Abstract
Due to the excessive use of disposable face masks during the COVID-19 pandemic, their accumulation has posed a great threat to the environment. In this study, we explored the fate of masks after being disposed in landfill. We simulated the possible process that masks would experience, including the exposure to sunlight before being covered and the contact with landfill leachate. After exposure to UV radiation, all three mask layers exhibited abrasions and fractures on the surface and became unstable with the increased UV radiation duration showed aging process. The alterations in chemical groups of masks as well as the lower mechanical strength of masks after UV weathering were detected to prove the happened aging process. Then it was found that the aging of masks in landfill leachate was further accelerated compared to these processes occurring in deionized water. Furthermore, the carbonyl index and isotacticity of the mask samples after aging for 30 days in leachate were higher than those of pristine materials, especially for those endured longer UV radiation. Similarly, the weight and tensile strength of the aged masks were also found lower than the original samples. Masks were likely to release more microparticles and high concentration of metal elements into leachate than deionized water after UV radiation and aging. After being exposed to UV radiation for 48 h, the concentration of released particles in leachate was 39.45 μL/L after 1 day and then grew to 309.45 μL/L after 30 days of aging. Seven elements (Al, Cr, Cu, Zn, Cd, Sb and Pb) were detected in leachate and the concentration of this metal elements increased with the longer aging time. The findings of this study can advance our understanding of the fate of disposable masks in the landfill and develop the strategy to address this challenge in waste management.
Keywords: Disposable face masks, Landfill leachate, Aging, Microparticles, Chemical pollutants
Graphical Abstract
1. Introduction
Since the outbreak of the SARS-CoV-2 (COVID-19) pandemic, more than 611 million people have been confirmed infected, and 6.5 million people claimed lives as of 23 September, 2022 [1]. To prevent transmission of the coronavirus infection, wearing personal protective equipment (PPE), such as face masks, are recommended. With the excessive demand for face masks, the global production reached previously unheard-of levels, increasing from 12,534.4 million pieces in 2019 to 37,8854.5 million pieces in 2020 and 402,138.1 million pieces in 2021, respectively [2], [3]. It is inevitable that mask wastes will become a new threat to the environment because of limited advancements in management and disposal [4], [5]. Disposable face masks are manufactured from a variety of polymers, such as polypropylene (the most common, PP) and/or polyethylene (PE), polyurethane (PUR), polystyrene (PS), polycarbonate (PC), and poly- acrylonitrile (PAN) [6], along with other chemicals (organophosphate ester compounds, phthalates amides, types of paraffin, olefins, etc.) [7]. In addition, plasticizers and stabilizers are often added to polymers used for disposable face masks to enhance their physical, mechanical, and chemical properties [8].
Landfilling and incineration are the common methods used to deal with municipal solid waste in many countries, and the overburdened medical waste often ends in landfills [9], [10]. According to recently published research reports, the majority of COVID-19 related waste, such as PPE, has been deposited in landfills; more than 28 million masks are disposed of in landfills in the United States annually, and approximately 3.5 million metric tons of masks ended up in landfills globally in the first year of the COVID-19 pandemic [4], [7]. Overloading landfills with PPE waste will pose certain concerns to the ecosystem, including space crush, plastic pollution, and leaching of toxic chemicals [11]. The accumulation of toxic substances in landfill leachate will lead to a serious burden on soil, surface water, and groundwater [12], resulting in severe water shortage [13], as landfill leachate may potentially filtrate into the soil and groundwater.
Plastic disposable face masks waste discarded in the environment is susceptible to ultraviolet (UV) photodegradation as well as physical and chemical changes during transportation induced by water flows and winds. As a result of that degradation in the natural terrestrial and aquatic area, microplastics (MPs) and nanoplastics (NPs), which have the dimensions of ≤ 5 mm and 1–100 nm, respectively, are released into the environment [14], [15], [16]. The number of MPs released from masks in various circumstances and treatments ranges from 1429 to 2.43 × 109 per mask [18], [17], due to the use of different quantification techniques, including metallographic microscopy, stereomicroscopy and field-emission scanning electron microscopy (SEM), etc. Because of their small size, the aggregation of MPs and NPs has been shown to pass through bio-membranes and penetrate the tissues and organs of humans and non-human organisms alike [19], [20]. According to Ma et al. [18], MPs are found in nasal mucus and might be ingested by marine species, which could have serious effects, such as inflammation of the alveola. However, the concentration limits of MPs and NPs, as well as how these fine particles may affect organisms, need further investigation.
A considerable amount of research has reported physical and chemical changes and the release of pollutants during the aging of disposable masks discarded in a variety of experimental settings, such as aqueous solution with UV pre-treatment, soil bury, and dumpsites [22], [21]. The released particles and leached hazardous chemicals can also have the negative effect on the wildlife [10], [23]. The physical characteristics of DFMs change as a result of aging and degradation. For example, Wang et al. [3] reported an 87.2% and 59.5% drop in the maximum load force of the outer and inner layers of masks, respectively, after 15 h of UV weathering. This is an example of how exposure to UV-radiation induces decreased tensile strength. As the mechanical properties of the plastic material decrease, fragmentation occurs under various conditions and may result in the generation of MPs [24], [25]. The physical changes during the aging process can also be evaluated by SEM micrographs and AFM. Physically degraded DFMs typically experience irreversible chemical changes along with diminished mechanical properties. Chemical changes can also be assessed through various technologies such as Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and Raman spectroscopy. For instance, Saliu et al. [26] reported that UV-lighted masks appear as broad peaks around 3400 cm-1 and 1700 cm-1 due to the oxidation of the PP, compared with pristine masks. In addition, there have been some studies on biological changes in DFM in natural environments. For instance, Liao et al. [27] found that bacterial diversity and community structure changed significantly in seawater immersed with the masks. With increasing time, the relative abundance of Flavobacteriaceae, Methyloligellaceae, Halieaceae, Methylophilaceae, and Coxiellaceae increased while that of Algiphilaceae and Saprospiraceae decreased. Ma et al. [28] found that polypropylene face masks had the highest alpha diversity of bacteria after 21 days of aging in seawater. Six exclusive families were found in the 21-day sample: Coleofasciculaceae, Tenderiaceae, Woeseiaceae, Nitrosopumilaceae, a no-rank family from the class Dadabacteriia, and an unclassified family from the order Gammaproteobacteria incertae sedis.
To the best of our knowledge, no study has been conducted on the aging and degradation of disposable masks in landfill leachate. Investigating this issue could help us understand it better and lead to the development of better management strategies for this type of waste in the future. Thus, the primary objective of this investigation was to ascertain how the physical and chemical characteristics of the masks change as they age in landfill leachate. The second objective of this study was to quantify the pollutants, including microparticles and metal elements, released into landfill leachate.
2. Experimental section
2.1. Materials
Commercially available surgical-grade disposable face masks made of non-woven PP were used and the masks had three layers, spun-bond inner and outer layers and melt-blown middle layer. Prior to aging studies, the ear loops and nose wire were detached. The remaining mask material was cut into small pieces (2.0 cm × 2.0 cm) and dried at 60 ℃ overnight to remove moisture.
2.2. Preparation of synthetic landfill leachate
An artificial leachate was synthesized to simulate landfill conditions according to previous studies with modifications by supplementing the phenolic compounds [29], [30]. The components of the synthetic leachate are shown in Table S1 (Supplementary). Before use, leachate was filtrated through a 0.22 µm membrane under vacuum using an Aldrich glass funnel and receiving flask.
2.3. UV pre-treatment for aging of the masks
The disposable masks were cut into 2.0 cm × 2.0 cm pieces to simulate the solar irradiation on the surface of landfills, and the small pieces were put in a glass Petri dish exposed to UV radiation for 4, 24, 36, and 48 h. The weathering of the masks was performed in a UV chamber (CL-3000 M Crosslinker, Analytik Jena, Germany) with a wavelength of 302 nm and UV intensity of 20 mW/cm2 at a distance of 0.5 in..
2.4. Aging and degradation testing
The aging of the disposable masks was evaluated in landfill leachate over a variety of time periods (i.e., 1, 3, 5, 7, 10, 20, and 30 days). A mixture of three pieces of masks and 100 mL were added to an Erlenmeyer flask. The flasks were sealed with aluminum foil and slowly agitated in a New Brunswick Innova 42 R incubator shaker (Eppendorf, USA) at 25 ℃ with 120 rpm so that the DFMs were always in full contact with leachate. Four different experimental conditions were used: (a) immersion of the mask pieces in leachate, (b) immersion of the mask pieces in DI water, (c) use of leachate alone without masks, and (d) use of DI water alone without masks. To keep the flasks from being exposed to external light, aluminum foil constantly covered them. The initial weight of the mask samples was recorded, and then they were removed from leachate, washed with DI water (About 1 L per sample) at 1, 3, 5, 7, 10, 20, and 30 days, and dried in an oven at 60 ℃ for a duration of 24 h to get rid of any impurities. The final weight of the test samples was measured to evaluate the reduction in weight of the disposable masks after aging. The effectiveness of aging was calculated depending on the change in weight (%) of the mask samples using the equation: weight change (%) = final weight reduction of sample after aging/initial weight of sample initial weight of sample × 100% [31].
