Abstract
Leptospira interrogans is an important mammalian pathogen. Transmission from an environmental source requires adaptations to a range of new environmental conditions in the organs and tissues of the infected host. Since many pathogenic bacteria utilize temperature to discern their environment and regulate the synthesis of appropriate proteins, we investigated the effects of temperature on protein synthesis in L. interrogans. Bacteria were grown for several days after culture temperatures were shifted from 30 to 37°C. Triton X-114 cellular fractionation identified several proteins of the cytoplasm, periplasm, and outer membrane for which synthesis was dependent on the culture temperature. Synthesis of a cytoplasmic protein of 20 kDa was switched off at 37°C, whereas synthesis of a 66-kDa periplasmic protein was increased at the higher temperature. Increased synthesis of a 25-kDa outer membrane protein was observed when the organisms were shifted from 30 to 37°C. A 36-kDa protein synthesized at 30 but not at 37°C was identified as LipL36, an outer membrane lipoprotein. In contrast, expression of another lipoprotein, LipL41, was the same at either temperature. Immunoblotting with convalescent equine sera revealed that some proteins exhibiting thermoregulation of synthesis elicited antibody responses during infection. Our results show that sera from horses which aborted as a result of naturally acquired infection with L. interrogans serovar pomona type kennewicki recognize periplasmic and outer membrane proteins which are differentially synthesized in response to temperature and which therefore may be important in the host-pathogen interaction during infection.
Leptospirosis, a worldwide zoonotic disease caused by several species of the spirochete genus Leptospira, affects a wide range of mammalian hosts, including humans, horses, dogs, pigs, cattle, and wildlife. Infection varies from subclinical infection to a potentially fatal disease with multiorgan involvement (9). Long considered to be endemic in tropical countries, leptospirosis has been increasingly recognized in more temperate countries, such as the United States, where outbreaks are associated with recreational exposure from natural water sources and exposure of inner-city residents to rat-infested alleys (1, 21, 22). Reservoirs of infection include chronically infected wild or domesticated carrier animals, and transmission is effected directly from contaminated urine or indirectly by entry from environments that permit leptospiral survival.
Transmission of leptospires requires that the bacteria adapt to and survive in a range of environments. Aside from vertebrate organs and tissues, leptospires survive in swamps, streams and rivers, and alkaline muds and soils (6, 9). Such a diverse lifestyle likely requires that Leptospira interrogans sense its environment and respond by synthesizing appropriate proteins and other cellular constituents necessary for survival in each habitat. Many pathogenic bacteria are known to respond to changes in temperature and pH experienced during entry into a host and accordingly modify synthesis of virulence factors and other proteins. Rhodococcus equi, Shigella flexneri, and other bacteria produce certain proteins only when cultured at temperatures comparable to those encountered during infection of mammals (12, 14, 20). Another spirochete, Borrelia burgdorferi, apparently also senses changes in temperature experienced during vertebrate infection (19). For example, the OspC surface protein is produced during transmission from the tick vector to a mammal. Consistent with this observation, in culture, very little OspC is synthesized at 23°C, whereas shifting the culture temperature from 23 to 35°C results in the synthesis of large amounts of this protein (15, 16).
We have found that L. interrogans also regulates the synthesis of proteins in response to in vitro temperature changes. Organisms cultured at 24 and 30°C show altered synthesis of proteins involved in infection when subsequently grown at 37°C, a temperature similar to that encountered during invasion of mammalian hosts.
MATERIALS AND METHODS
Bacteria.
An isolate of L. interrogans serovar pomona type kennewicki, kindly provided by Mike Donahue (Lexington Livestock Disease Diagnostic Center, University of Kentucky, Lexington), was cultured from the kidney of an aborted equine fetus in 1997. It was subsequently cloned from a single colony grown in Johnson-Harris bovine serum albumin Tween 80 medium (Bovuminar PLM-5 microbiological Medium; Intergen, Purchase, N.Y.) solidified with 1% Noble agar. The isolate, JEN4, was typed as L. interrogans serovar pomona type kennewicki by Carole Bolin (National Animal Disease Center, Ames, Iowa).
