Abstract
Plasma lipopolysaccharide (LPS)-binding protein (LBP) and membrane CD14 function to enhance the responses of monocytes to low concentrations of endotoxin. Surprisingly, recent reports have suggested that LBP or CD14 may be dispensable for macrophage responses to low concentrations of LPS or may even exert an inhibitory effect in the case of LBP. We therefore investigated whether LBP and CD14 participated in the response of mouse peritoneal exudate macrophages (PEM) to LPS stimulation. In the presence of a low amount of plasma (<1%) or of recombinant mouse or human LBP, PEM were found to respond to low concentrations of LPS (<5 to 10 ng/ml) in an LBP- and CD14-dependent manner. However, tumor necrosis factor production (not interleukin-6 production) by LPS-stimulated PEM was reduced when cells were stimulated in the presence of higher concentrations of plasma or serum (5 or 10%). Yet, the inhibitory effect of plasma or serum was not mediated by LBP. Taken together with previous results obtained with LBP and CD14 knockout mice in models of experimental endotoxemia, the present data confirm a critical part for LBP and CD14 in innate immune responses of both blood monocytes and tissue macrophages to endotoxins.
Lipopolysaccharide (LPS) has been shown to play a central role in the pathogenesis of severe sepsis and septic shock caused by gram-negative bacteria. LPS stimulates monocytes and macrophages to release proinflammatory mediators, such as cytokines. In circulating monocytes, this pathway is under the control of two proteins, plasma LPS-binding protein (LBP) and membrane CD14. Indeed, neutralizing anti-CD14 and anti-LBP antibodies have been shown to inhibit LPS-induced proinflammatory responses in vitro and in vivo (8, 9, 20, 21). Similar results have been obtained for mice with deletion of the CD14 or LBP genes (10, 14). When LPS is bound to LBP, manyfold smaller concentrations of LPS can activate monocytes through CD14 (7, 11, 30). Recent observations help in understanding the mechanism by which LPS-LBP engagement of CD14 leads to monocyte activation, since CD14 lacks a transmembrane domain. The recently identified Toll-like receptors (TLRs), and in particular TLR4, are likely candidates to transmit the LPS signal from CD14 to the cell (4, 12, 27, 29). At high concentrations of LPS, neither LBP nor CD14 is required for activation of circulating monocytes (7, 11, 30). It is not yet clear whether under these circumstances LPS stimulation occurs through TLRs or by another as yet unidentified pathway.
Whereas most studies indicating an important contribution of plasma LBP and membrane-bound CD14 in LPS-induced activation have been performed with circulating monocytes, other reports have suggested that macrophages may be activated by LPS without the participation of serum LBP or CD14 (13, 15, 16, 22, 26, 32). Furthermore, in contrast with what was shown to occur in human monocytes, purified or recombinant human LBP (rhLBP) was found to inhibit rather than to enhance tumor necrosis factor (TNF) production by mouse peritoneal exudate macrophages (PEM) stimulated with LPS (2, 3, 25). These unexpected observations raised some doubts about the well-documented contribution of LBP in amplifying the response of monocytes to LPS.
We thus investigated further the role played by LBP and CD14 in the activation of PEM by LPS. Experiments were performed with (i) recombinant mouse LBP (rmLBP), (ii) plasma from LBP knockout mice, (iii) plasma or serum of various sources (human, mouse, calf), and (iv) a neutralizing anti-CD14 monoclonal antibody.
MATERIALS AND METHODS
Sources of plasma and serum.
Heparinized human plasma and human serum derived from clotted whole blood were obtained from healthy human volunteers and were aliquoted and frozen at −80°C. Eight- to ten-week-old female OF1, NMRI, BALB/c, and C57BL/6J mice (Iffa Credo, Lyon, France) were bled to obtain heparinized plasma or serum, which was aliquoted and kept frozen at −80°C. Plasma or serum was also obtained from LBP+/− heterozygous and LBP−/− mice generated on a BALB/c background (14) (a kind gift of C. Schütt, Greifswald, Germany).
Plasma and serum were equivalent in their capacity to enhance LPS-induced responses, as shown by the fact that similar dilutions of plasma or serum induced similar TNF responses of monocytes in the presence of LPS (data not shown). Heat-inactivated (56°C for 45 min) plasma and serum were used in some experiments. LBP is stable under these conditions (7). Normal plasma (not heat-inactivated) was used in all experiments reported in the tables and figures.
rmLBP was cloned in baculovirus using the cotransfection method with Baculo-Gold (Pharmingen, San Diego, Calif.) and was expressed in SF9 insect cells and cultivated in Excell medium, as described previously (19, 20). Experiments were performed with cell culture supernatant or with purified rmLBP. In selected experiments, supernatants of insect SF9 cells transfected with the empty plasmid were used as controls for the LBP-containing cell culture supernatant. CHO cells transfected with the human LBP gene and secreting rhLBP were a kind gift of P. S. Tobias (Scripps, La Jolla, Calif.). The concentration of LBP in the supernatants of SF9 and CHO cells was measured by enzyme-linked immunosorbent assay, as previously described (19).