2.5. Characterization of physical and chemical changes of masks during aging in landfill leachate
The morphologies of the masks were observed with an S-3400 N scanning electron microscopy (SEM, Hita-chi, Japan) at an acceleration voltage of 5 kV and a working distance of 8 mm. The masks were placed on sample stages with conductive tapes and sputter coated with a 2 nm layer of gold before the measurements. The chemical changes of masks aging during landfill leachate were examined by the Fourier transform infrared spectroscopy (FTIR, Invenio, Bruker, USA). Spectra were obtained by recording 64 scans between 4000 and 400 cm-1 with a resolution of 4 cm-1. The background spectrum was obtained for each sample, and three measurements were taken for each. During the UV radiation and aging process, the carbonyl index (CI) and isotacticity (ISO) were calculated from the spectrum. The CI is usually used to characterize the oxidation level of polypropylene. ISO is defined as the length of the isotactic sequence of polypropylene, and it can be used to show the stability of the structure. The larger the ISO, the more unstable the polypropylene [32]. The CI values were acquired by calculating the adsorption of the ketone peak at 1715 cm-1 to the methylene peak at 1455 cm-1 [33]. ISO was determined by calculating the peak at 997 cm-1 to the peak at 972 cm-1 [34]. The FTIR spectroscopic imaging of three mask layers after aging were captured by FT-IR spectroscope (Cary 670, Agilent, USA) coupled with a microscope (Cary 620, Agilent, USA) within a spectral range of 4000–800 cm-1 using 4 cm-1 spectral resolution at the Canadian Light Source in Saskatoon, Canada. The mechanical tests were conducted using the Material Test System (Hoskin Scientific, Canada), and the stress-strain cures were obtained. The tensile strength at the break values were obtained from the stress–strain curves. The load cell used was 1 kN, and the displacement speed was set at 10 mm.min-1. For the measurement, the samples were cut into pieces 50 mm long and 20 mm wide. Every measurement was repeated three times. The surface wetting characteristics of all mask samples were observed with a contact angle analyzer (VCA Optima, AST Products, USA) at room temperature of 20 ℃. For each sample, after 10 s, two measurements were carried out to obtain the average.
The distribution of the microparticles released from the masks was obtained using a laser in-situ scattering and transmissometer analyzer (LISST-200X, Sequoia, USA). The detection range of the analyzer was 1–500 µm, and the concentration of particulate matter (μL/L) was obtained by calculating the equivalent volume of the irregular spheres. Samples of 20 mL were added to the small chamber of the LISST, and live data collecting mode was used to scan the samples. Each sample was scanned for 1 min, and a total of 60 data points for each size of particles were collected. The physical shape of the microplastics was also obtained with a microscope (Lumenera Infinity 3 camera, Lumenera Corporation, Canada) and SEM. Then 20 μL of water was carefully dropped on the sample holder. A 5-mL leachate sample of the mask was filtrated with a 0.22 µm nylon membrane and analyzed on an inductively coupled plasma mass spectrometer (ICP-MS) (7700, Agilent Technologies, CA), equipped with an SPS 4 autosampler for measuring the concentration of the following elements: Al, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Sr, Ag, Cd, Sb, and Pb. Before analysis, the leachate was stabilized for 24 h, and then was subsampled and acidified using 1 M nitric acid.
3. Results and discussion
3.1. Changes in the physicochemical and morphological characteristics of the masks exposed to UV radiation
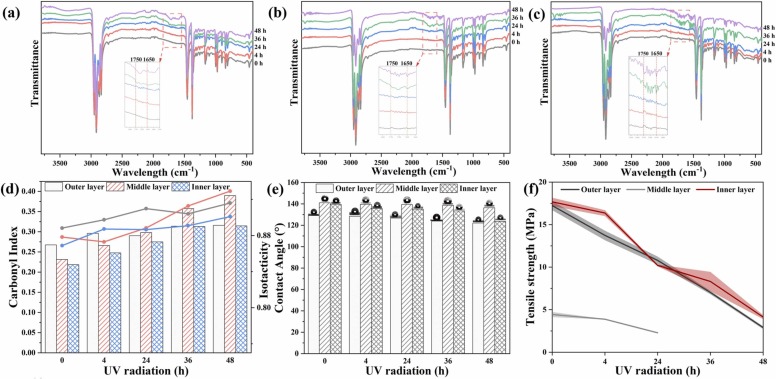
Due to the increasing waste and improper management strategies in landfills, a large amount of dumped waste at landfills is not covered on a daily basis, especially for uncontrolled open dumping in developing countries [35], [10]. This would expose disposable face masks to solar radiation for varying lengths of time, which could oxidize mask polymers, reduce mechanical properties, and accelerate the aging of masks during landfilling [36], [37]. UV radiation was thus used in the present study to simulate such exposure scenario in landfills. In the present study, disposable masks were characterized by ATR-FTIR spectroscopy to identify changes in the chemical groups during different weathering periods. Fig. 1 depicts the chemical nature of different layers of the pristine and radiated masks. For the peaks of the main groups, all three layers are similar; the peaks located at 2949, 2916, and 2866 cm-1 are attributed to asymmetric and symmetric stretching vibration of CH3, while the peak at 2837 cm-1 is due to CH3 groups. Absorbance peaks at 1456 and 1375 cm-1 are also symmetric deformations of CH3 groups [38]. Additional peaks appearing at 1165, 972, and 840 cm-1 are attributed to the bending vibration of tertiary carbon and C-H deformation, respectively [39]. It was noticed that all three layers exhibited some small peaks between 1750 and 1650 cm-1 after exposure to UV radiation. There are no discernible alterations in the ATR spectrum of the PP three layers exposed to UV-weathering for 24 h. Moreover, after 36 h of exposure to UV radiation, new peaks of all three layers emerged between 1750 and 1650 cm-1, respectively, indicating the formation of carbonyl (C O) and alkenyl (C C). Photochemical weathering not only leads to the change of mechanical performance, but also to the release of polymeric fragments and microplastics, chemical additives, and other byproducts [40].
Fig. 1.
FTIR analysis (Outer layer (a), Middle layer (b), Inner layer (c)), carbonyl index (d), hydrophilicity (e), and tensile strength (f) of the different layers of masks with different UV radiation period.
For more in-depth and quantitative analysis of the formation of C O, the CI was calculated. From the CI results, we found that the CI of the virgin outer layer is 0.26, which is higher than that of the middle and inner layers (0.23 and 0.22, respectively). After 48 h of exposure to UV radiation, the CI of the outer layer increased slightly to 0.32, indicating that the outer layer is more resistant to UV radiation than the inner and middle layers. With respect to middle layer, the CI fast increased from 0.23 to 0.39 and that of the inner layer changed from 0.22 to 0.31. In a previous study, it was reported that CI of PP increased from 0.2 to 0.7 for polyethylene (PE), polypropylene (PP), and expanded polystyrene (EPS) after 30 days of UV aging [33]. Zhu et al. [41] found that under simulated sunlight irradiation in aquatic environments, CI of polyethylene microplastics increased from 0.04 to 0.14 and from 0.14 to 0.33 after 60-d and 150-d irradiation, respectively. Wu et al. [42] observed that 20-day aging of polypropylene food packaging in artificial seawater with UV light resulted in the increased CI values from 0.1 to 0.6. The CI trends of the three layers were also supported by the ISO results. The middle layer showed a significant increase in the ISO, from 0.88 to 0.93, which means that the middle layer became more unstable after 48 h of UV radiation.
Under this circumstance, the hydrophilicity would be enhanced, and the contact angle would be reduced (Fig. 1e). The contact angle of the outer layer presented a slight decrease, from 129° to 122.1° after being subjected to UV radiation for 48 h. The contact angle of the middle layer showed a similar change after exposure to UV radiation for 48 h, decreasing slightly from 141° to 136.4°, which may be due to the surface of the middle having tight fiber space ( Fig. 2). In contrast, the contact angle of the inner layer showed a more obvious change, decreasing from 139.5° to 123.7°, indicating that the inner layer is the most adherable to water.
Fig. 2.
SEM images at 500 × magnification for the three mask layers after exposed to different durations of UV.
The influence of UV radiation on the mechanical performance of the masks was further investigated. The tensile strength reflects the mechanical properties. As shown in Fig. 1f, the general tendency for the three layers was that the tensile strength decreased as the UV radiation time increased. The original inner layer has the maximum tensile strength (17.68 MPa), followed by the outer layer (17.23 MPa), and the middle layer (4.43 MPa), attributed to the finer and irregular fibers in pore size and distribution produced by the melt-blowing process [43]. After 4 h of UV radiation, the tensile strength of the outer layer decreased by 20% to 13.74 MPa, while the middle layer dropped by 12% to 3.88 MPa; in contrast, the inner layer decreased marginally to 16.36 MPa. This could also be evidenced by SEM images that showed minor changes on the surface of the inner layer (Fig. 2). Furthermore, radiation exposure for more than 24 h dramatically reduced the tensile strength of the three layers, falling by 47%, 55%, and 46%, respectively. After 36 h of exposure to UV radiation, the middle layer was too brittle to be tested, whereas the tensile strength of the outer and inner layers decreased by 95% and 92%, respectively. After 48 h of radiation, it further dropped by 59% and 50%, respectively. These results are consistent with previous FTIR analysis results showing the middle layer with the highest ISO.