Triplicate cultures were maintained in Bovuminar PLM-5 medium and grown at 24, 30, and 37°C until the mid-logarithmic phase (approximately 106 bacteria/ml). An aliquot of each was then diluted 1:100 in fresh medium and either maintained at the same temperature or shifted to 24, 30, or 37°C. All cultures were grown to mid-logarithmic phase (approximately 5 to 7 days) and harvested at the same cell density by centrifugation. The bacteria were washed three times with phosphate-buffered saline (PBS) and lysed by being boiled for 5 min.
Gel electrophoresis and immunoblotting.
The protein concentrations of lysates were determined by the bicinchoninic assay (Pierce, Rockford, Ill.). Equal amounts of lysate protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were then silver stained (PlusOne silver stain kit; Amersham Pharmacia Biotech, Uppsala, Sweden). Alternatively, the separated proteins were transferred to nitrocellulose membranes (Schleicher & Scheull, Keene, N.H.) and blocked with 5% (wt/vol) nonfat dry milk in PBS–0.05% Tween 20. The membranes were individually incubated with serum samples from mares that had aborted following naturally acquired infection with L. interrogans serovar pomona type kennewicki kindly provided by Barbara Smith (Lexington Livestock Disease Diagnostic Center, University of Kentucky, Lexington). The sera were diluted 1:100 in PBS–0.05% Tween 20 and probed with protein G conjugated to horseradish peroxidase (Zymed, San Francisco, Calif.). Additionally, the membranes were incubated with antisera raised against spirochetal flagella (D. Blanco, University of California—Los Angeles) (4), GroEL (B. Adler, Monash University, Clayton Campus, Australia) (2), LipL36 and LipL41 (D. Haake, University of California—Los Angeles) (10, 17), or DnaK (J. Timoney) followed by incubation with horseradish peroxidase–goat anti-rabbit immunoglobulin G conjugate (Zymed). Antiserum raised against endoflagella of Treponema pallidum has previously been shown to cross-react with flagella of Leptospira (11). Antigen-antibody binding was detected by incubation in 10 mg of 4-chloro-1-naphthol (Sigma, St. Louis, Mo.) dissolved in 5 ml of methanol, 25 ml of PBS, and 50 μl of 30% hydrogen peroxide for approximately 10 min before being terminated by washing in distilled H2O.
Bacterial fractionation with Triton X-114.
Extraction and phase partitioning with Triton X-114 was performed as previously described (5). In brief, mid-logarithmic-phase cultures were harvested by centrifugation, washed once in ice-cold PBS, resuspended in ice-cold 2% (vol/vol) Triton X-114 (Sigma) in PBS, and gently rotated overnight at 4°C. Insoluble materials, including protoplasmic cylinders, were removed by centrifugation, and the supernatant wash phase was separated by warming the solution to 37°C for 15 min followed by centrifugation for 15 min at 25°C. The separated detergent phase was washed three times with ice-cold PBS, while the aqueous phase was washed three times by adding ice-cold 10% Triton X-114 to bring the final concentration of Triton X-114 to 2%, followed by warming to 37°C and centrifugation as described above. Methanol-chloroform precipitation was used to remove detergent contaminants.
RESULTS
Analysis of leptospiral whole-cell lysates.
L. interrogans JEN4 was cultured at 24, 30, and 37°C, passaged into fresh medium, and either maintained at its original culture temperature or shifted to one of the other temperatures. JEN4 grows well at each temperature, although somewhat faster growth was noted at 24 and 30°C than at 37°C. Equal amounts of total protein from lysates of cultures of L. interrogans shifted among 24, 30, and 37°C were separated by SDS-PAGE and silver stained. No differences in protein profiles were detected in any of these cultures.