Antibodies.
The neutralizing anti-mouse LBP monoclonal antibody (MAb) (clone M330-19) and the nonneutralizing anti-mouse LBP MAb (clone M306-5) have been previously described (20). These two rat MAbs (both immunoglobulin G2a isotypes) were purified by protein G chromatography, dialyzed into phosphate-buffered saline, and stored at −80°C. The LPS content of the anti-LBP MAbs was <5 pg/μg of protein.
4C1 is a newly developed rat antibody that neutralizes mouse CD14. 4C1 was found to block the binding of LPS to mouse macrophage RAW264.7 and to reduce LPS-mediated production of cytokines of these cells (1).
Macrophage isolation and culture conditions.
OF1 mice were injected intraperitoneally with 3 ml of 4% autoclaved Brewer thioglycolate (Difco Laboratories, Detroit, Mich.). PEM were collected 3 or 5 days after injection and were washed and used without further purification. More than 90% of the cells were macrophages by morphological examination. All experiments were carried out with both 3- and 5-day PEM, which gave similar results. For the sake of simplicity, only data concerning 3-day PEM are reported.
Cells (50,000/well) were plated into 96-well culture plates (Costar, Cambridge, Mass.) and were stimulated with the indicated concentrations of LPS (from Escherichia coli O111; Sigma, St. Louis, Mo.) in the presence of plasma or serum diluted in RPMI 1640 medium supplemented with l-glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml). Cells were incubated for 6 h at 37°C in 5% CO2 in a final volume of 200 μl. In some experiments, anti-LBP MAbs or anti-CD14 MAbs were added at a concentration of 10 μg/ml 10 min before adding LPS. In other experiments purified rmLBP, SF9 insect cells supernatant containing LBP, or control SF9 supernatant with the empty plasmid was added to the cell culture medium. Similarly, CHO cell supernatants secreting rhLBP or control supernatants were added to PEM. Supernatants of stimulated PEM were collected, and concentrations of TNF and of interleukin-6 (IL-6) were measured as described below.
TNF and IL-6 determination.
PEM culture supernatants were assayed for TNF by bioassay using WEHI clone 13 as targets and were assayed for IL-6 by bioassay using 7TD1 cells as targets, as described previously (11).
Presence of CD14.
Membrane-bound CD14 was assessed by incubating PEM with the anti-CD14 MAb 4C1 (1 μg/ml) or with an irrelevant MAb (1 μg/ml). After washing, cells were further incubated with goat F(ab)′2 anti-rat immunoglobulin G labeled with fluorescein isothiocyanate (Sigma), and binding of the antibodies was measured by flow cytometry.
Data and statistics.
Statistical analyses were done using the nonparametric analysis of variation (ANOVA) test on ranks for multiple comparisons.
RESULTS
Contribution of plasma in the response of PEM to endotoxin.
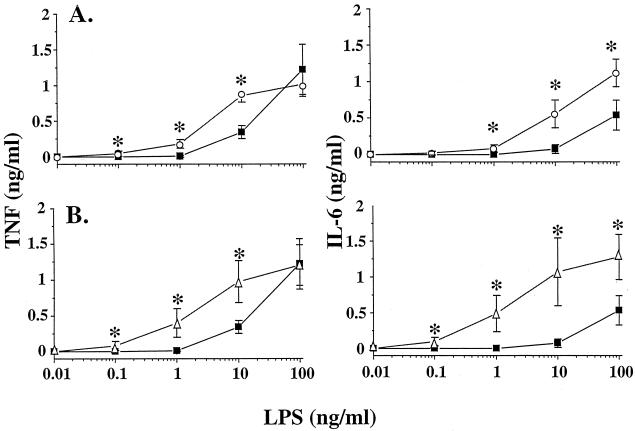
To examine the role of plasma in the response of macrophages to LPS, we stimulated PEM with increasing concentrations of LPS. The cells were cultured in plasma-free RPMI medium or in RPMI medium containing 0.2 or 1% mouse or human plasma. Cell culture supernatants were assessed for TNF and IL-6. In the absence of plasma, 10 ng of LPS/ml was necessary to induce cytokine production. The addition of 0.2% human or mouse plasma (Fig. 1) or 1% plasma (data not shown) resulted in increased production of TNF or IL-6 over that measured in plasma-free medium. The addition of 0.2% plasma markedly potentiated TNF or IL-6 production, especially at low concentrations of LPS. Under these conditions, PEM started to produce cytokines at concentrations of LPS as low as 100 pg/ml. At a concentration of 100 ng of LPS/ml, the production of TNF was similar for cells incubated in plasma-free medium or in medium enriched with 0.2% plasma. However, the addition of 0.2% plasma potentiated the IL-6 response at high concentrations of LPS.