The morphological characteristics of the three mask layers throughout the UV radiation process were investigated by SEM imaging. It was confirmed that the middle layer was unevenly thick, with the average diameter of the outer and inner layers was roughly 20 µm. Compared with the surface of the pristine samples, radiated three layers showed a major alteration. As depicted in Fig. 2, the fibers of the three layers exhibited smooth surfaces before UV radiation. After 36 h of UV radiation, the outer and inner layers appeared some minor damage or deformation on the surface, while the middle layer did not show obvious signs of abrasion. Once the masks were subjected to UV radiation for 48 h, erosion and fractures can be seen distinctly on the surface of the three layers. The middle layer is more sensitive to UV radiation; most of the fibers showed more severe corrosion and breakage and appeared to have certain small particles attached. The reason the middle layer is so vulnerable may be that it has smaller diameter fibers and a higher specific surface area.
3.2. Chemical changes in the composition of the masks during aging in simulated leachate
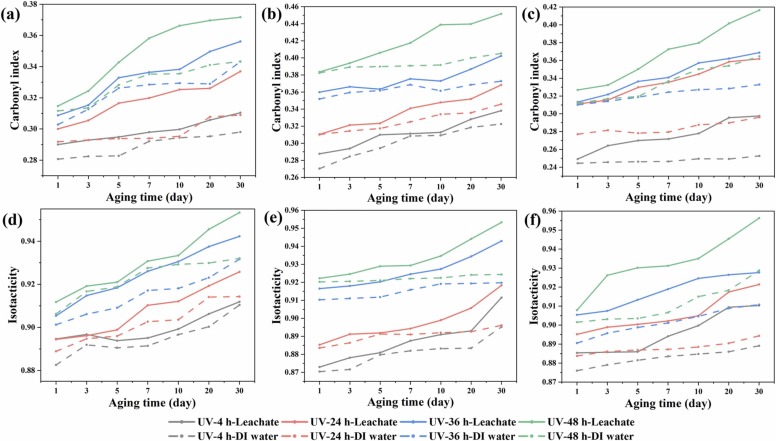
The CI results could indicate the level of aging of the masks in simulated leachate and DI water. As shown in Fig. 3(a-c), when the aging duration reached 30 days, the average CI results for the middle layer show more substantial changes when compared with the outer and inner layers aged in simulated leachate. The increase in the CI in the PP samples suggests that more generation of carbonyl (C O) and alkenyl (C C) groups, which may be due to the loss of volatile parts during the aging process [44], and further leading to chain fracture or crosslinking. It is worth noting that the disparity between CI outcomes widens when the exposure to UV radiation exceeds 24 h. However, the CI of all three layers did not show an obvious increase when they were aged in DI water. When the middle layer was exposed to UV radiation for 4 h, its CI increased from 0.29 to 0.34 after aging for 30 days in leachate, and the CI also increased from 0.27 to 0.32 after aging in DI water, which means the middle layer could also age in DI water. The main reason might be that the manufacturing process of the middle layer involves melt blowing and will become broken when exposed to UV radiation. When the middle layer was exposed to UV radiation for 24 h, its CI increased more noticeably from 0.31 to 0.37 after aging for 30 days in leachate. This result was also supported by the contact angle results (Fig. 7), which fell from 134.5° to 120.9° after 30 days of aging in leachate. Moreover, when the middle layer was subjected to UV radiation for 36 and 48 h, CI increased from 0.36 and 0.38–0.40 and 0.45, respectively, after it was aged for 30 days in leachate. However, the CI of the middle layer increased slightly from 0.35 and 0.38–0.37 and 0.40, respectively, after 30 days of aging in DI water.
Fig. 3.
CI and ISO results of three layers of masks after aging in simulated leachate and DI water. Outer layer (a, d), Middle layer (b, e), Inner layer (c, f).
Fig. 7.
Hydrophilicity and tensile strength of three mask layers after aging in simulated leachate and DI water. Outer layer (a, d), Middle layer (b, e), Inner layer (c, f).
The CI alterations of the inner layer showed a similar pattern to those of the middle layer and increased more pronouncedly as the aging duration rose. For instance, the CI of the inner layer that was exposed to UV radiation for 4 h increased from 0.25 to 0.30 when the layer was aged for 30 days in leachate. Moreover, the CI of the inner layer that was exposed to UV weathering for 48 h increased by 30%, from 0.32 to 0.42, after being aged for 30 days in leachate. However, the CI did not show an obvious change when the layer was aged in DI water. For the outer layer, when UV radiated for 4 h, the CI increased slightly from 0.29 to 0.31 after the layer was aged in leachate for 30 days, which is higher than that for the layer aged in DI water. The CI showed more significant changes with increasing length of exposure to UV radiation. For example, when the length of UV radiation exposure reached 24 h, the CI of the outer layer increased from 0.30 to 0.34, while the CI of the outer layer aged in DI water increased only from 0.30 to 0.31. Furthermore, the CI increased by 19%, from 0.31 to 0.37, when the UV radiation time was 48 h, whereas the CI increased only from 0.31 to 0.34 after the layer was aged for 30 days in DI water. The CI of the three layers is increasing in the long-term aging in simulated leachate. This indicates that the process of oxidation was taking place continuously [45]. The inner layer is more sensitive, as it is usually made softer during production so that it would fit over human flesh, compared with the outer layer. This may then result in chemical and physical alterations that cause embrittlement at the surface of polymers. The produced cracks and breaks could facilitate the dwelling of microbial communities on mask surface. The carbonyl chemical changes in the range of 1650–1750 cm-1 of the three mask layers after aging for 30 days in simulated leachate were further analyzed by FTIR mapping, were presented in a colormap spanning from low absorbance (blue) to high absorbance (red) ( Fig. 4). It is noticed that the strength and the range of the carbonyl groups of three mask layers increased after the samples were aged 30 days in leachate, which are consistent with previous carbonyl index analysis. The environmental processes can often be impacted by various factors [46], [47], [48], [49]. The cracks and flakes on the polymer surface are common patterns during degradation, facilitating the accessibility of ions to the internal layer and accelerating the aging process. In addition, chemical additives, such as stabilizers, may accelerate or slow down the aging process, and further affect the crosslinking or scission caused by UV irradiation [50]. Disposable masks contain many types of plasticizers (such as phthalates) to increase the flexibility of the masks. After UV irradiation, the plasticizer can be migrated from the inside [51]. This migration is a gradual outflow process, first from the inside of the material to the surface, while potentially changing the properties of the mask, leading to accelerated molecular mobility and facilitated physico-chemical. In addition, the chemical reactions of inorganic ions with the hydroxyl and carbonyl groups on the surface could help the –COO− anion formed from sodium carboxylate, which could further affect the aging process [52].
Fig. 4.
FTIR mapping of the carbonyl functional groups (1650–1750 cm-1) of three mask layers before (A–I) and after 30 days of aging in simulated leachate (a–i).
The ISO results of the three layers are consistent with the CI results. The ISO of all three layers increased with the longer aging durations, indicating that these layers become unstable compared with the unaged pristine parts. As shown in Fig. 3e, the ISO of the middle layer increased noticeably after being exposed to UV radiation for 4 h, going from 0.87 and 0.87–0.91 and 0.89, respectively, after being aged for 30 days in leachate and DI water. Additionally, when the middle layer was exposed to UV radiation for 24 h, the ISO went up from 0.89 and 0.88–0.92 and 0.90, respectively, after 30 days of aging in leachate and DI water, suggesting that the middle layer became more easily broken during aging. In contrast to the parts aged in leachate, the middle layer aged in DI water did not clearly exhibit an increase in ISO when exposed to UV radiation for 36 and 48 h. This phenomenon can be observed from the weight loss ( Fig. 5) of the middle layer after it was aged for 30 days. However, compared to the CI of the inner layer that was exposed to UV radiation for 4 and 24 h and rose from 0.89 and 0.90–0.91 and 0.92 after 30 days of aging in leachate (Fig. 3f), the CI of the parts aged in DI water did not exhibit obvious changes. When aged in DI water, only the UV exposure lasting more than 24 h would appear apparently to increase the ISO. Furthermore, after the inner layer was UV radiated for 48 h, the ISO rose considerably from 0.90 to 0.96 after 30 days of aging in leachate. However, for the outer layer (Fig. 3d), the ISO increased as the aging duration in leachate and DI water increased. For instance, when the outer layer was exposed to UV radiation for 4 and 24 h, the ISO grew from 0.89 and 0.89–0.91 and 0.93 after the layer was aged for 30 days in leachate, while the ISO increased from 0.88 and 0.89–0.91 and 0.91 after aging for 30 days in DI water, respectively. After the outer layer was exposed to UV radiation for a longer period, the ISO of the outer layer increased more significantly after being aged in leachate and DI water.
Fig. 5.
Weight loss of the three mask layers after aging in simulated leachate and DI water. Outer layer (a), Middle layer (b), Inner layer (c).
