants with recurrent CAP reported lower health-related quality of life than Control participants, including higher anxiety, depression, fatigue, sleep disturbance, and pain interference, and lower physical and social functioning. For most domains of health-related quality of life on the PROMIS measures, participants with remitted CAP reported intermediate scores between participants with recurrent CAP (lower quality of life) and participants in the Control group (higher quality of life). Moreover, average T-scores were generally within normal limits compared to the PROMIS calibration sample for CAP+, CAP−, and Control groups. Consistent with the high prevalence of anxiety disorders in earlier assessments of this cohort of pediatric patients with CAP,50 the group of patients with ongoing CAP at the 20-year follow-up reported anxiety symptoms in the moderate to severe range.
Even though pediatric patients with current CAP at this follow up in early to mid-adulthood reported higher anxiety and depressive symptoms and poorer functioning compared to those with remitted CAP or the Control group, their standardized scores on PROMIS domains were generally closer to average than those reported elsewhere for samples with clinically significant chronic pain.43,53 Participants with current CAP in the present study may not identify as chronic pain patients as CAP status was determined by their symptom reports on the Rome IV and Persistent Pain Questionnaires and not their health care use. Furthermore, a substantial proportion of participants in the original CAP cohort did not recall having a childhood chronic pain experience despite presenting to a tertiary pediatric GI clinic for recurrent abdominal pain. Pediatric patients with CAP who are treated in a gastroenterology clinic may not identify as having a chronic pain problem as do pediatric patients who receive care through a pediatric chronic pain clinic. In recent research evaluating parent-child reminiscing around surgery, parents have been identified as powerful change agents in the way a child is socialized around and remembers a painful event.41,42 The way in which parent-child dyads understand, name, and remember pain problems may have implications for longitudinal patterns of remission and recovery and is an important avenue for further research.
Approximately half of the patients with ongoing CAP at 20-year follow-up likely would meet criteria for widespread or generalized pain based on the number of non-abdominal chronic pain sites and reported number of chronic pain diagnoses from a medical provider. The biological mechanisms involved in the onset and maintenance of nociplastic or widespread pain (e.g., central sensitization) likely differ from those of more localized pain conditions. Thus, early detection of extraintestinal chronic pain and matching with appropriate treatment could improve long-term health and quality of life outcomes for this subset.
Consistent with past research,2,45 health-related anxiety, indexed by pain catastrophizing and body vigilance in this study, could be a potential mechanism of risk for health-related impairment among patients with CAP. Former pediatric patients with CAP showed similarly elevated pain catastrophizing relative to Control participants regardless of current chronic pain status. In addition, those with remitted CAP reported significantly higher body vigilance than the Control group despite similar levels of general anxiety on the PROMIS-29. Although patients with remitted CAP were functioning relatively well in adulthood, they may have heightened risk for future chronic pain problems if faced with a pain-inducing event (e.g., acute GI illness, fracture) given elevations on pain catastrophizing and bodily vigilance.
CAP and health-related anxiety within the individual may operate in a cycle, with greater pain increasing anxious thoughts and higher anxiety, in turn, exacerbating pain. Intergenerationally, having a parent with high health-related anxiety may increase the risk of the same anxious symptom interpretation style in offspring. Parents anxious about their own health are likely to have negative interpretations of their child’s symptoms and consult health services in response. Because children commonly learn behaviors through modeling their parents, this could be one pathway involved in the intergenerational transmission of CAP.18,20 For example, adolescents who observe more parent pain behaviors (e.g., grimacing, moving slowly) in turn appraise pain as more threatening, and experience more functional impairment.52
Consistent with this perspective, former CAP patients reported higher levels of emotional problems in their young children as compared to Control participants’ reports about their offspring. In contrast, former CAP patients reported lower levels of conduct problems as compared to reports of Control participants about their children. This latter finding is contrary to some prior work on offspring of parents with chronic pain.19 While speculative, lower conduct problems could reflect a child’s tendency towards overcontrol, a characteristic of pediatric anxiety disorders.13 The extent to which the intergenerational risk for the development of pediatric chronic pain is accounted for by intergenerational transmission of a tendency towards internalizing symptoms (e.g., anxiety and depression) is worthy of further research. Indeed, siblings of youth with CAP compared to siblings of youth in a control group have also been found to experience heightened internalizing symptoms,16 suggesting this may represent a familial pattern. Given that prospective studies have shown that child depressive symptoms predict the transition from pediatric acute to chronic pain,22,44 future research should evaluate shared or unique pathways accounting for the intergenerational transmission of risk for internalizing symptoms and chronic pain, and the potential bidirectional relation between them. This research should consider not only psychosocial risk pathways, but also genetic and neurobiological pathways that may be relevant.