FIG. 1.
TNF and IL-6 production by PEM stimulated with LPS in the presence of low doses of plasma. PEM (50,000 cells/well) were stimulated for 6 h with LPS in the presence of RPMI medium with no plasma (squares), RPMI medium containing 0.2% human plasma (circles, panel A), or 0.2% autologous mouse plasma (triangles, panel B). Data are the mean ± SD of five different experiments run in duplicates. ∗, P < 0.05 by ANOVA.
To determine if other sources of plasma or serum would also augment cytokine production after LPS challenge, experiments were repeated comparing native versus heat-inactivated plasma or serum from calves, humans, or mice. In all conditions, 0.2 or 1% of plasma or serum increased the production of TNF or IL-6 (data not shown), suggesting that this effect could be mediated by a protein such as heat-stable LBP.
Role of LBP present in plasma in the response of PEM to endotoxin.
We then investigated whether LBP could account for the enhancing effect of plasma. A neutralizing rat anti-mouse LBP MAb (clone M330-19), shown to prevent the binding of LPS to LBP (20), was used to inhibit LBP activity. A nonneutralizing anti-LBP MAb (clone M306-5) was used as the control (20). The addition of clone M306-5 to cells stimulated with LPS in the presence of 1% mouse plasma did not affect the production of TNF or of IL-6 (data not shown). The addition of 1% autologous (OF1) mouse plasma induced an increase of the TNF or IL-6 production over controls in plasma-free conditions, an effect that was largely inhibited by the neutralizing anti-LBP MAb (clone M330-19) (Fig. 2). LBP blockade completely suppressed cytokine production induced by 5 ng of LPS/ml. However, LBP blockade had no effect when the cells were stimulated with a high concentration of LPS (50 ng/ml). Similar results were obtained with the plasma or serum of OF1, NMRI, BALB/c or C57BL/6J mice (data not shown).
FIG. 2.
Effect of neutralization of mouse plasma LBP on the cytokine response of PEM to LPS. PEM (50,000 cells/well) were stimulated for 6 h with LPS in the presence of RPMI medium containing 1% autologous mouse plasma (A) or RPMI medium with no plasma (B) and in the presence of nonneutralizing anti-LBP MAb (clone M306-5) (squares) or neutralizing anti-LBP MAb (clone M 330-19) (circles). Data are the mean ± SD of five different experiments run in duplicates. ∗, P < 0.05 by ANOVA.
To further evaluate the contribution of LBP in the LPS-induced activation of PEM, similar experiments were performed with plasma of mice deficient in LBP (14). Plasma (1%) of heterozygous LBP+/− and knockout LBP−/− mice was added to PEM, and cells were then stimulated with increasing concentrations of LPS (Fig. 3). As anticipated, plasma of LBP+/− mice but not of LBP−/− mice increased cytokine production. This effect was observed at all LPS concentrations. In the presence of LBP−/− plasma, cytokine production by LPS-stimulated PEM was similar to that of cells cultivated in plasma-free conditions. The difference between these two plasmas was indeed due to the presence of LBP, as demonstrated by the suppression of enhanced cytokine production upon blocking LBP activity with the neutralizing anti-LBP MAb (clone M330-19) in LBP+/− plasma (data not shown). To ensure that the absence of LBP in LBP−/− plasma was the sole factor responsible for the lack of enhancing effect in the response of PEM to LPS, we reconstituted LBP−/− plasma with 10 ng of rmLBP/ml (corresponding to a 1% plasma concentration, as the normal mouse plasma level is 1 μg/ml). The addition of exogenous LBP to the LBP−/− plasma reconstituted the cytokine-enhancing effect that was blocked by neutralizing anti-LBP MAb (clone M330-19) (Table 1).
FIG. 3.
Effect of heterozygous LBP+/− mice and LBP−/− plasma on the cytokine response by LPS-stimulated PEM. PEM (50,000 cells/well) were stimulated for 6 h with LPS in the presence of RPMI medium containing either 1% LBP+/− plasma (squares) or 1% LBP−/− plasma (circles). Data are the mean ± SD of five different experiments run in duplicates. ∗, P < 0.05 by ANOVA.