Fig. 5 shows the weight loss results of the different layers after aging in leachate and DI water. There was a similar pattern for the weight loss percentage of the different aging durations. The three layers all showed progressive weight loss when the length of exposure to UV radiation reached 36 h. Compared with the outer and inner layers, the middle layer presented a greater weight loss, which also indicated that it was more oxidated during aging, as shown in Fig. 3b. All three layers lost more weight when the layer was aged in leachate, compared with DI water. At short length of exposure to UV radiation, the weight loss rate of the three layers was gradually increased after the layers were aged in leachate compared with DI water. The general trend was that the rate of weight loss increased with the prolonging in the length of exposure to UV radiation and the aging duration. For example, when UV radiation time was 4 h, the outer layer showed a slight increase to 1.7% after aging for 30 days in leachate. Additionally, the outer layer exposed to UV radiation for 24 and 48 h showed weight loss of 2.2% and 3% after aging for 30 days in leachate, respectively. For the middle layer, the largest weight loss was found in the middle layer exposed to UV radiation for 48 h, with a sharp weight loss of about 14% after 1 day of aging in leachate, which then tapered off for the rest of the aging period and rose to 26% after 30 days. This might be related to the instability of the middle layer, where some parts of the layer stuck to other parts during aging. The combined action of UV and salt ions in leachate, which could accelerate the surface degradation of the polypropylene membrane, may account for why the three layers lost more weight [53]. However, a corresponding larger weight loss was not presented in DI water. With respect to the inner layer, it showed less weight loss when UV radiation time was less than 24 h; on the other hand, there was a noticeable weight loss. For instance, after the inner layer was exposed to UV radiation for 4 h, the layer exhibited slight rise to 1.3% after being aged for 30 days in leachate, and it grew to 7% when the exposure to UV radiation lasted for 48 h after the layer was aged for 30 days in leachate. These findings are similar to Khoironi et al.’s [54] finding that polypropylene hardly degrades in the water environment due to its high hydrophobicity, molecular weight, and high surface roughness.
3.3. Morphological observation of masks impacted by simulated leachate
The surface morphological characteristics of the three mask layers during aging in leachate and DI water were further examined by SEM. Fig. 6 shows the surface characteristics of the three mask layers with various durations of exposure UV radiation after aging for 30 days in leachate and DI water. For the outer layer, when radiated for 4 h of UV, the surface changes after 30 days of aging in leachate were more noticeable than those observed after 30 days of aging in DI water. When the UV radiation exposure reached 36 h, the outer layer became rough and had cracks after 30 days of aging in leachate. Moreover, when the outer layer was exposed to UV weathering for 48 h, significant fractures and abrasion appeared after 30 days aging in leachate. Contrarily, when compared to the pristine sample, the outer layer did not show significant surface aging, regardless of how long it was aged in DI water. Instead, the abrasion on the surface was mostly caused by the impact of UV radiation.
Fig. 6.
SEM images at 500 × magnification for the three mask layers after 30 days of aging in simulated leachate and DI water with different UV weathering durations.
The middle layer showed more pronounced surface aging when aged in leachate. When subjected to UV radiation for 4 h, the middle layer displayed bends and wraps of the fibers after 30 days of aging in leachate, indicating some physical degradations on the surface. However, after aging in DI water, the middle layer did not exhibit any surface differences. In addition, after 36-h UV radiation, the middle layer showed varying degrees of aging and changes on the surface when aged in leachate and DI water, which led to easily produced small debris and microplastics. When being exposed to UV radiation for 48 h, the middle layer developed many fractures and splits and formed minute fragments of fibers of varying lengths that attached on the surface after aging for 30 days in leachate. Meanwhile, some fractures and cracks also appeared in the middle layer after it was aged for 30 days in DI water, resulting in a large specific surface area for water to interact with the fibers. Ions like sodium, calcium, and zinc seem to inhibit polymer chains from disengaging, and ultimately causes chain scission [55].
The inner layer showed a similar trend in surface characteristics as the outer layer. When being UV radiated for 4 h, the signs of surface abrasion appeared on the inner layer after it was aged for 30 days in leachate, but not after it was aged in DI water. As the length of exposure to UV radiation and aging duration prolonged, so did the severity of the surface deterioration on the surface of the inner layer. For instance, when the layer was exposed to UV radiation for 48 h, distinct rifts and erosion were clearly observed on the inner layer after aging for 30 days in leachate. For the inner layer aged in DI water, most of the fibers showed some abrasions, and some attached small particles appeared. The results of the SEM images of the surface degradation of the three layers are consistent with those of weight loss study. The middle layer presented the largest weight loss when aged in leachate, compared to the outer and inner layers. In general, those cracks and abrasion on the surface after aging in leachate had increased surface area and roughness compared to virgin samples, which could help on the growth of microbial communities. Furthermore, the micrometer-sized fragments can provide sites for microbial attachment, and thus enhance microorganisms-polymer interactions that may facilitate polymer degradation in long period [56].
3.4. Surface wettability and mechanical changes of masks impacted by simulated leachate
To further explore the aging of masks in leachate, the hydrophilicity of the surface of the three mask layers was studied. Fig. 7(a-c) illustrates how the contact angle of the three layers changed in distinct ways after 30-day aging in leachate and DI water. For outer, middle, and inner layer after aging, the contact angle decreased from 122.1°, 136.4°, 118.5–78.3°, 88.6°, 86.6 in leachate, and to 106°, 129.4°, 111.9° in DI water, respectively. The general trend is that the contact angle decreased with longer exposure to UV radiation and prolonged aging duration in leachate, which suggests that some hydrophilic chemical groups, such as carbonyl groups, were generated on the surface of the three layers. For the outer layer, when UV radiated for 4 h, the contact angle declined to 102.9° and 119.8° after the layer was aged for 30 days in leachate and DI water, respectively. Furthermore, when the exposure to UV radiation reached 24 h, the contact angle obviously fell to 89.3° after aging for 30 days in leachate, resulting in increased hydrophilicity. With prolonged exposure to UV radiation, the trend with decreasing contact angle continued after aging in leachate. When the outer layer was subjected to UV radiation for 48 h, the contact angle dropped to 78.3° after 30 days of aging in leachate, while the contact angle decreased slightly to 106° after the layer was aged for 30 days in DI water. There is a correlation of bacterial settlement with surface hydrophobicity, surface wettability, and molecular flexibility. A hydrophilic surface is favorable for microbial adherence, leading to an increase in surface roughness which would further improve the attachment of microbes [57].
The inner layer displayed a similar trend as the outer layer in contact angle after it was aged in leachate and DI water. For example, after being subjected to UV radiation for 4 and 24 h, the contact angle of the inner layer reduced to 104.1° and 96.1° with aging of 30 days in leachate, respectively. Meanwhile, the contact angle dropped to 130.9° and 125° after the layer was aged for 30 days in DI water. However, when the inner layer was exposed to UV radiation for 36 and 48 h, the contact angle declined to 96.4° and 86.6° after aging for 30 days in leachate, while it decreased to 115.2° and 111.9° after 30 days of aging in DI water, respectively.
In contrast, compared to the outer and inner layers, the middle layer was more water-resistant. When the middle layer was exposed to UV radiation for 4 and 24 h, the contact angle of the layer declined to 118.5° and 106.6° after it was aged for 30 days in leachate, respectively. However, the contact angle of the middle layer only fell slightly, to 133.4° and 132.1°, after 30 days of aging in DI water, respectively. Additionally, when the layer was subjected to UV weathering for 36 h, the contact angle dropped to 101.5° and 130.7° after aging for 30 days in leachate and DI water, respectively. Furthermore, when the middle layer was radiated for 48 h, the contact angle with aging of 30 days in leachate could not be tested as the middle layer had broken into numerous small fragments. In contrast, the contact angle decreased slightly to 129.4° after 30 days of aging in DI water. The contact angle results are comparable to the CI, where the CI of the three layers aged in leachate was higher than that of the three layers aged in DI water, leading to more hydrophilic chemical groups like carbonyl groups being generated on the surface of the three layers [52].
Mechanical properties, such as tensile strength, are widely used in the assessment of aging for polymers [58], Fig. 7(d-f) shows the changes in the tensile strength of the three layers after aging in leachate and DI water. Compared to the original sample layers, the tensile strength of the three layers decreased by different degrees after aging in leachate and DI water. For the outer layer, when radiated for 4 and 24 h, the tensile strength was reduced by 1.7 and 7.1 times, to 7.98 and 1.53 MPa, respectively, after aging for 30 days in leachate. Besides, the tensile strength of the outer layer declined by 1.3 and 2.4 times, to 10.54 and 4.54 MPa, after 30 days of aging in DI water, respectively. Moreover, when the outer layer was exposed to UV weathering for 36 and 48 h, the tensile strength declined by 4.8 and 7.8 times, to 1.46 and 0.37 MPa, respectively, which was close to 0, after the layer was aged for 30 days in leachate. Meanwhile, the tensile strength of the outer layer aged for 30 days in DI water was reduced by 2.5 and 2.4 times, to 2.77 and 1.19 MPa, respectively. The inner layer presented a similar pattern to the outer layer in terms of tensile strength. Under a radiation time of 4 and 24 h, the tensile strength of the inner layer was reduced by 2.3 and 4.4 times, to 7.27 and 2.33 MPa, after the layer was aged for 30 days in leachate, respectively. In comparison, the tensile strength of the inner layer decreased slightly by 1.5 times, to 10.55 and 6.93 MPa, after 30 days of aging in DI water, respectively. When the inner layer was exposed to UV weathering for 36 and 48 h, the tensile strength decreased further by 22.5 and 20.7 times to close to 0 after the layer was aged for 30 days in leachate. In comparison, the tensile strength of the inner layer declined by 3.5 and 4.4 times, to 2.4 and 0.93 MPa, after aging for 30 days in DI water. The more fragments could be released from masks due to the lower mechanical properties, which will increase surface roughness and favor the settlement of microorganism. Physical degradation of plastic is often a precondition for later biological degradation since the former could break the polymer backbone, and microorganisms have the potential to breakdown polymer only at chain length of oligomers [59], [60].
