Abstract
Purpose of Review
Congenital pseudarthrosis of the tibia (CPT) is a rare condition closely associated with neurofibromatosis type I. Affected children are born with anterolateral bowing of the tibia which progresses to pathologic fracture, pseudarthrosis, and high risk of refracture even after initial union has been attained. There is currently no consensus on the classification of this disease or consensus on its treatment. The purpose of this review is to (1) review the clinical presentation, etiology, epidemiology, classification, and natural history of congenital pseudarthrosis of the tibia and (2) review the existing trends in treatment of congenital pseudarthrosis of the tibia and its associated complications.
Recent Findings
Current treatment protocols focus primarily on combining intramedullary fixation with external or internal fixation to achieve union rates between 74 and 100%. Intramedullary devices should be retained as long as possible to prevent refracture. Cross-union techniques, though technically difficult, have a reported union rate of 100% and no refractures at mid- to long-term follow-up. Vascularized fibular grafting and induced membrane technique can be successful, but at the cost of numerous surgical procedures. Growth modulation is a promising new approach to preventing fracture altogether, though further study with larger patient series is necessary.
Summary
The primary consideration in treatment of CPT is expected union rate and refracture risk. Combined intramedullary and external or internal fixation, especially with cross-union techniques, show most promise. Perhaps most exciting is further research on preventing fracture through guided growth, which may reduce the morbidity of multiple surgical procedures which have been the mainstay of treatment for CPT thus far.
Keywords: Anterolateral, Tibia, Bowing, Congenital pseudoarthrosis of the tibia, Treatment
Introduction
Congenital pseudarthrosis of the tibia (CPT) is a rare condition with reported incidence of 1/60000 to 1/250000 live births [1–4]. Congenital pseudarthrosis of the tibia represents a continuum of disease beginning with anterolateral bowing of the tibia which progresses to pathologic tibial fracture with subsequent pseudoarthrosis which can be exceptionally difficult to manage. In certain severe cases, fracture and pseudarthrosis can be seen at birth [5].
Etiology and Epidemiology
CPT does not show a predilection for either gender or laterality. It is commonly associated with neurofibromatosis type 1 (NF1), is present in 2–6% of patients with NF1, and has been suggested as a diagnostic criterion for NF1 [3, 6–8]. Of patients afflicted by CPT, 40–84% carry a diagnosis of NF1 [3, 6–9]. Among CPT patients with NF1, 80% carry heterozygous NF1 pathologic variants, and many of these variants are novel [10]. Computational analysis continues to strengthen the hypothesis of genetic factors leading to CPT [11]. Furthermore, as reported by Zhu et al., no difference in clinical course exists between CPT in patients with a diagnosis of NF1 compared to patients with CPT and no concurrent diagnosis of NF1 [10].
The etiology of CPT is incompletely understood. Quantitative ultrasound of affected tibiae in CPT shows abnormal bone quality despite characteristic cortical thickening [12]. It has been postulated that the periosteum of patients with CPT plays a significant role in pseudarthrosis and the disease is perhaps a primary disease of the periosteum with the bone secondarily affected [13•]. Bone marrow–derived mesenchymal stem cells in CPT are not significantly different whether derived from the iliac crest or fracture site [14]. In CPT, the periosteum is described as thickened, relatively avascular, constrictive, and adherent to the tibia and fibula, potentially leading to attritional loss of bone with subsequent increased risk of fracture and refracture [13•, 15, 16]. Surgical pathology at the pseudarthrosis site shows fibrous hamartoma replacing periosteum [17–19]. There is increased osteoclastic activity and a dense fibroblastic hyperplasia in the periosteum of children with CPT, and some suggest that the diseased periosteum is what gives rise to the fibrous hamartoma seen at the pseudarthrosis site [5, 18, 20–22]. The decreased osteogenic potential in osteoprogenitor cells seen in CPT is independent of epidermal growth factor receptor activity, suggesting that therapeutic agents targeting this pathway may not be efficacious [23]. Animal studies further suggest decreased bone morphogenic protein (BMP) production and decreased response to BMP in periosteal cells in CPT which may further explain the weakening of bone and difficulty in achieving union following fracture [24, 25].
Classification
There are numerous classification systems for CPT, which are primarily descriptive and do not guide specific treatment, and which include the Anderson, Boyd, Crawford, Choi et al., and Paley classifications, with the Crawford classification most frequently utilized [5, 20, 26, 27, 28•]. The Crawford classification proposes 4 categories. Crawford type I CPT is the presence of anterolateral bowing of the tibia with a narrow canal secondary to increased cortical thickness. Type II patients are further divided into IIA, IIB, and IIC. Type IIA involves anterolateral bowing and widening of the tibial canal with tubulation defect. Type IIB involves anterolateral bowing and cystic lesion which signals impending fracture or healing from previous fracture. Type IIIC involves anterolateral bowing with frank fracture and pseudarthrosis with or without cystic change [26].
The El-Rosas-Paley classification system [28•] has recently been proposed to include classification driven treatment with 3 categories. Types 1 and 2, defined as a mobile pseudarthrosis with narrowed diaphyses either without or with prior, failed surgery, respectively, were treated with complete excision of adjacent periosteum, iliac crest autograft, and combined intramedullary (IM) and Ilizarov external fixation. Type 3 CPT, or those with stiff arthroses with hypertrophic diaphyses, were treated with a pre-fabricated Ilizarov external fixator for distraction and angular correction without open surgery [28•].
