Abstract
Patients with long COVID suffer from many neurological manifestations that persist for 3 months following infection by SARS-CoV-2. Autonomic dysfunction (AD) or dysautonomia is one complication of long COVID that causes patients to experience fatigue, dizziness, syncope, dyspnea, orthostatic intolerance, nausea, vomiting, and heart palpitations. The pathophysiology behind AD onset post-COVID is largely unknown. As such, this review aims to highlight the potential mechanisms by which AD occurs in patients with long COVID. The first proposed mechanism includes the direct invasion of the hypothalamus or the medulla by SARS-CoV-2. Entry to these autonomic centers may occur through the neuronal or hematogenous routes. However, evidence so far indicates that neurological manifestations such as AD are caused indirectly. Another mechanism is autoimmunity whereby autoantibodies against different receptors and glycoproteins expressed on cellular membranes are produced. Additionally, persistent inflammation and hypoxia can work separately or together to promote sympathetic overactivation in a bidirectional interaction. Renin-angiotensin system imbalance can also drive AD in long COVID through the downregulation of relevant receptors and formation of autoantibodies. Understanding the pathophysiology of AD post-COVID-19 may help provide early diagnosis and better therapy for patients.
Keywords: Autonomic dysfunction, Autonomic nervous system, COVID-19, Dysautonomia, Long COVID, Post-COVID
1. Introduction
From a mysterious case of pneumonia to a global pandemic, the coronavirus disease 2019 (COVID-19) or severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged as a threatening challenge that has greatly altered our lives. The virus shares 80 % of its genome with previous human coronaviruses (SARS-CoV) and binds the angiotensin-converting enzyme type 2 (ACE-2) receptor through its spike (S) glycoprotein to enter the cell by endocytosis (Gralinski and Menachery, 2020; Ou et al., 2020). Its entry also depends on transmembrane serine protease 2 (TMPRSS2) responsible for S protein priming (Hoffmann et al., 2020). Angiotensin-converting enzyme type 2 receptors are found in the lung alveolar epithelial cells and enterocytes of the small intestine (Hamming et al., 2004). They are located in the venous and arterial endothelial cells and arterial smooth muscle cells of most body organs comprising the stomach, brain, lymph nodes, bone marrow, spleen, kidneys, oral and nasal mucosa, nasopharynx, and skin (Hamming et al., 2004). The S1 motif of SARS-CoV-2 binds the cell surface neuropillin 1 (NRP1), which could explain its increased pathogenicity compared to SARS-CoV (Daly et al., 2020).
Multiple systems such as the nervous, gastrointestinal, endocrine, cardiovascular, renal, and respiratory systems are implicated in long COVID symptoms (Mehandru and Merad, 2022). These symptoms include brain fog, headache, myalgia, tingling, fatigue, chest pain, paraesthesia, shortness of breath, anosmia, and diarrhea among others (Aiyegbusi et al., 2021; Graham et al., 2021). A post-COVID-19 symptoms model classifies some patients' symptoms into a transition phase followed by three consecutive phases (Fernández-de-Las-Peñas et al., 2021). In the transition phase and phase 1, acute post-COVID-19 symptoms appear during weeks 4 to 12 (Fernández-de-Las-Peñas et al., 2021). Phase 2 is characterized by long post-COVID-19 symptoms arising during weeks 12 to 24 while phase 3 includes persistent symptoms that last beyond week 24 (Fernández-de-Las-Peñas et al., 2021). However, according to the WHO, a patient is diagnosed with post-COVID after 3 months post-infection where the symptoms persist for more than 2 months (WHO, 2021). Other terms include long COVID, long haul COVID-19, or post-acute sequelae of COVID-19 (PASC) (Al-Aly et al., 2021; Phillips and Williams, 2021). A cross-sectional study showed that PASC may persist in 52 % of patients 1 month after onset of symptoms (Sugiyama et al., 2022). Multiple case reports have indicated that autonomic dysfunction (AD) or dysautonomia is an important sequela in long COVID (Blitshteyn and Whitelaw, 2021; Dani et al., 2021b; Novak et al., 2022). An observational study noted that autonomic symptoms in the post-COVID-19 phase require monitoring even in patients without neurological manifestations (Buoite Stella et al., 2022). In this study, 61.1 % of patients with long COVID developed AD and scored more than 13.25 on the COMPASS-31 questionnaire (Buoite Stella et al., 2022). Autonomic dysfunction, which can be primary or secondary, refers to a malfunction in the autonomic nervous system (ANS) with orthostatic hypotension (OH) being the most critical complication (Reichgott, 1990). Its cause can be genetic like familial dysautonomia or acquired due to autoimmunity, traumatic injuries, infections, abnormal reflexia, and metabolic dysfunction among others (Sánchez-Manso et al., 2017). Although it is an overlooked condition that is sometimes underdiagnosed and undertreated, its occurrence as a post-COVID manifestation has highlighted its severity (Blitshteyn and Whitelaw, 2021; Sánchez-Manso et al., 2017). This review aims to highlight the mechanisms by which AD occurs in patients with long COVID. First, a brief overview of the ANS anatomy and AD will be provided followed by a compilation of clinical reports of patients with AD symptoms after COVID-19 infection. Second, the potential routes of SARS-CoV-2 entry into the ANS will be discussed along with mechanisms of AD onset post-COVID-19.
2. Overview of the autonomic nervous system
The ANS is the part of the peripheral nervous system (PNS) responsible for regulating involuntary physiological functions such as blood pressure, heart rate, digestion, respiration, and sexual arousal (Waxenbaum et al., 2022). The hypothalamus is one of the chief autonomic centers in the brain, which contains various nuclear groups, among which the paraventricular nucleus (PVN) plays a crucial role in controlling ANS functions (Miana Gabriela et al., 2018). This hypothalamic nucleus is extensively connected to several structures in the limbic system, brainstem, and spinal cord. Neurons in the PVN receive afferent inputs from several integrative centers in the hypothalamus (suprachiasmatic nucleus, median preoptic nucleus, subfornical organ, arcuate nucleus), and from different pre-autonomic nuclei in the pons (lateral parabrachial nucleus) and medulla (nucleus tractus solitarius, ventrolateral medulla, dorsal motor nucleus of the vagus) (Zeevalk, 2010). Efferently, neurons in the PVN project their axons to the median eminence in the hypothalamus, nucleus tractus solitarius (NTS) and rostral ventrolateral medulla, as well as to intermediolateral cell column in the spinal cord (Zeevalk, 2010).
The PVN is a pivotal hypothalamic nucleus that not only controls neuroendocrine and autonomic functions but also regulates the body's stress response (Busnardo et al., 2010; Herman et al., 2008). Moreover, the PVN controls the hypothalamic-pituitary-adrenal (HPA) axis through the release of corticotropin-releasing hormone (CRH) into the anterior pituitary via the portal vessels of the median eminence (Ferguson et al., 2008). It also exerts negative feedback control over the HPA through glucocorticoid receptors (GR) expressed by PVN CRH neurons (Herman and Tasker, 2016; Liposits et al., 1987). Its remarkable integrative function is attributed to GABAergic and glutamatergic interneurons that can be synaptically modulated by chronic stress resulting in various autonomic pathologies (Ferguson et al., 2008; Herman et al., 2008).
Other nuclei in the hypothalamus have also been implicated in the central autonomic nexus, such as the lateral hypothalamic area, the posterior hypothalamic nucleus, the dorsomedial nucleus, and the mammillary nucleus (Miana Gabriela et al., 2018). These nuclei are interconnected with the PVN, the dorsal motor nucleus of the vagus (DMV), NTS, the lateral and ventral medulla and intermediolateral spinal columns (Miana Gabriela et al., 2018).
As a regulator of autonomic activities, the hypothalamus is interconnected with autonomic centers via three major pathways: the medial forebrain bundle (MFB), the dorsal longitudinal fasciculus (DLF), and the mammillotegmental tract (MTT) (Bear et al., 2022). In a later section, the role of these fibers as potential routes by which the SARS-CoV-2 virus can reach the autonomic network is discussed.
Another chief regulator of the ANS is the brainstem. The latter is divided into three major regions: midbrain, pons, and medulla oblongata (Benghanem et al., 2020). The gray matter of the brainstem, especially the medulla oblongata, includes autonomic nuclei that are involved in respiratory, cardiocirculatory, immune, and digestive systems homeostasis (Benghanem et al., 2020; Martín-Gallego et al., 2017).
3. Autonomic dysfunction
Dysregulation in the autonomic reflex can occur as a presyncope or syncope that is caused by a decrease in brain perfusion (Arnold et al., 2017). Syncope caused by AD usually occurs as a result of central neuropathy or peripheral ganglionopathy including neurally mediated syncope like vasovagal, situational, and carotid sinus syncope in addition to OH and postural orthostatic tachycardia syndrome (POTS) (Arnold et al., 2017). Additionally, AD can cause other cardiovascular diseases like hypertension and heart failure (Shen and Zipes, 2014). Patients with these conditions show sympathetic nervous system (SNS) hyperactivity at rest with increased blood noradrenaline levels, which causes additional stress on the heart and kidneys (Shen and Zipes, 2014). Orthostatic hypotension, a major characteristic of AD, is a drop in systolic blood pressure by a minimum of 20 mmHg or in diastolic blood pressure by 10 mmHg (Metzler et al., 2013). This drop occurs within 3 min after standing up or a head-up tilt (Metzler et al., 2013). As a result of OH, other non-specific symptoms may occur due to a decrease in tissue and organ perfusion such as dizziness, dyspnea, generalized weakness, chronic fatigue, or visual acuity dysfunction (Milazzo et al., 2015). Patients with POTS suffer from impaired selective attention, processing speed, and executive function (Arnold et al., 2015). The cause of the resultant brain fog remains unknown, yet several mechanisms have been proposed including decreased cerebral blood flow velocity in the middle cerebral artery and elevated levels of norepinephrine (Arnold et al., 2015; Schroeder et al., 2002; Wells et al., 2020). Chronic fatigue syndrome is a complex disease with unknown etiology that exhibits ANS dysfunction (Hoad et al., 2008). In patients with chronic fatigue syndrome, symptoms of dysautonomia like OH include decreased cardiac adrenergic response, decreased blood pressure parameters, increased tonic sympathetic activity, and POTS (Hoad et al., 2008).
4. Clinical reports of autonomic dysfunction post-COVID-19
Twelve clinical reports have demonstrated the development of AD in long COVID (Table 1 ). A retrospective study that included 9 patients with PASC showed reduced orthostatic cerebral blood flow velocity with or without orthostatic tachycardia (Novak et al., 2022). This reduction (−20 %) was comparable to a group of patients with POTS caused by cerebral hypoperfusion (Novak et al., 2022). Several cases of young healthy females show the development of POTS 3 or 4 weeks following COVID-19 infection (Bosco and Titano, 2022; Kanjwal et al., 2020; Miglis et al., 2020; O'Sullivan et al., 2021). Common symptoms included fatigue, tachycardia, dyspnea, and tightness in the chest, which were alleviated by Ivabradine administration (Kanjwal et al., 2020; O'Sullivan et al., 2021). Lifestyle changes such as increasing fluid and sodium intake in addition to wearing compression socks helped patients improve as well (Bosco and Titano, 2022). Other hyperadrenergic symptoms were diarrhea, face flushing, restlessness, and tremors (Miglis et al., 2020).
Table 1.
