Abstract
Background and Aims
Major depressive disorder (MDD) is the fourth biggest health‐related concern that dramatically impacts individuals' mental and physical health. Alteration of serum proinflammatory cytokine levels may take part in the development and progression of MDD. We aimed to explore and compare the role of interleukin‐12 (IL‐12) in MDD patients and healthy controls (HCs) and its involvement with the disease severity.
Methods
The present study included 85 patients and 87 age‐sex matched HCs. A qualified psychiatrist utilized the diagnostic and statistical manual of mental disorders, fifth edition (DSM‐5) criteria to diagnose patients and evaluate HCs. We applied the Ham‐D rating scale to measure the severity of depression. Serum IL‐12 levels were measured using ELISA kits.
Results
We observed a notable increase in the serum levels of IL‐12 in MDD patients compared to HCs (164.27 ± 10.18 pg/ml and 82.55 ± 4.40 pg/ml; p < 0.001). Moreover, we noticed a positive correlation between serum IL‐12 levels and Ham‐D scores in MDD patients (r = 0.363; p = 0.001). Receiver operating characteristic analysis showed a good predictive performance (AUC = 0.871; p < 0.001) at the cut‐off point of 53.46 pg/ml for serum IL‐12.
Conclusion
The current study findings support that IL‐12 levels are involved with the pathogenesis and inflammatory process in MDD. At the same time, this involvement may make this cytokine eligible for the risk evaluation of MDD. However, we recommend further interventional studies to explore more accurate associations between IL‐12 and depressive disorder.
Keywords: cytokines, depression, depressive disorder, IL‐12, interleukin‐12, major depressive disorder
1. BACKGROUND
A distinct depressive episode that lasts at least 2 weeks characterizes major depressive disorder (MDD). It is a severe health issue that may last for a lifetime. MDD can induce a drastic change in interests, mood, and pleasure, as well as changes in cognitive and vegetative behaviors. 1 , 2 , 3 MDD is the fourth biggest global cause of life‐years adjusted for disability out of all medical illnesses significantly linked to disability, major death for patients, and suffering for patients with their family members. 4 , 5 , 6 According to an estimation, up to 50% of the 0.8 million suicides every year worldwide occurred during a depressed episode. 1 Compared to the mass population, individuals with MDD are prone to a 20‐fold higher risk of death due to suicide. 7 , 8 Approximately 37% of cases, MDD occurs due to genetic factors, whereas environmental factors such as physical, sexual, or emotional abuse during childhood are closely related to MDD development. 9 , 10 A study was conducted for 12 months to find the frequency of MDD patients in 28 countries. The report revealed that individuals suffering from MDD were the lowest in Japan (2.2%) and the highest in Brazil (10.4%). The study concluded that, on average, around 5.5% of people in developed countries were MDD patients compared to 5.9% in developing countries. 11 Every year, roughly 6% of adults globally are affected by MDD, with females experiencing it nearly twice as frequently as males. 12 , 13 As MDD is responsible for the deterioration of physical and mental health along with family and social life, it is significant to be concerned about the development and progression of MDD.
