Abstract
Background:
Prodromal multiple system atrophy (MSA) has been characterized mainly by retrospective chart reviews. Direct observation and tracking of prodromal markers in MSA have been very limited
Objective:
To report the baseline characteristics and evolution of prodromal markers of MSA as they were prospectively measured in patients with idiopathic/isolated REM sleep behavior disorder (iRBD)
Methods:
Patients with iRBD were evaluated as part of a comprehensive protocol repeated annually. The protocol included assessment of motor, sleep, psychiatric, and autonomic symptoms supplemented by motor examination, quantitative motor testing, neuropsychological examination, orthostatic blood pressure measurement, and tests of olfaction and color vision. Patients who eventually developed MSA were described and compared with those who phenoconverted to Lewy body disease (Parkinson’s disease and dementia with Lewy bodies).
Results:
Of 67 phenocoverters, 4 developed MSA-P and 63 developed Lewy body disease. An additional 2 MSA-C patients were seen at baseline, already with cerebellar signs. Compared to those with Lewy body disease, those with MSA-P were younger, had less severe loss of tonic REM sleep atonia, more insomnia symptoms, and better olfaction. Clinically-evident autonomic dysfunction was not invariable in prodromal stages, often developing proximate to or after motor phenoconversion. Of the autonomic symptoms, genitourinary dysfunction was the first to develop in all cases. Olfaction and cognition remained normal throughout the prodromal and clinical disease course, in clear contrast to patients with Lewy body disease.
Conclusion:
Prodromal MSA progresses rapidly, often without substantial autonomic dysfunction, and with preserved olfaction and cognition throughout its prodromal course.
Keywords: Multiple system atrophy, prodromal
INTRODUCTION
Multiple system atrophy (MSA), like all neurodegenerative conditions, has a prodromal stage, namely a period in which some clinical signs or symptoms of disease are evident but are not yet at threshold for clinical diagnosis. The ability to directly measure this stage has been extremely limited, mainly because there are few methods to reliably detect prodromal MSA. This represents a critical unmet need, as it will be crucial to intervene as early as possible with neuroprotective therapy once this becomes available.
Most of what is known about prodromal MSA comes from retrospective recall or chart review of clinical symptoms among those with clinical MSA [1]. Predominant symptoms of the prodromal state include autonomic failure and REM sleep behavior disorder (RBD). Most studies of prodromal MSA have come from autonomic laboratories; studies have suggested that approximately 4–20% of patients diagnosed with pure autonomic failure will develop MSA over the subsequent 4–10 years [2–5]. However, autonomic failure is not the only prodromal presentation of MSA. Large observational studies have found that that approximately 4–5% of patients with idiopathic/isolated RBD (iRBD) will ultimately develop MSA [6], providing a second avenue to directly examine prodromal MSA.
Retrospective surveys of RBD occurrence in MSA have been performed; approximately 30–40% of patients with MSA retrospectively recognize RBD symptoms prior to disease onset, with most of these noting that RBD was the initial symptom [7] (note that many more may have RBD but be unaware). Studies have suggested that the presence of RBD anticipating MSA also predicts poor prognosis, including earlier death [7, 8]. However, studies prospectively tracking prodromal MSA patients from iRBD have been extremely limited. One observational study found that MSA patients had normal olfaction at baseline, in distinction to those who developed Lewy body disease (i.e., Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) [9]. This study only assessed baseline measures and did not describe the evolution over time of prodromal MSA. Over the last 17 years in our center, we have been systematically tracking iRBD patients using a prospective annual follow-up protocol. During this period, we have directly observed 4 patients develop MSA-P from their prodromal state, plus two with MSA-C who already had mild cerebellar findings. We describe here the evolution of clinical and biomarker features of these patients, compare them to those who developed PD and DLB, and discuss the implications of our findings for development of potential prodromal MSA criteria.