Summary of clinical reports and case series of patients with autonomic dysfunction post-COVID-19. Abbreviations: POTS: postural orthostatic tachycardia syndrome; ECG: Electrocardiogram; OH: orthostatic hypotension; QSART: Quantitative Sudomotor Axon Reflex Testing; HRV: heart rate variability; SDNN: Standard Deviation of NN intervals; SDANN: Standard Deviation of the Average NN intervals; LF/HF: Low Frequency/High Frequency Ratio; SNS: sympathetic nervous system; PNS: peripheral nervous system; ANS: autonomic nervous system; NCS: Neurocardiogenic Syncope.
| Number of participants | Age and gender | Clinical findings post-COVID-19 infection | Reference |
|---|---|---|---|
| 1 | 36-year-old female |
3–4 weeks post-COVID-19 diagnosis: Fatigue, dizziness, headache, orthostatic chest pain, and palpitations Diagnosis of POTS |
(Kanjwal et al., 2020) |
| 1 | 26-year-old female |
Day 7 Shortness of breath, palpitations, burning chest pains upon inhalation, and anorexia Day 14 Fatigue, tachycardia, shortness of breath, exercise intolerance, chest pains, and insomnia Day 19 Orthostatic presyncope and lightheadedness Day 22 Pressured speech and restlessness Day 24 Adrenaline surges and restlessness Day 30 Worsening of orthostatic intolerance Day 45 Non-pruritic hives, facial flushing, dermatographia Day 107 (over 3 months after symptoms onset) Diagnosis of POTS |
(Miglis et al., 2020) |
| 1 | 58-year-old female | Headache, somnolence, and hypertensive palpitations at rest Diagnosis: acute dysautonomia with sympathetic overactivity |
(Abdelnabi et al., 2021) |
| 1 | 22-year-old female |
Day 21 post infection Chest tightness, palpitations, dyspnea, and fatigue upon mild exertion ECG: Sinus tachycardia Electrocardiography: Hyperdynamic left ventricle |
(O'Sullivan et al., 2021) |
| 1 | 67-year-old male | OH, lightheadedness, generalized weakness, shortness of breath, and anorexia Day 6 of hospitalization Syncopal episode |
(Suresh et al., 2021) |
| 1 | 27-year-old female |
5 weeks after infection: Headache, fatigue upon exertion, weakness, blurry vision, slow cognition, heart palpitations 2 months after initial symptoms: worsening initial symptoms, burning in lower extremities, muscle spasms and twitches 5 months after initial symptoms: worsening initial symptoms, diarrhea, extreme exhaustion, uncontrollable shaking Diagnosis of POTS |
(Bosco and Titano, 2022) |
| 27 | 59 % are females and 41 % are males with an average age of 30 years old |
Autonomic symptoms onset during infection: 41 % of patients Autonomic symptoms onset 0–122 days post-infection: 59 % of patients: lightheadedness (93 %), burning pain (11 %), orthostatic headache (22 %), orthostatic tachycardia (7 %), syncope (11 %), flushing (7 %), hyperhidrosis (11 %), and weight loss (7 %) Abnormal autonomic test (63 %), abnormal QSART (36 %), abnormal cardiovagal test (27 %), abnormal cardiovagal adrenergic function (7 %) |
(Shouman et al., 2021) |
| 40 | 57.5 % are females and 42.5 % are males with an average age of 55 years |
6 months post-discharge from COVID-19 hospitalization Fever (2.5 %), cough (30 %), breathing difficulties (40 %), diarrhea (20 %), muscle pain (25 %), ventilation dysfunction (17.5 %), diffuse dysfunction (55 %), and pulmonary fibrosis (57.5 %) Patients with Ventilation Dysfunction SDNN: 28.6 % had an abnormal HRV SDANN: 14.3 % had an abnormal circadian HRV LF/HF: 14.3 % had a SNS/PNS imbalance Patients with Diffuse Dysfunction SDNN: 40.91 % had an abnormal HRV SDANN: 30 % had an abnormal circadian HRV LF/HF: 9.1 % had a SNS/PNS imbalance Patients with Pulmonary Fibrosis SDNN: 38.1 % had an abnormal HRV SDANN: 14.3 % had an abnormal circadian HRV LF/HF: 4.5 % had a SNS/PNS imbalance |
(Bai et al., 2021) |
| 152 | Mild group: 30 patients who had COVID-19 without pneumonia 53.33 % are males and 46.67 % are females with an average age of 42 years Severe group: 45 patients who had COVID-19 with interstitial pneumonia 53.33 % are males and 46.67 % are females with an average age of 51 years Control group: 77 healthy controls matched by sex and age with infected groups |
Sympathetic function tests: OH:
Parasympathetic function tests: Heart rate response to Valsalva maneuver:
ANS Impairment Parasympathetic dysfunction
|
(Milovanovic et al., 2021) |
| 20 | 30 % are males and 70 % are females with average age of 40 years |
During COVID-19 Infection Acute respiratory syndrome (75 %), anosmia (50 %), ageusia (50 %), and pneumonia (10 %) 6–8 months Post-COVID-19 Infection: Fatigue, orthostatic intolerance, dizziness, postural tachycardia, and exercise intolerance POTS (75 %), NCS (15 %), and OH (10 %) |
(Blitshteyn and Whitelaw, 2021) |
| 9 | Females with average age of 35.8 years |
0.8 ± 0.3 years after a mild COVID-19 infection: 100 %: Autonomic symptoms, gastrointestinal symptoms, pain, brain fog, fatigue, and dyspnea Mean Reduction in Orthostatic cerebral blood flow velocity: −20 % Dysautonomia: 100 % Small fiber neuropathy: 89 % Presence of inflammatory markers: 67 % |
(Novak et al., 2022) |
| 218 | 112 patients who recovered from COVID-19: 61 % females, 39 % males Average age of 40 years 106 healthy controls: 60 % females, 40 % males Average age of 46 years |
3.7 ± 1.7 months after COVID-19 infection: Symptoms: Fatigue (73.2 %), headache (66 %), anosmia (65.1 %), ageusia (59.8 %), fever (58.9 %), cough (48.2 %), pharyngitis (47.4 %), myalgia (46.4 %), dyspnea (29 %), rhinitis (25.9), diarrhea (21.8 %). Significant averages of SCOPA-AUT autonomic symptoms: -COVID-19 group: Pupillomotor 0.4 ± 0.7 Sudomotor 3.1 ± 2.5 Urinary 3.6 ± 3.2 -Control group: Pupillomotor 0.6 ± 0.7 Sudomotor 1.7 ± 1.8 Urinary 2.5 ± 2.2 |
(Erdal et al., 2022) |
Dysautonomia following COVID-19 can also be presented through labile blood pressure, episodes of hypertension, and OH (Suresh et al., 2021). A study showed that heart rate variability may be used as an indicator for AD in COVID-19 patients and may be associated with pulmonary fibrosis sequelae in patients within 6 months post-SARS-CoV-22 infection (Bai et al., 2021). In fact, normalized high frequency component of blood pressure variability marker (HF-nu-sBP), which regulates systolic blood pressure and illustrates parasympathetic activity, significantly increased in both mild and severe cases of COVID-19 when compared to the control group (Milovanovic et al., 2021). Levels of normalized low frequency component of blood pressure variability marker (LF-nu-dBP), which regulates diastolic blood pressure and illustrates sympathetic activity, significantly decreased in both COVID-19 groups when compared to healthy individuals (Milovanovic et al., 2021). These results demonstrate that sympathetic impairment is associated with COVID-19 infection; thus, the compensatory activity of the parasympathetic nervous system to modulate blood pressure (Milovanovic et al., 2021).
5. Mechanisms of autonomic dysfunction by SARS-CoV-2
The pathophysiology of AD following COVID-19 is largely speculative due to the lack of investigative clinical studies in patients exhibiting symptoms of ANS dysfunction. Many previous reviews have attempted to decipher the possible mechanisms behind AD onset post COVID-19 (Al-Kuraishy et al., 2021; Dani et al., 2021a; Hassani et al., 2021). In this section, we will attempt to identify more specific targets used by SARS-CoV-2 in each suggested mechanism.
5.1. Viral invasion of autonomic centers
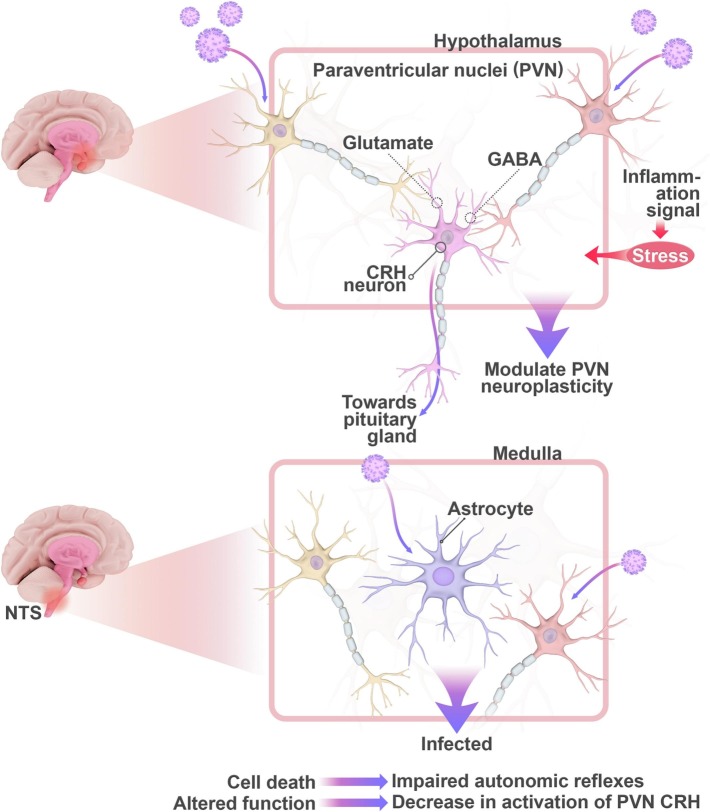
Viruses can enter the nervous system through the hematogenous or transneuronal route (Lima et al., 2020) (Table 2 ). Targeted neurons may exhibit altered function, lysis, or act as viral reservoirs (Price and Notkins, 1977). These virus-induced changes can impact the components of the autonomic network leading to symptoms of dysautonomia. Possible targets that can lead to dysautonomia include autonomic centers like the hypothalamus and medulla oblongata in the brainstem (Martin-Gallego et al., 2016; Nosaka, 1966) (Fig. 1 ).
Table 2.
Direct of routes of entry by SARS-CoV-2 into the autonomic nervous system. Different cranial nerves can act as access points by SARS-CoV-2 to reach the hypothalamus or brainstem. The virus can reach the autonomic nervous system through the blood by crossing the blood-brain-barrier or the circumventricular organs. Abbreviations: ANS: autonomic nervous system; ACE-2: angiotensin-converting enzyme type 2; BBB: blood-brain barrier; CVOs: circumventricular organs; MFB: medial forebrain bundle; NTS: nucleus tractus solitarius; NRP1: neuropillin 1; PVN: paraventricular nucleus; TMPRSS2: transmembrane serine protease 2.
| Route of entry | Anatomical site of entry | Findings suggesting entry through the route | Reference | Suggested pathway to ANS invasion |
|---|---|---|---|---|
| Neuronal | Olfactory nerve | Autopsy of patients with COVID-19 showed viral RNA of SARS-CoV-2 in the olfactory mucosa and olfactory bulb. Spike protein was colocalized with neuronal cells In macaques, SARS-CoV-2 was detected in the olfactory cortex preferentially in neurons |
Meinhardt et al., 2021 Beckman et al., 2022 |
Olfactory epithelium → olfactory nerve → olfactory cortex → MFB → brainstem |
| Ocular nerve | In patients with COVID-19, SARS-CoV-2 was detected in the retina Patients with COVID-19 had increased retinal nerve fiber layer thickness |
Casagrande et al., 2020 Burgos-Blasco et al., 2021 |
Retina → ocular nerve → retinohypothalamic tract → suprachiasmatic nucleus of the hypothalamus | |
| Trigeminal nerve | Oral mucosa, taste buds, salivary gland, and trigeminal ganglion express ACE-2 receptors and TMPRSS2 | Park et al., 2022 | Nociceptors in the oral cavity → trigeminal nerve → trigeminal ganglia → trigeminal spinal nucleus caudalis in the medulla | |
| Glossopharyngeal and vagus, nerves | High expression of ACE-2, NRP1, and TMPRSS2, in the vagus and the glossopharyngeal nerves | Vitale-Cross et al., 2022 | Taste sensory fibers in oral cavity → glossopharyngeal and vagus nerves → NTS Respiratory tract epithelium → vagus nerve → vagal ganglia → NTS and the nucleus ambiguous |
|
| Hematogenous | BBB | Postmortem examination of a COVID-19 patient showed viral-like particles in the capillary endothelium of the frontal lobe | Paniz-Mondolfi et al., 2020 | Viral hijacked leukocytes or transcellular/paracellular migration across BBB → hypothalamus and brainstem |
| CVOs | High expression of ACE-2 in CVOs and PVN | Ong et al., 2022 | Subfornical zone, organum vasculosum, median eminence, and area postrema → PVN of hypothalamus |
Fig. 1.