In MDD, several biological processes are involved in the pathophysiology and disease progression. 14 These biological processes that may contribute to the genesis of depression include neurotrophic growth, hypothalamic‐pituitary (HPA) axis, and vitamin D and inflammation. Significant differences have been identified in terms of their mechanisms through case‐control studies. 15 , 16 , 17 , 18 , 19 Nevertheless, it is yet uncertain whether improper regulations in these systems become more prominent when MDD advances into a condition with numerous episodes or chronicity. 14 An increase in inflammation may play a crucial role in the genesis of depression by lowering the generation of monoamines and a newer generation of tryptophan catabolites that are potentially toxic to the brain. 20 , 21 Cytokines are also involved with depression as MDD exhibits similar nature to “sickness behavior,” a typical reaction to inflammatory cytokines. 22 Cytokines are glycoproteins released by lymphoid and non‐lymphoid cells that regulate immune responses. It has been proposed that improper regulation in the genesis of cytokines or altered cytokines level may strongly impact the development and progression of MDD. There are two types of cytokines which are proinflammatory and anti‐inflammatory, classified according to their functions in the inflammatory system. As proinflammatory and anti‐inflammatory cytokines are seemed to be altered in MDD demonstrated by their abnormal levels in cerebrospinal fluid and blood, it has been proposed that altered cytokines levels are associated with the pathogenesis of MDD. 23 , 24 , 25 , 26
Interleukin‐12 (IL‐12) is a cytokine, heterodimeric in nature, which is initially released by macrophages and monocytes. 27 , 28 IL‐12 has a vital role in exhibiting cell‐mediated immunity by causing T helper 1 (Th1) immune responses. 29 , 30 IL‐12 exhibits these immune responses by increasing the cytotoxic effect of natural killer (NK) cells, producing cytotoxic T cells, and stimulating the production of interferon‐gamma (IFN‐γ), where IFN‐γ produces from T cells and NK cells which remained at both activation and resting state. Therefore, IL‐12 is a cytokine of proinflammatory type. 31 , 32 It has been found that a depressed episode causes an increase in proinflammatory cytokine levels. The increase in proinflammatory cytokine levels can occur before a depressive episode, which may remain the same after the depressive symptoms have subsided. 22 IL‐12 is created in the initial stage of inflammation and infection, causing inflammation. 33 IL‐12 may have a role in regulating other cytokines; therefore, it can be assumed that similar to other proinflammatory cytokines, improper regulated IL‐12 might be involved with MDD. 34 The elevated IL‐12 in the MDD population may be more proof that the inflammatory response system is activated during MDD. 34 , 35 , 36
Thus, to find out the involvement of proinflammatory cytokines in MDD, finding out the role of IL‐12 in the progression of MDD could be a practical approach. Here we aimed to inspect the serum IL‐12 level in MDD patients and healthy controls (HCs). Further, if any alteration would arise, we intended to investigate the involvement of altered serum IL‐12 levels with the severity of depression.
2. MATERIALS AND METHODS
2.1. Study population
We included 85 MDD patients and 87 HCs from the psychiatry department of BSMMU (Bangabandhu Sheikh Mujib Medical University), Dhaka, Bangladesh. HCs were recruited from different areas of Dhaka matched by age and sex. A qualified psychiatrist performed patient diagnosis and evaluated HCs using the diagnostic and statistical manual of mental disorders, fifth edition (DSM‐5) criteria. We applied a 17‐item Ham‐D rating scale to estimate the severity of depression. Also, we used a structured questionnaire to record demographic information and the history of study participants. We included participants aged between 18 and 60 years in this study. We considered MDD patients for individuals with depressive symptoms for a minimum of two consecutive weeks. Subjects having a history of cardiovascular disease, hypertension, epilepsy, hepatic failure, or renal failure were excluded from this study. Patients receiving any treatment that would alter the concentration of serum IL‐12 levels were also eliminated from this study. Individuals with mental retardation and other comorbid psychiatric conditions were considered as additional exclusion criteria. We also excluded participants with a history of substance misuse or dependence, severe organic illnesses, an abnormal body mass index (BMI), or infections. The sociodemographic characteristics of the study participants were documented using a prestructured and well‐designed questionnaire.
2.2. Blood sample collection
Five milliliters of blood from each participant's cephalic vein were collected and put into falcon conical tubes. Then, the supernatant was separated by centrifuging the blood samples at 1000g for 15 min. As soon as the serum samples were collected, they were kept at −80°C until the measurement of serum IL‐12 concentrations.