METHODS
All patients were enrolled in an ongoing prospective study of iRBD, which has been described in detail elsewhere [10, 11]. Briefly, all patients met criteria for polysomnography (PSG)-confirmed iRBD, defined with standard ICSD-III criteria [12], and were free of parkinsonism or dementia at baseline. Patients were then followed on an annual basis, with a systematic protocol that included motor markers (Unified Parkinson Disease Rating Scale, timed-up and go, alternate tap test, Purdue Pegboard), cognitive evaluation (Montreal Cognitive Assessment and an extensive neuropsychological battery [13]), clinical measures of autonomic function (orthostatic, urinary, erectile, and bowel symptoms on the Unified Multiple System Atrophy Rating Scale [14], systolic blood pressure drop measured manually in the supine and standing position after 1 min), special senses (olfaction with the cross-cultural 12-item version of the University of Pennsylvania Smell Identification Test (UPSIT) test [15], and color discrimination with the Farnsworth-Munsell 100 Hue test (FM-100) [16]), other sleep measures (Insomnia severity Index [17], Epworth sleepiness scale [18], Pittsburgh Sleep Quality Index [19], PSG indices of REM sleep atonia [20, 21]), and depression and anxiety measures (Beck depression and Anxiety Inventories [22, 23]). Other additional diagnostic procedures including neuroimaging [24] and skin biopsy [25] were more recently added to the protocol and assessed on a subset. All participants provided written informed consent and ethics approval was obtained from the local research ethics board.
Patients were then annually assessed with the same protocol and examined for development of defined neurodegenerative syndromes. Outcomes of interest included dementia for parkinsonism (first by UK brain bank [26], then by Movement Disorders Society criteria [27]) or dementia (using DLB criteria [28, 29]). If patients developed parkinsonism, the most likely diagnosis was defined according to best clinical impression, based upon all diagnostic information available at final clinical visit.
RESULTS
Four patients developed MSA-P, all without parkinsonism at baseline examination. Details of each case are provided.
Case descriptions
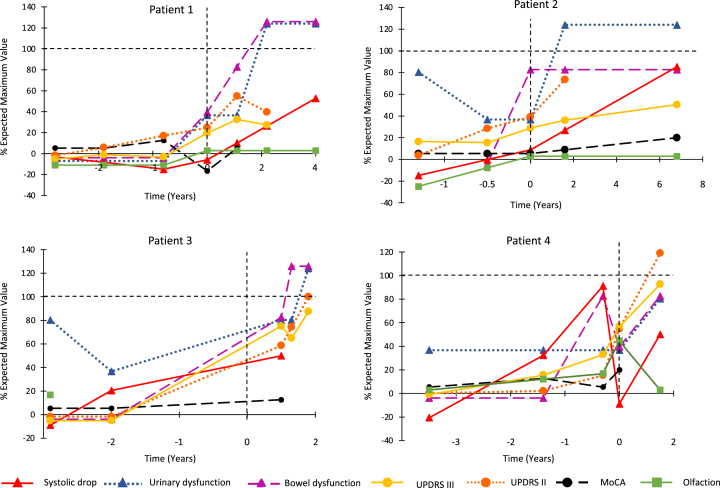
Please see Table 1 for details of all baseline variables and Fig. 1 for changes over time.
Table 1.
Description of baseline measures in patients who developed MSA-P
| Variable | Case 1 | Case 2 | Case 3 | Case 4 |
| Sex | M | F | M | F |
| Duration of RBD symptoms | 4 | 2 | 6 | 6 |
| Prodromal Interval Baseline to MSA (y) | 2.5 | 1.3 | 2.8 | 2.6 |
| Family History | ||||
| Parkinsonism | No | No | No | No |
| Dementia | No | Yes | No | Yes |
| RBD Symptoms | No | No | No | No |
| Sleep Variables | ||||
| Tonic REM % | 43 | 45 | 25 | 52 |
| Phasic REM % | 34 | 39 | 14 | 32 |
| Apnea-Hypopnea Index | 0.9 | 12.9 | N/A | 0.8 |
| Other Sleep Symptoms (self-report) | Insomnia | Insomnia | Insomnia | Mild Somnolence |
| Insomnia Severity Index | 15 | 23 | 18 | 9 |
| Epworth Sleepiness Scale | 3 | 7 | 7 | 9 |
| Pittsburgh Sleep Quality Index | 10 | 13 | 10 | 6 |
| Motor Variables | ||||
| UPDRS Part III | 1 | 9 | 0 | 0 |
| UPDRS Part II | 2 | 3 | 0 | 0 |
| Alternate Tap Test (normal ∼> 175) | 239 | 197 | 194 | 162 |
| Purdue PegBoard (normal ∼> 10) | 12 | 13 | 13 | 11.5 |
| Timed Up and Go (s) | 5 | 7 | 5.5 | 8.5 |
| Cognition | ||||
| MoCA / 30 | 27 | 27 | 27 | 27 |
| MCI – neuropsychologic exam | Normal | Normal | Normal | MCI multiple domain |
| Autonomic function | ||||
| Orthostatic Symptom Score | 1 | 0 | 0 | 0 |
| Systolic Blood Pressure drop (mmHg) | 2 | –2 | 0 | –4 |
| Urinary Dysfunction Score | 0 | 2 | 2 | 1 |
| Erectile Dysfunction Score | 0 | N/A | 1 | N/A |
| Constipation Score | 0 | 0 | 0 | 0 |
| Bowel movement Frequency / week | 7 | 7 | 7 | 10 |
| Beck Depression Inventory | N/A | 9 | 10 | 11 |
| Beck Anxiety Inventory | 9 | 2 | 1 | N/A |
| UPSIT result | 11 (Normal) | 12 (Normal) | 9 (Normal) | 10 (Normal) |
| Color Vision (FM-100 score) | Normal (8) | Abnormal (164) | Abnormal (168) | Normal (128) |
MoCA, Montreal Cognitive Assessment; MCI, mild cognitive impairment; UPSIT, University of Pennsylvania Smell Identification Test (12-item cross-cultural version); FM-100, Farnsworth-Munsell 100 Hue test; UPDRS, Unified Parkinson Disease Rating Scale; RBD, REM sleep behavior disorder; MSA, multiple system atrophy.