Natural History
Excepting severe cases in which fracture and pseudoarthrosis is seen time of birth, (Boyd type 1) or even in utero, CPT begins with anterolateral bowing of the tibia, noticeable at birth [7, 20, 25]. This typically progresses to pathologic fracture of the tibia, with or without concurrent fibular fracture, following little or no trauma, and pseudarthrosis, which can be extremely challenging to obtain union in and is subject to high risk of refracture even after union is obtained [2, 7]. Progressive diaphyseal varus deformity of the tibia can also lead to significant compensatory ankle valgus, with associated proximal fibular migration and subsequent limitations in limb function [5, 29, 30]. Special attention should be paid to the development of proximal tibial valgus and ankle valgus as there exists a significant relationship between the two [31•]. Even after union of the pathologic tibial fracture, patients may still have very limited function and significant pain in the affected limb, even in absence of refracture, and some may still undergo amputation to address this [7, 25, 32, 33]. For reasons not entirely clear, once skeletal maturity has been reached, the risk of refracture normalizes and fracture is seen after appropriately significant trauma [20].
Patients with anterolateral bowing of the tibia should be carefully examined for duplication of the ipsilateral hallux as this is recognized as a separate form of anterolateral bowing of the tibia which is benign, does not progress to pseudarthrosis, and resolves spontaneously [34–36].
Treatment
While historically, radiographic union alone has been used as the measure of successful treatment of CPT, more recent literature supports a more patient centered goal of treatment. The authors suggest that the goal of treatment of CPT should be a functional lower extremity that is not pain limited, achieved through restoring equal limb lengths, rotational profile, and alignment while protecting against refracture through the age of skeletal maturity [13•, 25, 37••, 38]. This treatment goal must be weighed against strategies including non-operative treatment prior to fracture and pseudarthrosis and operative treatment, with or without medical augmentation, following fracture and pseudarthrosis formation.
Non-operative Management
Non-operative management of CPT should be employed in the years prior to fracture and pseudarthrosis as earlier age of first fracture in CPT has been correlated inferior outcomes [39]. Bracing does not alter the natural course of the disease but does delay its course to fracture. Even following operative management, many surgeons continue to recommend bracing until the age of skeletal maturity to help mitigate risk of refracture.
Medical Management and Biologic Adjuncts
Bisphosphonates have been suggested as an adjunct to surgical treatment. Paley, in his cross-union protocol, utilizes bisphosphonate therapy pre- and post-operatively, particularly as the pre-operative bisphosphonate use is postulated to prevent post-operative resorption of autologous bone graft and improve cross-union success [13•, 40].
Evidence regarding use of recombinant bone morphogenic protein (rhBMP) is not currently conclusive. In some studies, rhBMP appears to reduce the time to union when union is achieved, but does not increase the rate of union [41–43]. While it is difficult to truly ascertain the utility of rhBMP in CPT, its use is touted as relatively safe and not prone to excessive complications [44]. Some postulate that the efficacy of rhBMP may be seen more fully in combination with medical therapy. Indeed, in a murine model, BMP-2 combined with zoledronic acid yielded greater bone formation than controls and BMP-2 alone [24]. This combination of BMP and bisphosphonates is also included in Paley’s cross-union protocol [13•, 40].
A recent study by Memeo et al. focused on the effect of bone marrow aspirate on healing in CPT. At 2 years follow-up, bone marrow aspirate concentrate sped healing by 1.7 months, which finding was significant; further study is needed to determine the effect of this increased speed of healing and perceived morbidity of CPT and its treatment [45].
Intramedullary Fixation
Rigid intramedullary (IM) fixation of CPT was first introduced by Charnley in 1956 and has remained a common surgical treatment for this condition ever since [46]. In this technique, the pseudarthrosis is resected and the fracture stabilized with IM fixation [17, 33, 46, 47•]. Both fixed length IM devices and lengthening, telescopic devices, such as the Fassier-Duval rod, are commonly used [17, 33, 47•, 48]. Currently, the use of intramedullary fixation without additional fixation methods is a less commonly employed practice. Laufer et al., reported, at mean 7.4 years follow-up, patients treated with IM fixation alone had a 60% union rate [49]. Singer and Johnston, reporting on 34 CPT patients at skeletal maturity, found a 97.1% union rate and 39.4% risk of refracture, with all but 3 refractures successfully treated with either external fixator or repeat IM rodding [47•]. An additional point of consideration when removing or exchanging IM constructs, especially in pre-operative planning, is the possibility of bony overgrown of IM fixation, particularly Fassier-Duval rods, for which custom trephines may be required for extraction [48].
External Fixation
Hinged external fixation was also a mainstay of CPT treatment initially. Similarly, to IM fixation, it is now most commonly used in combination with other fixation constructs, primarily due to concerns for refracture if IM implants are not employed to provide internal, rigid support. Nonetheless, El-Rosas recently reported on treating CPT patients with stiff hypertrophic pseudarthroses with external fixator alone without operative fixation and reported a 100% union rate and 4% refracture rate at an average 3-year follow-up [28•]. However, external fixation alone was only indicated in stiff hypertrophic pseudarthroses and these findings should not be generalized to all cases of CPT.
Combination Techniques
Most recent publications on the treatment of CPT now focus on combination techniques which commonly employ IM fixation, external fixation, and excision of the pseudarthrosis site (Fig. 1) [28, 38, 50–53]. While most of the IM fixation utilized in these constructs are traditional Fassier-Duval rods or Charnley rods, the use of titanium elastic nails (TENS) has recently been described [29]. Bone grafting is commonly performed in these combination protocols as well, with corticocancellous autograft often reported [38, 51]. With the use of combination techniques, while Ilizarov fixators are removed at time of fracture union, many authors recommend retention of IM rods to decrease risk of refracture [28•, 29, 50–52]. Primary union rates in these combination techniques ranges from 74 to 100% with reported refracture rates between 0 and 45% [28, 29, 50–52]. Shah et al. reported that use of BMP, combined IM rodding, and Ilizarov fixation were associated with significantly higher risk of treatment failure; however this finding has not been replicated in other studies [54].