2.3. Analysis of serum samples
All the reagents and samples were brought to room temperature on the day of analysis. Serum samples were vortexed to produce homogeneous serum samples after. We used commercial ELISA kits to measure the serum IL‐12 levels according to the instructions by the manufacturer. In brief, about 100 µl of standards and samples were diluted before being put to the wells of microplates that were previously coated with capture antibody, followed by incubation at 37°C for 90 min after sealing each plate with a plate sealer properly. After incubation, the liquid was removed from each well, and 100 µl of detecting antibody was added. Once more, plates were sealed with plate sealers and incubated for 60 min at 37°C. After 60 min, the liquid was discarded again and using phosphate buffer solution, the plates were washed three times. Then, the avidin‐biotin‐peroxidase complex was prepared, and 100 µl was added to each well, followed by 40 min of incubation at room temperature. Later, after discarding the liquid and then washing plates with wash buffer five times, a chemical agent for color development was added, which was about 90 μl, and incubated again at room temperature for 30 min in the dark. In the end, within 30 min after adding 100 µl of stop solution to each well, with the help of a microplate reader, the absorbance was measured at 450 nm. By using a standard curve, the serum concentration of IL‐12 of both MDD and HCs was measured and expressed in pg/ml.
2.4. Statistical analysis
The SPSS (version 25.0) was used to conduct the necessary statistical analyses. Descriptive statistics were performed for sociodemographic variables, clinical features, and laboratory findings. The results were reported as mean ± SEM. We applied the independent sample t‐test and Fisher's exact test to differentiate between the groups for continuous and categorical variables. The differences between the serum IL‐12 levels of MDD patients and the serum IL‐12 levels of HCs were shown in box‐plot graphs. We performed a correlation study to find associations between serum IL‐12 levels and Ham‐D scores in depression. The receiver operating characteristic (ROC) curve analysis was performed to determine the diagnostic or predictive value of altered IL‐12 levels. A p Value of ≤0.05 was deemed statistically significant.
3. RESULTS
3.1. General description of the study population
In this study, each participant was assigned a category in Table 1 based on their sociodemographic profiles and biophysical traits. Both MDD population and HCs were found similar regarding their age (MDD patients: 32.09 ± 0.991 years, HCs: 32.23 ± 0.994 years; p = 0.923) and BMI (MDD patients: 23.48 ± 0.434 kg/m2, HCs: 23.94 ± 0.419 kg/m2; p = 0.454). We observed the majority of the study population were young adults between 26 and 35 years old (MDD patients: 35.29%, HCs: 40.23%). The proportion of females was higher than males among the MDD patients and HCs. The majority of the study population have normal BMI (kg/m2) and have no smoking habits (MDD patients: 81.18%, HCs: 74.71%). More than 95% of the MDD patients were from medium economic backgrounds, whereas more than half of the HCs were from low economic classes. Moreover, above 57% of the MDD patients lived in rural areas, but more than 75% of the HCs lived in urban areas. Around 52% of MDD patients gained a secondary level of education, whereas around an equal proportion of HCs had at least a graduation degree.
Table 1.