Strengths of the current study include the longitudinal design following both a clinical and control cohort over a 20-year period. Nevertheless, limitations of the study highlight important directions for future research. First, we were only able to reach and enroll approximately one-fourth of participants from T2, primarily due to the absence of accurate contact information. In the follow-up period from T2 to T3, most participants were in early adulthood and likely experiencing many important life transitions (e.g., college, employment, moves, and marriage) that could have accounted for less accurate contact information. Despite loss of participants from T2 to T3, the similarity in key variables at T1 and T2 between participants and non-participants in T3 suggests that the sample studied here was generally representative of the earlier, larger cohorts. Nonetheless, a number of factors could have contributed to the attrition rate which might impact the interpretation of our results, including decreased motivation to participate once pain has resolved, difficulty participating due to severity of pain course, or systemic factors impacting stability of contact information. Moreover, due to recruitment challenges, we were unable to enroll as many parents as needed to comprehensively evaluate intergenerational risks. The extent to which the COVID-19 pandemic and related challenges affected recruitment rates is also unknown. Thus, results from the current study should primarily be considered hypothesis generating and suggestive of future directions that could enhance longitudinal work. The subset of analyses that focused on parents and offspring should be considered preliminary as sample sizes were quite small for hypothesis testing.
Another potential limitation is that we could not differentiate between persistent versus recurrent symptoms of CAP or the extent to which interventions could have affected the course of chronic pain. That is, the T2 and T3 evaluations were a snapshot in time and did not provide information about the extent of CAP or interventions experienced between time points. Thus, future prospective studies of children with CAP should track their physical and emotional symptoms and interventions received continuously or at least at multiple points over time. By so doing, we will be able to determine the extent to which pediatric CAP patients’ patterns of remission, recurrence, persistence, and treatment response predict the quality of their subsequent health and functioning.
More longitudinal cohort studies following children with chronic pain from childhood into adulthood are needed to comprehensively assess both long-term outcomes and how the experience of pediatric chronic pain may be associated with future parenting behaviors and offspring functioning. These studies ideally will be multisite, include a more diverse population in terms of race, ethnicity, and socioeconomic status; and maintain at least annual contact during cohort participation to enhance retention and participation. Few studies have attempted to evaluate children at potentially high-risk for developing chronic pain in early childhood to better identify intergenerational and individual predictors of childhood chronic pain development. The ultimate goals of longitudinal cohort studies are to both add to the body of literature examining the long-term public health significance of pediatric pain as well as to identify salient factors for targeted interventions. It is important that longitudinal studies consider both patient registries of treatment-seeking youth and youth with chronic pain from the community who are not treatment-seeking in order to more fully capture the diversity of trajectories and outcomes.
In summary, former pediatric patients with recurrent CAP had poorer psychological and physical outcomes in early to mid-adulthood than did those with remitted CAP who, in turn, had poorer outcomes than those who had not experienced CAP as a child. Although outcomes for patients with remitted or recurrent CAP were poorer as compared to the Control group, self-reported mental health and functioning impairments generally fell within normal limits when compared to a nationally representative calibration sample. In addition, offspring of former pediatric patients with CAP had more emotional problems, which is consistent with previous theoretical frameworks suggesting that emotional distress is a potential pathway underlying the intergenerational risk for chronic pain.56 Further research is needed to disentangle which factors are most predictive of persistent CAP across multiple developmental periods and how to optimize interventions to address the unique needs of each. Comprehensive interventions taking into account multiple domains of both physical and mental health symptoms and functioning could have significant impacts both on longitudinal outcomes for these patients as well as have potential preventive effects to reduce chronic pain risks for future offspring.