Direct invasion of the hypothalamus or the medulla by SARS-CoV-2 through the neuronal or hematogenous routes can induce autonomic dysfunction. At the level of the hypothalamus, PVN plasticity may be modulated directly or indirectly by targeting GABAergic or glutamatergic interneurons. These interneurons synapse with the CRH neurons that connect to the pituitary gland. Inflammatory signals can also be translated into stress signals that overwhelm the PVN resulting in neuroinflammation and autonomic disruption. At the level of the medulla, viral invasion of GABAergic interneurons and astrocytes in the NTS can modulate normal autonomic function and/or cause cell death. Abbreviations: PVN: Paraventricular Nuclei; CRH: Corticotropin Releasing Hormone; NTS: nucleus tractus solitarius.
5.1.1. Direct routes of SARS-CoV-2 entry into the autonomic system
5.1.1.1. Neuronal route
Viruses are considered neuroinvasive if they can be carried by motor proteins across neurons through retrograde or anterograde axonal transport to reach nearby cells (Taylor and Enquist, 2015). The olfactory receptor neurons (ORNs) are possible targets of such invasion demonstrated in patients with COVID-19 (Meinhardt et al., 2021). A study on rhesus monkeys showed SAR-CoV-2 invades neurons in the frontal lobe following entry via the nasal olfactory epithelium (Beckman et al., 2022). With age, the virus further spreads in the olfactory cortex (Beckman et al., 2022). The dendrites of the ORNs are exposed to the outside, which makes them easy entry point for viruses to invade the CNS (Mori et al., 2005). Through that same pathway, we hypothesize that SARS-CoV-2 can spread to the brainstem through the MFB. The MFB establishes major hypothalamic connections with the olfactory bulb, septal nuclei, prefrontal cortex, and the dopaminergic neurons of the ventral tegmental area (Koob et al., 2013). Therefore, it possibly poses a direct pathway for SARS-CoV-2 from the olfactory epithelium to the hypothalamus.
However, immunolabeling has revealed that ACE2 receptors are expressed more abundantly on the apical side of sustentacular cells of the olfactory epithelium compared to the respiratory epithelium and are not found on ORNs (Chen et al., 2020). Similarly, another study showed that ACE-2 receptors and TMPRSS2 protein were mainly located in sustentacular cells while TMPRSS2 was detected in both neural and non-neural olfactory cells (Bilinska et al., 2020). An examination of infected hamster and human olfactory epithelia indicated that SARS-CoV-2 preferentially invades the sustentacular cells and not the olfactory neurons causing a downregulation in the expression of olfactory receptors and their signaling genes (Zazhytska et al., 2022). It is also suggested that local inflammation in the upper respiratory tract causes axonal and microvascular damage in the olfactory tract and bulb (Ho et al., 2022). In this study, olfactory pathology did not correlate with the presence of SARS-CoV-2 in the olfactory bulb, so direct viral invasion of ORNs was excluded (Ho et al., 2022). These results oppose the viral neurotropism of SARS-CoV-2 as a cause for olfactory dysregulation. Therefore, more research is required to investigate whether direct viral invasion of the ORNs is possible to allow access to the ANS and to understand how SARS-CoV-2 can cause neuronal damage indirectly. The role of age and comorbid diseases should be investigated as factors that may facilitate the spread of the virus in the brain.
Other cranial nerves may act as viral entry points into the central nervous system (CNS) (Bauer et al., 2022). In a case series, COVID-19 patients displayed ocular abnormalities (Wu et al., 2020). Severe acute respiratory syndrome coronavirus-2 has been detected in the retina of deceased COVID-19 patients (Casagrande et al., 2020). The involvement of the optic nerve was suspected. Upon examination of retinal nerve fiber layer thickness in 8 COVID-19 patients, an increase was noted in 7 patients suggesting possible optic nerve inflammation caused by the virus (Burgos-Blasco et al., 2021). The retinohypothalamic tract allows entry into the suprachiasmatic nucleus of the hypothalamus (Miana Gabriela et al., 2018). It can be hypothesized that the virus may use this route to affect circadian function. There is little evidence to explain how the virus impacts the optic nerve and whether it uses it for transneuronal entry to the brain.
The oral mucosa, taste buds, salivary gland, and trigeminal ganglion express ACE-2 receptors and TMPRSS2 (Park et al., 2022). The trigeminal ganglia transmit oral facial nociception to the trigeminal spinal nucleus caudalis in the medulla (Bista and Imlach, 2019). This suggests that the trigeminal nerve can be another possible point of entry into the ANS (Park et al., 2022). Ageusia is an early symptom in COVID-19 patients that appears alongside anosmia (CDC, 2020). The cranial nerves involved in the sense of taste are the facial, glossopharyngeal, and vagus nerves that connect taste receptors to the brain through the NTS (Mennella et al., 2017). Both the vagus and the glossopharyngeal nerves express ACE-2, NRP1, and TMPRSS2, which makes them potential sites of entry to the NTS in the brainstem (Vitale-Cross et al., 2022). The vagus nerve creates a link between the lung and the brainstem nuclei (Baker and Lui, 2021). The vagal ganglia project to both the NTS and the nucleus ambiguus, known to regulate respiratory rhythm (Baker and Lui, 2021). We can postulate that the virus uses the vagal nerve to spread from the respiratory tract to the vagal ganglia, NTS, and nucleus ambiguous. Viral infiltration may cause respiratory dysfunction, only worsening the already existing respiratory manifestations of SARS-CoV-2.
5.1.1.2. Hematological route
The CNS is immune privileged due to protection offered by physical barriers like the blood-brain barrier (BBB) or immunological barriers in order to evade inflammation (Forrester et al., 2018). Nonetheless, this privilege can be exploited by the virus to invade the CNS (Forrester et al., 2018). For example, the BBB can be breached through viral infection of the endothelial cells lining it or by using leukocytes as “Trojan horses” to cross it (Salinas et al., 2010). Postmortem examination of a COVID-19 patient revealed the presence of viral-like particles in the capillary endothelium of the frontal lobe with active budding across the basolateral membrane of the endothelial cells (Paniz-Mondolfi et al., 2020). This observation supports the possible hematogenous spread of the virus to access the CNS through the infected endothelial cells. Moreover, endothelial cells of the BBB possess tight junctions that limit the paracellular transport of molecules (Sandoval and Witt, 2008). These tight junctions are mainly comprised of the transmembrane proteins: claudin, occludin, and junction adhesions molecules (JAM) (Sandoval and Witt, 2008). Viruses can damage these tight junctions through different ways to cross the BBB. For example, West Nile virus (WNV) is able to degrade claudin-1 and JAM-1 by endocytosis once it infects epithelial/endothelial cells (Xu et al., 2012). Another study noted that WNV upregulates the expression of matrix metalloproteinase 9 (MMP9) that in turn disrupts the BBB by damaging the basement membrane to enter the brain (Wang et al., 2008). It is worth investigating whether SARS-CoV-2 impacts tight junction proteins to cross the BBB or utilizes a transcellular route to access the CNS. Alternatively, viruses can use immune cells to cross the BBB through the “Trojan horse” mechanism, which is used by the human immunodeficiency virus-1 (HIV-1) (Kramer-Hämmerle et al., 2005). Viruses can increase the expression of intercellular adhesion molecule-1 (ICAM-1) on the surface of endothelial cells to facilitate the transmigration of leukocytes to the CNS, the site of inflammation (Dietrich, 2002). Viral particles of SARS have been detected in the monocytes, granulocytes, and lymphocytes of SARS patients with T cells as the most infected immune cells (Gu et al., 2005).
Another possible gateway for the virus is through the circumventricular organs (CVOs). Molecules in the blood stream can communicate with the brain through CVOs without crossing the BBB (Ganong, 2000). Once termed “the windows of the brain”, CVOs are characterized by their high capillary permeability and the absence of a BBB (Duvernoy and Risold, 2007; Gross et al., 1987). Circumventricular organs are so called since they surround the third ventricle (neurohypophysis, vascular organ of the lamina terminalis, subfornical organ, pineal gland and subcommissural organ) and the fourth ventricle (area postrema) (Duvernoy and Risold, 2007). They function to regulate molecular transport, carry out an immunological response against invaders, and interact with autonomic centers like the hypothalamus (Siso et al., 2010). Various chemicals signals are communicated with the hypothalamus and the brainstem through interconnections to regulate salt and water balance, cardiovascular function, immunomodulation and energy metabolism (Benarroch, 2011). However, this comes hand in hand with decreased protection and increased exposure to viral particles in the blood.
The hypothalamus is exposed to CVOs that include the subfornical zone, organum vasculosum, median eminence, and area postrema (AP) (Card and Rinaman, 2002). The AP is considered the “emetic chemoreceptor trigger zone” (MacDougall and Sharma, 2022). The neurocircuit of the AP also includes the dorsal vagal complex (DVC), NTS and DMV (Mirza and Das, 2021). It has been proposed that the high expression of ACE-2 in CVOs and PVN might be utilized by SARS-CoV-2 to gain entry into the hypothalamus (Ong et al., 2022). Hence, the presence of the virus in any of these structures could elicit signs of nausea and vomiting observed in patients with COVID-19 (Livanos et al., 2021). The median eminence is one particularly permeable region, found adjacent to the arcuate nucleus (ARC) in the hypothalamus (Rodríguez et al., 2010). The relatively increased permeability enables the median eminence to access the portal blood, while the ARC accesses the CSF, providing them with a somewhat “private milieu” (Rodríguez et al., 2010). Also, the organum vasculosum and the subfornical zone govern important physiological functions. The organum vasculosum is located along the rostral wall of the third ventricle and is in charge of fluid homeostasis (Benarroch, 2011). On the other hand, the subfornical zone is located on the anterodorsal wall of the third ventricle and senses circulating hormones that send signals regarding fluid balance (Benarroch, 2011). We propose that through invading these fenestrated locations, the virus can target autonomic centers in the brain.
5.1.2. Hypothalamic impairment
Input to the hypothalamus is neural or hematogenous; thus, its connections can be utilized by SARS-CoV-2 to invade different nuclear groups resulting in loss of control over autonomic functions. As mentioned before, the PVN plays an important role in controlling the body's stress response. It has been suggested that direct binding of SARS-CoV-2 to ACE-2 receptors in the PVN circuitry can underlie daytime fluctuations in fatigue states, alertness, and anxiety following COVID-19 (Rosenzweig et al., 2020). Resident GABAergic neurons in the PVN express ACE-2 receptors (Mukerjee et al., 2019). It can be hypothesized that chronic stress induced by long COVID can modulate the plasticity of PVN interneurons. Another viewpoint posits that SARS-CoV-2 may indirectly impair PVN function by translating inflammatory signals into stress signals that overwhelm the PVN resulting in neuroinflammation and autonomic disruption (Mackay, 2021).
Various studies that have documented hypothalamic involvement in patients with COVID-19 and the correlation between severity of prognosis and the attenuation of hypothalamic-pituitary response (Alzahrani et al., 2021; Pascual-Goñi et al., 2020; Zheng et al., 2021). While the acute stage of virus infection leads to HPA hyperactivation and hypercorticolism, hypocorticolism has been reported as a more delayed symptom of COVID-19 (Leow et al., 2005; Vassiliou et al., 2021). Hypothalamic-pituitary-adrenal hyporesponsiveness post-COVID may be explained by impaired functioning of adrenergic and catecholaminergic neurons in the nucleus of NTS and ventrolateral medulla oblongata of the brainstem responsible for directly activating PVN CRH neurons (Aguilera and Liu, 2012). Indirect impairment of GABAergic and glutamatergic interneurons may indirectly damage PVN CRH neurons as well leading to decreased CRH release (Aguilera and Liu, 2012). We hypothesize that the plasticity of the PVN can also be utilized by SARS-CoV-2 to modulate the function of medial PVN CRH neurons resulting in GR upregulation and HPA suppression. Further investigation is required to understand the interplay between impaired autonomic function and the possible maladaptive plasticity of the PVN in long COVID.