TABLE 1.
Adding recombinant mouse LBP to LBP−/− plasma augments cytokine production by PEM stimulated with LPSa
| Conditions | Mean ± SDb for:
|
|
|---|---|---|
| TNF (pg/ml)c | IL-6 (pg/ml)c | |
| Plasma-free medium | 30 ± 15 | 20 ± 10 |
| 1% LBP−/− plasma | 20 ± 5 | 30 ± 10 |
| 1% LBP−/− plasma + rmLBP | 1,500 ± 120c | 1,300 ± 250c |
| 1% LBP−/− plasma + rmLBP + anti-LBP | 40 ± 25 | 35 ± 25 |
PEM (50,000 cells/well) were stimulated for 6 h with 5 ng of LPS/ml in the presence of 1% LBP−/− plasma, 1% LBP−/− plasma plus 10 ng of rmLBP/ml, or 1% LBP−/− plasma plus 10 ng of rmLBP/ml and 10 μg of neutralizing anti-LBP MAb (clone M330-19)/ml.
Data are from three different experiments run in duplicates.
P < 0.05 by ANOVA.
To determine whether higher doses of LBP in plasma might have an inhibitory effect on the LPS-induced production of TNF, as suggested by previous studies (2, 3, 18, 25), we compared the cytokine production of PEM stimulated with LPS in the presence of 1% autologous plasma or 1% autologous plasma spiked with 100 ng of rmLBP/ml (i.e., a 10-fold increase of LBP concentration). As shown in Table 2, the addition of a 10-fold excess of LBP marginally affected the response, whereas blockade of LBP activity with anti-LBP MAb suppressed the response to LPS.
TABLE 2.
Effect of anti-LBP MAb or rmLBP on TNF production by LPS-stimulated PEM cultured with 1% autologous plasmaa
| Conditions | Mean ± SDb for TNF (pg/ml) |
|---|---|
| Plasma-free medium | 30 ± 20 |
| 1% plasma | 1,100 ± 250c |
| 1% plasma + anti-LBP MAb | 20 ± 25 |
| 1% plasma + rmLBP | 700 ± 350c |
PEM (50,000 cells/well) were stimulated for 6 h with 5 ng of LPS/ml in plasma-free medium or in the presence of (i) 1% autologous plasma, (ii) 1% autologous plasma plus 10 μg of anti-LBP MAb (clone M330-19)/ml, or (iii) 1% autologous OF1 plasma plus 100 ng of rmLBP/ml.
Data are from two different experiments run in duplicates.
P < 0.05 by ANOVA.
Role of recombinant LBP in the response of PEM to endotoxin.
Having shown that LBP controls the cytokine response of PEM under conditions of low doses of both plasma and LPS, we next investigated the role of rmLBP in culture conditions carried out in the absence of plasma. Various doses of purified rmLBP or dilutions of a titrated supernatant of SF9 cells containing LBP or the empty vector (used as a control) were added to PEM, which were then stimulated with LPS (Fig. 4). In the presence of plasma-free medium or of control SF9 supernatant, PEM did not produce TNF after stimulation with 1 ng of LPS/ml. rmLBP (purified or cell culture supernatant) enhanced the TNF response by LPS-stimulated PEM. No difference in the level of TNF produced was obtained with concentrations of rmLBP from 3 pg/ml to 100 ng/ml. A high dose of LBP did not inhibit TNF production.
FIG. 4.
Effect of recombinant mouse LBP on TNF production by PEM stimulated with LPS. PEM (50,000 cells/well) were stimulated for 6 h with 1 ng of LPS/ml in the presence of increasing concentrations of purified rmLBP (squares) or of SF9 supernatant containing known concentrations of LBP (circles). TNF was not detected in cultures containing control SF9 supernatant or RPMI medium (data not shown). Data are the mean ± SD of four different experiments run in duplicates.
Recombinant human and mouse LBPs were found to be equipotent. PEM were stimulated with 1 ng of LPS/ml in the presence of 3 ng/ml of titrated supernatants containing rmLBP or rhLBP. TNF production (the mean ± the standard deviation [SD] of three different experiments) was 2,550 ± 865 using rmLBP and 2,620 ± 1,145 using rhLBP.
Role of CD14 in the response of PEM to endotoxin.
PEM express high levels of membrane CD14, as revealed by the binding of the anti-CD14 MAb 4C1. By flow cytometry, 3-day PEM that have reacted with an irrelevant MAb express 3.5 fluorescence units, whereas cells that have reacted with the anti-CD14 MAb 4C1 express 32.5 ± 5.6 fluorescence units (mean of five different determinations).