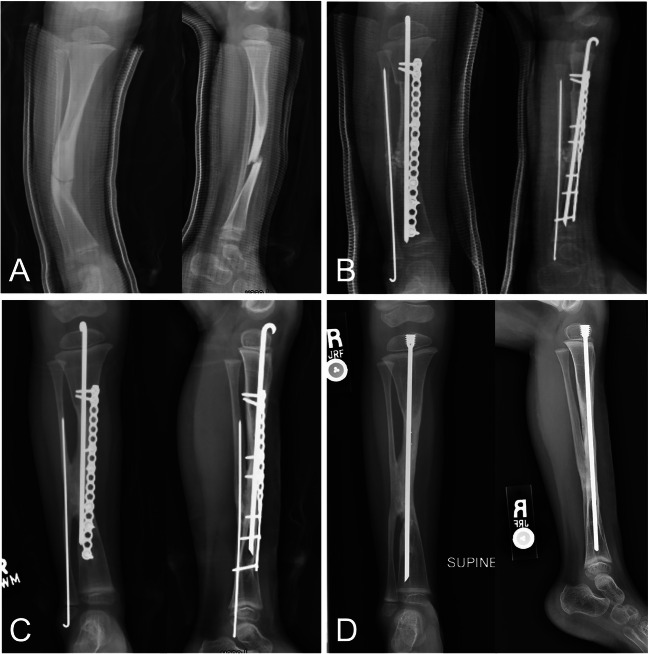
Fig. 1.
Anterior and lateral radiographic views of the tibia in a child with neurofibromatosis type 1 and anterolateral tibial bowing showing A fracture through anterior lateral tibial bow. B Reconstruction with resection of fracture/pseudoarthrosis, proximal tibia osteotomy, iliac crest bone and periosteal grafting, intramedullary and plate fixation for planned tibia and fibula cross-union. C Full healing and cross-union after reconstruction. D Rod exchange to protect area of prior fracture/pseudoarthrosis during growth
Given the diseased nature of periosteum in CPT, some protocols have focused on IM fixation, Ilizarov fixator, pseudarthrosis excision, and autogenous periosteal grafting. Unlike other recent studies, IM fixation was removed after union in one periosteal grafting protocol and retention of implants was not discussed in the other [55]. Union rates for combination fixation with periosteal grafting is between 67 and 88.2%, with refracture rate reported in only one series, with a 8.5-year refracture rate of 29.4% [53, 55].
Perhaps the ultimate culmination of combining multiple fixation techniques are the cross-bridging techniques reported by Paley et al. and Choi et al. In his technique, Paley advocates for IM rodding of both tibia and fibula, malleable plating of the tibia, an extensive autogenous bone graft spanning the two bones, rhBMP2 and zoledronic acid administered both pre- and post-operatively [37••, 40]. Choi et al. describe a similar cross-union technique, the so-called 4-in-1 osteosynthesis in which the pseudarthrosis is excised, the tibia stabilized with IM fixation which spans the ankle and circular Ilizarov fixator, the fibular pseudarthrosis is converged on the tibia, and autogenous bone graft with rhBMP-2 is applied [56]. Both techniques are reported to have 100% primary union rate with 0% refracture at greater than 7 years of follow-up [37••, 50, 56]. It is important to note that ankle spanning fixation is correlated to decreased ankle push off and that the detriment of this gait derangement must be weighed carefully when considering the necessity of ankle spanning fixation [57]. Union and refracture rate for these studies are shown in Table 1.
Table 1.
Union and refracture rates reported for reconstruction of congenital pseudoarthrosis of the tibia
| Author | Treatment | Union rate | Refracture rate |
|---|---|---|---|
| Wang et al. | Titanium elastic nails, Ilizarov fixator | 88.2% | 11.8% |
| Liu et al. | Fassier-Duval rods, Ilizarov fixator | 100% | 0% |
| Kesirreddy et al. | IM rod, Ilizarov fixator, corticocancellous bone graft | 74% | 45% |
| El-Rosasy | IM rod, Ilizarov fixator | 100% | 4% |
| Popkov et al. | IM rod, Ilizarov fixator | 100% | 0% |
| Yan et al. | IM rod, Ilizarov fixator, corticocancellous bone graft | 92.2% | Not reported |
| Kocaoglu [55] et al. | IM rod, Ilizarov fixator, periosteal graft | 88.2% | 29.4% |
| Zargarbashi et al. | IM rod, Ilizarov fixator, periosteal graft | 67% | Not reported |
| Shannon et al. | Paley cross-union | 100% | 0% |
| Liu et al. | 4-in-1 Osteosynthesis | 100% | 0% |
| Choi et al. | 4-in-1 Osteosynthesis | 100% | 0% |
Vascularized Fibular Graft and Masquelet Technique
(VFG) has remained a popular surgical treatment of CPT. Primary union rates in patients using VFG range between 90 and 100% [58–60]. Use of VFG is reported to lead to union between 2 and 4 months post-operatively [59]. Rates of tibial shaft deformity remain high, occurring in over 50% of patients, following this type of treatment and are correlated with untreated fibular pseudarthrosis [58]. Refracture rate is similarly high, between 53.8 and 57.1% in long-term follow-up [58, 60]. Regarding the timing of VFG, authors have recommended that VGF be performed prior to the age of 3 years old to limit leg length discrepancy as patients near skeletal maturity [58].