Supplementary Material
Funding
This work was supported by the National Institutes of Health [R01 HD23264, T32 GM 108554, K23 HD104183, UL1 TR000445]; and the International Association for the Study of Pain [Early Career Grant].
Footnotes
Conflicts of Interest
The authors have no relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramowitz JS, Deacon BJ, Valentiner DP: The Short Health Anxiety Inventory: Psychometric Properties and Construct Validity in a Non-clinical Sample. Cogn Ther Res 31:871–83, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayonrinde OT, Ayonrinde OA, Adams LA, Sanfilippo FM, O’ Sullivan TA, Robinson M, Oddy WH, Olynyk JK: The relationship between abdominal pain and emotional wellbeing in children and adolescents in the Raine Study. Sci Rep 10:1646, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baber KF, Anderson J, Puzanovova M, Walker LS: Rome II versus Rome III classification of functional gastrointestinal disorders in pediatric chronic abdominal pain. J Pediatr Gastroenterol Nutr 47:299, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruehl S, Chung OY: Parental history of chronic pain may be associated with impairments in endogenous opioid analgesic systems. Pain 124:287–94, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bruehl S, France CR, France J, Harju A, al’Absi M: How accurate are parental chronic pain histories provided by offspring? Pain 115:390–7, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R: The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63:1179–94, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen MF, Mortensen O: Long-term prognosis in children with recurrent abdominal pain. Arch Dis Child 50:110–4, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claar RL, Walker LS, Smith CA: Functional Disability in Adolescents and Young Adults with Symptoms of Irritable Bowel Syndrome: The Role of Academic, Social, and Athletic Competence. J Pediatr Psychol 24:271–80, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cservenka A, Stein H, Wilson AC, Nagel BJ: Neurobiological phenotypes of familial chronic pain in adolescence: a pilot fMRI study. J Pain 16:913–25, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber J, Walker LS, Zeman J: Somatization symptoms in a community sample of children and adolescents: Further validation of the Children’s Somatization Inventory. Psychol Assess 3:588–95, 1991. [Google Scholar]
- 11.Gatchel RJ: Comorbidity of Chronic Pain and Mental Health Disorders: The Biopsychosocial Perspective. Am Psychol 59:795–805, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Gieteling MJ, Bierma-Zeinstra SMA, Passchier J, Berger MY: Prognosis of chronic or recurrent abdominal pain in children. J Pediatr Gastroenterol Nutr 47:316–26, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert K, Perino MT, Myers MJ, Sylvester CM: Overcontrol and neural response to errors in pediatric anxiety disorders. J Anxiety Disord 72:102224, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman R: Psychometric Properties of the Strengths and Difficulties Questionnaire. J Am Acad Child Adolesc Psychiatry 40:1337–45, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Goodman R: The Strengths and Difficulties Questionnaire: A Research Note. J Child Psychol Psychiatry 38:581–6, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Guite JW, Lobato DJ, Shalon L, Plante W, Kao BT: Pain, disability, and symptoms among siblings of children with functional abdominal pain. J Dev Behav Pediatr 28:2–8, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heathcote LC, Williams SE, Smith AM, Sieberg CB, Simons LE: Parent Attributions of Ambiguous Symptoms in Their Children: A Preliminary Measure Validation in Parents of Children with Chronic Pain. Children 5:76, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins KS, Birnie KA, Chambers CT, Wilson AC, Caes L, Clark AJ, Lynch M, Stinson J, Campbell-Yeo M: Offspring of Parents with Chronic Pain: A Systematic Review and Meta-Analysis of Pain, Health, Psychological, and Family Outcomes. Pain 156:2256–66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins KS, Chambers CT, Rosen NO, Sherry S, Mohammadi S, Lynch M, Campbell-Yeo M, Clark AJ: Testing the intergenerational model of transmission of risk for chronic pain from parents to their children: an empirical investigation of social transmission pathways. PAIN 160:2544–53, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Hoftun GB, Romundstad PR, Rygg M: Association of Parental Chronic Pain With Chronic Pain in the Adolescent and Young Adult: Family