PEM were pretreated with the neutralizing anti-CD14 MAb 4C1 and were then stimulated with increasing concentrations of LPS in plasma-free medium or in medium containing 1% mouse plasma. As shown in Fig. 5, anti-CD14 MAb completely suppressed TNF and IL-6 responses of cells stimulated with 1 ng of LPS/ml irrespective of the culture conditions (with or without plasma). In the presence of plasma, the anti-CD14-mediated inhibition of cytokine production was less effective at 5 ng of LPS/ml and was ineffective at 100 ng of LPS/ml. However, in plasma-free conditions, CD14 blockade suppressed cytokine production even at the highest concentrations of LPS.
FIG. 5.
Effect of anti-CD14 MAb on cytokine production by PEM stimulated with LPS. PEM (50,000 cells/well) were pretreated with 10 μg of the anti-CD14 MAb/ml (circles) or with an irrelevant MAb (squares) and were stimulated for 6 h with LPS in the presence of RPMI medium containing 1% OF1 autologous mouse plasma (A) or RPMI medium with no plasma (B). Data are the mean ± SD of five different experiments run in duplicates. ∗, P < 0.05 by ANOVA.
Response of PEM in the presence of high concentrations of plasma.
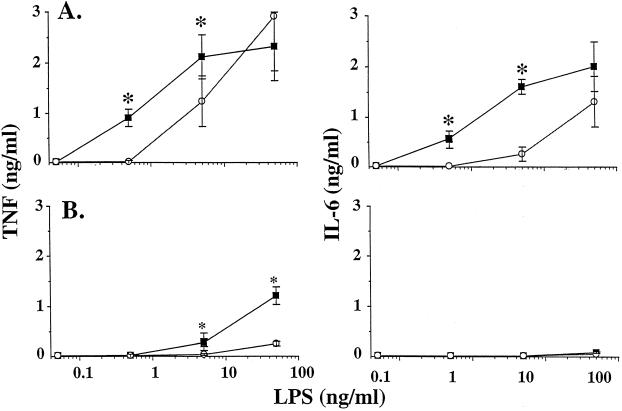
Whereas the addition of low doses of plasma (1% or less) enhanced the LPS-induced cytokine responses of PEM, the addition of 5 or 10% plasma decreased the TNF response as compared to that obtained with plasma-free medium. This was observed with human plasma and mouse plasma of various sources (autologous OF1 plasma as in Fig. 6 or heterologous NMRI, BALB/c, or C57BL/6J plasma [data not shown]). In contrast to TNF, IL-6 production was similar whether the cells were stimulated with LPS in the presence or in the absence of 5% human or mouse plasma.
FIG. 6.
TNF and IL-6 production by PEM stimulated with LPS in the presence of high doses of plasma. PEM (50,000 cells/well) were stimulated for 6 h with LPS in the presence of RPMI medium with no plasma (squares), RPMI medium containing 5% human plasma (circles, panel A) or RPMI medium containing 5% autologous mouse plasma (triangles, panel B). Data are the mean ± SD of five different experiments run in duplicates. ∗, P < 0.05 by ANOVA.
Finally, to investigate whether LBP played a role in experiments carried out with high doses of plasma, we stimulated PEM in the presence of 10% mouse plasma, which was shown to suppress TNF production of PEM stimulated by LPS. As shown in Table 3, the addition of 200 ng of LBP/ml (which represents a fourfold increase in the concentration of LBP over that present in 10% normal mouse plasma) did not restore the LPS-induced TNF response of PEM stimulated in the absence of LBP. The addition of the neutralizing anti-LBP antibody also did not modify the response of PEM to LPS, suggesting that the inhibitory effect of a high concentration of plasma was not mediated by LBP.
TABLE 3.
Effect of anti-LBP MAb or rmLBP on TNF production by LPS-stimulated PEM cultured with 10% autologous plasmaa
| Conditions | Mean ± SDb for TNF (pg/ml) |
|---|---|
| 1% plasma | 1,100 ± 250c |
| 10% plasma | 20 ± 20 |
| 10% plasma + anti-LBP MAb | 20 ± 15 |
| 10% plasma + rmLBP | 20 ± 25 |
PEM (50,000 cells/well) were stimulated for 6 h with 5 ng of LPS/ml in the presence of (i) 1% (as control) or 10% autologous plasma, (ii) 10% autologous plasma plus 10 μg of anti-LBP MAb (clone M330-19)/ml, or (iii) 10% autologous plasma plus 100 ng rmLBP/ml.
Data are from two different experiments run in duplicates. Cells stimulated with LPS in plasma-free medium released less than 30 pg/ml of TNF.