Similar to VFG is induced membrane, or Masquelet, technique. Though less commonly reported in the extant literature, induced membrane technique has been described as primarily a salvage technique in recalcitrant cases of CPT [61–63]. Patients being treated by Masquelet technique should be advised of its equivalent outcome to VFG and the high number of surgical procedures inherent to this method, 5 procedures on average, similar to VFG [63]. Even following union, procedures may be required to correct angular, rotation, or length discrepancies [61]. Patients and surgeons alike should also be aware that radiographic evidence of union after induced membrane technique may take on average, 25.3 weeks to manifest and should not be discouraged or prematurely reoperate if evidence is not seen sooner [62].
Guided Growth
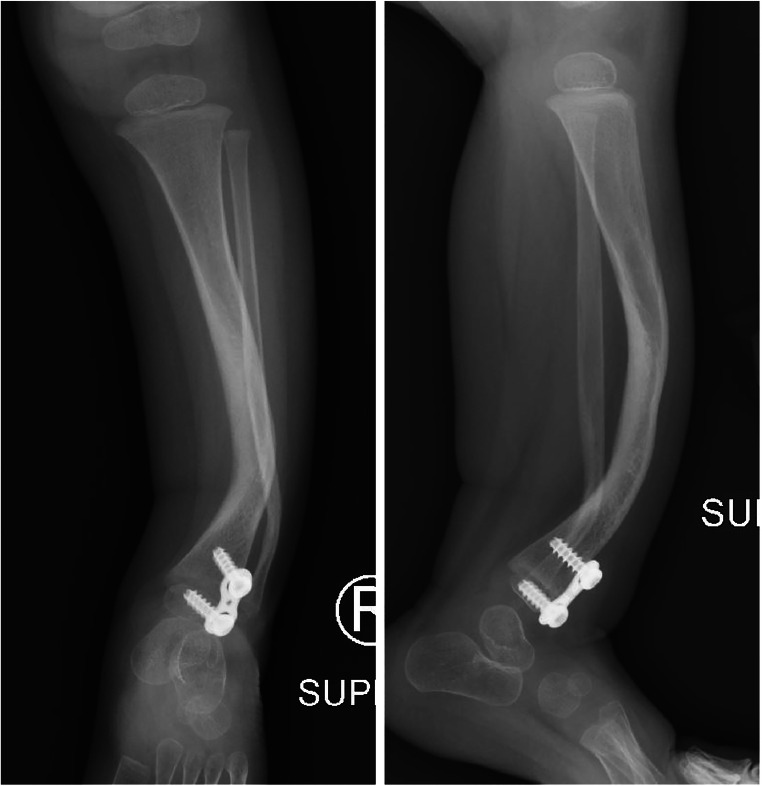
Given the difficulty in treating the fracture and pseudarthrosis in CPT, there is considerable interest in preventing fracture altogether, although this has proven difficult to achieve. Recent study, however, has published promising results on the use of guided growth to prevent progression and even improve anterolateral bowing of the tibia and thus fracture and pseudarthrosis formation (Fig. 2). This technique was first reported in a case report by Kennedy et al., who described the use of tension band plates at 33 months of age, with plates placed on the proximal medial tibia and anterolateral distal tibia. Plates were removed at 50 months of age, and the patient remained without fracture at 4-years follow-up [64]. Subsequently, Laine et al. reported a series of 10 patients treated with anterior lateral distal tibial–guided growth prior to tibial fracture and pseudarthrosis with growth modulation beginning at a mean age of 2.6 years [65••]. At a mean of 5.9 years follow-up, no patients studied had sustained fracture. The authors emphasize the need for obliquely oriented imaging to capture the plane of deformity and plan implant positioning. Further study will be required to determine whether these encouraging findings are durable through skeletal maturity.
Fig. 2.
Distal tibia anterior lateral tension band plate hemiepiphysiodesis for anterolateral tibial bowing
Limb Lengthening
Due to the pathologic nature of the affected tibiae in CPT and the needed resection of the pseudarthrosis site, limb length discrepancy (LLD) is common in CPT. The question of when to lengthen an affected limb and where to perform an osteotomy, especially given the dreaded fear of pseudarthrosis, has always been a topic of interest. One recent report proposes an average LLD of 5.44 cm [66]. There are differing opinions on timing of lengthening, with some authors advocating lengthening only after solid union of the pseudarthrosis is obtained, while others report that lengthening may be performed concurrently with treatment of the pseudarthrosis without added complication [66, 67]. Regarding location of osteotomy for lengthening, authors agree that the osteotomy should be made proximally, at least in the metaphysis, but that it can also be placed through the proximal tibial physis itself or immediately distal via subphyseal distraction osteotomy [66, 67]. The use of proximal tibial physeal or subphyseal distraction osteotomy is not found to have any higher complication rate compared to standard metaphyseal distraction osteotomy; however, it is important to note that the small number of patients studied likely represents insufficient power to definitively declare that no difference in complications exists between the two osteotomy sites [67].
Treatment of Fibular Pathology
Whilst the congenital pseudarthrosis of the tibia treatment has primarily focused on the tibia, the influence of fibular pathology cannot be ignored. Paley asserts that proximal migration of the fibula must be addressed in the treatment of CPT [13•]. Patients with an intact fibula have significantly lower incidence of proximal tibial valgus, ankle valgus, and relative shortening of the fibula relative to the tibial length compared to those with fibular pseudarthroses [31•]. Decreased fibular length correlates independently with ankle valgus [31•]. Given the significant correlation with greater ankle valgus in fibulas with pseudarthrosis, some authors recommend cross-union surgery in cases of fibular pseudarthrosis to better stabilize the fibula [53]. Finally, compared to patients with intact fibulas, patients with pseudarthrosis of the fibula were significantly more likely experience refracture (27.3%), again emphasizing the importance of treating fibular pathology when present [68].