5.1.3. Medulla oblongata damage
Autopsy studies have revealed the presence of SARS-CoV-2 viral proteins in the medulla oblongata either in neuronal and glial cells or in the vagus and glossopharyngeal nerves (Bulfamante et al., 2021; Matschke et al., 2020). Histopathological assessment of the brainstem highlighted an increase in neuronal damage along with an increase in ionized calcium binding adaptor molecule (Iba1) expression indicative of microglial activation (Bulfamante et al., 2021). Hence, it has been suggested that long COVID can be accompanied by brainstem dysfunction caused by progressive neuroinflammation and neurodegeneration (Yong, 2021). Brainstem dysfunction may occur as a result of direct viral invasion via ACE2 and NRP1 receptors, neuroinflammation through microglial/astrocytic activation and leukocyte infiltration, and finally by vascular activation (Yong, 2021).
As discussed in the previous section, the AP exposes the NTS to chemical signals in the blood that may activate the vomiting reflex (Cutsforth-Gregory and Benarroch, 2017). The medulla and pons exhibit the highest expression of ACE-2 receptors in the brain (Lukiw et al., 2020). It can be hypothesized that viral invasion of medullary nuclei and consequent cell lysis may lead to impaired autonomic reflexes. Moreover, GABAergic interneurons receive converging excitatory signals from multiple descending pathways or primary afferents of the NTS and send out inhibitory signals to modulate medullary reflexes (McDougall and Andresen, 2012). Any disruption at the level of the NTS GABAergic integrative network may impair these autonomic reflexes (Mei et al., 2003; Travagli et al., 2006; Wasserman et al., 2002). Astrocytes in the NTS release glutamate that acts on vagal afferents via NMDA receptors containing GluN2B and GluN2C/D subunits to modulate NTS excitability (Vance et al., 2015). Examination of the postmortem tissues of patients with COVID-19 has shown that astrocytes are predominantly infected by SARS-CoV-2 through NRP1 receptors (Crunfli et al., 2021). These infected astrocytes exhibited changes in glucose and glutamine metabolism which in turn caused neuronal dysfunction and altered plasticity (Crunfli et al., 2021). Neurotoxic factors were shown to be released by infected astrocytes leading to changes in cortical thickness in COVID-19 patients through promoting progressive neurodegeneration (Crunfli et al., 2021). Thus, it can be hypothesized that direct infection of NTS astrocytes can alter normal autonomic reflexes by promoting neuronal death or altered function in other medullary nuclei. We suggest that local GABAergic interneurons in the NTS along with astrocytes can be targeted by SARS-CoV-2 thus rewiring the interconnections of the medulla.
5.2. Autoimmunity
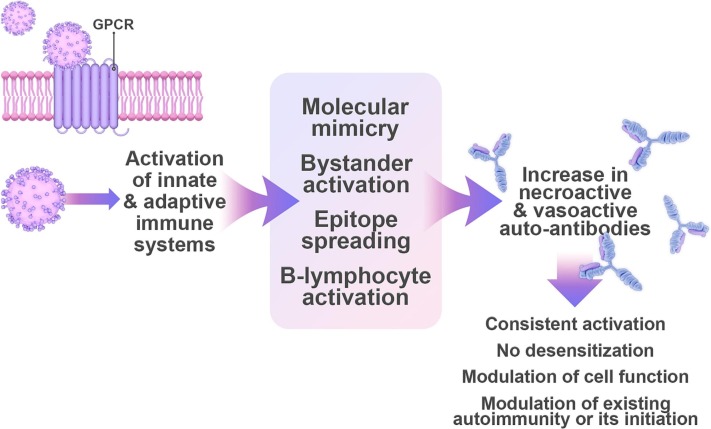
One of the mechanisms behind a common form of AD, POTS, has been attributed to the formation of autoantibodies suggesting an autoimmune basis (Gunning et al., 2019) (Fig. 2 ). Receptor autoantibodies act as agonists and activate their corresponding receptors in a consistent fashion preventing normal desensitization (Wallukat et al., 1991). They can also modulate the function of cells and even trigger synaptic changes between neuronal connections (Dalmau et al., 2017). Specifically, patients with POTS exhibit high levels of G-protein-coupled adrenergic A1 receptor and muscarinic acetylcholine M4 receptor autoantibodies (Gunning et al., 2019). Autoantibodies against adrenergic A2, B1, and B2 receptors along with muscarinic receptors are only observed in patients who express antibodies against adrenergic A1 receptors (Gunning et al., 2019). Measuring the activity of autoantibodies against different subtypes of G-protein-coupled receptors (GPCRs) in the serum of patients with POTS revealed that the activity of adrenergic A1 receptors correlated with POTS symptoms more significantly than adrenergic B2 receptors, muscarinic M2 receptors, and opioid receptor-like 1 (Kharraziha et al., 2020). As such, the activity of adrenergic A1 receptors may act as a vital diagnostic marker for POTS (Kharraziha et al., 2020).
Fig. 2.
Autoimmunity is one mechanism by which SARS-CoV-2 might trigger autonomic dysfunction. Activation of the innate and adaptive immune responses contributes to inflammation and autoimmunity. The production of neuroactive and vasoactive autoantibodies leads to persistent activation of their corresponding receptors. Mechanisms of virus-induced autoimmunity include molecular mimicry, bystander activation, epitope spreading, and B lymphocyte immortalization Autoimmunity caused by long COVID impacts cell function, exacerbate immune-related symptoms, or initiate new symptoms of autonomic dysfunction. Abbreviations: GPCR: G-protein-coupled receptor.
There is an interesting interplay between viral infections and autoimmune disorders be it the modulation of existing autoimmune disorders like MS and rheumatoid arthritis or their initiation (Smatti et al., 2019). In fact, patients with long COVID have surprisingly high levels of autoantibodies against GPCRs (Wallukat et al., 2021). These patients suffer from long COVID symptoms that include fatigue, attention deficit, tachycardia, hypertension, but most importantly POTS and dysautonomia (Wallukat et al., 2021). They also have variable levels of autoantibodies against adrenergic A1 receptors, adrenergic B2 receptors, muscarinic M2 receptors, nociceptin receptors, endothelin receptors, MAS receptors, angiotensin II AT1 receptor, but only the autoantibodies against adrenergic B2 and muscarinic M2 receptors are present in all recovered patients (Wallukat et al., 2021). Overall, the study demonstrated that the corresponding vasoactive changes induced by these autoantibodies in addition to inflammatory or ischemic mediators are responsible for the post-COVID-19 symptoms (Wallukat et al., 2021). Another study that utilized Rapid Extracellular Antigen Profiling to check for autoantibodies against 2770 proteins in patients with COVID-19 identified the presence of autoantibodies against orexin receptors (HCRT2R) in the hypothalamus that correlated negatively with the Glasgow Coma Scale scores (Wang et al., 2021). These receptors are GPCRs distributed throughout the PVN, tuberomammillary nuclei, and autonomic nuclei of the medulla to mediate arousal and the stress response (Grimaldi et al., 2014). They receptors exert autonomic control by regulating arterial blood pressure, cardiovagal response, cardiovascular reflexes, respiratory function, and gastrointestinal function (Grimaldi et al., 2014). Even though the study found autoantibodies against HCRT2R in 8 out of 194 COVID-19 patients, it is worth checking the involvement of this autoantibody in COVID-19 patients with dysautonomia. Another noteworthy antibody, which was found to be elevated in patients with long COVID (n = 9), is trisulfated heparin disaccharide (TS-HDS) antibody (44 %) (Novak et al., 2022). Trisulfated heparin disaccharide is a constituent of heparin and heparan sulfate located in peripheral nerves (Levine et al., 2020). Increased expression of TS-HDS autoantibodies is a marker of small fiber neuropathy (Levine et al., 2020). In the aforementioned study, dysautonomia without orthostatic hypotension was detected in all tested patients with long COVID and 78 % exhibited small fiber neuropathy with chronic mild pain (Novak et al., 2022). Therefore, it is hypothesized that vasculitis induced by TS-HDS antibodies can cause small fiber neuropathy and consequently dysautonomia (Novak et al., 2022). A larger sample size is required to assess this mechanism especially given the prevalence of small fiber neuropathy in patients with long COVID (Oaklander et al., 2022).
In patients with an immune-mediated inflammatory disease, SARS-CoV-2 infection may induce the reemergence of immune-related symptoms or introduce new immune-mediated inflammatory diseases (Winchester et al., 2021). For example, a patient with a history of small fiber neuropathy and orthostatic cerebral hypoperfusion syndrome, which developed due to Lyme disease treatment, experienced an exacerbation of symptoms post-COVID-19 infection (Novak, 2020). The patient had central symptoms including brain fog, fatigue, and orthostatic dizziness along with peripheral symptoms of painful burning sensation in the extremities that were resolved by immunotherapy (Novak, 2020). Therefore, it was probable that the symptoms of AD following COVID-19 infection reemerged via an autoimmune mechanism (Novak, 2020). Another beneficial impact of immunotherapy was demonstrated by the use of BC 007 to neutralize autoantibodies against GPCRs in a patient with long COVID resulting in attenuated fatigue and capillary impairment (Hohberger et al., 2021).
The mechanism by which SARS-CoV-2 is activating autoantibodies is not clear, yet proposed mechanisms of virus-induced autoimmunity include molecular mimicry, bystander activation, epitope spreading, and B lymphocyte immortalization (Hui-Yuen et al., 2014; Smatti et al., 2019). An indirect cause of autoimmunity is persistent inflammation and immune activation (Mobasheri et al., 2022). A dysregulated immune system may perpetuate inflammation resulting in organ damage affecting the cardiovascular, renal, pulmonary, digestive, and nervous systems (Liu et al., 2021). Both the innate and adaptive immune responses contribute to autoimmunity (Liu et al., 2021). After antigen presenting cells phagocytize viral particles, they are presented to CD4+ T cells triggering their activation (Mobasheri et al., 2022). Activated CD4+ T cells release pro-inflammatory cytokines and promote antibody secretion by B cells (Mobasheri et al., 2022). Autoimmunity can result from molecular mimicry whereby the similarity between the self and viral antigens causes the attack of host surface proteins (Cusick et al., 2012). It is suggested that SARS-CoV-2 can induce an autoimmune response via molecular mimicry (Liu et al., 2021). A link between autoinflammation and autoimmunity is represented by the mixed pattern disease (Rodríguez et al., 2020). Evidence has recently shown that POTS may in fact be a mixed pattern inflammatory disease mediated by T cells (Gunning et al., 2022). This was based on a study that showed a platelet delta granule storage pool deficiency in patients with POTS and GPCR autoantibodies suggesting innate system activation (Gunning et al., 2021; Gunning et al., 2022). It remains to be investigated whether the innate and adaptive immune systems contribute to autoantibody production against adrenergic and cholinergic receptors following SARS-CoV-2 infection.
