Figure 4.
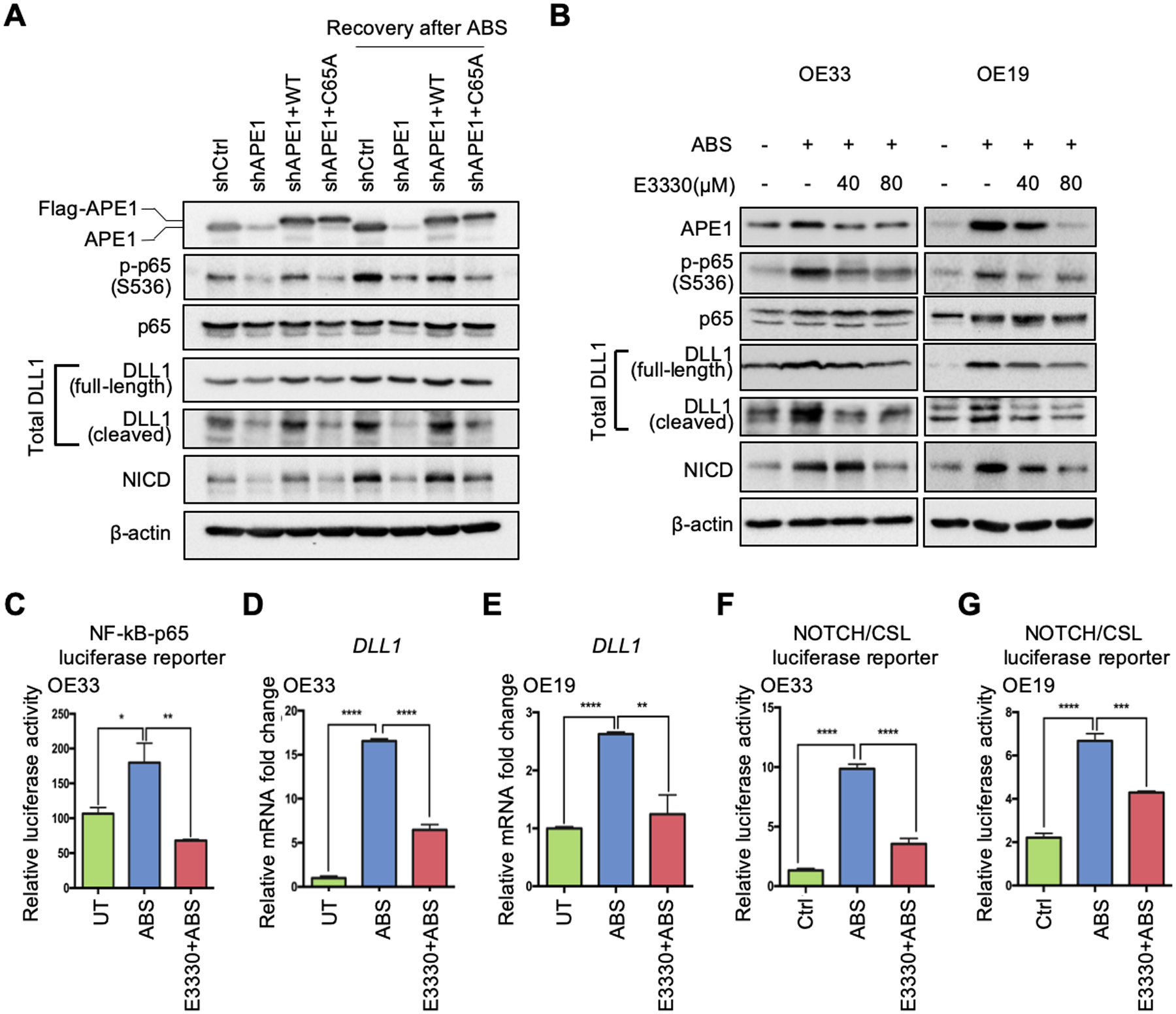
APE1 regulates NF-κB-DLL1-NOTCH axis through its redox function in response to ABS. (A) The APE1 stable silencing OE33 cells (shAPE1) were transfected with APE1-wild-type (WT) overexpression plasmid or APE1-redox-deficient-mutant (C65A) plasmid in the absence or presence of ABS exposure for Western blots. (B) Western blots were used to detect protein levels of interested genes in the condition of ABS treatment with or without APE1 redox inhibitor, E3330, in OE33 (left) and OE19 (right) cells. (C) OE33 cells were maintained with 80 μM E3330 before and after ABS exposure, and then NF-κB-p65 activity luciferase reporter assay was performed. (D) and (E) qRT-PCR was used to detect DLL1 mRNA change in the condition of ABS exposure with or without E3330 application (80 μM) in OE33 (D) and OE19 (E) cells. (F) and (G) Luciferase reporter assays were performed to examine NOTCH transcriptional activity in the condition of ABS treatment with or without E3330 in OE33 (F) and OE19 (G) cells. Statistical data are shown as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 as calculated by t test for two group comparisons.