Fig. 1.
Combined progression trajectory of motor and non-motor manifestations from prodromal stages. For each marker, the 0 point is set as the normal control values, selected from our cohort study. The 100% indicates severe abnormality of the marker, as assessed as the worst 1 standard deviation of patients with advanced Parkinson’s disease and RBD, taken from a previous study [31].
Case 1 was a man who presented with a clinical history of RBD for 4 years prior to polysomnography. Baseline evaluation revealed normal motor function, cognition, and olfaction. Regarding autonomic findings, he had only equivocal orthostatic symptoms, without objective blood pressure drop. Over the following 3 years, the evaluation remained entirely normal, with his final pre-diagnostic visit showing a UPRDS of 0, no orthostatic hypotension, no erectile dysfunction, a Montreal Cognitive Assessment (MoCA) of 26, and normal olfaction. Of note, he had a skin biopsy [25], which was negative and MRI 6 months prior to conversion showed a right swallow-tail sign [30], with no other abnormalities.
Six months after the previous normal examination, he called to request urgent follow-up for rapid deterioration. Upon this evaluation, UPDRS was now 12, with left sided rigidity and bradykinesia. He now had new erectile dysfunction, urinary frequency with mild retention symptoms, and constipation. There was still no orthostatic hypotension (blood pressure 143/78 lying to 144/78 standing). He received levodopa with clear, but moderate benefit that only became evident at doses of 600 mg per day. Over the next 3 years, he has required high doses of levodopa for symptom control (1500 mg per day) and experienced on-off fluctuations and dyskinesia (dyskinesia include the face). Balance has remained normal. As of last visit, he had evident orthostatic hypotension (systolic drop = 20 mm Hg at 1 min, despite taking fludrocortisone), severe urinary retention requiring catherization (prostate examination was normal), severe bowel dysfunction often requiring manual disimpaction because of sphincter dyssynergia, and complete erectile dysfunction. There was severe bulbar dysfunction, with frequent choking. Exam revealed polyminimyoclonus and brisk reflexes. Cognition and olfaction have remained normal throughout.
Case 2 was a woman who presented with a clinical history of RBD for 2 years. On baseline examination, UPDRS was 9, with mild increase in bradykinesia scores, with no rigidity, tremor, or postural instability. However, quantitative motor tests were normal, as was cognition, olfaction, and orthostatic blood pressure. After a 1.3-year follow-up parkinsonism progressed, and at diagnosis, UPDRS was 15. At diagnosis, there was no orthostatic drop. However, urinary and fecal incontinence developed, and a sphincter EMG suggested findings consistent with MSA. MRI was unremarkable (this did not include neuromelanin or susceptibility weighted imaging). She received levodopa with symptomatic benefit, including fluctuations. She experienced facial dyskinesia/dystonia as a side effect. Postural instability with repeated falls developed 2 years after diagnosis. She was followed clinically at another institution but returned for examination 6 years post diagnosis (she has since died). At this time there was a 32 mmHg systolic drop, and she had severe urinary retention requiring catheterization. She continued to have response to levodopa with facial dystonia/dyskinesia as a side effect. Olfaction and cognition were normal throughout.