5.3. Persistent inflammation, hypoxia, and sympathetic overactivation
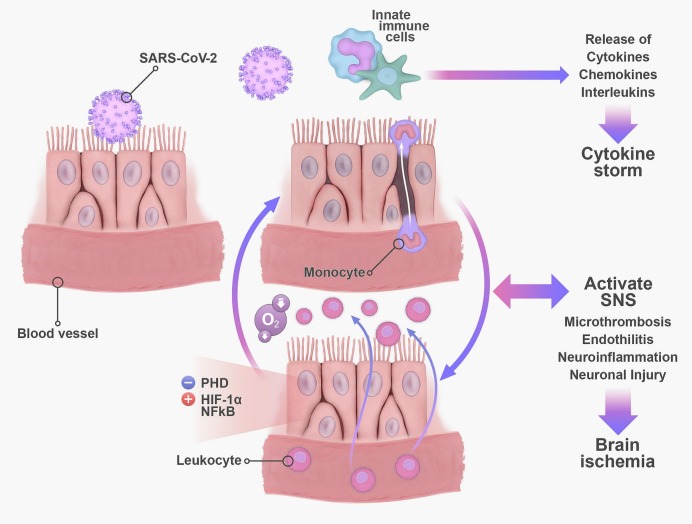
Along with autoimmunity, persistent systemic inflammation may play a central role in the underlying pathophysiology of long COVID (Mehandru and Merad, 2022). Coronavirus disease 2019 is associated with an exaggerated inflammatory response that is characterized by a cytokine storm (Ragab et al., 2020). The innate immune response releases inflammatory cytokines which in turn signal the transport of immune cells to the site of injury exacerbating tissue damage (Ragab et al., 2020). These cytokines include interferons, tumor necrosis factor α (TNF α), colony stimulating factors, chemokines, and interleukins (IL) (Coperchini et al., 2020). Seven to eleven months following COVID-19, patients exhibit an upregulation in pro-inflammatory mediators such as TNF α, IL-6, IL-13, IL-17A, and IL1-β along with a sustained increase in naïve B cells and effector T cells (Acosta-Ampudia et al., 2022). There is a bidirectional interplay between inflammation and SNS activation (Pongratz and Straub, 2014). Acute inflammation triggers the SNS to adopt a pro-inflammatory response, which then switches to an anti-inflammatory response (Pongratz and Straub, 2014). However, with chronic inflammation, the SNS pro-inflammatory response persists (Pongratz and Straub, 2014). A chronic state of systemic inflammation potentiates the activities of the SNS and HPA axis resulting in chronic inflammatory effects like increased cardiovascular risk and hypertension (Pongratz and Straub, 2014). Hypoxia is another mediator of sympathetic overactivation, which has been suggested to cause AD in patients with COVID-19 (Al-Kuraishy et al., 2021; Porzionato et al., 2020). This sympathetic overactivation triggers pro-inflammatory cytokine release and organ damage further worsening this vicious cycle (Al-Kuraishy et al., 2021; Porzionato et al., 2020). In COVID-19, pneumonia causes silent hypoxia, a condition in which oxygen saturation levels drop without breathing problems (Levitan, 2020; Rahman et al., 2021). This occurs through lung alveolar epithelial injury and surfactant dysfunction that causes alveoli and air sacs to collapse decreasing oxygen levels (Ochs et al., 2021).
Interestingly, hypoxia and inflammation work together in a positive feedback loop involving the enzyme prolylhydroxylase (PHD) and with the ability to exacerbate capillary damage in COVID-19 (Bartels et al., 2013; Østergaard, 2021) (Fig. 3 ). An increase in pro-inflammatory cells and ILs damages capillary endothelial cells and mitochondrial function in addition to promoting an influx of leukocytes and increased oxygen consumption by inflammatory cells (Yang and Dunn, 2019). Consequently, the decrease in oxygen supply in the affected tissues promotes hypoxia by inhibiting PHD and allowing the activation of hypoxia inducible factor 1 alpha (HIF-1α) and nuclear factor kappa B (NF-κB), a master inducer of inflammation (Bartels et al., 2013). Both HIF-1α and NF-κB are important transcriptional regulators of immune cells that establish communication between immunity and inflammation (Taylor and Colgan, 2017). This hypoxia-inflammation crosstalk can be responsible for persistent COVID-19 symptoms and microvascular disruptive processes like microthrombosis and endotheliitis (Østergaard, 2021). In fact, an examination of the brains of SARS-CoV-2 infected non-human primates revealed significant neuroinflammation, neuronal injury and death, and microhemorrhages related to hypoxia/ischemia along with upregulated HIF-1α (Rutkai et al., 2022). These results were consistent with autopsy examinations of patients with COVID-19 that revealed brain ischemic injury (Fabbri et al., 2021; Matschke et al., 2020).
Fig. 3.
Persistent inflammation, hypoxia, and sympathetic overactivation can result in autonomic dysfunction in long COVID. The activation of the innate immune cells following SARS-CoV-2 infection results in a cytokine storm characterized by the great release of interleukins and chemokines. As more leukocytes migrate to the site of infection, more oxygen is consumed leading to a hypoxic state. The decrease in oxygen supply in the affected tissues promotes hypoxia by inhibiting PHD and allowing the activation of HIF-1α and NF-κB, a master inducer of inflammation. This positive loop promotes SNS overactivation that can eventually induce neuroinflammation and cell death. Abbreviations: PHD: Prolylhydroxylase; NF-κB: nuclear factor kappa B; HIF-1α: hypoxia inducible factor 1 alpha; SNS: sympathetic nervous system.
5.4. Renin-angiotensin system imbalance
The binding of SARS-CoV-2 to ACE-2 receptors indicates an interaction between COVID-19 and the renin-angiotensin system (RAS) (Khazaal et al., 2022). This system plays an important role in the maintenance of blood pressure and volume thus impacting the cardiovascular, renal, and pulmonary systems (Khazaal et al., 2022). Patients with COVID-19 display AD one year after infection characterized by blunted heart rate recovery (HRR), and exaggerated blood pressure response to exercise (EBPR) in addition to high levels of uric acid (Inanc and Sabanoglu, 2022). This increases the risk of patients with long COVID to develop cardiovascular disease and other metabolic disorders (Inanc and Sabanoglu, 2022). As a regulator of cardiovascular functions, RAS activation contributes to AD pathophysiology (Díaz et al., 2020). There exists a positive feedback loop between the ANS and RAS whereby angiotensin peptides can bind to receptors expressed in the medulla, sympathetic preganglionic neurons, sympathetic ganglia, nerve terminals, or vagal afferents that in turn control neurotransmitter release at the level of organs involved in the synthesis of these angiotensin peptides (Miller and Arnold, 2019). Increased RAS activation can therefore enhance SNS tone and decrease PNS tone resulting in cardiovascular and autonomic dysregulation (Miller and Arnold, 2019). Renin-angiotensin system imbalance has been suggested to be a driving factor of COVID-19 pathophysiology (Rysz et al., 2021). Given the important role of RAS in autonomic regulation and the observed cardiovascular/metabolic dysfunction in patients with long COVID, RAS imbalance may play a role in the development of AD post-COVID-19.
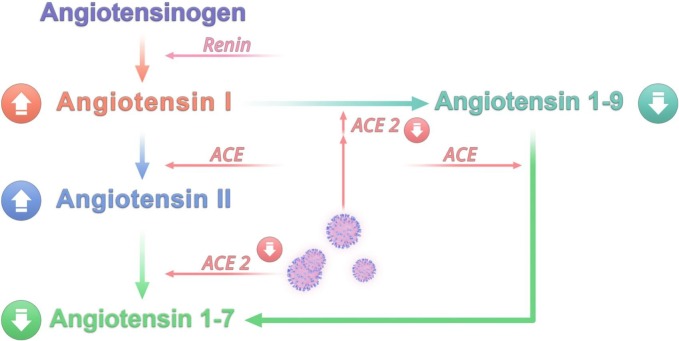
In the RAS pathway, renin converts angiotensinogen (AGT) into angiotensin I (Ang I) (El-Arif et al., 2021). Angiotensin converting enzyme (ACE) transforms Ang I into Angiotensin II (Ang II) (El-Arif et al., 2021). In turn, ACE-2 cleaves Ang I and Ang II into Ang 1–9 and Ang 1–7 respectively (El-Arif et al., 2021). Renin-angiotensin system balance is controlled by anti-inflammatory and pro-inflammatory pathways (El-Arif et al., 2021). The binding of Ang II to angiotensin type-1 receptor (AT1R) promotes vasoconstriction, oxidative stress, apoptosis, fibrosis, and inflammation (El-Arif et al., 2021). However, Ang II and Ang 1–7 support the protective anti-inflammatory pathway by binding to angiotensin type-2 receptor (AT2R) and GPCR Mas (MasR) respectively (El-Arif et al., 2021). This pathway results in vasodilation and protects against the detrimental effects of AT1R activation (El-Arif et al., 2021). Different organs express AT1R such as the lungs, heart, brain, kidneys, and blood vessels (Su et al., 2021). Several brain regions contain AT1R including hypothalamic and brainstem nuclei such as PVN, NTS, supraoptic nuclei, median preoptic nucleus, and rostral ventrolateral medulla in addition to CVOs like the organum vasculosum and subfornical organs (Su et al., 2021). Its activation induces sympathetic effects that include different kinds of hypertension depending on CNS localization (Su et al., 2021).
The RAS imbalance in patients with COVID-19 is triggered by the downregulation of ACE-2 receptors upon SARS-CoV-2 infection (Silhol et al., 2020). It is characterized by the accumulation of Ang I and Ang II along with a drop in the levels of Ang 1–9 and Ang 1–7 (Silhol et al., 2020) (Fig. 4 ). This has been supported by a study on patients with COVID-19 who displayed high levels of Ang II that were associated with viral load (Liu et al., 2020). Consequently, AT1R is hyperactivated by Ang II triggering the pro-inflammatory pathway and sympathetic effects. Studies examining RAS imbalance in patients with COVID-19 exhibit conflicting results. In one study, it was shown that patients with COVID-19 had elevated levels of Ang II and low levels of Ang 1–7 despite high ACE-2 activity compared to control subjects (Amezcua-Guerra et al., 2022). There was also a significant increase in Ang II/Ang 1–7 ratio that correlated with decreased ACE-2 activity (Amezcua-Guerra et al., 2022). Patients who died had a significantly low Ang II/Ang 1–7 ratio (Amezcua-Guerra et al., 2022). Measuring ACE-2 activity in the tissues rather than in the blood may better reflect ACE-2 function and explain the contradictory results regarding ACE-2 activity in this study (Amezcua-Guerra et al., 2022). However, other studies demonstrated an increase in Ang 1–7 and a decrease in Ang II (Reindl-Schwaighofer et al., 2021; Valle Martins et al., 2021). In one of these studies, the Ang 1–7/Ang II ratio was increased from early to late in patients with severe COVID reflecting enhanced ACE-2 activity and a shift towards a preferential protective effect of RAS during late stages of COVID-19 (Reindl-Schwaighofer et al., 2021). Unfortunately, the other study did not provide a measurement of ACE-2 activity nor Ang 1–7/Ang II ratio to explain the observed increase in Ang 1–7 and decrease in Ang II (Valle Martins et al., 2021). In a clinical trial, Ang 1–7 (TXA 127) was administered to patients with severe COVID-19 to test its ability to reduce multi-organ failure (Wagener et al., 2022). However, the study was terminated due the high discharge rate of patients with only 3 remaining patients who completed the 10-day schedule of TXA 127 administration (Wagener et al., 2022). No adverse effects were reported, yet no change in outcome was detected due to the small sample size (Wagener et al., 2022). Larger clinical trials that account for the length of hospital stay upon deciding the duration of drug administration are needed. The conflicting results regarding the change in angiotensin levels, Ang II/Ang 1–7 ratio, and ACE-2 activity highlight the need for further clinical studies with improved methods to assess the true activity of ACE-2. This could help investigate the role of the RAS imbalance in AD during long COVID.
Fig. 4.
An imbalance in the renin-angiotensin system can be responsible for autonomic dysfunction following SARS-CoV-2 infection. The downregulation of ACE-2 upon SARS-CoV-2 cell entry in addition to the formation of autoantibodies against ACE-2 and AT1R are important contributors to such imbalance. Consequently, levels of angiotensin I and angiotensin II increase while levels of angiotensin 1–9 and angiotensin 1–7 drop. This results in the hyperactivation of AT1R responsible for mediating the pro-inflammatory pathway and sympathetic effects. Abbreviations: ACE: angiotensin-converting enzyme.