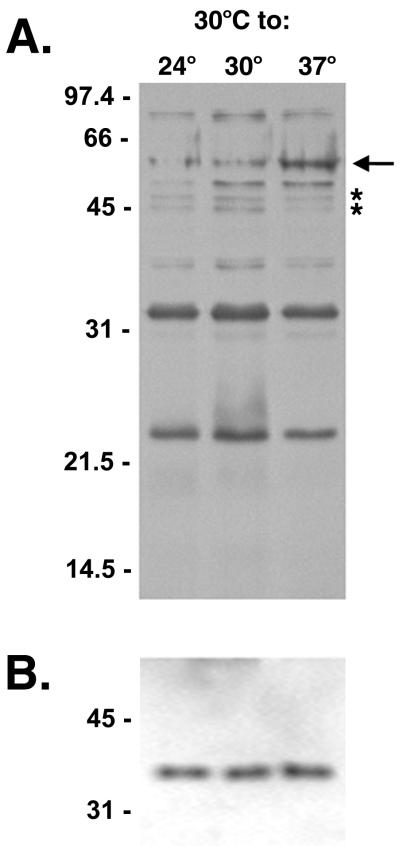
Each lysate was also immunoblotted with serum from a mare naturally infected with L. interrogans serovar pomona (Fig. 1A). Several antigenic proteins were observed to be differentially synthesized in response to temperature shift. Three antigens of approximately 24, 34, and 78 kDa were each recognized with equal intensities at all three temperatures. An antigen with an apparent molecular mass of 57 kDa was present in greater amounts after a temperature shift from 30 to 37°C. Conversely, antigens of approximately 45 and 47 kDa were present in greater amounts in the bacteria maintained at 24 and 30°C than in those shifted to 37°C. Lysates were also probed with antiserum directed against the non-temperature-regulated flagellar protein as an internal standard to verify the amounts of protein loaded (Fig. 1B) (16).
FIG. 1.
(A) Antigens in lysates of L. interrogans serovar pomona type kennewicki JEN4 grown at 30°C and shifted to 24, 30, or 37°C. The lysates were separated by SDS-PAGE and immunoblotted with serum from a convalescent mare. A 57-kDa antigen apparently upregulated at 37°C is indicated with an arrow. The asterisks indicate antigens present in greater amounts at 24 and 30°C than at 37°C. (B) To establish that equivalent amounts of protein were loaded in the gel shown in panel A, lysates were probed with antiserum to endoflagella of T. pallidum, a non-temperature-regulated protein. Molecular mass markers are indicated in kilodaltons.
Temperature-regulated proteins are not heat shock proteins.
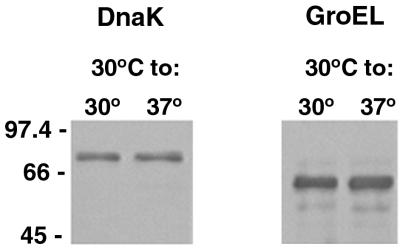
In our studies, bacteria were grown for several days after culture temperatures were shifted, so the observed differentially synthesized proteins would not be expected to be heat shock proteins. To confirm this, lysates separated by SDS-PAGE were immunoblotted with antisera specific for the leptospiral heat shock proteins DnaK and GroEL (Fig. 2). No differences between the expression levels of DnaK and GroEL in organisms maintained at 30°C and those in organisms shifted from 30 to 37°C were detected. Further, we confirmed that the upregulated 57-kDa antigen seen in Fig. 1A was not GroEL, as the two proteins have different apparent molecular masses that were evident when the proteins were examined on the same immunoblot (data not shown).
FIG. 2.
Immunoblot analysis of leptospiral heat shock proteins DnaK and GroEL in lysates of L. interrogans JEN4 expressed at 30 and 37°C following SDS-PAGE. Molecular mass markers are indicated in kilodaltons.
Cellular localization of temperature-regulated proteins.
Bacteria were fractionated with Triton X-114 to obtain protoplasmic cylinder, periplasmic, and outer membrane protein fractions. The protoplasmic cylinder comprises the cytoplasm, the inner membrane, and the anchored periplasmic flagella (9). The detergent-soluble phase contains outer membrane constituents, while periplasmic proteins separate into the aqueous phase (13).