Amputation
Once a mainstay of CPT management, amputation is less commonly employed as a primary procedure, but remains a viable option in select patients who have failed other management. Westberry et al. report that of 17 patients managed with Boyd amputation and rush rod stabilization of the pseudarthrosis or transtibial amputation above the level of pseudarthrosis, 92% of patients treated by Boyd amputation achieved union within 2 procedures and all patients functioned well with prostheses [69]. As some families may be attracted to amputation given its potential “one and done” appeal, patients must be counseled that additional procedures may still be required following amputation and transtibial amputees may experience overgrowth requiring further surgical management. A recently described pedicled composite calcaneal flap may address tibial overgrowth in CPT patients treated by transtibial amputation and obviated the need for reoperation in this patient population; however, further study is needed given the small number of patients included in the existing study [70]. Given the improved success of combined treatment approaches, we recommend that amputation be reserved for rare cases of salvage after failed reconstruction.
Conclusion
Congenital pseudarthrosis of the tibia remains an exceptionally difficult condition to treat, with both primary union and prevention of refracture being the primary obstacles. Goals of treatment should be a stable, functional limb with a concurrent goal to reduce the morbidity of frequent reoperation which can be all too common in CPT treatment. Currently, the existing literature would suggest that with a combination of pseudoarthrosis and periosteal resection, autologous bone grafting, and fixation including a combination of either IM rod and external fixation or IM rod and internal plate fixation, union rates greater than 75% and up to 100% can be expected. Refracture rate remains highly variable but can still be as high as 45% even with combination technique. Retention of IM rods may help prevent refracture. Cross-union techniques have a reported 100% union rate and zero refractures, even at greater than 7-year follow-up. VFG and Masquelet technique are valid primary and salvage treatments but require multiple operations to succeed. Future directions should include further research on guided growth, and its role in prevent fracture outright which may completely change the course of this disease.
Declarations
Conflict of Interest
Matthew J. Siebert and Christopher A. Makarewich declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Pediatric Orthopedics
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Horn J, Steen H, Terjesen T. Epidemiology and treatment outcome of congenital pseudarthrosis of the tibia. J Child Orthop. 2013;7(2):157–166. doi: 10.1007/s11832-012-0477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hefti F, Bollini G, Dungl P, Fixsen J, Grill F, Ippolito E, Romanus B, Tudisco C, Wientroub S. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000;9(1):11–15. doi: 10.1097/01202412-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Crawford AH, Jr, Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop. 1986;6(1):72–88. doi: 10.1097/01241398-198601000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Andersen KS. Occurrence of congenital tibial pseudoarthrosis in Denmark 1940-1965. Nord Med. 1971;86(47):1395. [PubMed] [Google Scholar]
- 5.El-Rosasy MA, Paley D. In: Congenital pseudarthrosis of the tibia. Limb lengthening and reconstruction surgery. Herzenberg JE, editor. CRC Press; 2006. pp. 511–520. [Google Scholar]
- 6.Vitale MG, Guha A, Skaggs DL. Orthopaedic manifestations of neurofibromatosis in children: an update. Clin Orthop Relat Res. 2002;401:107–118. doi: 10.1097/00003086-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Vander Have KL, Hensinger RN, Caird M, Johnston C, Farley FA. Congenital pseudarthrosis of the tibia. J Am Acad Orthop Surg. 2008;16(4):228–236. doi: 10.5435/00124635-200804000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Crawford AH, Schorry EK. Neurofibromatosis update. J Pediatr Orthop. 2006;26(3):413–423. doi: 10.1097/01.bpo.0000217719.10728.39. [DOI] [PubMed] [Google Scholar]
- 9.Van Royen K, Brems H, Legius E, Lammens J, Laumen A. Prevalence of neurofibromatosis type 1 in congenital pseudarthrosis of the tibia. Eur J Pediatr. 2016;175(9):1193–1198. doi: 10.1007/s00431-016-2757-z. [DOI] [PubMed] [Google Scholar]
- 10.Zhu G, Zheng Y, Liu Y, Yan A, Hu Z, Yang Y, Xiang S, Li L, Chen W, Peng Y, Zhong N, Mei H. Identification and characterization of NF1 and non-NF1 congenital pseudarthrosis of the tibia based on germline NF1 variants: genetic and clinical analysis of 75 patients. Orphanet J Rare Dis. 2019;14(1):221. doi: 10.1186/s13023-019-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlier A, Brems H, Ashbourn JM, Nica I, Legius E, Geris L. Capturing the wide variety of impaired fracture healing phenotypes in Neurofibromatosis Type 1 with eight key factors: a computational study. Sci Rep. 2016;7:20010. doi: 10.1038/srep20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson DA, Hanson H, Stevens A, Carey J, Viskochil D, Sheng X, Wheeler K, Slater H. Quantitative Ultrasound and Tibial Dysplasia in Neurofibromatosis Type 1. J Clin Densitom. 2018;21(2):179–184. doi: 10.1016/j.jocd.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 13.• Paley D. Congenital pseudarthrosis of the tibia: biological and biomechanical considerations to achieve union and prevent refracture. J Child Orthop. 2019;13(2):120–33. Reviews the pathophysiology of CPT and describes the factors important for obtaining bony union. [DOI] [PMC free article] [PubMed]
- 14.Dilogo IH, Mujadid F, Nurhayati RW, Kurniawan A. Evaluation of bone marrow-derived mesenchymal stem cell quality from patients with congenital pseudoarthrosis of the tibia. J Orthop Surg Res. 2018;13(1):266. doi: 10.1186/s13018-018-0977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mc ER. Congenital pseudo-arthrosis of the tibia; the findings in one case and a suggestion as to possible etiology and treatment. Q Bull Northwest Univ Med Sch. 1949;23(4):413–423. [PMC free article] [PubMed] [Google Scholar]
- 16.Codivilla A. On the cure of the congenital pseudoarthrosis of the tibia by means of periosteal transplantation. JBJS. 1906;2(2):163–169. [Google Scholar]