The formation of ACE-2 and AT1R autoantibodies can also play a role in RAS imbalance post-COVID-19. Studies have shown elevated levels of ACE-2 and AT1R autoantibodies in patients with COVID-19 potentiating disease severity and inflammation (Jiang et al., 2021; Rodriguez-Perez et al., 2021). Patients with POTS also possess AT1R autoantibodies with a high activity capable of inhibiting Ang II activity and consequent vasoconstriction (Yu et al., 2018). These autoantibodies impair the normal response to upright posture by affecting vascular function (Yu et al., 2018). Up to our knowledge, there are no studies that investigated the association between AT1R autoantibodies and AD onset post-COVID-19. Such work would help diagnose AD in patients with long COVID by measuring the levels of RAS enzymes as useful biomarkers in addition to improving therapy via AT1R blockers.
6. Conclusion
The emergence of AD as one of many complications of long COVID has highlighted the importance of understanding AD pathophysiology. This can be challenging since the symptoms of AD are multifaceted, and dysregulation in the ANS can occur either at the level of the autonomic centers, the sympathetic and parasympathetic connections, or the target organs. In this review, it is hypothesized that direct invasion of the hypothalamus or the medulla by SARS-CoV-2 can induce AD. This direct mode of invasion may occur via neuronal routes or through the blood. At the level of the invaded autonomic centers, the plasticity of the PVN network may be modulated directly or indirectly by targeting GABAergic or glutamatergic interneurons or astrocytes. However, emerging studies are proposing that SARS-CoV-2 may cause neurological manifestations such as AD indirectly rather than directly via neuronal invasion (Ho et al., 2022; Spudich and Nath, 2022). Inflammation and immune activation may be the main inducers (Spudich and Nath, 2022). Indeed, one possible indirect mechanism is through autoimmunity in which neuroactive and vasoactive autoantibodies are produced resulting in persistent activation of their corresponding receptors. Autoimmunity caused by long COVID can also impact cell function, exacerbate immune-related symptoms, or initiate new symptoms of AD. Moreover, the interaction between persistent systemic inflammation, hypoxia, and SNS overactivation can play a major role in AD onset. Renin-angiotensin system imbalance is a possible inducer of AD in long COVID capable of triggering pro-inflammatory pathway with SNS effects. The downregulation of ACE-2 upon SARS-CoV-2 cell entry in addition to the formation of autoantibodies against ACE-2 and AT1R are important contributors to such imbalance. Further research is needed to investigate the effect of the above mechanisms on AD development post-COVID-19. Understanding AD in patients with long COVID would help in diagnosis and treatment. It is worth studying the effect of comorbidities such as diabetes or hypertension on AD post-COVID given the serious complications that may ensue.
Declaration of competing interest
None.
Acknowledgements
We would like to thank Mrs. Alia Trabolsi for her help in editing the paper.
Data availability
No data was used for the research described in the article.
References
- Abdelnabi M., Eshak N., Almaghraby A. COVID-19 Associated Dysautonomia in a Non-Critically Ill COVID-19 Patient. Am. J. Med. Sci. 2021;362(6):619–620. doi: 10.1016/j.amjms.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Ampudia Y., Monsalve D.M., Rojas M., Rodríguez Y., Zapata E., Ramírez-Santana C., Anaya J.M. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J. Infect. Dis. 2022;225:2155–2162. doi: 10.1093/infdis/jiac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G., Liu Y. The molecular physiology of CRH neurons. Front. Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyegbusi O.L., Hughes S.E., Turner G., Rivera S.C., McMullan C., Chandan J.S., Haroon S., Price G., Davies E.H., Nirantharakumar K., Sapey E., Calvert M.J., Group T.L.C.S. Symptoms, complications and management of long COVID: a review. J.R.Soc.Med. 2021;114:428–442. doi: 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- Al-Kuraishy H.M., Al-Gareeb A.I., Qusti S., Alshammari E.M., Gyebi G.A., Batiha G.E.-S. COVID-19-induced dysautonomia: a menace of sympathetic storm. ASN Neuro. 2021;13 doi: 10.1177/17590914211057635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani A.S., Mukhtar N., Aljomaiah A., Aljamei H., Bakhsh A., Alsudani N., Elsayed T., Alrashidi N., Fadel R., Alqahtani E. The impact of COVID-19 viral infection on the hypothalamic-pituitary-adrenal axis. Endocr. Pract. 2021;27:83–89. doi: 10.1016/j.eprac.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua-Guerra L.M., Del Valle L., González-Pacheco H., Springall R., Márquez-Velasco R., Massó F., Brianza-Padilla M., Manzur-Sandoval D., González-Flores J., García-Ávila C., Juárez-Vicuña Y., Sánchez-Muñoz F., Ballinas-Verdugo M.A., Basilio-Gálvez E., Paez-Arenas A., Castillo-Salazar M., Cásares-Alvarado S., Hernández-Diazcouder A., Sánchez-Gloria J.L., Tavera-Alonso C., Gopar-Nieto R., Sandoval J. The prognostic importance of the angiotensin II/angiotensin-(1–7) ratio in patients with SARS-CoV-2 infection. Ther. Adv. Respir. Dis. 2022;16 doi: 10.1177/17534666221122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A.C., Haman K., Garland E.M., Raj V., Dupont W.D., Biaggioni I., Robertson D., Raj S.R. Cognitive dysfunction in postural tachycardia syndrome. Clin. Sci. 2015;128:39–45. doi: 10.1042/CS20140251. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A.C., Ng J., Lei L., Raj S.R. Autonomic dysfunction in cardiology: pathophysiology, investigation, and management. Can. J. Cardiol. 2017;33:1524–1534. doi: 10.1016/j.cjca.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T., Zhou D., Yushanjiang F., Wang D., Zhang D., Liu X., Song J., Zhang J., Hou X., Ma Y. Alternation of the autonomic nervous system is associated with pulmonary sequelae in patients with COVID-19 after six months of discharge. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.805925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E., Lui F. StatPearls Publishing; 2021. Neuroanatomy, Vagal Nerve Nuclei, StatPearls [Internet] [PubMed] [Google Scholar]
- Bartels K., Grenz A., Eltzschig H.K. Hypoxia and inflammation are two sides of the same coin. Proc. Natl. Acad. Sci. 2013;110:18351–18352. doi: 10.1073/pnas.1318345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L., Laksono B.M., de Vrij F.M.S., Kushner S.A., Harschnitz O., van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022;45:358–368. doi: 10.1016/j.tins.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear M.H., Reddy V., Bollu P.C. StatPearls Publishing LLC.; Treasure Island (FL): 2022. Neuroanatomy, Hypothalamus, StatPearls. StatPearls Publishing Copyright © 2022. [PubMed] [Google Scholar]
- Beckman D., Bonillas A., Diniz G.B., Ott S., Roh J.W., Elizaldi S.R., Schmidt B.A., Sammak R.L., Van Rompay K.K.A., Iyer S.S., Morrison J.H. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 2022;41 doi: 10.1016/j.celrep.2022.111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. Circumventricular organs: receptive and homeostatic functions and clinical implications. Neurology. 2011;77:1198–1204. doi: 10.1212/WNL.0b013e31822f04a0. [DOI] [PubMed] [Google Scholar]
- Benghanem S., Mazeraud A., Azabou E., Chhor V., Shinotsuka C.R., Claassen J., Rohaut B., Sharshar T. Brainstem dysfunction in critically ill patients. Crit. Care. 2020;24:5. doi: 10.1186/s13054-019-2718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bista P., Imlach W.L. Pathological mechanisms and therapeutic targets for trigeminal neuropathic pain. Medicines. 2019;6 doi: 10.3390/medicines6030091. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitshteyn S., Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol. Res. 2021;69:205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco J., Titano R. Severe post-COVID-19 dysautonomia: a case report. BMC Infect. Dis. 2022;22:1–4. doi: 10.1186/s12879-022-07181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfamante G., Bocci T., Falleni M., Campiglio L., Coppola S., Tosi D., Chiumello D., Priori A. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J. Neurol. 2021;268:4486–4491. doi: 10.1007/s00415-021-10604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoite Stella A., Furlanis G., Frezza N.A., Valentinotti R., Ajcevic M., Manganotti P. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: a prospective multidomain observational study. J. Neurol. 2022;269:587–596. doi: 10.1007/s00415-021-10735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Blasco B., Güemes-Villahoz N., Donate-Lopez J., Vidal-Villegas B., García-Feijóo J. Optic nerve analysis in COVID-19 patients. J. Med. Virol. 2021;93:190–191. doi: 10.1002/jmv.26290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnardo C., Tavares R.F., Resstel L.B., Elias L.L., Correa F.M. Paraventricular nucleus modulates autonomic and neuroendocrine responses to acute restraint stress in rats. Auton. Neurosci. 2010;158:51–57. doi: 10.1016/j.autneu.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Card J.P., Rinaman L. In: Encyclopedia of the Human Brain. Ramachandran V.S., editor. Academic Press; New York: 2002. Hypothalamus; pp. 525–535. [Google Scholar]
- Casagrande M., Fitzek A., Püschel K., Aleshcheva G., Schultheiss H.-P., Berneking L., Spitzer M.S., Schultheiss M. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul. Immunol. Inflamm. 2020:1–5. doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- CDC . 2020. Symptoms of Coronavirus | CDC. @CDCgov. [Google Scholar]
- Chen M., Shen W., Rowan N.R., Kulaga H., Hillel A., Ramanathan M., Jr., Lane A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunfli F., Carregari V.C., Veras F.P., Vendramini P.H., Valença A.G.F., Antunes A.S.L.M., Brandão-Teles C., da Silva Zuccoli G., Reis-de-Oliveira G., Silva-Costa L.C. 2021. SARS-CoV-2 infects brain astrocytes of COVID-19 patients and impairs neuronal viability. MedRxiv, 2020.2010. 2009.20207464. [Google Scholar]
- Cusick M.F., Libbey J.E., Fujinami R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012;42:102–111. doi: 10.1007/s12016-011-8294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsforth-Gregory J.K., Benarroch E.E. Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology. 2017;88:1187–1196. doi: 10.1212/WNL.0000000000003751. [DOI] [PubMed] [Google Scholar]
- Dalmau J., Geis C., Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol. Rev. 2017;97:839–887. doi: 10.1152/physrev.00010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani M., Dirksen A., Taraborrelli P., Torocastro M., Panagopoulos D., Sutton R., Lim P.B. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin. Med. 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani M., Dirksen A., Taraborrelli P., Torocastro M., Panagopoulos D., Sutton R., Lim P.B. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin.Med. 2021;21 doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz H.S., Toledo C., Andrade D.C., Marcus N.J., Del Rio R. Neuroinflammation in heart failure: new insights for an old disease. J. Physiol. 2020;598:33–59. doi: 10.1113/JP278864. [DOI] [PubMed] [Google Scholar]
- Dietrich J.-B. The adhesion molecule ICAM-1 and its regulation in relation with the blood–brain barrier. J. Neuroimmunol. 2002;128:58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- Duvernoy H.M., Risold P.-Y. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res. Rev. 2007;56:119–147. doi: 10.1016/j.brainresrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- El-Arif G., Farhat A., Khazaal S., Annweiler C., Kovacic H., Wu Y., Cao Z., Fajloun Z., Khattar Z.A., Sabatier J.M. The renin-angiotensin system: a key role in SARS-CoV-2-induced COVID-19. Molecules. 2021;26:6945. doi: 10.3390/molecules26226945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdal Y., Atalar A.C., Gunes T., Okluoglu T., Yavuz N., Emre U. Autonomic dysfunction in patients with COVID‑19. Acta Neurol. Belg. 2022;122(4):885–891. doi: 10.1007/s13760-022-01899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri V.P., Foschini M.P., Lazzarotto T., Gabrielli L., Cenacchi G., Gallo C., Aspide R., Frascaroli G., Cortelli P., Riefolo M., Giannini C., D'Errico A. Brain ischemic injury in COVID-19-infected patients: a series of 10 post-mortem cases. Brain Pathol. 2021;31:205–210. doi: 10.1111/bpa.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.V., Latchford K.J., Samson W.K. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets. 2008;12:717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Cuadrado M.L., Florencio L.L. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int. J. Environ. Res. Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester J.V., McMenamin P.G., Dando S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018;19:655–671. doi: 10.1038/s41583-018-0070-8. [DOI] [PubMed] [Google Scholar]