Linkage Data From the HUNT Study. JAMA Pediatr 167:61–9, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Holley AL, Wilson AC, Palermo TM: Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain 158:794–801, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooten WM: Chronic Pain and Mental Health Disorders. Mayo Clin Proc 91:955–70, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Horst S, Shelby G, Anderson J, Acra S, Polk DB, Saville BR, Garber J, Walker LS: Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin Gastroenterol Hepatol 12:2026–32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotopf M, Carr S, Mayou R, Wadsworth M, Wessely S: Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. BMJ 316:1196–200, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA: Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr 129:220–6, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Incledon E, O’Connor M, Giallo R, Chalkiadis GA, Palermo TM: Child and Family Antecedents of Pain During the Transition to Adolescence: A Longitudinal Population-Based Study. J Pain 17:1174–82, 2016. [DOI] [PubMed] [Google Scholar]
- 28.Irwin DE, Stucky BD, Thissen D, DeWitt EM, Lai JS, Yeatts K, Varni JW, DeWalt DA: Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Qual Life Res 19:585–94, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalomiris AE, Ely SL, Love SC, Mara CA, Cunningham NR: Child-Focused Cognitive Behavioral Therapy for Pediatric Abdominal Pain Disorders Reduces Caregiver Anxiety in Randomized Clinical Trial. J Pain 23:810–21, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knook LME, Lijmer JG, Konijnenberg AY, Taminiau B, Engeland H van: The Course of Chronic Pain With and Without Psychiatric Disorders: A 6-Year Follow-Up Study From Childhood to Adolescence and Young Adulthood. J Clin Psychiatry 73:0–0, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Levy RL, Whitehead WE, Walker LS, Von Korff M, Feld AD, Garner M, Christie D: Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol 99:2442–51, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Love SC, Mara CA, Kalomiris AE, Cunningham NR: The influence of caregiver distress and child anxiety in predicting child somatization in youth with functional abdominal pain disorders. Children 6:134, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch-Jordan AM, Peugh J, Cunningham NR, Trygier JR, Kashikar-Zuck S: Maternal Protective Parenting Accounts for the Relationship Between Pain Behaviors and Functional Disability in Adolescents. Clin J Pain 34:1089–95, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magni G, Pierri M, Donzelli F: Recurrent abdominal pain in children: a long term follow-up. Eur J Pediatr 146:72–4, 1987. [DOI] [PubMed] [Google Scholar]
- 35.McWilliams LA, Kowal J, Wilson KG: Development and evaluation of short forms of the Pain Catastrophizing Scale and the Pain Self-efficacy Questionnaire. Eur J Pain 19:1342–9, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle: International Association for the Study of Pain, 1994. [Google Scholar]
- 37.Mulvaney S, Lambert EW, Garber J, Walker LS: Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry 45:737–44, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Øster J: Recurrent Abdominal Pain, Headache and Limb Pains in Children and Adolescents. Pediatrics 50:429–36, 1972. [PubMed] [Google Scholar]
- 39.Palsson OS, Whitehead WE, van Tilburg MAL, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SS, Sperber A, Spiegel B, Tack J, Vanner S, Walker LS, Whorwell P, Yang Y: Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology S0016–58085:00180–3, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Palsson OS, Whitehead WE, van Tilburg MAL, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SSC, Sperber A, Spiegel B, Tack J, Vanner S, Walker LS, Whorwell P, Yang Y: Development and Validation of the Rome IV Diagnostic Questionnaire for Adults. Gastroenterology 150:1481–91, 2016. [DOI] [PubMed] [Google Scholar]
- 41.Pavlova M, Graham SA, Jordan A, Chorney J, Vinall J, Rasic N, Brookes J, Hoy M, Yunker WK, Noel M: Socialization of pain memories: Parent-child reminiscing about past painful and sad events. J Pediatr Psychol 44:679–91, 2019. [DOI] [PubMed] [Google Scholar]