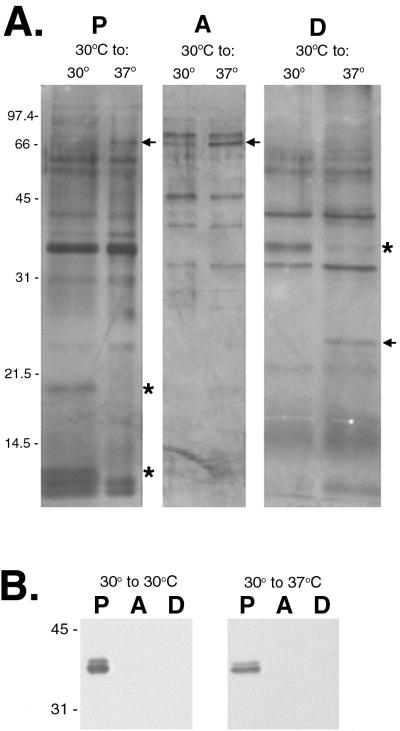
These three fractions were separated by SDS-PAGE and silver stained, revealing several differentially synthesized proteins (Fig. 3A). In the protoplasmic cyclinder, a protein with a molecular mass of ∼20 kDa was downregulated when the bacteria were shifted from 30 to 37°C. Conversely, a protein of ∼66 kDa, not apparent at 30°C, was detected when the organisms were shifted to 37°C. An upregulated protein of ∼66 kDa is also present in the aqueous phase after a temperature shift from 30 to 37°C. Analysis of the hydrophobic proteins of the outer membrane in the detergent phase showed a protein of ∼36 kDa present at 30°C but absent when the bacteria were shifted to 37°C. In addition, a protein of ∼25 kDa was synthesized in greater amounts at 37 than at 30°C. Since leptospiral flagella are anchored to the protoplasmic cyclinder, flagella were found only in this fraction (Fig. 3B).
FIG. 3.
(A) Silver stain of Triton X-114 extracts of L. interrogans JEN4 at 30 or 37°C separated by SDS-PAGE and showing proteins in fractions consisting of protoplasmic cylinder (P), aqueous phase (A), and detergent phase (D). (B) Fractions probed with antiserum to T. pallidum endoflagella to demonstrate that the protoplasmic cylinder was intact. Molecular mass markers are indicated in kilodaltons. Upregulated proteins are indicated with arrows; downregulated proteins are indicated with asterisks.
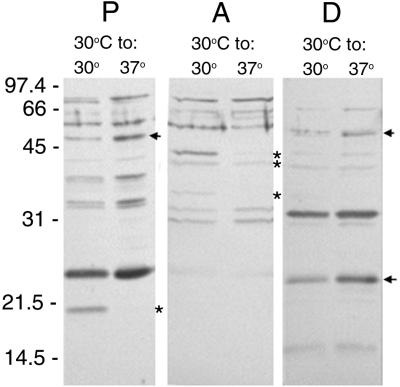
We next evaluated the cellular localization of differentially synthesized leptospiral antigens by immunoblotting with naturally infected equine serum. Serum from a convalescent mare recognized an ∼20-kDa antigen produced at 30 but not at 37°C in the protoplasmic fractions (Fig. 4). Aqueous-phase proteins at approximate molecular weights of 36, 41.5, and 44 were produced by bacteria grown at 30°C but not on those shifted to 37°C. Several proteins in the detergent phase were reactive at both temperatures, but levels of ∼25 and ∼55-kDa antigens increased when the bacteria were shifted from 30 to 37°C.
FIG. 4.
Triton X-114 extract of L. interrogans JEN4 at 30 and 37°C immunoblotted with serum from a convalescent mare following SDS-PAGE. Fractions consisting of protoplasmic cylinder (P), aqueous phase (A), and detergent phase (D) are reactive. Molecular mass markers are indicated in kilodaltons. Upregulated antigens are indicated with arrows; downregulated antigens are indicated with asterisks.
These immunoblot analyses also demonstrated that neither the upregulated 66-kDa protein of the aqueous phase nor the downregulated 36-kDa protein of the detergent phase observed by silver staining were reactive with convalescent equine sera (compare Fig. 3A and 4). In contrast, the 25-kDa detergent phase observed by silver stain to increase after a temperature shift from 30 to 37°C is similar in size to an antigen reactive with convalescent serum, indicating synthesis of this protein during infection of the mare.
Synthesis of LipL36 is temperature regulated.
LipL36 and LipL41 are lipoproteins originally characterized in Leptospira kirschneri (10, 17). LipL36 is found exclusively in the outer membrane, whereas LipL41 occurs in both the outer and inner membranes. LipL41 and LipL36 are solubilized by Triton X-114 and partition into the hydrophobic, detergent phase (10, 17).