- Ganong W.F. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000;27:422–427. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 "long haulers". Ann.Clin.Transl.Neurol. 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12 doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi D., Silvani A., Benarroch E.E., Cortelli P. Orexin/hypocretin system and autonomic control: new insights and clinical correlations. Neurology. 2014;82:271–278. doi: 10.1212/WNL.0000000000000045. [DOI] [PubMed] [Google Scholar]
- Gross P.M., Weindl A., Knigge K.M. Peering through the windows of the brain. J. Cereb. Blood Flow Metab. 1987;7:663–672. doi: 10.1038/jcbfm.1987.120. [DOI] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning W.T., III, Kvale H., Kramer P.M., Karabin B.L., Grubb B.P. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning W.T., 3rd, Stepkowski S.M., Kramer P.M., Karabin B.L., Grubb B.P. Inflammatory biomarkers in postural orthostatic tachycardia syndrome with elevated G-protein-coupled receptor autoantibodies. J. Clin. Med. 2021;10 doi: 10.3390/jcm10040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning W.T., Kramer P.M., Cichocki J.A., Karabin B.L., Khuder S.A., Grubb B.P. Platelet storage pool deficiency and elevated inflammatory biomarkers are prevalent in postural orthostatic tachycardia syndrome. Cells. 2022;11 doi: 10.3390/cells11050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M., Lely A., Navis G.v., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani M., Fathi Jouzdani A., Motarjem S., Ranjbar A., Khansari N. How COVID-19 can cause autonomic dysfunctions and postural orthostatic syndrome? A review of mechanisms and evidence. Neurol. Clin. Neurosci. 2021;9:434–442. doi: 10.1111/ncn3.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Tasker J.G. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front. Endocrinol. 2016;7:137. doi: 10.3389/fendo.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Flak J., Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog. Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.Y., Salimian M., Hegert J., O'Brien J., Choi S.G., Ames H., Morris M., Papadimitriou J.C., Mininni J., Niehaus P., Burke A., Canbeldek L., Jacobs J., LaRocque A., Patel K., Rice K., Li L., Johnson R., LeFevre A., Blanchard T., Shaver C.M., Moyer A., Drachenberg C. Postmortem assessment of olfactory tissue degeneration and microvasculopathy in patients with COVID-19. JAMA Neurol. 2022;79:544–553. doi: 10.1001/jamaneurol.2022.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoad A., Spickett G., Elliott J., Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. QJM. 2008;101:961–965. doi: 10.1093/qjmed/hcn123. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohberger B., Harrer T., Mardin C., Kruse F., Hoffmanns J., Rogge L., Heltmann F., Moritz M., Szewczykowski C., Schottenhamml J., Kräter M., Bergua A., Zenkel M., Gießl A., Schlötzer-Schrehardt U., Lämmer R., Herrmann M., Haberland A., Göttel P., Müller J., Wallukat G. Case report: neutralization of autoantibodies targeting G-protein-coupled receptors improves capillary impairment and fatigue symptoms after COVID-19 infection. Front. Med. 2021;8 doi: 10.3389/fmed.2021.754667. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui-Yuen J., Koganti S., Bhaduri-McIntosh S. Monoclonal Antibodies. Springer; 2014. Human B cell immortalization for monoclonal antibody production; pp. 183–189. [DOI] [PubMed] [Google Scholar]
- Inanc I.H., Sabanoglu C. Autonomic dysfunction and metabolic disorders as the possible sequelae of COVID-19 infection. Eur. Rev. Med. Pharmacol. Sci. 2022;26:5587–5595. doi: 10.26355/eurrev_202208_29431. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Duffy F., Hadlock J., Raappana A., Styrchak S., Beck I., Mast F.D., Miller L.R., Chour W., Houck J., Armistead B., Duvvuri V.R., Yeung W., Haglund M., Wallner J., Wallick J.A., Hardy S., Oldroyd A., Ko D., Gervassi A., Murray K.M., Kaplan H., Aitchison J.D., Heath J.R., Sather D.N., Goldman J.D., Frenkel L., Harrington W.E. Angiotensin II receptor I auto-antibodies following SARS-CoV-2 infection. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0259902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjwal K., Jamal S., Kichloo A., Grubb B.P. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J. Innov. Card. Rhythm Manag. 2020;11:4302–4304. doi: 10.19102/icrm.2020.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharraziha I., Axelsson J., Ricci F., Di Martino G., Persson M., Sutton R., Fedorowski A., Hamrefors V. Serum activity against g protein–coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.015989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaal S., Harb J., Rima M., Annweiler C., Wu Y., Cao Z., Abi Khattar Z., Legros C., Kovacic H., Fajloun Z. The pathophysiology of long COVID throughout the renin-angiotensin system. Molecules. 2022;27:2903. doi: 10.3390/molecules27092903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Everitt B.J., Robbins T.W. In: Fundamental Neuroscience. Fourth edition. Squire L.R., Berg D., Bloom F.E., du Lac S., Ghosh A., Spitzer N.C., editors. Academic Press; San Diego: 2013. Chapter 41 - reward, motivation, and addiction; pp. 871–898. [Google Scholar]
- Kramer-Hämmerle S., Rothenaigner I., Wolff H., Bell J.E., Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Leow M.K.S., Kwek D.S.K., Ng A.W.K., Ong K.C., Kaw G.J.L., Lee L.S.U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin. Endocrinol. 2005;63:197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T.D., Kafaie J., Zeidman L.A., Saperstein D.S., Massaquoi R., Bland R.J., Pestronk A. Cryptogenic small-fiber neuropathies: serum autoantibody binding to trisulfated heparan disaccharide and fibroblast growth factor receptor-3. Muscle Nerve. 2020;61:512–515. doi: 10.1002/mus.26748. [DOI] [PubMed] [Google Scholar]
- Levitan R.M. Pulse oximetry as a biomarker for early identification and hospitalization of COVID-19 pneumonia. Acad. Emerg. Med. 2020;27:785–786. doi: 10.1111/acem.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M., Siokas V., Aloizou A.M., Liampas I., Mentis A.A., Tsouris Z., Papadimitriou A., Mitsias P.D., Tsatsakis A., Bogdanos D.P., Baloyannis S.J., Dardiotis E. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr. Treat. Options Neurol. 2020;22:37. doi: 10.1007/s11940-020-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liposits Z., Uht R., Harrison R., Gibbs F., Paull W., Bohn M. Ultrastructural localization of glucocorticoid receptor (GR) in hypothalamic paraventricular neurons synthesizing corticotropin releasing factor (CRF) Histochemistry. 1987;87:407–412. doi: 10.1007/BF00496811. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021;33:155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livanos A.E., Jha D., Cossarini F., Gonzalez-Reiche A.S., Tokuyama M., Aydillo T., Parigi T.L., Ladinsky M.S., Ramos I., Dunleavy K. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160(2435–2450) doi: 10.1053/j.gastro.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw W.J., Pogue A., Hill J.M. SARS-CoV-2 infectivity and neurological targets in the brain. Cell. Mol. Neurobiol. 2020:1–8. doi: 10.1007/s10571-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M.R., Sharma S. StatPearls Publishing LLC.; Treasure Island (FL): 2022. Physiology, Chemoreceptor Trigger Zone, StatPearls. StatPearls Publishing Copyright © 2022</span>. [PubMed] [Google Scholar]
- Mackay A. A paradigm for post-Covid-19 fatigue syndrome analogous to ME/CFS. Front. Neurol. 2021;1334 doi: 10.3389/fneur.2021.701419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gallego A., Andrade-Andrade I., Dawid-Milner M., Domínguez-Páez M., Romero-Moreno L., González-García L., Carrasco-Brenes A., Segura-Fernández-Nogueras M., Ros-López B., Arráez-Sánchez M. Autonomic dysfunction elicited by a medulla oblongata injury after fourth ventricle tumor surgery in a pediatric patient. Auton. Neurosci. 2016;194:52–57. doi: 10.1016/j.autneu.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Martín-Gallego A., González-García L., Carrasco-Brenes A., Segura-Fernández-Nogueras M., Delgado-Babiano A., Ros-Sanjuán A., Romero-Moreno L., Domínguez-Páez M., Dawid-Milner M.S., Arráez-Sánchez M.A. Brainstem and autonomic nervous system dysfunction: a neurosurgical point of view. Acta Neurochir. Suppl. 2017;124:221–229. doi: 10.1007/978-3-319-39546-3_34. [DOI] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S.J., Andresen M.C. Low-fidelity GABA transmission within a dense excitatory network of the solitary tract nucleus. J. Physiol. 2012;590:5677–5689. doi: 10.1113/jphysiol.2012.241976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022:1–9. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L., Zhang J., Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am. J. Phys. Regul. Integr. Comp. Phys. 2003;285:R1276–R1286. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Mennella J., Bobowski N., Liem D.G. Taste And Smell. 2017. pp. 58–64. [Google Scholar]
- Metzler M., Duerr S., Granata R., Krismer F., Robertson D., Wenning G.K. Neurogenic orthostatic hypotension: pathophysiology, evaluation, and management. J. Neurol. 2013;260:2212–2219. doi: 10.1007/s00415-012-6736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miana Gabriela P., Carmen C., Iulian O. In: Hypothalamus in Health And Diseases. Stavros J.B., Jan Oxholm G., editors. IntechOpen; Rijeka: 2018. Anatomy and function of the hypothalamus. pp. Ch. 1. [Google Scholar]
- Miglis M.G., Prieto T., Shaik R., Muppidi S., Sinn D.-I., Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 2020;30:449–451. doi: 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo V., Di Stefano C., Milan A., Ravera A., Sobrero G., Sabia L., Veglio F., Maule S. Cardiovascular complications in patients with autonomic failure. Clin. Auton. Res. 2015;25:133–140. doi: 10.1007/s10286-015-0275-0. [DOI] [PubMed] [Google Scholar]
- Miller A.J., Arnold A.C. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin. Auton. Res. 2019;29:231–243. doi: 10.1007/s10286-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic B., Djajic V., Bajic D., Djokovic A., Krajnovic T., Jovanovic S., Verhaz A., Kovacevic P., Ostojic M. Assessment of autonomic nervous system dysfunction in the early phase of infection with SARS-CoV-2 virus. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.640835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M., Das J.M. StatPearls [Internet] StatPearls Publishing; 2021. Neuroanatomy, area postrema. [PubMed] [Google Scholar]
- Mobasheri L., Nasirpour M.H., Masoumi E., Azarnaminy A.F., Jafari M., Esmaeili S.-A. SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154 doi: 10.1016/j.cyto.2022.155873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Nishiyama Y., Yokochi T., Kimura Y. Olfactory transmission of neurotropic viruses. J.Neurovirol. 2005;11:129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- Mukerjee S., Gao H., Xu J., Sato R., Zsombok A., Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74:1181–1191. doi: 10.1161/HYPERTENSIONAHA.119.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka S. Hypertension induced by extensive medial anteromedian hypothalamic destruction in the rat. Jpn. Circ. J. 1966;30:509–523. doi: 10.1253/jcj.30.509. [DOI] [PubMed] [Google Scholar]
- Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. Eneurologicalsci. 2020;21 doi: 10.1016/j.ensci.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Mukerji S.S., Alabsi H.S., Systrom D., Marciano S.P., Felsenstein D., Mullally W.J., Pilgrim D.M. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann. Neurol. 2022;91:367–379. doi: 10.1002/ana.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaklander A.L., Mills A.J., Kelley M., Toran L.S., Smith B., Dalakas M.C., Nath A. Peripheral neuropathy evaluations of patients with prolonged long COVID. NeurologyNeuroimmunol.Neuroinflamm. 2022;9 doi: 10.1212/NXI.0000000000001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs M., Timm S., Elezkurtaj S., Horst D., Meinhardt J., Heppner F.L., Weber-Carstens S., Hocke A.C., Witzenrath M. Collapse induration of alveoli is an ultrastructural finding in a COVID-19 patient. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.04165-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong W.Y., Satish R.L., Herr D.R. ACE2, circumventricular organs and the hypothalamus, and COVID-19. Neuromol.Med. 2022:1–11. doi: 10.1007/s12017-022-08706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol.Rep. 2021;9 doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan J.S., Lyne A., Vaughan C.J. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.C., Bang S.Y., Lee H.W., Choi K.U., Kim J.M., Shin S.C., Cheon Y.I., Sung E.S., Lee M., Lee J.C., Kim H.S., Lee B.J. ACE2 and TMPRSS2 immunolocalization and oral manifestations of COVID-19. Oral Dis. 2022;28(Suppl. 2):2456–2464. doi: 10.1111/odi.14126. [DOI] [PubMed] [Google Scholar]
- Pascual-Goñi E., Fortea J., Martínez-Domeño A., Rabella N., Tecame M., Gómez-Oliva C., Querol L., Gómez-Ansón B. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurology® Neuroimmunol.Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S., Williams M.A. Confronting our next national health disaster—long-haul Covid. N. Engl. J. Med. 2021;385:577–579. doi: 10.1056/NEJMp2109285. [DOI] [PubMed] [Google Scholar]
- Pongratz G., Straub R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014;16:1–12. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzionato A., Emmi A., Barbon S., Boscolo-Berto R., Stecco C., Stocco E., Macchi V., De Caro R. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J. 2020;287:3681–3688. doi: 10.1111/febs.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.W., Notkins A.L. Viral infections of the autonomic nervous system and its target organs: pathogenetic mechanicsms. Med. Hypotheses. 1977;3:33–36. doi: 10.1016/0306-9877(77)90049-4. [DOI] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Tabassum T., Araf Y., Al Nahid A., Ullah M.A., Hosen M.J. Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol. Biol. Rep. 2021;48:3863–3869. doi: 10.1007/s11033-021-06358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichgott M.J. Clinical Methods: The History, Physical, And Laboratory Examinations. 3rd edition. 1990. Clinical evidence of dysautonomia. [Google Scholar]
- Reindl-Schwaighofer R., Hödlmoser S., Eskandary F., Poglitsch M., Bonderman D., Strassl R., Aberle J.H., Oberbauer R., Zoufaly A., Hecking M. ACE2 elevation in severe COVID-19. Am. J. Respir. Crit. Care Med. 2021;203:1191–1196. doi: 10.1164/rccm.202101-0142LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez E.M., Blázquez J.L., Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., Gershwin M.E., Selmi C., Anaya J.M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Perez A.I., Labandeira C.M., Pedrosa M.A., Valenzuela R., Suarez-Quintanilla J.A., Cortes-Ayaso M., Mayán-Conesa P., Labandeira-Garcia J.L. Autoantibodies against ACE2 and angiotensin type-1 receptors increase severity of COVID-19. J. Autoimmun. 2021;122 doi: 10.1016/j.jaut.2021.102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig I., Mitrečić D., Petanjek Z., Duffy B., Young A.H., Nesbitt A.D., Morrell M.J. Does damage to hypothalamic paraventricular nucleus underlie symptoms of ultradian rhythm disorder and an increased anxiety in coronavirus disease 2019? Croat.Med.J. 2020;61:377. doi: 10.3325/cmj.2020.61.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkai I., Mayer M.G., Hellmers L.M., Ning B., Huang Z., Monjure C.J., Coyne C., Silvestri R., Golden N., Hensley K., Chandler K., Lehmicke G., Bix G.J., Maness N.J., Russell-Lodrigue K., Hu T.Y., Roy C.J., Blair R.V., Bohm R., Doyle-Meyers L.A., Rappaport J., Fischer T. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 2022;13:1745. doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysz S., Al-Saadi J., Sjöström A., Farm M., Campoccia Jalde F., Plattén M., Eriksson H., Klein M., Vargas-Paris R., Nyrén S. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 2021;12:1–12. doi: 10.1038/s41467-021-22713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S., Schiavo G., Kremer E.J. A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nat. Rev. Microbiol. 2010;8:645–655. doi: 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- Sánchez-Manso J.C., Gujarathi R., Varacallo M. 2017. Autonomic Dysfunction. [PubMed] [Google Scholar]
- Sandoval K.E., Witt K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Schroeder C., Tank J., Boschmann M., Diedrich A., Sharma A.M., Biaggioni I., Luft F.C., Jordan J. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105:347–353. doi: 10.1161/hc0302.102597. [DOI] [PubMed] [Google Scholar]
- Shen M.J., Zipes D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- Shouman K., Vanichkachorn G., Cheshire W.P., Suarez M.D., Shelly S., Lamotte G.J., Sandroni P., Benarroch E.E., Berini S.E., Cutsforth-Gregory J.K., Coon E.A., Mauermann M.L., Low P.A., Singer W. Autonomic dysfunction following COVID-19 infection: an early experience. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society. 2021;31(3):385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol F., Sarlon G., Deharo J.-C., Vaïsse B. Downregulation of ACE2 induces overstimulation of the renin–angiotensin system in COVID-19: should we block the renin–angiotensin system? Hypertens. Res. 2020;43:854–856. doi: 10.1038/s41440-020-0476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siso S., Jeffrey M., Gonzalez L. Sensory circumventricular organs in health and disease. Acta Neuropathol. 2010;120:689–705. doi: 10.1007/s00401-010-0743-5. [DOI] [PubMed] [Google Scholar]
- Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S., Nath A. Nervous system consequences of COVID-19. Science. 2022;375:267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- Su C., Xue J., Ye C., Chen A. Role of the central renin-angiotensin system in hypertension. Int. J. Mol. Med. 2021;47:1–16. doi: 10.3892/ijmm.2021.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A., Miwata K., Kitahara Y., Okimoto M., Abe K., E B., Ouoba S., Akita T., Tanimine N., Ohdan H., Kubo T., Nagasawa A., Nakanishi T., Takafuta T., Tanaka J. Long COVID occurrence in COVID-19 survivors. Sci.Rep. 2022;12:6039. doi: 10.1038/s41598-022-10051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K., Alam M.D.U., Satkovich E. COVID-19-associated dysautonomia. Cureus. 2021;13 doi: 10.7759/cureus.17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.T., Colgan S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.P., Enquist L.W. Axonal spread of neuroinvasive viral infections. Trends Microbiol. 2015;23:283–288. doi: 10.1016/j.tim.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli R.A., Hermann G.E., Browning K.N., Rogers R.C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle Martins A.L., da Silva F.A., Bolais-Ramos L., de Oliveira G.C., Ribeiro R.C., Pereira D.A.A., Annoni F., Diniz M.M.L., Silva T.G.F., Zivianni B., Cardoso A.C., Martins J.C., Motta-Santos D., Campagnole-Santos M.J., Taccone F.S., Verano-Braga T., Santos R.A.S. Increased circulating levels of angiotensin-(1–7) in severely ill COVID-19 patients. ERJ Open Res. 2021;7 doi: 10.1183/23120541.00114-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance K.M., Rogers R.C., Hermann G.E. NMDA receptors control vagal afferent excitability in the nucleus of the solitary tract. Brain Res. 2015;1595:84–91. doi: 10.1016/j.brainres.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliou A.G., Athanasiou N., Keskinidou C., Jahaj E., Tsipilis S., Zacharis A., Botoula E., Diamantopoulos A., Ilias I., Vassiliadi D.A., Tsagarakis S., Kotanidou A., Dimopoulou I. Increased glucocorticoid receptor alpha expression and signaling in critically ill coronavirus disease 2019 patients. Crit. Care Med. 2021;49:2131–2136. doi: 10.1097/CCM.0000000000005097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale-Cross L., Szalayova I., Scoggins A., Palkovits M., Mezey E. SARS-CoV-2 entry sites are present in all structural elements of the human glossopharyngeal and vagal nerves: clinical implications. EBioMedicine. 2022;78 doi: 10.1016/j.ebiom.2022.103981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener G., Goldklang M.P., Gerber A., Elisman K., Eiseman K.A., Fonseca L.D., D'Armiento J.M. A randomized, placebo-controlled, double-blinded pilot study of angiotensin 1–7 (TXA-127) for the treatment of severe COVID-19. Crit. Care. 2022;26:229. doi: 10.1186/s13054-022-04096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallukat G., Morwinski M., Kowal K., Förster A., Boewer V., Wollenberger A. Autoantibodies against the β-adrenergic receptor in human myocarditis and dilated cardiomyopathy: β-adrenergic agonism without desensitization. Eur. Heart J. 1991;12:178–181. doi: 10.1093/eurheartj/12.suppl_d.178. [DOI] [PubMed] [Google Scholar]
- Wallukat G., Hohberger B., Wenzel K., Fürst J., Schulze-Rothe S., Wallukat A., Hönicke A.-S., Müller J. Functional autoantibodies against G-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J.Transl.Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- Wang P., Dai J., Bai F., Kong K.-F., Wong S.J., Montgomery R.R., Madri J.A., Fikrig E. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J. Virol. 2008;82:8978–8985. doi: 10.1128/JVI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman A.M., Ferreira M., Jr., Sahibzada N., Hernandez Y.M., Gillis R.A. GABA-mediated neurotransmission in the ventrolateral NTS plays a role in respiratory regulation in the rat. Am. J. Phys. Regul. Integr. Comp. Phys. 2002;283:R1423–R1441. doi: 10.1152/ajpregu.00488.2001. [DOI] [PubMed] [Google Scholar]
- Waxenbaum J.A., Reddy V., Varacallo M. StatPearls Publishing LLC.; Treasure Island (FL): 2022. Anatomy, Autonomic Nervous System, StatPearls. StatPearls Publishing Copyright © 2022</span>. [PubMed] [Google Scholar]
- Wells R., Malik V., Brooks A.G., Linz D., Elliott A.D., Sanders P., Page A., Baumert M., Lau D.H. Cerebral blood flow and cognitive performance in postural tachycardia syndrome: insights from sustained cognitive stress test. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Vol. 2022. 2021. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester N., Calabrese C., Calabrese L. The intersection of COVID-19 and autoimmunity: what is our current understanding? Pathog.Immun. 2021;6:31. doi: 10.20411/pai.v6i1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Waeckerlin R., Urbanowski M.D., Van Marle G., Hobman T.C. West Nile virus infection causes endocytosis of a specific subset of tight junction membrane proteins. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0037886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Dunn J.F. Multiple sclerosis disease progression: contributions from a hypoxia-inflammation cycle. Mult. Scler. 2019;25:1715–1718. doi: 10.1177/1352458518791683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S.J. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem. Neurosci. 2021;12:573–580. doi: 10.1021/acschemneuro.0c00793. [DOI] [PubMed] [Google Scholar]
- Yu X., Li H., Murphy T.A., Nuss Z., Liles J., Liles C., Aston C.E., Raj S.R., Fedorowski A., Kem D.C. Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazhytska M., Kodra A., Hoagland D.A., Frere J., Fullard J.F., Shayya H., McArthur N.G., Moeller R., Uhl S., Omer A.D., Gottesman M.E., Firestein S., Gong Q., Canoll P.D., Goldman J.E., Roussos P., tenOever B.R., Jonathan B.O., Lomvardas S. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185:1052–1064.e1012. doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevalk G.D. In: Comprehensive Toxicology. Second edition. McQueen C.A., editor. Elsevier; Oxford: 2010. 13.02 - fundamentals of the structure and function of the nervous system; pp. 3–27. [Google Scholar]
- Zheng J., Cui Z., Shi N., Tian S., Chen T., Zhong X., Qiu K., Zhang J., Zeng T., Chen L. Suppression of the hypothalamic-pituitary-thyroid axis is associated with the severity of prognosis in hospitalized patients with COVID-19. BMC Endocr. Disord. 2021;21:1–9. doi: 10.1186/s12902-021-00896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.