P < 0.05 by ANOVA.
DISCUSSION
Circulating monocytes and tissue macrophages play a central role in the mediation of the biological effects of LPS, releasing a large array of mediators and cytokines. The present findings provide further evidence that LBP and CD14 mediate responses of activated peritoneal exudate macrophages to low concentrations of LPS, as has been described for circulating monocytes. In agreement with previous results obtained with circulating monocytes, we observed that the response of PEM was LBP- and CD14-dependent at low concentrations of LPS (<5 to 10 ng/ml). Indeed, recombinant LBP or the presence of low doses of plasma (<1%) increased the production of TNF and of IL-6 by LPS-stimulated PEM. Under similar plasma conditions, antibody-mediated blockade of LBP or CD14 suppressed the LPS response. However, it was also clear that PEM responded to high concentrations of LPS in the absence of LBP or CD14, as previously reported for other monocytic cell types (7, 11, 30).
CD14 blockade was more effective in plasma-free conditions than in the presence of plasma at high LPS concentrations. No explanation is available for the observation that CD14 blockade still diminished the TNF response in plasma-free conditions but not in plasma conditions, when the cells were stimulated with 50 ng of LPS/ml. Experiments were done with 10 μg of anti-CD14 MAb/ml, which is a large excess of MAb compared to soluble CD14 present in 1% plasma as well to CD14 expressed in macrophages. The fact that the TNF response was abolished at 1 ng of LPS/ml and was reduced at 5 ng of LPS/ml in both plasma-free and plasma conditions indicates that the problem was not the concentration of the anti-CD14 MAb.
It is now clearly established that CD14 and LBP are important partners in the LPS response of a large variety of monocytes and macrophages, including circulating human, rabbit, and calf alveolar macrophages (17, 28, 31), human or rabbit peritoneal or alveolar macrophages (6, 23, 24), and mouse whole blood (7). Macrophages that are derived from different tissues or that are at different stages of differentiation might exhibit variable responses to LPS. In fact, several investigators have hypothesized the existence of signaling pathways other than LBP/CD14 in macrophages. Recent reports have challenged the concept that serum proteins (LBP) and CD14 are required for the LPS-induced responses of macrophages: (i) both CD14- and LBP-dependent and CD14- and LBP-independent responses have been implicated in the response of bovine macrophages to LPS (5, 16); (ii) an established human monocytic cell line, derived from THP-1 cells, was found to respond to LPS when grown in the absence of serum proteins for more than 20 generations (22); (iii) CHO cells not expressing CD14 but transfected with CD11c/CD18 respond to LPS, although at higher concentrations of LPS than CHO cells transfected with CD14 (13); and (iv) in the absence of serum, mouse thioglycolate-elicited peritoneal macrophages were found to express high levels of TNF and IL-1β mRNA upon stimulation with a wide range of low concentrations of LPS (25). In fact, the addition of rhLBP to these cells did not enhance but actually decreased TNF and IL-1β mRNA while increasing that of interferon-inducible protein 10 (IP-10) (25). This observation was confirmed by Amura et al., who showed that rhLBP suppressed in a dose-dependent manner LPS-induced TNF production but not NO production by mouse peritoneal exudate macrophages (2, 3). These findings suggested the possibility that the modulatory properties of LBP and CD14 vary with the cellular targets, with the source of LBP or with the mediator under investigation. In the present study, no difference was observed between rmLBP and rhLBP, a finding which contrasted with the inhibitory role of rhLBP (2, 3, 25). Different PEM (from OF1 mice in the present study versus C3H/OuJ or C3HeB/FeJ mice) or different preparations of LPS (rough versus smooth) could account for these differences. All these data illustrate the difficulty of studying thioglycolate-induced PEM, all the more since various preparations of commercial thioglycolate may induce various populations of PEM. The time at which elicited macrophages were harvested (3- or 5-day PEM) did not play a significant role, as observed in the present study and in previous reports (2, 3, 25).
Yet, serum or plasma LBP was quite effective in enhancing cytokine production in response to low concentrations of LPS. Importantly, in the present study, TNF production was undetectable in serum-free medium under low LPS conditions and the addition of plasma or purified LBP allowed TNF production. Mouse plasma contains 1 to 2 μg of LBP/ml (8). Thus, the present experiments showing a potentiating effect of 0.2 or 1% mouse plasma were conducted with concentrations of LBP in the range of 4 to 20 ng/ml. Identical results were obtained with similar concentrations of rmLBP. rmLBP did not exert an inhibitory effect at concentrations as high as 100 ng/ml (corresponding to the concentration of LBP present in 10% plasma). However, in the presence of 5 and 10% plasma, the TNF response of PEM to LPS was reduced and was not influenced by the addition of exogenous rmLBP or by the blockade of endogenous LBP with anti-LBP antibody. Thus, factors other than LBP are clearly involved in the suppression of LPS-induced TNF response at high doses of plasma. The mechanisms underlying these inhibitory effects remain to be identified. Of note, the TNF but not the IL-6 response of PEM to LPS was reduced when cells were stimulated in the presence of 5% plasma or serum.