Socio‐demographic profile of the study population
| Characteristics | MDD patients (n = 85) Mean ± SEM | Healthy controls (n = 87) Mean ± SEM | p Value |
|---|---|---|---|
| Age in years | 32.09 ± 0.991 | 32.23 ± 0.994 | 0.923 |
| 18−25 | 26 (30.59%) | 23 (26.44%) | |
| 26−35 | 30 (35.29%) | 35 (40.23%) | |
| 36−45 | 25 (29.41%) | 18 (20.69%) | |
| 46−60 | 4 (4.71%) | 11 (12.64%) | |
| Sex | 0.062 | ||
| Male | 40 (47.06%) | 34 (39.08%) | |
| Female | 45 (52.94%) | 53 (60.92%) | |
| Marital status | 0.277 | ||
| Married | 50 (58.82%) | 44 (50.57%) | |
| Unmarried | 35 (41.18%) | 43 (49.43%) | |
| BMI (kg/m2) | 23.48 ± 0.434 | 23.94 ± 0.419 | 0.454 |
| Below 18.5 (CED) | 8 (9.20%) | 17 (19.54%) | |
| 18.5–25 (normal) | 47 (54.02%) | 47 (54.02%) | |
| Above 25 (obese) | 30 (34.48%) | 23 (26.44%) | |
| Education level | 0.001 | ||
| Illiterate | 5 (5.88%) | 4 (4.60%) | |
| Primary level | 14 (16.47%) | 4 (4.60%) | |
| Secondary level | 44 (51.77%) | 30 (34.48%) | |
| Graduate and above | 22 (25.88%) | 49 (56.32%) | |
| Occupation | <0.001 | ||
| Business | 5 (5.88%) | 17 (19.54%) | |
| Service | 12 (14.12%) | 11 (12.64%) | |
| Housewife | 35 (41.18%) | 15 (17.24%) | |
| Unemployed | 7 (8.24%) | 1 (1.15%) | |
| Student | 1 (1.17%) | 24 (27.59%) | |
| Others | 25 (29.41%) | 19 (21.84%) | |
| Economic impression | <0.001 | ||
| High | 0 (0.00%) | 14 (16.09%) | |
| Medium | 81 (95.29%) | 29 (33.33%) | |
| Low | 4 (4.71%) | 44 (50.58%) | |
| Smoking habit | 0.307 | ||
| Yes | 16 (18.82%) | 22 (25.29%) | |
| No | 69 (81.18%) | 65 (74.71%) | |
| Residence area | <0.001 | ||
| Rural | 49 (57.65%) | 20 (22.99%) | |
| Urban | 36 (42.35%) | 67 (77.01%) | |
| Previous history of MDD | <0.001 | ||
| Yes | 44 (51.76%) | 0 (0.00%) | |
| No | 41 (48.24%) | 87 (100.00%) | |
| Family history of MDD | <0.001 | ||
| Yes | 25 (29.41%) | 3 (3.45%) | |
| No | 60 (70.59%) | 84 (96.55%) |
Abbreviations: BMI, body mass index; CED, chronic energy deficiency; MDD, major depressive disorder; SEM, standard error mean.
3.2. Clinical outcome and laboratory findings
The clinical features and laboratory findings of MDD patients and HCs have been demonstrated in Table 2 and Figure 1. DSM‐5 score of MDD patients and HCs were 7.18 ± 0.18 and 2.00 ± 0.13, respectively (p < 0.001). Also, the Ham‐D scores of MDD patients and HCs were 17.94 ± 0.46 and 5.67 ± 0.36, respectively (p < 0.001). We found higher IL‐12 levels (164.27 ± 10.18 pg/ml) in MDD participants than HCs (82.55 ± 4.40 pg/ml). Moreover, significant differences in serum IL‐12 levels were observed between the groups for both males and females. To determine the relationship between serum IL‐12 levels and Ham‐D scores among MDD patients, Spearman's correlation test was used. A positive correlation was observed between IL‐12 levels and Ham‐D scores in the case of MDD patients (r = 0.363; p = 0.001). When the ROC curve for serum IL‐12 levels was depicted, the cut‐off point was identified at 53.46 pg/ml for diagnostic purposes (Figure 2). A significant area under the ROC curve (AUC) was observed, which was 0.871 (p < 0.001). As per ROC analysis, the sensitivity and specificity were 84.5%, and 86.2%, respectively, whereas positive predictive value, and negative predictive value were 82.3%, and 82.6%, respectively (Table 3).
Table 2.