- 17.Johnston CE. Congenital pseudarthrosis of the tibia: results of technical variations in the Charnley-Williams procedure. JBJS. 2002;84(10):1799–1810. doi: 10.2106/00004623-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Ippolito E, Corsi A, Grill F, Wientroub S, Bianco P. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B. 2000;9(1):3–10. doi: 10.1097/01202412-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Cho T-J, Seo J-B, Lee HR, Yoo WJ, Chung CY, Choi IH. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. JBJS. 2008;90(12):2735–2744. doi: 10.2106/JBJS.H.00014. [DOI] [PubMed] [Google Scholar]
- 20.Boyd HB. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 1982;166:5–13. doi: 10.1097/00003086-198206000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Hermanns-Sachweh B, Senderek J, Alfer J, Klosterhalfen B, Büttner R, Füzesi L, Weber M. Vascular changes in the periosteum of congenital pseudarthrosis of the tibia. Pathol Res Pract. 2005;201(4):305–312. doi: 10.1016/j.prp.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Yu H, Liu Y, Ye W, Zhu G, Yan A, Tan Q, Mei H. Serum-derived exosomes from neurofibromatosis type 1 congenital tibial pseudarthrosis impaired bone by promoting osteoclastogenesis and inhibiting osteogenesis. Exp Biol Med (Maywood). 2021;246(2):130–141. doi: 10.1177/1535370220962737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tahaei SE, Couasnay G, Ma Y, Paria N, Gu J, Lemoine BF, Wang X, Rios JJ, Elefteriou F. The reduced osteogenic potential of Nf1-deficient osteoprogenitors is EGFR-independent. Bone. 2018;106:103–111. doi: 10.1016/j.bone.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindeler A, Ramachandran M, Godfrey C, Morse A, McDonald M, Mikulec K, Little DG. Modeling bone morphogenetic protein and bisphosphonate combination therapy in wild-type and Nf1 haploinsufficient mice. J Orthop Res. 2008;26(1):65–74. doi: 10.1002/jor.20481. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson DA, Little D, Armstrong L, Crawford AH, Eastwood D, Friedman JM, Greggi T, Gutierrez G, Hunter-Schaedle K, Kendler DL, Kolanczyk M, Monsell F, Oetgen M, Richards BS, Schindeler A, Schorry EK, Wilkes D, Viskochil DH, Yang FC, Elefteriou F. Approaches to treating NF1 tibial pseudarthrosis: consensus from the Children’s Tumor Foundation NF1 Bone Abnormalities Consortium. J Pediatr Orthop. 2013;33(3):269–275. doi: 10.1097/BPO.0b013e31828121b8. [DOI] [PubMed] [Google Scholar]
- 26.Crawford AH. Neurofibromatosis in children. Acta Orthop Scand. 1986;57(sup218):8-60. [PubMed]
- 27.Andersen K. Congenital pseudarthrosis of the leg. Late results. J Bone Joint Surg Am Vol. 1976;58(5):657–662. doi: 10.2106/00004623-197658050-00013. [DOI] [PubMed] [Google Scholar]
- 28.• El-Rosasy MA. Congenital pseudarthrosis of the tibia: the outcome of a pathology-oriented classification system and treatment protocol. J Pediatr Orthop B. 2020;29(4):337-47. Presents a treatment based classification system with outcomes of the treatment protocol. [DOI] [PubMed]
- 29.Wang X, Shi L, Zhang R, Wang W, Wang F, Wang M, Xu Z, Zuo R, Xu J, Kang Q. Efficacy of the "Eiffel tower" double titanium elastic nailing in combined management of congenital pseudarthrosis of the tibia: preliminary outcomes of 17 cases with review of literature. BMC Musculoskelet Disord. 2021;22(1):490. doi: 10.1186/s12891-021-04382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah H, Doddabasappa SN, Joseph B. Congenital pseudarthrosis of the tibia treated with intramedullary rodding and cortical bone grafting: a follow-up study at skeletal maturity. J Pediatr Orthop. 2011;31(1):79–88. doi: 10.1097/BPO.0b013e318202c45d. [DOI] [PubMed] [Google Scholar]
- 31.• Deng H, Mei H, Wang E, Li Q, Zhang L, Canavese F, et al. The association between fibular status and frontal plane tibial alignment post-union in congenital pseudarthrosis of the tibia. J Child Orthop. 2021;15(3):261-9. Evaluates the effect of fibular healing on deformity recurrence in CPT. [DOI] [PMC free article] [PubMed]
- 32.Khan T, Joseph B. Controversies in the management of congenital pseudarthrosis of the tibia and fibula. Bone Joint J. 2013;95(8):1027–1034. doi: 10.1302/0301-620X.95B8.31434. [DOI] [PubMed] [Google Scholar]
- 33.Dobbs MB, Rich MM, Gordon JE, Szymanski DA, Schoenecker PL. Use of an intramedullary rod for treatment of congenital pseudarthrosis of the tibia: a long-term follow-up study. JBJS. 2004;86(6):1186–1197. doi: 10.2106/00004623-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Kitoh H, Nogami H, Hattori T. Congenital anterolateral bowing of the tibia with ipsilateral polydactyly of the great toe. Am J Med Genet. 1997;73(4):404–407. doi: 10.1002/(SICI)1096-8628(19971231)73:4<404::AID-AJMG6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Manner H, Radler C, Ganger R, Grossbötzl G, Petje G, Grill F. Pathomorphology and treatment of congenital anterolateral bowing of the tibia associated with duplication of the hallux. J Bone Joint Surg Br Vol. 2005;87(2):226–230. doi: 10.1302/0301-620X.87B2.15132. [DOI] [PubMed] [Google Scholar]
- 36.Bressers M, Castelein RM. Anterolateral tibial bowing and duplication of the hallux: a rare but distinct entity with good prognosis. J Pediatr Orthop B. 2001;10(2):153–157. [PubMed] [Google Scholar]
- 37.•• Shannon CE, Huser AJ, Paley D. Cross-union surgery for congenital pseudarthrosis of the tibia. Children. 2021;8(7):547. Describes a relatively large series using arguably the most successful surgical reconstruction technique to date. [DOI] [PMC free article] [PubMed]
- 38.Kesireddy N, Kheireldin RK, Lu A, Cooper J, Liu J, Ebraheim NA. Current treatment of congenital pseudarthrosis of the tibia: a systematic review and meta-analysis. J Pediatr Orthop B. 2018;27(6):541–550. doi: 10.1097/BPB.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 39.Murray HH, Lovell WW. Congenital pseudarthrosis of the tibia. A long-term follow-up study. Clin Orthop Relat Res. 1982;166:14–20. doi: 10.1097/00003086-198206000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Paley D. Congenital Pseudarthrosis of the Tibia: Combined Pharamcologic and Surgical 1 Treatment Using Biphosphonate Intravenous Infusion and Bone Morphogenic Protein 2 with Periosteal and Cancellous Autogenous Bone Grafting, Tibio-fibular cross union, 3 Intramedullary Rodding and External Fixation. 4. 2012.