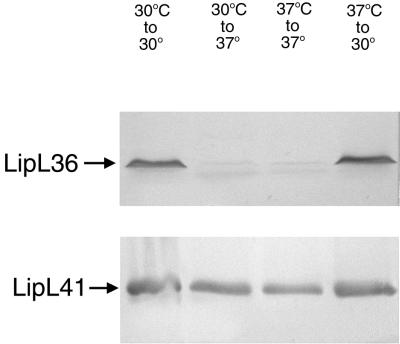
Since we observed the decrease of a 36-kDa detergent phase protein when the organisms were shifted from 30 to 37°C, immunoblotting with LipL36 antiserum was performed to assess whether this thermoregulated protein was LipL36 (Fig. 5A). LipL36 was expressed at 30°C but was switched off when cultures were shifted to 37°C. LipL36 remained switched off at 37°C, but shifting the cultures from 37 back to 30°C restored expression of LipL36. In contrast, expression of the outer membrane lipoprotein LipL41 was unaffected by these temperature changes (Fig. 5B).
FIG. 5.
Lysates of JEN4 grown at 30°C, grown at 30°C and shifted to 37°C, grown at 37°C and maintained at 37°C, or grown at 37°C and shifted to 30°C. Following SDS-PAGE, the lysates were immunoblotted with LipL36- and LipL41-specific antisera.
DISCUSSION
L. interrogans can adapt to a range of different environments, including temperature change. Our results show that sera from horses which aborted as a result of naturally acquired infection with L. interrogans serovar pomona type kennewicki recognize periplasmic and outer membrane proteins which are differentially synthesized in response to temperature and which therefore may be important in the host-pathogen interaction during infection. In contrast to classic heat shock studies, our observations are based on shifting cultures from 30 to 37°C and growing them for several days, simulating conditions that would be encountered during the infection of hosts from an environmental source.
Although silver staining did not reveal any significant differences among the protein profiles of lysed organisms shifted among 24, 30, and 37°C, treatment with Triton X-114 revealed several proteins differentially synthesized at 30 and 37°C. One such protein is LipL36, a 36-kDa leptospiral outer membrane lipoprotein first identified in L. kirschneri (10). LipL36 is solubilized by Triton X-114 extraction and partitions exclusively into the hydrophobic, detergent phase. Expression of LipL36 in culture is growth phase dependent. In early-logarithmic-phase cultures, LipL36 is one of the most abundant L. kirschneri proteins; however, beginning in mid-logarithmic phase, expression drops to a low level. Immunohistochemical studies confirmed that LipL36 is not expressed in infected hamster kidney tissue (3). Our results confirmed that its expression in isolate JEN4 was switched off after a temperature shift from 30 to 37°C, a finding that agrees with the observed failure to detect its expression at the higher body temperature of infected hamsters. When organisms were shifted back to 30°C from 37°C, LipL36 was again synthesized, confirming its thermoregulation. This suggests a functional role for LipL36 in adapting to new environments after bacteria are shed from an infected host. A second surface protein, LipL41, has also been characterized in L. kirschneri and is conserved among pathogenic Leptospira species (17). In contrast to LipL36, LipL41 was expressed in infected hamster kidney tissue. We have confirmed that JEN4 also expresses LipL41 and that expression is unaffected when the bacteria are shifted to 37°C. Silver staining of cellular fractions also revealed reduced levels of a 20-kDa cytoplasmic protein when cultures were shifted from 30 to 37°C. In contrast, levels of a cytoplasmic protein of ∼66 kDa, a protein of ∼25 kDa of the detergent phase, and an ∼66-kDa protein of the aqueous phase were increased upon temperature change.
Immunoblot analysis of lysed organisms with sera from naturally infected equines revealed that L. interrogans JEN4 expressed several antigenic proteins whose synthesis appeared to be affected by temperature shift. A reactive band with an apparent molecular mass of 57 kDa was present in larger amounts after a temperature shift from 30 to 37°C. In contrast, three immunoreactive bands of approximately 24, 34, and 78 kDa were each present in equal amounts at all three temperatures. Immunoblotting also revealed higher densities of reactive bands of approximately 45 and 47 kDa in bacteria grown at 24 and 30°C than in those grown at 37°C, indicating that their synthesis is also temperature dependent. While it is possible that the decreased synthesis of antigens at 37°C is a result of degradation due to higher temperature, the organisms remained motile and morphologically intact at each temperature. Further, the observed antibody responses by infected mares indicate that the antigens must be present for a sufficient time at the normal equine body temperature of 38.5°C to stimulate a response.