These results illustrated that the mechanical performance of the outer and inner layers decreased considerably after aging in leachate. On the contrary, the middle layer presented a large sensitivity to UV radiation and leachate and displayed a massive deterioration in mechanical performance over 30 days of aging, which is consistent with our previous study in which the middle layer was easily broken into fragments [3]. After the middle layer was exposed to UV radiation for 24 h, the tensile strength of the middle layer was too low to be tested.
3.5. Analysis of components released from masks into simulated leachate
The particles released from masks into leachate and DI water were tested by microscopy for initial observations ( Fig. 8). We found that microplastics existed in two forms: fibrous and rod-shaped. Fibrous-shaped microplastics are longer and narrower than rod-shaped microplastics, which are shorter and thicker. More in-depth analysis of the particles released from the masks into leachate and DI water was conducted using SEM-EDS. It was confirmed that microplastics released into leachate and DI can be classified as microplastics (<1 mm) and nanoplastics (0.1–1 µm). From Fig. 8c, we can see that there are some tangled fibers and finer particles emitted from the masks. The size range of the fibrous particles appeared to be wider, frequently spanning from as little as 25 µm to several millimeters (2.5 mm). The elemental compositions of the particles released from the mask were further examined through EDS analysis. It is worth noting that the high percentage of carbon in these fibrous and rod-shaped particles indicates that they are likely plastics [38].
Fig. 8.
Microscopy (a-b) and SEM (c-f) images of microplastics released from the masks. Images are at 500 × magnification.
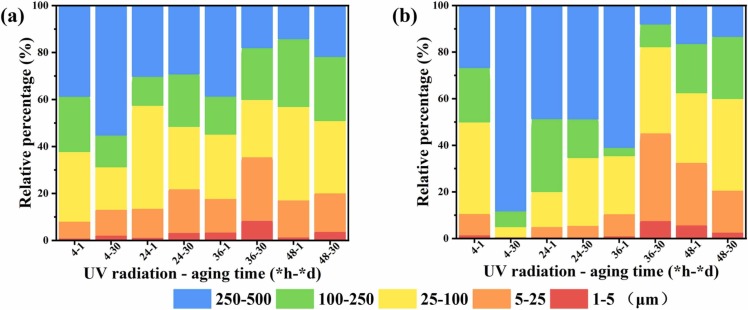
Under various environmental circumstances, the pollutant behaviors may vary [61], [62], [63], [64]. The microparticles released from the masks into leachate and DI water were further studied. Fig. 9 depicts the distribution of particles released in the range between 1 and 500 µm. It was found that the number of particles released from masks in leachate was higher than in DI water, with the increase in the length of UV radiation and the aging duration. Regarding the masks exposed to UV radiation for 4 h, two main different particle size distribution ranges were observed in leachate and DI water: 5–450 and 20–450 µm. After 1 day of aging, the total concentration of microplastics released into leachate and DI water was 0.75 and 0.35 μL/L and then increased by approximately 11 and 4 times, to 8.12 and 1.48 μL/L, after 30 days of aging, respectively. Microparticles in size ranges of 25–100, 100–250, and 250–500 µm released from leachate and DI water accounted for 30%, 24%, 39%, and 39%, 23%, 27% of the total concentration ( Fig. 10), respectively. When the masks were radiated with 24 h of UV, the concentration of microplastics released from the masks after 1 day of aging in leachate was 0.95 μL/L, which is similar to that for 4 h of exposure to UV radiation. Although the concentration of microplastics increased by around 20 times to 19.46 μL/L after 30 days of aging in leachate, the concentration of microplastics released in DI water increased only slightly, from 0.25 to 4.2 μL/L. The size ranges of 5–25, 25–100, 100–250, and 250–500 µm in leachate accounted for 19%, 27%, 22%, and 29% of the total particle size range, whereas the concentration of the same size in DI water took up 5%, 29%, 17%, and 49% of the total particle size range. This means that a larger number of smaller microplastics are released in leachate, which will result in more pollutants in landfills.
Fig. 9.
Size distribution of the microparticles released from masks into simulated leachate (a, c, e, g) and DI water (b, d, f, h).
Fig. 10.
The volume percentage of microparticles released from the masks into simulated leachate (a) and DI water (b).
When exposure to UV weathering for 36 h, the concentration of particles released in leachate was 6.38 μL/L after 1 day of aging, and then increased significantly by 30 times to 192.88 μL/L, after 30 days, with the particle sizes of 5–25, 25–100, 100–250, and 250–500 µm accounting for 27%, 24%, 22%, and 18% of the total, respectively. For the particles released in DI water, the concentration increased similarly by 30 times, from 1.50 to 37.18 μL/L, which is far less than that in leachate. The size ranges of 5–25, 25–100, 100–250, and 250–500 µm in DI water made up 38%, 37%, 10%, and 8%. At 48 h of exposure to UV radiation, the concentration of released particles in leachate was 39.45 μL/L after 1 day and then grew to 309.45 μL/L after 30 days of aging. More than 80% of the particles had a size distribution between 25 and 500 µm. Additionally, the microparticles released in leachate showed a unimodal pattern with the increase in concentration. For DI water, when the masks were aged from 1 day to 30 days, the total concentration of particles went from 2.91 to 84.68 μL/L. The percentage of particle sizes fall into the 5–25, 25–100, 100–250, and 250–500 µm ranges in DI water was 18%, 39%, 27%, and 13%, respectively. Disposable masks became fragile as a result of aging in leachate, with the release of irregular fragments and microplastics. The difference in such release before and after aging indicates that the environmental conditions have a significant impact on masks. This also demonstrates that if masks enter the environment as a result of improper disposal, they will have a significant impact on the environment.
In addition to the release of microparticles, the release of metal elements from masks during aging in simulated leachate was further analyzed by ICP-MS. Table 1 shows the metal elements from the masks released into leachate and DI water. We observed that seven elements (Al, Cr, Cu, Zn, Cd, Sb and Pb) in leachate and DI water, which is similar to previous research on the detectable elements from masks [38], [65], but we found the presence of Al and Cr elements. In the previous studies, Bussan et al. [65] determined the total concentrations of 12 metals elements (Cu, Pb, As, Cd, Sb, Hg, Zn, Ni, Cr, Tl, Se, Mn) being present in 24 commercial face masks after a complete digestion. Cu was detected in the range of 2.24–410 μg g−1; Sb was detected with a range of 0.97–90.18 μg g−1; Pb was detected ranged from 0.15 to 13.33 μg g−1; Zn was ranging from 15.93 to 56.80 μg g−1. Sullivan et al. [38] traced heavy metals of 7 kinds of face mask after submerged in 1.5 L DI water for 4 h. They found that Pb concentration was up to 6.79 µg/L and confirmed Cd up to 1.92 µg/L, Sb up to 393 µg/L and Cu up to 4.17 µg/L. Idowu et al. [66] monitored the release of toxic metals from medical face masks degraded under normal outdoor environmental conditions. The mean concentrations of As, Cd, Pb, Sn, Cu and Fe were 0.22 ± 0.042, 0.35 ± 0.21, 1.72 ± 0.23, 3.21 ± 0.07, 6.31 ± 0.47 and 139.86 ± 33.52 mg kg− 1 of face masks, respectively. Jemec Kokalj et al. [67] analyzed metal content of the source medical masks and reported that the highest values were Ti (88.9 ± 16.8 mg kg-1), Al (39.6 ± 4.82 mg kg-1), Cu (38.9 ± 1.88 mg kg-1) and Pb (9.56 ± 0.16 mg kg-1). It is interesting that after various lengths of exposure to UV radiation and 30 days of aging, the total concentration of these elements in leachate was higher than that released into DI water other than the Al element, which could be due to a stronger adsorption of Al on the aged plastic [68]. Ashton et al. [69] also found that aged plastics would adsorb higher concentration of Al, compared with other elements (Mn, Cu, Zn, Pb, Ag, Cd, Co, Cr, Mo, Sb, Sn, U). Cu had the concentration of metal elements released into leachate and DI water after 30 days of aging, with 4, 24, 36, and 48 h of UV radiation, where the concentrations in leachate were 5.91, 14.19, 16.08, and 29.65 ppb, which is higher than the concentrations in DI water, which were 3.45, 8.90, 15.30, and 16.08 ppb, respectively. Copper is a well-known environmental pollutant that can cause toxicity in a variety of organisms, including humans [70]. In addition to Cu, the weathered masks released Zn at concentrations of 2.44, 4.23, 7.63, and 16.15 ppb in leachate with different lengths of exposure to UV radiation, while the concentrations in DI water was 1.33, 1.63, 2.94, and 3.59 ppb after 30 days of aging. Noticeably, Cd and Pb were detected in leachate, where their concentrations were below 1 ppb, although they hardly ever were released into DI water. After 30 days of aging, the Sb element concentrations in leachate and DI water were comparable. Antimony and lead can cause respiratory problems, pneumoconiosis, and carcinogenic and toxicological effects on organisms, and even low exposure to them can have adverse effects on humans, when exposure in amounts greater than 8.87 mg.m−3 and 50 μg.m−3, respectively [71], [72]. Even though these metal additives are helpful in the functionality of polymer products, the presence of anions, such as Cl- and SO4 2-, in leachate may accelerate the release of metal elements from the plastics [73], potentially contaminating soil. Bussan et al. [65] analyzed the release of metal elements in the saline leachate solution and found masks leached out 16 times more Pb, and about 3 times more Sb than in the water leachate. In conclusion, the combined action of UV and inorganic ions in leachate, which could accelerate the surface aging and degradation of the polypropylene masks. Further research can be taken to study the mechanisms and kinetics of plastic–metal interactions and the effects of different environmental variables on these processes in the landfill environment.