Case 3 was a man who presented after a clinical history of RBD for 6 years. Baseline motor, olfaction, orthostatic, and cognitive examinations were normal. The following year, evaluation was unchanged except for a 10 mm HG systolic drop. After this visit, he stated that he felt too well to continue follow-up. However, three years later he returned because of a symptomatic deterioration that had started one year prior. At this time, he had developed urinary frequency and retention, severe erectile dysfunction, and constipation. Orthostatic blood pressure drop was 20 mm Hg without symptoms of orthostatic hypotension. MRI demonstrated an equivocal hot cross bun sign. UPDRS was 39, with rigidity, bradykinesia, and postural instability, without rest tremor. Levodopa was started with moderate benefit and mild wearing off. As of last visit he had developed repeated falls (which had started within the first year) with severe swallowing difficulty and a UPDRS of 45 despite 1500 mg levodopa per day, severe orthostatic symptoms, constipation and erectile dysfunction, with urinary incontinence and retention. Cognition and olfaction remained normal.
Case 4 was a woman who presented with a 6-year history of RBD symptoms. Baseline evaluation revealed UPDRS of 0, with slight slowing observed on the alternate tap test. Neuropsychological exam suggested possible multiple domain mild cognitive impairment (MCI) with impaired attention, executive functions, and verbal learning (although subjective cognitive complaints were equivocal, and there were substantial language and education barriers). There was no orthostatic blood pressure drop, and olfaction was normal. The following year, UPDRS was 9, with subtle bradykinesia and no rigidity or postural stability, with subtle urinary frequency and an orthostatic blood pressure drop of 14. One year afterwards, UPDRS increased to 16, this time with equivocal rigidity, an orthostatic drop of 24 mm Hg and new mild constipation. The following year, UPDRS was 30, now with clear rigidity, allowing full diagnosis of unequivocal parkinsonism. MRI was unremarkable. There was modest levodopa response, with some off periods and no dyskinesia. However, she became wheelchair bound within 2 years. In the final visit before death, UPRDS was 46, with severe orthostatic hypotension symptoms. She had both urinary and fecal incontinence. Inspiratory stridor was present. Cognition testing revealed no substantial worsening over the years (MoCA = 25, Mini-Mental State Examination = 28 with equivocal cognitive complaint) and olfaction remained normal.
MSA-cerebellar
Of note, two cases with previously-undiagnosed possible MSA-C have also been seen at our center. However, in both cases, they did not meet inclusion criteria, because both had mild cerebellar signs on baseline examination. Without defined thresholds for clinically-significant cerebellar dysfunction (unlike is the case for parkinsonism and dementia), these cases cannot be considered to have clearly been prodromal. Therefore, they are not included in this study. However, of note, one of the cases had clear autonomic dysfunction and orthostatic hypotension at baseline (64 mm Hg systolic drop). 5 years later, the patient was wheel-chair bound. The second had clear cerebellar ataxia at baseline with urinary frequency (no retention) and severe erectile dysfunction but had no orthostatic hypotension (with blood pressure measurements at 1 and 3 min standing). One year later, there was clear progression of cerebellar findings, with a new asymptomatic 19-mm Hg systolic drop.
Prodromal MSA versus Lewy body disease
We compared the patients developing MSA-P to 63 iRBD patients who developed Lewy body disease (Table 2). Noting the limited statistical power and exploratory nature of the analysis, we found that the MSA patients at baseline were significantly younger, had less severe loss of tonic REM sleep atonia, more insomnia symptoms, and normal olfaction. In terms of autonomic function, MSA convertors at baseline actually had less orthostatic blood pressure systolic drop and less constipation but had more urinary dysfunction. Point estimates of measures of both cognitive and motor testing suggested better baseline function in MSA, although differences were not significant.
Table 2.