- 41.Richards BS, Oetgen ME, Johnston CE. The use of rhBMP-2 for the treatment of congenital pseudarthrosis of the tibia: a case series. J Bone Joint Surg Am. 2010;92(1):177–185. doi: 10.2106/JBJS.H.01667. [DOI] [PubMed] [Google Scholar]
- 42.Richards BS, Anderson TD. rhBMP-2 and Intramedullary Fixation in Congenital Pseudarthrosis of the Tibia. J Pediatr Orthop. 2018;38(4):230–238. doi: 10.1097/BPO.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 43.Hissnauer TN, Stiel N, Babin K, Rupprecht M, Hoffmann M, Rueger JM, Stuecker R, Spiro AS. Bone morphogenetic protein-2 for the treatment of congenital pseudarthrosis of the tibia or persistent tibial nonunion in children and adolescents: A retrospective study with a minimum 2-year follow-up. J Mater Sci Mater Med. 2017;28(4):60. doi: 10.1007/s10856-017-5868-9. [DOI] [PubMed] [Google Scholar]
- 44.Stiel N, Hissnauer TN, Rupprecht M, Babin K, Schlickewei CW, Rueger JM, Stuecker R, Spiro AS. Evaluation of complications associated with off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in pediatric orthopaedics. J Mater Sci Mater Med. 2016;27(12):184. doi: 10.1007/s10856-016-5800-8. [DOI] [PubMed] [Google Scholar]
- 45.Memeo A, Verdoni F, Minoli CF, Voto A, D'Amato RD, Formiconi F, et al. Effectiveness of bone marrow aspirate concentrate (BMAC) as adjuvant therapy in the surgical treatment of congenital pseudoarthrosis of the tibia: a retrospective comparative study. J Biol Regul Homeost Agents. 2020;34(4 Suppl. 3):431-40. Congress of the Italian Orthopaedic Research Society. [PubMed]
- 46.Charnley J. Congenital pseudarthrosis of the tibia treated by intramedullary nail. J Bone Joint Surg Am. 1956;38-a(2):283-90. [PubMed]
- 47.• Singer D, Johnston CE. Congenital Pseudarthrosis of the Tibia: Results, at Skeletal Maturity, of the Charnley-Williams Procedure. JB JS Open Access. 2019;4(2):e0004. Long term study demonstrating the importance of maintaining intramedullary rod fixation to prevent refracture. [DOI] [PMC free article] [PubMed]
- 48.McClure PK, Franzone JM, Herzenberg JE. Challenges with Fassier-Duval rod exchanges in congenital pseudarthrosis of the tibia: explant roadblock and solution. J Pediatr Orthop B. 2022;31(1):e95–e100. doi: 10.1097/BPB.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 49.Laufer A, Frommer A, Gosheger G, Roedl R, Schiedel F, Broeking JN, et al. Reconstructive Approaches in Surgical Management of Congenital Pseudarthrosis of the Tibia. J Clin Med. 2020;9(12). [DOI] [PMC free article] [PubMed]
- 50.Liu Y, Yang G, Zhu G, Tan Q, Wu J, Liu K, Tang J, Mei H. Application of the "telescopic rod" in a combined surgical technique for the treatment of congenital pseudarthrosis of the tibia in children. J Orthop Surg Res. 2021;16(1):532. doi: 10.1186/s13018-021-02649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan A, Mei HB, Liu K, Wu JY, Tang J, Zhu GH, Ye WH. Wrapping grafting for congenital pseudarthrosis of the tibia: A preliminary report. Medicine (Baltimore). 2017;96(48):e8835. doi: 10.1097/MD.0000000000008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popkov D, Popkov A, Dučić S, Lazović M, Lascombes P. Combined technique with hydroxyapatite coated intramedullary nails in treatment of anterolateral bowing of congenital pseudarthrosis of tibia. J Orthop. 2020;19:189–193. doi: 10.1016/j.jor.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zargarbashi R, Bagherpour A, Keshavarz-Fathi M, Panjavi B, Bagherpour ZM. Prognosis of congenital pseudarthrosis of the tibia: effect of site of tibial pseudarthrososis and fibular involvement. J Pediatr Orthop. 2021. [DOI] [PubMed]
- 54.Shah H, Joseph B, Nair BVS, Kotian DB, Choi IH, Richards BS, Johnston C, Madhuri V, Dobbs MB, Dahl M. What factors influence union and refracture of congenital pseudarthrosis of the tibia? A multicenter long-term study. J Pediatr Orthop. 2018;38(6):e332–e3e7. doi: 10.1097/BPO.0000000000001172. [DOI] [PubMed] [Google Scholar]
- 55.Kocaoğlu M, Eralp L, Bilen FE, Civan M. Congenital pseudarthrosis of the tibia: Results of circular external fixation treatment with intramedullary rodding and periosteal grafting technique. Acta Orthop Traumatol Turc. 2020;54(3):245–254. doi: 10.5152/j.aott.2020.03.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi IH, Lee SJ, Moon HJ, Cho T-J, Yoo WJ, Chung CY, Park MS. “4-in-1 osteosynthesis” for atrophic-type congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2011;31(6):697–704. doi: 10.1097/BPO.0b013e318221ebce. [DOI] [PubMed] [Google Scholar]