- 42.Pavlova M, Orr SL, Noel M: Parent–Child Reminiscing about Past Pain as a Preparatory Technique in the Context of Children’s Pain: A Narrative Review and Call for Future Research. Children 7:130, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pope JE, Fishman MA, Gunn JA, Cotten BM, Hill MM, Deer TR: Cross-Validation of the Foundation Pain Index with PROMIS-29 in Chronic Pain Patients. J Pain Res 14:2677–85, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabbitts JA, Palermo TM, Zhou C, Meyyappan A, Chen L: Psychosocial Predictors of Acute and Chronic Pain in Adolescents Undergoing Major Musculoskeletal Surgery. J Pain 21:1236–46, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramchandani PG, Fazel M, Stein A, Wiles N, Hotopf M: The impact of recurrent abdominal pain: predictors of outcome in a large population cohort. Acta Paediatr 96:697–701, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramchandani PG, Stein A, Hotopf M, Wiles NJ: Early Parental and Child Predictors of Recurrent Abdominal Pain at School Age: Results of a Large Population-Based Study. J Am Acad Child Adolesc Psychiatry 45:729–36, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Salkovskis PM, Rimes KA, Warwick HMC, Clark DM: The Health Anxiety Inventory: development and validation of scales for the measurement of health anxiety and hypochondriasis. Psychol Med 32:843–53, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Scherrer KH, Ziadni MS, Kong J-T, Sturgeon JA, Salmasi V, Hong J, Cramer E, Chen AL, Pacht T, Olson G, Darnall BD, Kao M-C, Mackey S: Development and validation of the Collaborative Health Outcomes Information Registry body map. Pain Rep 6:e880, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmulson MJ, Drossman DA: What Is New in Rome IV. J Neurogastroenterol Motil 23:151–63, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shelby GD, Shirkey KC, Sherman AL, Beck JE, Haman K, Shears AR, Horst SN, Smith CA, Garber J, Walker LS: Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics, 132:475–482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherman AL, Bruehl S, Smith CA, Walker LS: Individual and Additive Effects of Mothers’ and Fathers’ Chronic Pain on Health Outcomes in Young Adults With a Childhood History of Functional Abdominal Pain. J Pediatr Psychol 38:365–75, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stone AL, Bruehl S, Smith CA, Garber J, Walker LS: Social learning pathways in the relation between parental chronic pain and daily pain severity and functional impairment in adolescents with functional abdominal pain. Pain 159:298–305, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone AL, Holley AL, Dieckmann NF, Wilson AC: Use of the PROMIS-29 to Identify Subgroups of Mothers with Chronic Pain. Health Psychol 38:422–30, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone AL, Walker LS, Guest Editors: Gerhardt Cynthia A. Deborah CAB Wiebe J and Holmbeck Grayson N: Adolescents’ Observations of Parent Pain Behaviors: Preliminary Measure Validation and Test of Social Learning Theory in Pediatric Chronic Pain. J Pediatr Psychol 42:65–74, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone AL, Walker LS, Heathcote LC, Hernandez JM, Basch MC, Wilson AC, Simons LE: Somatic symptoms in pediatric patients with chronic pain: Proposed clinical reference points for the Children’s Somatic Symptoms Inventory (formerly the Children’s Somatization Inventory). J Pain 20:932–40, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone AL, Wilson AC: Transmission of risk from parents with chronic pain to offspring: an integrative conceptual model. Pain 157:2628–39, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J: A classification of chronic pain for ICD-11. Pain 156:1003–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker LS, Dengler-Crish CM, Rippel S, Bruehl S: Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain 150:568–72, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker LS, Garber J, Smith CA, Van Slyke DA, Claar RL: The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. J Consult Clin Psychol, 2001. [PMC free article] [PubMed] [Google Scholar]
- 60.Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA: Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 153:1798–806, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker LS, Smith CA, Garber J, Van Slyke DA: Development and validation of the pain response inventory for children. Psychol Assess 9:392, 1997. [Google Scholar]
- 62.Wallis EM, Fiks AG: Nonspecific Abdominal Pain in Pediatric Primary Care: Evaluation and Outcomes. Acad Pediatr 15:333–9, 2015. [DOI] [PubMed] [Google Scholar]
- 63.Youssef NN, Atienza K, Langseder AL, Strauss RS: Chronic Abdominal Pain and Depressive Symptoms: Analysis of the National Longitudinal Study of Adolescent Health. Clin Gastroenterol Hepatol 6:329–32, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.