Baseline variables in MSA versus Lewy body disease
| Variable | MSA | Lewy body | p |
| (n = 4) | disease | (uncorrected) | |
| (N = 63) | |||
| Age | 54.3±7.8 | 69.5±7.9 | < 0.001 |
| Sex (% female) | 50 | 30.5 | 0.58 |
| Duration of RBD symptoms | 4.5±1.9 | 7.4±8.3 | 0.49 |
| Age of RBD onset | 49.8±7.6 | 61.1±10.0 | 0.03 |
| Follow-up Interval baseline to disease (y) | 2.3±0.7 | 3.3±2.7 | 0.46 |
| Total interval symptoms to disease | 6.8±2.6 | 10.7±8.5 | 0.37 |
| Family History (first degree relative) | |||
| Parkinsonism (%) | 0 | 7.9 | 1.0 |
| Dementia (%) | 50 | 14.3 | 0.12 |
| RBD Symptoms (%) | 0 | 7.9 | 1.0 |
| Sleep Variables | |||
| Tonic REM % | 42±12 | 62±26 | 0.13 |
| Phasic REM % | 30±11 | 38±18 | 0.32 |
| Apnea-Hypopnea Index | 4.7±6.7 | 11.5±16.1 | 0.47 |
| Insomnia Severity Index | 16.3±5.9 | 9.7±5.2 | 0.024 |
| Epworth Sleepiness Scale | 6.5±2.5 | 6.6±4.6 | 0.96 |
| Pittsburgh Sleep Quality Index | 9.8±3.1 | 7.3±3.9 | 0.93 |
| Motor Variables | |||
| UPDRS Part III | 2.5±4.4 | 6.7±5.2 | 0.12 |
| UPDRS Part II | 1.3±1.5 | 2.2±2.2 | 0.52 |
| Alternate Tap Test (normal ∼> 175) | 196±32 | 164±32 | 0.053 |
| Purdue PegBoard (normal ∼> 10) | 12.4±0.75 | 10.5±2.1 | 0.066 |
| Timed Up and Go (s) | 6.5±1.6 | 7.2±1.6 | 0.41 |
| Cognition | |||
| MoCA | 27.0±0 | 24.7±3.3 | 0.16 |
| MCI – neuropsychologic exam (%) | 25 | 47 | 0.61 |
| Autonomic function | |||
| Orthostatic Symptom Score | 0.25±0.5 | 0.35±0.6 | 0.73 |
| Systolic Blood Pressure drop (mmHg) | –1.0±2.6 | 17.0±16 | 0.024 |
| Urinary Dysfunction Score | 1.25±0.96 | 0.40±0.58 | < 0.01 |
| Erectile Dysfunction Score | 2±0.75 | 1.6±1.35 | 0.36 |
| (n = 2) | (n = 45) | ||
| Constipation Score | 0±0 | 0.92±0.90 | 0.046 |
| Bowel movement Frequency (per week) | 7.8±1.5 | 6.3±2.8 | 0.32 |
| Bowel movement Frequency < 7 (%) | 0 | 51 | 0.12 |
| Beck Depression Inventory | 10±1.0 | 9.2±6.5 | 0.83 |
| Beck Anxiety Inventory | 4±4.4 | 8.3±8.2 | 0.38 |
| Olfaction – UPSIT result | 10.5±1.3 | 6.1±2.4 | < 0.01 |
| UPSIT % expected for age/sex | 100±13 | 64±25 | < 0.01 |
| Color Vision (FM-100 score) | 125±59.5 | 194±99.4 | 0.17 |
| FM-100 % expected for age | 131±72 | 138±61 | 0.84 |
p values are calculated using t-test and Fisher exact test. They are presented unadjusted for multiple comparisons; given this, and given the low sample size, they are included for information only, and should be considered exploratory in nature. MoCA, Montreal Cognitive Assessment; MCI, mild cognitive impairment; UPSIT, University of Pennsylvania Smell Identification Test (12-item cross-cultural version); FM-100, Farnsworth-Munsell 100 Hue test; UPDRS, Unified Parkinson Disease Rating Scale; RBD, REM sleep behavior disorder; MSA, multiple system atrophy.
Patterns of evolution are illustrated in Fig. 1, mapped using methodology similar to that of a previously-published evaluation of the entire cohort [31]. Although sample size precludes direct statistical analysis of prodromal intervals in MSA, substantial differences with Lewy body disease are evident, notably normal olfaction at all intervals, normal cognition, an unclear/highly variable autonomic interval with absent orthostatic hypotension before diagnosis, and a shorter motor interval (e.g., UPDRS Part III interval =≅2 years in MSA on average, versus 6.5 years in the entire cohort).
DISCUSSION
The prospective nature of our cohort enabled us to directly and comprehensively measure the emergence of MSA-P from its prodromal stages. We report here the evolution of 4 prodromal MSA-P patients, tracked 1.3–6 years before phenoconversion to parkinsonism. Although conclusions are obviously limited by the low number of cases, we note several themes, with potential implications for early MSA diagnosis and potential future MSA criteria:
-
1.
Motor progression is rapid. In some cases, patients converted very quickly from apparent normality to clear parkinsonism. This was most notable in Case 1, who went from a UPDRS of 0 to 12 in 6 months. At baseline, the MSA patients showed generally better motor function than Lewy body patients, despite a nominally shorter interval between baseline and disease. We have previously estimated the interval of motor dysfunction in RBD phenoconvertors, finding that in Lewy body disease, UPDRS deviates from normal values at approximately 7 years before diagnosis [31]. In MSA, however, the interval was clearly shorter, and would correspond to approximately 2 years (given the low sample size, this estimate should be considered imprecise). This may have implications for prodromal diagnosis; if the interval is short, identifiable prodromal motor MSA will be very uncommon and therefore difficult to find.