It could be speculated that if circulating monocytes are adapted to respond to LPS in the presence of 100% plasma, tissue macrophages and, in particular, PEM may not be capable of responding to LPS in the presence of high doses of plasma, at least ex vivo. During severe acute-phase responses, LBP concentrations did not exceed 50 ng/ml in the peritoneal cavity (our unpublished observations). Similarly, protein concentrations were approximately 1% of those measured in plasma. This indicates that in vitro studies with macrophages are likely more relevant when macrophages are stimulated in the presence of low amounts of plasma. This also suggests that in vivo PEM are in a situation in which mechanisms of responses are LBP and CD14 dependent.
In summary, the present study indicates that the response of PEM to low concentrations of LPS is LBP and CD14 dependent. These observations confirm and extend previous results obtained in mouse models of endotoxemia, for which it is not yet clear whether circulating monocytes or tissue macrophages are the major targets of LPS. Mice treated with anti-LBP antibodies, LBP knockout mice, and CD14 knockout mice were shown to be resistant to LPS-induced cytokine production and resistant to injections of low concentrations of LPS (8–10, 14, 21). Yet all these mice succumb to high concentrations of LPS. These observations appear to rule out an important contribution of LBP- and CD14-independent mechanisms in the activation of monocytes and macrophages by low concentrations of LPS (up to 100 ng/mouse). However, they do not rule out the possibility that cells may be activated by LBP- and CD14-independent mechanisms in the presence of high concentrations of LPS (from 1 μg to 1 mg/mouse).
ACKNOWLEDGMENTS
This study was supported by grant no. 32-55829.98 to D.H. and grants no. 32-489/6.96 and no. 32-49129.96 to T.C. from the Fonds National Suisse de la Recherche.
We thank M. Knaup for technical assistance.
REFERENCES
- 1.Adachi Y, Satokawa C, Saeki M, Ohno N, Tamura H, Tanaka S, Yadomae T. Inhibition by a CD14 monoclonal antibody of lipopolysaccharide binding to murine macrophages. J Endotoxin Res. 1999;5:139–146. [Google Scholar]
- 2.Amura C R, Chen L C, Hirohashi N, Lei M G, Morrison D C. Two functionally independent pathways for lipopolysaccharide-dependent activation of mouse peritoneal macrophages. J Immunol. 1998;159:5079–5083. [PubMed] [Google Scholar]
- 3.Amura C R, Kamei T, Ito N, Soares M J, Morrison D C. Differential regulation of lipopolysaccharide (LPS) activation pathways in mouse macrophages by LPS-binding proteins. J Immunol. 1998;161:2552–2560. [PubMed] [Google Scholar]
- 4.Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 5.Dean D F, Bochsler P N, Caroll R C, Olchowy F W, Neilsen N R, Slauson D O. Signaling pathways for tissue factor expression in lipopolysaccharide-stimulated bovine alveolar macrophages. Am J Vet Res. 1998;59:445–451. [PubMed] [Google Scholar]
- 6.Dentener M A, Bazil V, Von Asmuth E J U, Ceska M, Buurman W A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–2891. [PubMed] [Google Scholar]
- 7.Gallay P, Carrel S, Glauser M P, Barras C, Ulevitch R J, Tobias P S, Baumgartner J D, Heumann D. Purification and characterization of murine lipopolysaccharide-binding protein. Infect Immun. 1993;61:378–383. doi: 10.1128/iai.61.2.378-383.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallay P, Heumann D, Le Roy D, Barras C, Glauser M P. Lipopolysaccharide-binding protein as a major plasma protein responsible for endotoxemic shock. Proc Natl Acad Sci USA. 1993;90:9935–9938. doi: 10.1073/pnas.90.21.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallay P, Heumann D, Le Roy D, Barras C, Glauser M P. Mode of action of anti-lipopolysaccharide binding protein (LBP) antibodies for prevention of endotoxemic shock in mice. Proc Natl Acad Sci USA. 1994;91:7922–7926. doi: 10.1073/pnas.91.17.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Sliver J, Stewart C L, Goyert S M. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 11.Heumann D, Gallay P, Barras C, Zaech P, Ulevitch R J, Tobias P S, Glauser M P, Baumgartner J D. Control of LPS binding and LPS-induced TNF secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–3512. [PubMed] [Google Scholar]