Clinical profile and laboratory findings of the study population
| Parameters | MDD patients (n = 85) Mean ± SEM | Healthy controls (n = 87) Mean ± SEM | p Value |
|---|---|---|---|
| Age (years) | 32.09 ± 0.991 | 32.23 ± 0.994 | 0.923 |
| Male (P/C:40/34) | 28.75 ± 1.02 | 33.76 ± 1.63 | 0.009 |
| Female (P/C:45/53) | 35.07 ± 1.52 | 31.25 ± 1.24 | 0.052 |
| BMI (Kg/m2) | 23.48 ± 0.434 | 23.94 ± 0.419 | 0.454 |
| Male (P/C:40/34) | 24.04 ± 0.57 | 23.78 ± 0.58 | 0.757 |
| Female (P/C:45/53) | 22.98 ± 0.64 | 24.06 ± 0.59 | 0.226 |
| DSM‐5 score | 7.18 ± 0.18 | 2.00 ± 0.13 | <0.001 |
| Male (P/C:40/34) | 6.90 ± 0.28 | 1.71 ± 0.19 | <0.001 |
| Female (P/C:45/53) | 7.42 ± 0.24 | 2.22 ± 0.17 | <0.001 |
| Ham‐D score | 17.94 ± 0.46 | 5.67 ± 0.36 | <0.001 |
| Male (P/C:40/34) | 17.25 ± 0.69 | 4.38 ± 0.33 | <0.001 |
| Female (P/C:45/53) | 18.55 ± 0.61 | 6.67 ± 0.51 | <0.001 |
| Serum IL‐12 (pg/ml) | 164.27 ± 10.18 | 82.55 ± 4.40 | <0.001 |
| Male (P/C:40/34) | 167.63 ± 16.32 | 76.68 ± 6.05 | <0.001 |
| Female (P/C:45/53) | 161.29 ± 12.79 | 86.31 ± 6.09 | <0.001 |
Abbreviations: BMI, body mass index; DSM‐5, diagnostic and statistical manual for mental disorders, 5th edition; Ham‐D, 17‐item Hamilton depression rating scale; IL‐12, interleukin‐12; MDD, major depressive disorder; P/C, patients/control; SEM, standard error mean.
Figure 1.
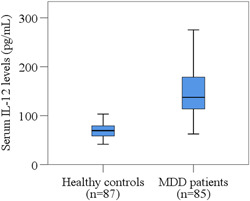
Distribution of serum IL‐12 levels in MDD patients and healthy controls. Boxplot graphs showing the median, maximum and minimum value range. IL‐12, Interleukin‐12; MDD, major depressive disorder.
Figure 2.
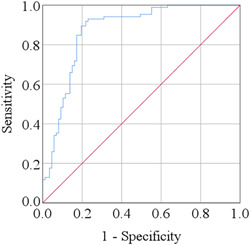
Receiver operating characteristic (ROC) curve for serum IL‐12. The cut‐off point was detected as 53.46 pg/ml.
Table 3.
Receiver operating characteristic curve analysis of serum interleukin‐12
| Parameters | Cut‐off value (pg/ml) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | 95% CI | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||||
| Interleukin‐12 | 53.46 | 84.5 | 86.2 | 82.3 | 82.6 | 0.871 | 0.815 | 0.926 | <0.001 |
Abbreviations: AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
4. DISCUSSION
Depression is a common neuropsychiatric disorder characterized by a wide variety of symptoms, including impaired mood and cognitive skills. It has a complex etiology caused by the combination of environmental, biological, and genetic variables and frequently coexists with other diseases. 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 The significant comorbidity of persistent and chronic MDD with other medical conditions, particularly inflammatory diseases, raises the possibility that inflammation plays a role in the emergence or continuation of MDD. 46 , 47 In the course of inflammatory events, cytokines start an immunological response in the brain. Several studies indicate a connection between proinflammatory cytokines and MDD. 48 , 49 , 50 , 51 , 52 , 53 IL‐12 is a proinflammatory cytokine that seems to be involved with MDD. 34
In our study, cytokine marker IL‐12 levels in serum were investigated in MDD patients in comparison with HCs. We found a significant increase in the serum levels of IL‐12 in MDD patients than HCs. A positive correlation was established between IL‐12 serum levels and the severity of depression as a higher concentration of IL‐12 and a higher Ham‐D score were observed in respected patients. The observation of elevated serum levels of IL‐12 in MDD patients raises the possibility that IL‐12 may have a role in the immune system's activation during that condition. There is no substantial proof that considerable alterations in humoral immunity and cell‐mediated immunity may occur in MDD. It is known that antigen‐presenting cells are the leading producers of IL‐12, which has immunoregulatory effects on T cells and NK cells. IL‐12 also controls the equilibrium between type I helper T cells, which secrete IL‐2 and IFN‐γ, and type 2 helper T cells, which produce IL‐4 and IL‐10. 27 , 29 Therefore, the result of elevated IL‐12 in our depressed individuals may be seen as a solid indication that the inflammatory response system is activated during MDD. Moreover, our analysis found significantly higher serum IL‐12 levels among men with MDD (167.63 ± 16.32 pg/ml) compared to women with MDD (161.29 ± 12.79 pg/ml). Thus, according to our findings, we may conclude that men are more prone to MDD.