-
2.
Clinically-evident orthostatic hypotension is uncommon. Although all patients ultimately developed symptoms of orthostatic hypotension with corresponding blood pressure drops, these were not observed in the prodromal state, or even at the time of parkinsonism diagnosis. This is especially notable given that the majority of prodromal MSA patients previously described have presented to autonomic laboratories with pure/primary autonomic failure. Although it should be emphasized that these were clinical office assessments, generally measured at only 1 min of standing, it does suggest that clinically-evident orthostatic hypotension is not an invariate feature of prodromal MSA, and that the clinical spectrum of prodromal MSA needs to be expanded.
-
3.
Erectile dysfunction is not invariably present before diagnosis. Although both men ultimately developed severe erectile dysfunction by the time of parkinsonism diagnosis, neither of them noticed substantial dysfunction at baseline. Note that sexual dysfunction in the female participants was not systematically queried (although Case 2 did spontaneously report difficulties at baseline examination). This suggests that erectile dysfunction need not be required to fulfill a diagnosis of prodromal MSA.
-
4.
Bladder symptoms may precede orthostatic symptoms. In each case, symptoms of urinary frequency and urinary retention developed before orthostatic symptoms. This suggests the importance of systematic bladder assessment in the diagnosis of prodromal MSA.
-
5.
Cognitive tests are generally normal in prodromal stages. Only 1 of the 4 prodromal MSA cases, demonstrated MCI. Of note, even this diagnosis was uncertain; there were substantial education and language issues which may have been insufficiently adjusted for, and her cognitive defects did not substantially progress over the subsequent 6 years. The other three retained normal cognition at last follow-up. By contrast, most Lewy body phenoconvertors have detectible cognitive impairment at baseline. Moreover, most PD-first convertors with 3 years of parkinsonism phenoconversion (and by definition all of the DLB convertors) demonstrate cognitive impairment [32, 33]. Therefore, cognitive impairment is uncommon is prodromal MSA.
-
6.
Sleep parameters are not more severe. Although RBD is extremely common in MSA and can be very severe, we saw no evidence of more severe atonia loss compared to Lewy body convertors (note, of course, that all patients by definition have RBD, so PD/DLB/MSA patients without RBD are not included here). We had also not clinically noted more severe RBD symptoms in these patients (no published RBD symptom severity scales have been validated, so we were unable to quantify severity further). There were increased insomnia symptoms, but with substantial overlap between groups. There was no increased apnea, and no patient had a history of inspiratory stridor at the time of phenoconversion. Therefore, no objective or subjective sleep measures appear to be able to differentiate prodromal MSA from Lewy body disease with high sensitivity and specificity.
-
7.
Olfaction remains a reliable differentiating marker. There were clear differences in olfactory function between those developing MSA and those developing Lewy body disease. Indeed, all 4 MSA patients retained normal olfaction even after several years of established disease. By contrast, of 63 Lewy body convertors, only 3 had normal olfaction at time of parkinsonism/dementia diagnosis. This suggests that hyposmia can be used to indicate a reduced likelihood of possible prodromal MSA.
It is important to emphasize that we are observing one pathway of prodromal MSA, namely that which emerges from prodromal RBD. Most observations of early/prodromal MSA come from clinics specializing in autonomic dysfunction [2–4]. These prior studies have been either retrospective or included a follow-up examination that is less comprehensive than the current study, making direct comparison to our findings impossible. Regardless, our findings emphasize the importance of avoiding over-reliance on one pathway to make prodromal diagnosis (whether that be from autonomic clinics or sleep/RBD clinics); in particular, our finding that clinically-evident autonomic function is not an invariable feature of early MSA would have not been possible to observe using autonomic clinic-based studies.
Some limitations should be noted. The primary limitation is a small sample size, which limits statistical analysis. This reflects the relative rarity of MSA, with the inherent difficulty in detecting its prodromal stage. This limitation is counterbalanced by the fact that there is no published prospective study of prodromal MSA that has assessed patients with such a comprehensive testing protocol. Another important limitation is that the diagnosis of MSA cannot be directly confirmed. In many cases, ancillary diagnostic investigations were conducted, but these were at the discretion of the clinical team (once patients phenoconvert there is no formal research follow-up protocol, although we did follow patients whenever possible). Autonomic testing was very limited in our protocol to simple clinical measures; it is possible that numerous abnormalities would have been documented with a detailed laboratory-based autonomic protocol. Finally, we are missing a 3-year period of prodromal data from one patient, who had elected to not follow with us until he had symptoms.