Immunoblot analysis of Triton X-114 fractions further indicated temperature-dependent synthesis of proteins produced during natural infection. Convalescent sera recognized a 20-kDa protein of the protoplasmic cylinder produced by the bacteria grown at 30°C but not those grown at 37°C and which corresponds in size to the apparently downregulated 20-kDa protein observed by silver staining. Since antibodies to this antigen were induced, it must have been synthesized during infection. Antigens of ∼25 and 55 kDa apparently upregulated after a temperature shift from 30 to 37°C were identified in the detergent phase. The 55-kDa antigen may be the same as the 57-kDa antigen recognized by convalescent sera in whole-cell lysates, because treatment with Triton X-114 may affect the apparent molecular mass. Characterization of these proteins will be important, as they are potentially surface targets for protective antibodies.
Stamm et al. have demonstrated that L. interrogans exhibits a rapid induction of synthesis of specific proteins as a consequence of a sudden increase in temperature (18). Seven heat shock proteins, including GroEL and DnaK, were identified 1 h after a temperature increase from 30 to 37 or 42°C. Immunoblot analysis of JEN4 shifted from 30 to 37°C revealed no differential expression of GroEL or DnaK after several days at 37°C, indicating that observed changes in protein levels were due to long-term accumulation through several cycles of cell division at the higher temperature.
We have demonstrated a distinct change in the protein profiles of cultured organisms shifted from 30 to 37°C. Other effects, including changes in lipopolysaccharide (LPS), are possible and are being investigated. In Bordetella pertussis, temperature is a modulator of LPS expression independent of the BvgAS sensory system (20a). It will be interesting to explore this possibility in Leptospira, particularly as LPS is considered a major protective antigen.
Transmission of Leptospira is effected by shedding from chronically infected animals. The existence of these persistently infected hosts may be explained in part by evasion of the host immune response by downregulation of outer membrane protein expression. Faine reported that Leptospira in the urine or kidneys of carrier mice was resistant to the action of antibody in the serum or urine of the host animal (8). We observed diminished expression of at least three aqueous-phase proteins reactive with convalescent serum after a temperature shift from 30 to 37°C. While most aqueous-phase proteins are soluble periplasmic proteins, at least one transmembrane porin has been shown to partition into the aqueous phase (13). Although the presence of antibody suggests exposure of the proteins to the host immune response, elevated temperature may also affect antigenic structure, resulting in lower affinity for antibodies. In support of this hypothesis, Ellis et al. have observed morphological changes during the subculture of freshly isolated strains of L. interrogans serovar hardjo which possibly alter the configuration of surface antigens and thus facilitate leptospiral survival in the presence of specific antibodies (7). In either case, it provides a means to delay complement activation and evade the immune response.
Identification of the thermoregulated proteins of Leptospira, and elucidation of the mechanisms by which protein synthesis is regulated, will undoubtedly provide important insights into the pathogenesis of leptospirosis.
ACKNOWLEDGMENTS
This work was funded by the Keeneland Association. J. E. Nally is a recipient of a Maxwell and Muriel Gluck Fellowship in Equine Studies.
We thank M. Donahue for providing the Leptospira isolate, C. Bolin for serotyping, and B. Smith, B. Adler, D. Blanco, and D. Haake for antisera. We also thank K. Babb and A. Sinai for technical advice.