- 12.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 13.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack R S, Fan X L, Bernheiden M, Rune G, Ehlers M, Weber A, Kirsch G, Mentel R, Fürll B, Freudenberg M, Schmitz G, Stelter F, Schütt C. Lipopolysaccharide-binding protein is required to combat a Gram-negative bacterial infection. Nature. 1997;389:742–745. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 15.Jahr T G, Sundan A, Lichenstein H S, Espevik T. Influence of CD14, LBP and BPI in the monocyte response to LPS of different polysaccharide chain length. Scand J Immunol. 1995;42:119–127. doi: 10.1111/j.1365-3083.1995.tb03634.x. [DOI] [PubMed] [Google Scholar]
- 16.Jungi T W, Sager H, Adler H, Brcic M, Pfister H. Serum factors, cell membrane CD14, and β2 integrins are not required for activation of bovine macrophages by lipopolysaccharide. Infect Immun. 1997;65:3577–3584. doi: 10.1128/iai.65.9.3577-3584.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khemlani L S, Yang Z, Bochsler P N. Identification and characterization of a bovine lipopolysaccharide-binding protein. J Leukoc Biol. 1994;56:784–791. doi: 10.1002/jlb.56.6.784. [DOI] [PubMed] [Google Scholar]
- 18.Lamping N, Dettmer R, Schröder W J, Pfeil D, Hallatschek W, Schumann R R. LPS-binding protein protects mice from septic shock caused by LPS or Gram-negative bacteria. J Clin Investig. 1998;101:2065–2071. doi: 10.1172/JCI2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lengacher S, Jongeneel C V, Le Roy D, Lee J D, Kravtchenko V, Ulevitch R J, Glauser M P, Heumann D. Reactivity of murine and human recombinant LPS-binding protein (LBP) with LPS and Gram negative bacteria. J Inflamm. 1996;47:165–172. [PubMed] [Google Scholar]
- 20.Le Roy D, Di Padova F, Tees R, Lengacher S, Landmann R, Glauser M P, Calandra T, Heumann D. Monoclonal antibodies to murine lipopolysaccharide (LPS)-binding protein (LBP) protect mice from lethal endotoxemia by blocking either the binding of LPS to LBP or the presentation of LPS/LBP complexes to CD14. J Immunol. 1999;162:7454–7460. [PubMed] [Google Scholar]
- 21.Leturcq D, Moriarty A M, Talbott G, Winn R K, Martin T R, Ulevitch R J. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Investig. 1996;98:1533–1538. doi: 10.1172/JCI118945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynn W A, Liu Y, Golenbock D T. Neither CD14 nor serum is absolutely necessary for activation of mononuclear phagocytes by bacterial lipopolysaccharide. Infect Immun. 1993;61:4452–4461. doi: 10.1128/iai.61.10.4452-4461.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin T R, Mathison J C, Tobias P S, Leturcq D J, Moriarty A M, Maunder R J, Ulevitch R. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J Clin Investig. 1992;90:2209–2219. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathison J C, Wolfson E, Steinemann S, Tobias P S, Ulevitch R J. Lipopolysaccharide (LPS) recognition in macrophages. Participation of LPS-binding protein and CD14 in LPS-induced adaptation in rabbit peritoneal exudate macrophages. J Clin Investig. 1993;92:2053–2059. doi: 10.1172/JCI116801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perera P Y, Qureshi N, Christ W J, Stütz P, Vogel S N. Lipopolysaccharide and its analog antagonists display differential factor dependencies for induction of cytokine in murine macrophages. Infect Immun. 1998;66:2562–2569. doi: 10.1128/iai.66.6.2562-2569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera P Y, Vogel S N, Detore G R, Haziot A, Goyert S M. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol. 1997;158:4422–4429. [PubMed] [Google Scholar]
- 27.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton P, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 28.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 29.Shimazu R, Akashi S, Ogata H, Nagai Y F K, Miyake K, Imoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulevitch R J, Tobias P S. Recognition of endotoxin by cells leading to transmembrane signaling. Curr Biol. 1994;6:125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 31.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 32.Wurfel M M, Monks B G, Ingalls R R, Dedrick R L, Delude R, Zhou D, Lamping N, Schumann R R, Thieringer R, Fenton M J, Wright S D, Golenbock D. Targeted deletion of the lipopolysaccharide (LPS)-binding protein gene leads to profound suppression of LPS responses ex vivo, whereas in vivo responses remain intact. J Exp Med. 1997;186:2051–2056. doi: 10.1084/jem.186.12.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]