Several studies have been carried out to find out the involvement of proinflammatory cytokines, particularly IL‐12, with MDD. According to research that made similar conclusions to ours, MDD patients had considerably greater levels of IL‐12 than HCs, demonstrating the activation of inflammatory systems during depressive episodes. 54 A case‐control study has been carried out between 30 depressive patients and 30 controls, and it has been found that, when compared to HCs, MDD patients had considerably greater amounts of IL‐12 in their plasma. 34 These two individual studies not only found higher serum levels of IL‐12 but also found a decrease in serum IL‐12 levels after treating MDD patients with sertraline, an antidepressant drug. These findings enhanced the association of higher IL‐12 levels with MDD. According to several findings, it has been demonstrated that improper regulation of immune mediators, such as an increase in IL‐1Ra, IL‐1, IL‐2, IL‐6, soluble IL‐2R, soluble IL‐6R, IFN‐, and IL‐12, is linked to major depression. These alterations have been examined in light of the inconsistency between specific proinflammatory and anti‐inflammatory cytokines and the type I helper T cells and type II helper T cells imbalance in major depression. 35 Furthermore, an increased supply of these proinflammatory cytokines may not only activate T cells and B cells but might also impact the brain and cause numerous depressive symptoms, such as lack of appetite, listlessness, and sleep disorders. 55 Thus, the involvement of IL‐12 with MDD has also been established from previous findings.
In our study, we performed a ROC analysis of serum IL‐12 to quantify the predictive performance of altered IL‐12 levels. The accuracy of the test was measured with AUC. The ROC−AUC test has the following accuracy: 0.9–1.0 = excellent, 0.8−0.9 = good, 0.7−0.8 = fair, 0.6‐0.7 = poor and <0.6 = not useful. 56 We found a significant AUC in our current investigation, and the value was 0.871 (p < 0.001). The cut‐off value was identified as 53.46 pg/ml. Similar markers were identified in major depression by several prior investigations, but their diagnostic accuracy was not assessed. 34 , 42 The current study is distinctive in this regard when compared to earlier investigations.
Moreover, as per our concern, our study is the first attempt to investigate the association of serum IL‐12 levels with MDD among the Bangladeshi population, where age and sex were precisely matched for patients and HCs. Simultaneous measurements of IL‐12 in MDD patients and HCs were performed under identical experimental circumstances. The study findings regarding higher IL‐12 serum levels in MDD patients compared to HCs would play a vital role in the sector of biological psychiatry for accurate diagnosis and treatment of MDD. Though, the present study has a few limitations. Firstly, we evaluated the serum IL‐12 levels once MDD patients and HCs were enrolled. Secondly, we did not consider the study population's food habits, sleeping patterns, usage of current medications, or tobacco use, 15 , 57 , 58 which might have been confounding variables for IL‐12 analysis.