REFERENCES
- 1.Anderson D C, Folland D S, Fox M D, Patton C M, Kaufmann A F. Leptospirosis: a common-source outbreak due to leptospires of the grippotyphosa serogroup. Am J Epidemiol. 1978;107:538–544. doi: 10.1093/oxfordjournals.aje.a112573. [DOI] [PubMed] [Google Scholar]
- 2.Ballard S A, Segers R P, Bleumink-Pluym N, Fyfe J, Faine S, Adler B. Molecular analysis of the hsp (groE) operon of Leptospira interrogans serovar copenhageni. Mol Microbiol. 1993;8:739–751. doi: 10.1111/j.1365-2958.1993.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 3.Barnett J K, Barnett D, Bolin C A, Summers T A, Wagar E A, Cheville N F, Hartskeerl R A, Haake D A. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853–861. doi: 10.1128/iai.67.2.853-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco D R, Champion C I, Miller J N, Lovett M A. Antigenic and structural characterization of Treponema pallidum (Nichols strain) endoflagella. Infect Immun. 1988;56:168–175. doi: 10.1128/iai.56.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt M E, Riley B S, Radolf J D, Norgard M V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diesch S L, McCulloch W F, Braun J L, Crawford R P., Jr Environmental studies on the survival of leptospires in a farm creek following a human leptospirosis outbreak in Iowa. Wildl Dis. 1969;5:166–173. doi: 10.7589/0090-3558-5.3.166. [DOI] [PubMed] [Google Scholar]
- 7.Ellis W A, Hovind-Hougen K, Moller S, Birch-Andresen A. Morphological changes upon subculturing of freshly isolated strains of Leptospira interrogans serovar hardjo. Zentbl Bakteriol Mikrobiol Hyg A. 1983;255:323–335. [PubMed] [Google Scholar]
- 8.Faine S. The growth of Leptospira australis B in the kidneys of mice in the incipient experimental carrier state. J Hyg Camb. 1962;60:435–442. doi: 10.1017/s0022172400020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. 2nd ed. Melbourne, Australia: MediSci; 1999. [Google Scholar]
- 10.Haake D A, Martinich C, Summers T A, Shang E S, Pruetz J D, McCoy A M, Mazel M K, Bolin C A. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect Immun. 1998;66:1579–1587. doi: 10.1128/iai.66.4.1579-1587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake D A, Walker E M, Blanco D R, Bolin C A, Miller M N, Lovett M A. Changes in the surface of Leptospira interrogans serovar grippotyphosa during in vitro cultivation. Infect Immun. 1991;59:1131–1140. doi: 10.1128/iai.59.3.1131-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konkel M E, Tilly K. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2000;2:157–166. doi: 10.1016/s1286-4579(00)00272-0. [DOI] [PubMed] [Google Scholar]
- 13.Maher P A, Singer S J. Anomalous interaction of the acetylcholine receptor protein with the nonionic detergent Triton X-114. Proc Natl Acad Sci USA. 1985;82:958–962. doi: 10.1073/pnas.82.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurelli A T, Blackmon B, Curtiss R D. Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984;43:195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obonyo M, Munderloh U G, Fingerle V, Wilske B, Kurtti T J. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J Clin Microbiol. 1999;37:2137–2141. doi: 10.1128/jcm.37.7.2137-2141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang E S, Summers T A, Haake D A. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 1996;64:2322–2330. doi: 10.1128/iai.64.6.2322-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamm L V, Gherardini F C, Parrish E A, Moomaw C R. Heat shock response of spirochetes. Infect Immun. 1991;59:1572–1575. doi: 10.1128/iai.59.4.1572-1575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takai S, Iie M, Watanabe Y, Tsubaki S, Sekizaki T. Virulence-associated 15- to 17-kilodalton antigens in Rhodococcus equi: temperature-dependent expression and location of the antigens. Infect Immun. 1992;60:2995–2997. doi: 10.1128/iai.60.7.2995-2997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.van den Akker W M R. Lipopolysaccharide expression within the genus Bordetella: influence of temperature and phase variation. Microbiology. 1998;144:1527–1535. doi: 10.1099/00221287-144-6-1527. [DOI] [PubMed] [Google Scholar]
- 21.Vinetz J M, Glass G E, Flexner C E, Mueller P, Kaslow D C. Sporadic urban leptospirosis. Ann Intern Med. 1996;125:794–798. doi: 10.7326/0003-4819-125-10-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Yeruham I, Bernstein M, Perl S, Irlin S, Cohen A, Yacobson B, Machnai B. Outbreak of acute febrile illness among athletes participating in triathlons–Wisconsin and Illinois, 1998. Morb Mortal Wkly Rep. 1998;47:585–588. . (Erratum, 47:619.) [PubMed] [Google Scholar]