- 57.Karol LA, Haideri NF, Halliday SE, Smitherman TB, Johnston CE. 2nd. Gait analysis and muscle strength in children with congenital pseudarthrosis of the tibia: the effect of treatment. J Pediatr Orthop. 1998;18(3):381–386. doi: 10.1097/01241398-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 58.El-Gammal TA, El-Sayed A, Kotb MM, Saleh WR, Ragheb YF, Refai OA, et al. Crawford type IV congenital pseudarthrosis of the tibia: treatment with vascularized fibular grafting and outcome at skeletal maturity. J Pediatr Orthop. 2021;41(3):164–170. doi: 10.1097/BPO.0000000000001751. [DOI] [PubMed] [Google Scholar]
- 59.Soldado F, Barrera-Ochoa S, Bergua-Domingo JM, Domenech P, Corona PS, Knorr J. Bone nonunion management in children with a vascularized tibial periosteal graft. Microsurgery. 2020;40(7):760–765. doi: 10.1002/micr.30655. [DOI] [PubMed] [Google Scholar]
- 60.Van Den Heuvel SCM, Winters HAH, Ultee KH, Zijlstra-Koenrades N, Sakkers RJB. Combined massive allograft and intramedullary vascularized fibula transfer: the Capanna technique for treatment of congenital pseudarthrosis of the tibia. Acta Orthop. 2020;91(5):605–610. doi: 10.1080/17453674.2020.1773670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollon T, Sales de Gauzy J, Pham T, Thévenin Lemoine C, Accadbled F. Salvage of congenital pseudarthrosis of the tibia by the induced membrane technique followed by a motorised lengthening nail. Orthop Traumatol Surg Res. 2018;104(1):147-153. [DOI] [PubMed]
- 62.Meselhy MA, Elhammady AS, Singer MS. Outcome of induced membrane technique in treatment of failed previously operated congenital pseudarthrosis of the tibia. Orthop Traumatol Surg Res. 2020;106(5):813–818. doi: 10.1016/j.otsr.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 63.Vigouroux F, Mezzadri G, Parot R, Gazarian A, Pannier S, Chotel F. Vascularised fibula or induced membrane to treat congenital pseudarthrosis of the Tibia: a multicentre study of 18 patients with a mean 9.5-year follow-up. Orthop Traumatol Surg Res. 2017;103(5):747–753. doi: 10.1016/j.otsr.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Kennedy J, O'Toole P, Baker JF, Moore D. Guided growth: a novel treatment for anterolateral bowing of the tibia. J Pediatr Orthop. 2017;37(5):e326–e3e8. doi: 10.1097/BPO.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 65.•• Laine JC, Novotny SA, Weber EW, Georgiadis AG, Dahl MT. Distal Tibial Guided Growth for Anterolateral Bowing of the Tibia: Fracture May Be Prevented. J Bone Joint Surg Am. 2020;102(23):2077-86. Case series of a novel technique for fracture prevention and deformity correction involving minimally invasive guided growth. [DOI] [PubMed]
- 66.Balci H, Bayram S, Pehlivanoglu T, Anarat FB, Eralp L, Şen C, et al. Effect of lengthening speed on the quality of callus and complications in patients with congenital pseudarthrosis of tibia. Int Orthop. 2021;45(6):1517–1522. doi: 10.1007/s00264-021-05011-7. [DOI] [PubMed] [Google Scholar]
- 67.Jang WY, Choi YH, Park MS, Yoo WJ, Cho TJ, Choi IH. Physeal and subphyseal distraction osteogenesis in atrophic-type congenital pseudarthrosis of the tibia: efficacy and safety. J Pediatr Orthop. 2019;39(8):422–428. doi: 10.1097/BPO.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 68.Liu YX, Mei HB, Zhu GH, He RG, Liu K, Tang J, Wu JY, Ye WH, Hu X, Tan Q, Yan A, Huang SX, Tan XQ, Lei T. Relationship between postoperative complications and fibular integrity in congenital pseudarthrosis of the tibia in children. World J Pediatr. 2017;13(3):261–266. doi: 10.1007/s12519-016-0074-2. [DOI] [PubMed] [Google Scholar]
- 69.Westberry DE, Carpenter AM, Tisch J, Wack LI. Amputation outcomes in congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2018;38(8):e475–ee81. doi: 10.1097/BPO.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 70.Mongon MLD, Ribera FC, de Souza AMA, Sposito AL, Belangero WD, Livani B. Pedicled sensate composite calcaneal flap in children with congenital tibial pseudoarthrosis. J Pediatr Orthop. 2017;37(4):e271–e2e6. doi: 10.1097/BPO.0000000000000942. [DOI] [PubMed] [Google Scholar]