5. CONCLUSION
An association between altered serum levels of IL‐12 and MDD would be assumed according to the present study results. It can also predict that the higher serum IL‐12 levels in MDD patients might be involved in the pathophysiology and the severity of depression. Therefore, we would like to propose IL‐12 as a potential candidate for the risk assessment of depressive disorder. However, we recommend more interventional research with more homogeneous samples and repeated assessments of serum IL‐12 levels to produce more accurate results.
AUTHOR CONTRIBUTIONS
Zabun Nahar: Formal analysis; writing – original draft. Nisat Sal‐Sabil: Conceptualization; formal analysis. Md. Sohan: Writing – original draft; writing – review and editing. MMA Shalahuddin Qusar: Supervision; writing – review and editing. Md. Rabiul Islam: Formal analysis; supervision; writing – review and editing.
CONFLICT OF INTEREST
The authors declares no conflict of interests.
ETHICS STATEMENT
The study protocol was approved by the Research Ethics Committee, University of Asia Pacific (Ref: UAP/REC/2021/102). The study was performed in accordance with the Declaration of Helsinki. We briefed about the objective and purpose of this study to the participants and obtained a written consent from each participant before participation. The consent was given by their legal guardians in the case where an individual's thinking capacity was suspected to be impaired.
Md. Rabiul Islam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
TRANSPARENCY STATEMENT
The lead author Md. Rabiul Islam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
We thank to the study participants and their relatives for their participation and cooperation to this study. Also, we would like to thank all physicians and administrative staffs at the department of psychiatry, BSMMU, for their cooperation and administrative support to this study. This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Nahar Z, Sal‐Sabil N, Sohan M, Qusar MS, Islam MR. Higher serum interleukin‐12 levels are associated with the pathophysiology of major depressive disorder: a case‐control study results. Health Sci Rep. 2022;6:e1005. 10.1002/hsr2.1005
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials.
REFERENCES
- 1. Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. 10.1038/nrdp.2016.65 [DOI] [PubMed] [Google Scholar]
- 2. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587‐596. 10.1097/PSY.0b013e318148c19a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahman S, Shanta AA, Daria S, et al. Increased serum resistin but not G‐CSF levels are associated in the pathophysiology of major depressive disorder: findings from a case‐control study. PLoS One. 2022;17(2):e0264404. 10.1371/journal.pone.0264404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Insel TR, Charney DS. Research on major depression: strategies and priorities. JAMA. 2003;289(23):3167‐3168. 10.1001/jama.289.23.3167 [DOI] [PubMed] [Google Scholar]
- 5. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349(9063):1436‐1442. 10.1016/S0140-6736(96)07495-8 [DOI] [PubMed] [Google Scholar]
- 6. Emon MPZ, Das R, Nishuty NL, Shalahuddin Qusar MMA, Bhuiyan MA, Islam MR. Reduced serum BDNF levels are associated with the increased risk for developing MDD: a case‐control study with or without antidepressant therapy. BMC Res Notes. 2020;13(1):83. 10.1186/s13104-020-04952-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daria S, Proma MA, Shahriar M, Islam SMA, Bhuiyan MA, Islam MR. Serum interferon‐gamma level is associated with drug‐naïve major depressive disorder. SAGE Open Med. 2020;8:205031212097416. 10.1177/2050312120974169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chesney E, Goodwin GM, Fazel S. Risks of all‐cause and suicide mortality in mental disorders: a meta‐review. World Psychiatry. 2014;13(2):153‐160. 10.1002/wps.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li M, D'Arcy C, Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta‐analysis, and proportional attributable fractions. Psychol Med. 2016;46(4):717‐730. 10.1017/S0033291715002743 [DOI] [PubMed] [Google Scholar]