Table 1.
Lists some of the main metal elements released from masks after 30 days of aging in simulated leachate and DI water (μg.kg-1).
| Sample | Al | Cr | Cu | Zn | Cd | Sb | Pb |
|---|---|---|---|---|---|---|---|
| UV-4 h-leachate | 1.76 ± 0.02 | 0.92 ± 0.01 | 5.91 ± 0.01 | 2.44 ± 0.08 | 0.01 ± 0.001 | 0.17 ± 0.02 | 0.13 ± 0.008 |
| UV-4 h-DI water | 14.1 ± 0.24 | 0.10 ± 0.42 | 3.45 ± 0.02 | 1.33 ± 0.03 | N.D* | 0.15 ± 0.01 | 0.01 ± 0.001 |
| UV-24 h-leachate | 2.55 ± 0.03 | 1.03 ± 0.01 | 14.19 ± 0.02 | 4.23 ± 0.16 | 0.17 ± 0.02 | 0.31 ± 0.05 | 0.35 ± 0.008 |
| UV-24 h-DI water | 20.99 ± 0.86 | 0.15 ± 0.01 | 8.90 ± 0.16 | 1.63 ± 0.04 | N.D* | 0.32 ± 0.09 | 0.01 ± 0.001 |
| UV-36 h-leachate | 2.42 ± 0.02 | 1.52 ± 0.01 | 16.08 ± 0.07 | 7.63 ± 0.24 | 0.21 ± 0.01 | 0.48 ± 0.02 | 0.72 ± 0.040 |
| UV-36 h-DI water | 35.10 ± 0.16 | 0.16 ± 0.01 | 15.30 ± 0.49 | 2.94 ± 0.08 | N.D* | 0.43 ± 0.02 | 0.02 ± 0.002 |
| UV-48 h-leachate | 2.44 ± 0.01 | 1.54 ± 0.01 | 29.65 ± 1.71 | 16.15 ± 0.90 | 0.19 ± 0.02 | 0.47 ± 0.05 | 1.01 ± 0.008 |
| UV-48 h-DI water | 41.10 ± 0.08 | 0.20 ± 0.01 | 16.08 ± 0.82 | 3.59 ± 0.09 | N.D* | 0.54 ± 0.02 | 0.02 ± 0.001 |
N.D* not detected.
4. Conclusions and recommendations for future efforts
In this study, we explored the aging of disposable face masks in landfill leachate. The results demonstrated how UV radiation caused oxidation of the three mask layers, as well as changes in physicochemical properties. After exposure to UV radiation, all three layers exhibited abrasions and fractures on the surface and became unstable, which, in turn, increased the aging of the masks in landfill leachate. Furthermore, the CI kept rising after 30 days of aging in leachate, suggesting that oxidation occurred in the presence of leachate. The combined action of UV and inorganic ions in leachate could accelerate the surface aging and degradation of the polypropylene membrane. The ISO results also confirmed that the three layers of masks became more unstable compared to the pristine samples. In addition, when the masks were aged in leachate, the SEM images clearly displayed distinct erosion and fractures on the surfaces of three layers, with the middle layer presenting obvious morphological alterations compared to the outer and inner layers. Compared to the mechanical performance of the three layers aged in DI water, the masks aged in leachate also exhibited a larger drop in tensile strength, demonstrating that they had undergone more aging. From the analysis of the pollutants released from masks during aging, especially microplastics and metal elements, we found that the total concentration of the microparticles released into leachate was higher than into DI water due to the increased fractures of plastics during aging. Additionally, the masks aged in leachate were likely to release metal elements. After UV irradiation, the additives can be gradually released, which while potentially changing the mechanical properties of the mask.
The use of disposable face masks will continue to increase. It is expected to make further efforts in order to address the challenges of mask waste management. Some recommendations for future work are as follows: (i) A large quantity of used disposable masks currently end in landfills. Our results show that the microparticles and metal ions can be released to the leachate during mask aging. A well-designed landfill with appropriate liners and leachate collection system can help minimize the risks of leachate leaking into the surrounding environment. Future research can also be conducted to achieve the simultaneous removal of microplastics and other pollutants from leachate. (ii) The majority of masks in the market are made of synthetic polymers such as polypropylene and polyethylene, which are usually difficult to be degraded. There is an increasing interest in the use of more environmentally friendly and biodegradable materials such as poly (l-lactic acid) and polyhydroxyalkanoate in the manufacturing of disposable masks. It is expected to develop more degradable masks which can also meet the performance requirement of filtration efficiency. (iii) To reduce the mask disposal in landfills, the government regulations can be developed and implemented to separate the waste masks from the regular stream of municipal solid wastes. The enhanced public awareness will also facilitate the appropriate disposal of mask wastes. (iv) Based on the good source sorting, mask wastes produced from mask manufacturing and consumption can be further recycled and reused. There have been some successful examples for the recycling and reuse of mask wastes in construction materials and energy recovery. It is expected to explore a wider range of mask reuse and recycle strategies, which will make the life cycle of mask products more sustainable.
CRediT authorship contribution statement
Linxiang Lyu: Conceived and designed the analysis; collected the data; performed the analysis; wrote the paper. Zheng Wang: Collected the data. Monisha Bagchi: Conceived and designed the analysis; revised the paper. Zhibin Ye: Revised the paper. Ahmed Soliman: Revised the paper. Ashutosh Bagchi: Revised the paper. Nektaria Markoglou: Revised the paper. Jianan Yin: Collected the data. Chunjiang An: Conceived and designed the analysis; revised the paper. Xiaohan Yang: Collected the data. Huifang Bi: Collected the data. Mengfan Cai: Collected the data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada. The authors are also grateful to the editor and the anonymous reviewers for their insightful comments and suggestions.
Environmental implication
The use of disposable face masks as preventive measures significantly increased in the past years. Overloading landfills with disposable face mask wastes will raise certain environmental concerns. This study investigated the aging and degradation of disposable masks in landfill leachate. The change of physical and chemical characteristics of the masks in landfill leachate were comprehensively studied. The pollutants, including microparticles and metal elements, released into landfill leachate were analyzed. The findings of this study showed masks would age more and release more pollutants in landfill leachate. The findings can help address the challenges of mask waste management.
Editor: Dr. R. Teresa
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2022.130671.
Appendix A. Supplementary material
Supplementary material
.
Data availability
The data are included in the manuscript.
References
- 1.WHO Coronavirus (covid-19) dashboard, 2022. 〈https://covid19.who.int〉. (accessed 23 September 2022).
- 2.Statista, 2022, Face masks - worldwide | Statista Market Forecast. 〈https://www.statista.com/outlook/cmo/tissue-hygiene-paper/face-masks/worldwide〉. (accessed 5 November 2022).