In summary, by systematically observing MSA emerging from prodromal stages of RBD, we have observed a prodromal state characterized by rapid motor progression, without inevitable clinical autonomic dysfunction, and with normal olfaction and cognition. These findings may have implications for detection of early stages of MSA and for development of prodromal MSA criteria.
ACKNOWLEDGMENTS
This study was funded by the Fonds de Recherche du Québec – Santé, the W. Garfield Weston Foundation, and the Canadian Institute of Health Research.
CONFLICT OF INTEREST
Dr. Postuma reports grants and personal fees from Fonds de Recherche du Québec – Santé, the Canadian Institute of Health Research, Parkinson Canada, the W. Garfield Weston Foundation, the Michael J. Fox Foundation, the Webster Foundation, and Roche, and personal fees from Takeda, Roche/Prothena, Teva Neurosciences, Novartis Canada, Biogen, Boehringer Ingelheim, Theranexus, GE HealthCare, Jazz Pharmaceuticals, Abbvie, Jannsen, and Otsuko, outside the submitted work. Dr. Gagnon received grants from the Fonds de Recherche du Québec – Santé, the W. Garfield Weston Foundation, and the Canadian Institutes of Health Research. He holds a Canada Research Chair in Cognitive Decline in Pathological Aging. Dr Montplaisir received personal compensation as consultant (Impax pharma, Servier, Jazz pharma, Merk, Valeant), speaker (Valeant), and received financial support for research activities from Merck, GlaxoSmithKline is funded by grants from the Fonds de Recherche du Québec – Santé, the W. Garfield Weston Foundation, and the Canadian Institutes of Health Research. A Pelletier has nothing to disclose.
REFERENCES
- [1]. McKay JH, Cheshire WP (2018) First symptoms in multiple system atrophy. Clin Auton Res 28, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Kaufmann H, Norcliffe-Kaufmann L, Palma JA, Biaggioni I, Low PA, Singer W, Goldstein DS, Peltier AC, Shibao CA, Gibbons CH, Freeman R, Robertson D (2017) Natural history of pure autonomic failure: A United States prospective cohort. Ann Neurol 81, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Gibbons CH, Freeman R (2015) Clinical implications of delayed orthostatic hypotension: A 10-year follow-up study. Neurology 85, 1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Coon EA, Mandrekar JN, Berini SE, Benarroch EE, Sandroni P, Low PA, Singer W (2020) Predicting phenoconversion in pure autonomic failure. Neurology 95, e889–e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Giannini G, Calandra-Buonaura G, Asioli GM, Cecere A, Barletta G, Mignani F, Ratti S, Guaraldi P, Provini F, Cortelli P (2018) The natural history of idiopathic autonomic failure: The IAF-BO cohort study. Neurology 91, e1245–e1254. [DOI] [PubMed] [Google Scholar]
- [6]. Postuma RB, Iranzo A, Hu M, Hogl B, Boeve B, Manni R, Oertel W, Arnulf I, Ferini-Strambi L, Puligheddu M, Antelmi E, Cochen de Cock V, Arnaldi D, Mollenhauer B, Videnovic A, Sonka K, Jung KH, Kunz D, Dauvilliers Y, Provini F, Lewis SJ, Buskova J, Pavlova M, Heidbreder A, Montplaisir J, Santamaria J, Barber TR, Leu-Semenescu S, Plazzi G, Nobili F, Sixel-Doring F, Dusek P, Bes F, Cortelli P, Ehgoetz Martens K, Gagnon JF, Gaig C, Zucconi M, Mahlknecht P, Holzknecht E, Boeve AR, Teigen L, Toscano G, Mayer G, Morbelli S, Dawson BK, Pelletier A, RBDSG (2019) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behavior disorder: A multicenter study. Brain 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Giannini G, Provini F, Cortelli P, Calandra-Buonaura G (2021) REM sleep behaviour disorder in multiple system atrophy: From prodromal to progression of disease. Front Neurol 12, 677213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Giannini G, Mastrangelo V, Provini F, Droghini A, Cecere A, Barletta G, Mignani F, Guaraldi P, Cortelli P, Calandra-Buonaura G (2020) Progression and prognosis in multiple system atrophy presenting with REM behavior disorder. Neurology 94, e1828–e1834. [DOI] [PubMed] [Google Scholar]
- [9]. Stefani A, Ferini-Strambi L, Postuma RB, Iranzo A, Videnovic A, Hogl B, Wenning GK (2020) Olfaction in patients with isolated REM sleep behavior disorder who eventually develop multiple system atrophy. Sleep 43, zsz303. [DOI] [PubMed] [Google Scholar]