- 3.Wang Z., An C., Chen X., Lee K., Zhang B., Feng Q. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J Hazard Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.126036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z., Wang J., Yang X., Huang Q., Zhu K., Sun Y., Van Hulle S., Jia H. Generation of environmental persistent free radicals (EPFRs) enhances ecotoxicological effects of the disposable face mask waste with the COVID-19 pandemic. Environ Pollut. 2022;301 doi: 10.1016/j.envpol.2022.119019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizarro-Ortega C.I., Dioses-Salinas D.C., Fernández Severini M.D., Forero López A.D., Rimondino G.N., Benson N.U., Dobaradaran S., De-la-Torre G.E. Degradation of plastics associated with the COVID-19 pandemic. Mar Pollut Bull. 2022;176 doi: 10.1016/j.marpolbul.2022.113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvaranjan K., Navaratnam S., Rajeev P., Ravintherakumaran N. Environmental challenges induced by extensive use of face masks during COVID-19: a review and potential solutions. Environ Chall. 2021;3 doi: 10.1016/j.envc.2021.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muensterman D.J., Cahuas L., Titaley I.A., Schmokel C., De la Cruz F.B., Barlaz M.A., Carignan C.C., Peaslee G.F., Field J.A. Per- and polyfluoroalkyl substances (PFAS) in facemasks: potential source of human exposure to PFAS with implications for disposal to landfills. Environ Sci Technol Lett. 2022;9:320–326. doi: 10.1021/acs.estlett.2c00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jędruchniewicz K., Ok Y.S., Oleszczuk P. COVID-19 discarded disposable gloves as a source and a vector of pollutants in the environment. J Hazard Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.125938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z.K., Feng Q., Yue R., Chen Z., Moselhi O., Soliman A., Hammad A., An C. Construction, renovation, and demolition waste in landfill: a review of waste characteristics, environmental impacts, and mitigation measures. Environ Sci Pollut Res. 2022;29:46509–46526. doi: 10.1007/s11356-022-20479-5. [DOI] [PubMed] [Google Scholar]
- 10.Patrício Silva A.L., Prata J.C., Mouneyrac C., Barcelò D., Duarte A.C., Rocha-Santos T. Risks of COVID-19 face masks to wildlife: present and future research needs. Sci Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanapalli K.R., Sharma H.B., Ranjan V.P., Samal B., Bhattacharya J., Dubey B.K., Goel S. Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan H., Cousins I.T., Zhang C., Zhou Q. Perfluoroalkyl acids in municipal landfill leachates from china: occurrence, fate during leachate treatment and potential impact on groundwater. Sci Total Environ. 2015;524–525:23–31. doi: 10.1016/j.scitotenv.2015.03.111. [DOI] [PubMed] [Google Scholar]
- 13.Nie S., Huang C.Z., Huang W.W., Liu J. A non-deterministic integrated optimization model with risk measure for identifying water resources management strategy. J Environ Inf. 2021;38:41–55. doi: 10.3808/jei.202100459. [DOI] [Google Scholar]
- 14.Feng Q., Chen Z., Greer C.W., An C., Wang Z. Transport of microplastics in shore substrates over tidal cycles: roles of polymer characteristics and environmental factors. Environ Sci Technol. 2022;56:8187–8196. doi: 10.1021/acs.est.2c01599. [DOI] [PubMed] [Google Scholar]
- 15.Patrício Silva A.L., Prata J.C., Duarte A.C., Barcelò D., Rocha-Santos T. An urgent call to think globally and act locally on landfill disposable plastics under and after COVID-19 pandemic: pollution prevention and technological (bio) remediation solutions. Chem Eng J. 2021;426 doi: 10.1016/j.cej.2021.131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., An C., Feng Q., Boufadel M., Ji W. Aggregation of microplastics and clay particles in the nearshore environment: characteristics, influencing factors, and implications. Water Res. 2022 doi: 10.1016/j.watres.2022.119077. [DOI] [PubMed] [Google Scholar]
- 17.Chen X., Chen X., Liu Q., Zhao Q., Xiong X., Wu C. Used disposable face masks are significant sources of microplastics to environment. Environ Pollut. 2021;285 doi: 10.1016/j.envpol.2021.117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J., Chen F., Xu H., Jiang H., Liu J., Li P., Chen C.C., Pan K. Face masks as a source of nanoplastics and microplastics in the environment: quantification, characterization, and potential for bioaccumulation. Environ Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117748. [DOI] [PubMed] [Google Scholar]
- 19.Huang D., Chen H., Shen M., Tao J., Chen S., Yin L., Zhou W., Wang X., Xiao R., Li R. Recent advances on the transport of microplastics/nanoplastics in abiotic and biotic compartments. J Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2022.129515. [DOI] [PubMed] [Google Scholar]
- 20.Kik K., Bukowska B., Sicińska P. Polystyrene nanoparticles: sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114297. [DOI] [PubMed] [Google Scholar]
- 21.Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar Pollut Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng Q., An C., Chen Z., Yin J., Zhang B., Lee K., Wang Z. Investigation into the impact of aged microplastics on oil behavior in shoreline environments. J Hazard Mater. 2022;421 doi: 10.1016/j.jhazmat.2021.126711. [DOI] [PubMed] [Google Scholar]
- 23.Rocha-Santos T., Duarte A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal Chem. 2015;65:47–53. doi: 10.1016/j.trac.2014.10.011. [DOI] [Google Scholar]
- 24.De-la-Torre G.E., Dioses-Salinas D.C., Pizarro-Ortega C.I., Fernández Severini M.D., Forero López A.D., Mansilla R., Ayala F., Castillo L.M.J., Castillo-Paico E., Torres D.A., Mendoza-Castilla L.M., Meza-Chuquizuta C., Vizcarra J.K., Mejía M., De La Gala J.J.V., Ninaja E.A.S., Calisaya D.L.S., Flores-Miranda W.E., Rosillo J.L.E., Espinoza-Morriberón D., Gonzales K.N., Torres F.G., Rimondino G.N., Ben-Haddad M., Dobaradaran S., Aragaw T.A., Santillán L. Binational survey of personal protective equipment (PPE) pollution driven by the COVID-19 pandemic in coastal environments: abundance, distribution, and analytical characterization. J Hazard Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.128070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., An C., Lee K., Chen X., Zhang B., Yin J., Feng Q. Physicochemical change and microparticle release from disposable gloves in the aqueous environment impacted by accelerated weathering. Sci Total Environ. 2022;832 doi: 10.1016/j.scitotenv.2022.154986. [DOI] [PubMed] [Google Scholar]
- 26.Saliu F., Veronelli M., Raguso C., Barana D., Galli P., Lasagni M. The release process of microfibers: from surgical face masks into the marine environment. Environ Adv. 2021;4 doi: 10.1016/j.envadv.2021.100042. [DOI] [Google Scholar]
- 27.Liao J., Ji S., Chi Y. Effects of discarded masks on the offshore microorganisms during the COVID-19 pandemic. Toxics. 2022;10:426. doi: 10.3390/toxics10080426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J., Chen F., Xu H., Liu J., Chen C.C., Zhang Z., Jiang H., Li Y., Pan K. Fate of face masks after being discarded into seawater: aging and microbial colonization. J. Hazard. Mater. 2022;129084 doi: 10.1016/j.jhazmat.2022.129084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dan A., Oka M., Fujii Y., Soda S., Ishigaki T., Machimura T., Ike M. Removal of heavy metals from synthetic landfill leachate in lab-scale vertical flow constructed wetlands. Sci Total Environ. 2017;584–585:742–750. doi: 10.1016/j.scitotenv.2017.01.112. [DOI] [PubMed] [Google Scholar]
- 30.Grossule V., Fang D., Yue D., Lavagnolo M.C., Raga R. Preparation of artificial MSW leachate for treatment studies: testing on black soldier fly larvae process. Waste Manag Res. 2021 doi: 10.1177/0734242X211066702. [DOI] [PubMed] [Google Scholar]
- 31.Stelzer-Braid S., Oliver B.G., Blazey A.J., Argent E., Newsome T.P., Rawlinson W.D., Tovey E.R. Exhalation of respiratory viruses by breathing, coughing, and talking. J Med Virol. 2009;81:1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 32.Chammingkwan P., Yamaguchi F., Terano M., Taniike T. Influence of isotacticity and its distribution on degradation behavior of polypropylene. Polym Degrad Stab. 2017;143:253–258. doi: 10.1016/j.polymdegradstab.2017.07.024. [DOI] [Google Scholar]
- 33.Song Y.K., Hong S.H., Jang M., Han G.M., Jung S.W., Shim W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ Sci Technol. 2017;51:4368–4376. doi: 10.1021/acs.est.6b06155. [DOI] [PubMed] [Google Scholar]
- 34.Longo C., Savaris M., Zeni M., Brandalise R.N., Grisa A.M.C. Degradation study of polypropylene (PP) and bioriented polypropylene (BOPP) in the environment. Mater Res. 2011;14:442–448. doi: 10.1590/S1516-14392011005000080. [DOI] [Google Scholar]
- 35.Ferronato N., Torretta V. Waste mismanagement in developing countries: a review of global issues. Int J Environ Res Public Health. 2019;16:1060. doi: 10.3390/ijerph16061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akarsu C., Madenli Ö., Deveci E.Ü. Characterization of littered face masks in the southeastern part of turkey. Environ Sci Pollut Res. 2021;28:47517–47527. doi: 10.1007/s11356-021-14099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prata J.C., Silva A.L.P., Duarte A.C., Rocha-Santos T. Disposable over reusable face masks: public safety or environmental disaster? Environments. 2021;8:31. doi: 10.3390/environments8040031. [DOI] [Google Scholar]
- 38.Sullivan G.L., Delgado-Gallardo J., Watson T.M., Sarp S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks - linked to the COVID-19 pandemic. Water Res. 2021;196 doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaczmarek H., Ołdak D., Malanowski P., Chaberska H. Effect of short wavelength uv-irradiation on ageing of polypropylene/cellulose compositions. Polym Degrad Stab. 2005;88:189–198. doi: 10.1016/j.polymdegradstab.2004.04.017. [DOI] [Google Scholar]
- 40.Gewert B., M. Plassmann M., MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts. 2015;17:1513–1521. doi: 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- 41.Zhu K., Jia H., Sun Y., Dai Y., Zhang C., Guo X., Wang T., Zhu L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: roles of reactive oxygen species. Water Res. 2020;173 doi: 10.1016/j.watres.2020.115564. [DOI] [PubMed] [Google Scholar]
- 42.Wu X., Liu P., Shi H., Wang H., Huang H., Shi Y., Gao S. Photo aging and fragmentation of polypropylene food packaging materials in artificial seawater. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116456. [DOI] [PubMed] [Google Scholar]
- 43.Ju J.T.J., Boisvert L.N., Zuo Y.Y. Face masks against COVID-19: standards, efficacy, testing and decontamination methods. Adv Colloid Interface Sci. 2021;292 doi: 10.1016/j.cis.2021.102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouillon C., Bussiere P.-O., Desnoux E., Collin S., Vial C., Therias S., Gardette J.-L. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation. Polym Degrad Stab. 2016;128:200–208. doi: 10.1016/j.polymdegradstab.2015.12.011. [DOI] [Google Scholar]
- 45.Potrykus M., Redko V., Głowacka K., Piotrowicz-Cieślak A., Szarlej P., Janik H., Wolska L. Polypropylene structure alterations after 5 years of natural degradation in a waste landfill. Sci Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143649. [DOI] [PubMed] [Google Scholar]
- 46.Dong G., Chen B., Liu B., Hounjet L.J., Cao Y., Stoyanov S.R., Yang M., Zhang B. Advanced oxidation processes in microreactors for water and wastewater treatment: development, challenges, and opportunities. Water Res. 2022;211 doi: 10.1016/j.watres.2022.118047. [DOI] [PubMed] [Google Scholar]
- 47.Hong S., Chon T.S., Joo G.J. Spatial distribution patterns of Eurasian otter (Lutra lutra) in association with environmental factors unravelled by machine learning and diffusion kernel method. J Environ Inf. 2021;37:130–141. doi: 10.3808/jei.202000443. [DOI] [Google Scholar]
- 48.Safaei S.H., Young S., Samimi Z., Parvizi F., Shokrollahi A., Baniamer M. Technology development for the removal of COVID-19 pharmaceutical active compounds from water and wastewater: a review. J Environ Inf. 2022;40:141–156. doi: 10.3808/jei.202200480. [DOI] [Google Scholar]
- 49.Zhao S., Huang G., An C., Wei J., Yao Y. Enhancement of soil retention for phenanthrene in binary cationic gemini and nonionic surfactant mixtures: characterizing two-step adsorption and partition processes through experimental and modeling approaches. J Hazard Mater. 2015;286:144–151. doi: 10.1016/j.jhazmat.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 50.White J.R., Shyichuk A.V. Effect of stabilizer on scission and crosslinking rate changes during photo-oxidation of polypropylene. Polym Degrad Stab. 2007;92:2095–2101. doi: 10.1016/j.polymdegradstab.2007.07.013. [DOI] [Google Scholar]
- 51.Ito M., Nagai K. Analysis of degradation mechanism of plasticized PVC under artificial aging conditions. Polym Degrad Stab. 2007;92:260–270. doi: 10.1016/j.polymdegradstab.2006.11.003. [DOI] [Google Scholar]
- 52.Gryta M., Grzechulska-Damszel J., Markowska A., Karakulski K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J Membr Sci. 2009;326:493–502. doi: 10.1016/j.memsci.2008.10.022. [DOI] [Google Scholar]
- 53.Carneiro J.R., Almeida P.J., Lopes M., de L. Laboratory evaluation of interactions in the degradation of a polypropylene geotextile in marine environments. Adv Mater Sci Eng. 2018;2018 doi: 10.1155/2018/9182658. [DOI] [Google Scholar]
- 54.Khoironi A., Hadiyanto H., Anggoro S., Sudarno S. Evaluation of polypropylene plastic degradation and microplastic identification in sediments at tambak lorok coastal area, semarang, indonesia. Mar Pollut Bull. 2020;151 doi: 10.1016/j.marpolbul.2019.110868. [DOI] [PubMed] [Google Scholar]
- 55.Scheirs J. John Wiley & Sons,; 2000. Compositional and Failure Analysis of Polymers: A Practical Approach. [Google Scholar]
- 56.Mercier A., Gravouil K., Aucher W., Brosset-Vincent S., Kadri L., Colas J., Bouchon D., Ferreira T. Fate of eight different polymers under uncontrolled composting conditions: relationships between deterioration, biofilm formation, and the material surface properties. Environ Sci Technol. 2017;51:1988–1997. doi: 10.1021/acs.est.6b03530. [DOI] [PubMed] [Google Scholar]
- 57.Bhagwat G., O’Connor W., Grainge I., Palanisami T. Understanding the fundamental basis for biofilm formation on plastic surfaces: role of conditioning films. Front Microbiol. 2021:12. doi: 10.3389/fmicb.2021.687118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu B., Wang G.-X., Huang D., Ren Z.-L., Wang X.-W., Wang P.-L., Zhen Z.-C., Zhang W., Ji J.-H. Comparison of PCL degradation in different aquatic environments: effects of bacteria and inorganic salts. Polym Degrad Stab. 2018;150:133–139. doi: 10.1016/j.polymdegradstab.2018.02.002. [DOI] [Google Scholar]
- 59.Liu P., Zhan X., Wu X., Li J., Wang H., Gao S. Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere. 2020;242 doi: 10.1016/j.chemosphere.2019.125193. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y., Liu W., Zhang Z., Grossart H.-P., Gadd G.M. Microplastics provide new microbial niches in aquatic environments. Appl Microbiol Biotechnol. 2020;104:6501–6511. doi: 10.1007/s00253-020-10704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song P., Huang G., Hong Y., An C., Xin X., Zhang P. A biophysiological perspective on enhanced nitrate removal from decentralized domestic sewage using gravitational-flow multi-soil-layering systems. Chemosphere. 2020;240 doi: 10.1016/j.chemosphere.2019.124868. [DOI] [PubMed] [Google Scholar]
- 62.Sundar S., Mishra A.K., Shukla J.B. Effects of mitigation options on the control of methane emissions caused by rice paddies and livestock populations to reduce global warming: a modeling study and comparison with environmental data. J Environ Inf. 2021;38:106–115. doi: 10.3808/jei.202000447. [DOI] [Google Scholar]
- 63.Wei H.W., Hassan M., Che Y., Peng Q.K., Wang Q., Su Y.L., Xie B. Spatio-temporal characteristics and source apportionment of water pollutants in upper reaches of maotiao river, southwest of china, from 2003 to 2015. J Environ Inf. 2021;37:93–106. doi: 10.3808/jei.201900415. [DOI] [Google Scholar]
- 64.Xin X., Huang G., An C., Feng R. Interactive toxicity of triclosan and nano-TiO2 to green alga Eremosphaera viridis in Lake Erie: a new perspective based on fourier transform infrared spectromicroscopy and synchrotron-based x-ray fluorescence imaging. Environ Sci Technol. 2019;53:9884–9894. doi: 10.1021/acs.est.9b03117. [DOI] [PubMed] [Google Scholar]
- 65.Bussan D.D., Snaychuk L., Bartzas G., Douvris C. Quantification of trace elements in surgical and kn95 face masks widely used during the SARS-COVID-19 pandemic. Sci Total Environ. 2022;814 doi: 10.1016/j.scitotenv.2021.151924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Idowu G.A., Olalemi A.O., Aiyesanmi A.F. Environmental impacts of COVID-19 pandemic: release of microplastics, organic contaminants and trace metals from face masks under ambient environmental conditions. Environ Res. 2023;217 doi: 10.1016/j.envres.2022.114956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jemec Kokalj A., Dolar A., Drobne D., Škrlep L., Škapin A.S., Marolt G., Nagode A., van Gestel C.A.M. Effects of microplastics from disposable medical masks on terrestrial invertebrates. J Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2022.129440. [DOI] [PubMed] [Google Scholar]
- 68.Tabatabaei M., Hosseinzadeh-Bandbafha H., Yang Y., Aghbashlo M., Lam S.S., Montgomery H., Peng W. Exergy intensity and environmental consequences of the medical face masks curtailing the COVID-19 pandemic: malign bodyguard? J Clean Prod. 2021;313 doi: 10.1016/j.jclepro.2021.127880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashton K., Holmes L., Turner A. Association of metals with plastic production pellets in the marine environment. Mar Pollut Bull. 2010;60:2050–2055. doi: 10.1016/j.marpolbul.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Keller A.A., Adeleye A.S., Conway J.R., Garner K.L., Zhao L., Cherr G.N., Hong J., Gardea-Torresdey J.L., Godwin H.A., Hanna S., Ji Z., Kaweeteerawat C., Lin S., Lenihan H.S., Miller R.J., Nel A.E., Peralta-Videa J.R., Walker S.L., Taylor A.A., Torres-Duarte C., Zink J.I., Zuverza-Mena N. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact. 2017;7:28–40. doi: 10.1016/j.impact.2017.05.003. [DOI] [Google Scholar]
- 71.Pandey G., Madhuri S. Heavy metals causing toxicity in animals and fishes. Res J Anim Vet Fish Sci. 2014;2:17–23. [Google Scholar]
- 72.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. In: Molecular, clinical and environmental toxicology: volume 3: environmental toxicology, experientia supplementum. Luch A., editor. Springer; Basel: 2012. Heavy metal toxicity and the environment; pp. 133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Malack M.H. Migration of lead from unplasticized polyvinyl chloride pipes. J Hazard Mater. 2001;82:263–274. doi: 10.1016/S0304-3894(00)00366-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data are included in the manuscript.