- [10]. Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY (2015) Parkinson risk in idiopathic REM sleep behavior disorder: Preparing for neuroprotective trials. Neurology 84, 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Postuma RB, Gagnon JF, Vendette M, Montplaisir J (2009) Markers of neurodegeneration in idiopathic REM sleep behavior disorder and Parkinson disease. Brain 132, 2298–2307. [DOI] [PubMed] [Google Scholar]
- [12]. American Academy of Sleep Medicine (2014) International Classification of Sleep Disorders - 3, American Academy of Sleep Medicine, Westchester, IL. [Google Scholar]
- [13]. Genier Marchand D, Postuma RB, Escudier F, De Roy J, Pelletier A, Montplaisir J, Gagnon JF (2018) How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol 83, 1016–1026. [DOI] [PubMed] [Google Scholar]
- [14]. Wenning GK, Tison F, Seppi K, Sampaio C, Diem A, Yekhlef F, Ghorayeb I, Ory F, Galitzky M, Scaravilli T, Bozi M, Colosimo C, Gilman S, Shults CW, Quinn NP, Rascol O, Poewe W; Multiple System Atrophy Study Group (2004) Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 19, 1391–1402. [DOI] [PubMed] [Google Scholar]
- [15]. Doty RL, Marcus A, Lee WW (1996) Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 106(3 Pt 1), 353–356. [DOI] [PubMed] [Google Scholar]
- [16]. Farnsworth D (1943) The Farnsworth-Munsell 100-Hue and Dichotomous Tests for Color Vision. J Opt Soc Am 33, 568–578. [Google Scholar]
- [17]. Bastien CH, Vallieres A, Morin CM (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2, 297–307. [DOI] [PubMed] [Google Scholar]
- [18]. Johns MW (1991) A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14, 540–545. [DOI] [PubMed] [Google Scholar]
- [19]. Buysse DJ, Reynolds CF Jr., Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- [20]. Postuma RB, Gagnon JF, Pelletier A, Montplaisir JY (2017) Insomnia and somnolence in idiopathic RBD: A prospective cohort study. NPJ Parkinsons Dis 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Postuma RB, Gagnon JF, Rompre S, Montplaisir J (2010) Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology 74, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56, 893–897. [DOI] [PubMed] [Google Scholar]
- [23]. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- [24]. Rahayel S, Postuma RB, Montplaisir J, Genier Marchand D, Escudier F, Gaubert M, Bourgouin PA, Carrier J, Monchi O, Joubert S, Blanc F, Gagnon JF (2018) Cortical and subcortical gray matter bases of cognitive deficits in REM sleep behavior disorder. Neurology 90, e1759–e1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Al-Qassabi A, Tsao TS, Racolta A, Kremer T, Canamero M, Belousov A, Santana MA, Beck RC, Zhang H, Meridew J, Pugh J, Lian F, Robida MD, Ritter M, Czech C, Beach TG, Pestic-Dragovich L, Taylor KI, Zago W, Tang L, Dziadek S, Postuma RB (2021) Immunohistochemical detection of synuclein pathology in skin in idiopathic rapid eye movement sleep behavior disorder and parkinsonism. Mov Disord 36, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55, 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, W. O, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem B, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30, 1591–1600. [DOI] [PubMed] [Google Scholar]
- [28]. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 65, 1863–1872. [DOI] [PubMed] [Google Scholar]
- [30]. Mahlknecht P, Krismer F, Poewe W, Seppi K (2017) Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson’s disease. Mov Disord 32, 619–623. [DOI] [PubMed] [Google Scholar]
- [31]. Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB (2019) Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 142, 2051–2067. [DOI] [PubMed] [Google Scholar]
- [32]. Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB (2015) New clinical subtypes of Parkinson disease and their longitudinal progression: A prospective cohort comon with other phenotypes. JAMA Neurol 72, 863–873 paris. [DOI] [PubMed] [Google Scholar]
- [33]. Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, Panisset M, Gagnon JF (2012) Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: A prospective study. Mov Disord 27, 720–726. [DOI] [PubMed] [Google Scholar]