- 10. Flint J, Kendler KS. The genetics of major depression [published correction appears in Neuron]. Neuron. 2014;81(3):484‐503. 10.1016/j.neuron.2014.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrari AJ, Somerville AJ, Baxter AJ, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43(3):471‐481. 10.1017/S0033291712001511 [DOI] [PubMed] [Google Scholar]
- 12. Bromet E, Andrade LH, Hwang I, et al. Cross‐national epidemiology of DSM‐IV major depressive episode. BMC Med. 2011;9:90. 10.1186/1741-7015-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seedat S, Scott KM, Angermeyer MC, et al. Cross‐national associations between gender and mental disorders in the world health organization world mental health surveys. Arch Gen Psychiatry. 2009;66(7):785‐795. 10.1001/archgenpsychiatry.2009.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verduijn J, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman ATF, Penninx BWJH. Pathophysiology of major depressive disorder: mechanisms involved in etiology are not associated with clinical progression. Transl Psychiatry. 2015;5(9):e649. 10.1038/tp.2015.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Islam MR, Ali S, Karmoker JR, et al. Evaluation of serum amino acids and non‐enzymatic antioxidants in drug‐naïve first‐episode major depressive disorder. BMC Psychiatry. 2020;20(1):333. 10.1186/s12888-020-02738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milaneschi Y, Hoogendijk W, Lips P, et al. The association between low vitamin D and depressive disorders. Mol Psychiatry. 2014;19(4):444‐451. 10.1038/mp.2013.36 [DOI] [PubMed] [Google Scholar]
- 17. Vogelzangs N, Duivis HE, Beekman ATF, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2(2):e79. 10.1038/tp.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molendijk ML, Bus BAA, Spinhoven P, et al. Serum levels of brain‐derived neurotrophic factor in major depressive disorder: state‐trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16(11):1088‐1095. 10.1038/mp.2010.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vreeburg SA, Hoogendijk WJG, van Pelt J, et al. Major depressive disorder and hypothalamic‐pituitary‐adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617‐626. 10.1001/archgenpsychiatry.2009.50 [DOI] [PubMed] [Google Scholar]
- 20. Hallberg L, Janelidze S, Engstrom G, Wisén AGM, Westrin Å, Brundin L. Exercise‐induced release of cytokines in patients with major depressive disorder. J Affect Disord. 2010;126(1‐2):262‐267. 10.1016/j.jad.2010.02.133 [DOI] [PubMed] [Google Scholar]
- 21. Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595‐606. 10.1038/mp.2012.33 [DOI] [PubMed] [Google Scholar]
- 22. Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24(6):519‐525. 10.1097/YCO.0b013e32834b9db6 [DOI] [PubMed] [Google Scholar]
- 23. Petralia MC, Mazzon E, Fagone P, et al. The cytokine network in the pathogenesis of major depressive disorder. Close to translation? Autoimmun Rev. 2020;19(5):102504. 10.1016/j.autrev.2020.102504 [DOI] [PubMed] [Google Scholar]
- 24. Das R, Emon MPZ, Shahriar M, et al. Higher levels of serum IL‐1β and TNF‐α are associated with an increased probability of major depressive disorder. Psychiatry Res. 2021;295:113568. 10.1016/j.psychres.2020.113568 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt FM, Lichtblau N, Minkwitz J, et al. Cytokine levels in depressed and non‐depressed subjects, and masking effects of obesity. J Psychiatr Res. 2014;55:29‐34. 10.1016/j.jpsychires.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 26. Nothdurfter C, Milenkovic VM, Sarubin N, et al. The cytokine IL‐17A as a marker of treatment resistance in major depressive disorder? Eur J Neurosci. 2021;53(1):172‐182. 10.1111/ejn.14636 [DOI] [PubMed] [Google Scholar]
- 27. Gately MK, Renzetti LM, Magram J, et al. The interleukin‐12/interleukin‐12‐receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495‐521. 10.1146/annurev.immunol.16.1.495 [DOI] [PubMed] [Google Scholar]
- 28. D'Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176(5):1387‐1398. 10.1084/jem.176.5.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stern AS, Magram J, Presky DH. Interleukin‐12 an integral cytokine in the immune response. Life Sci. 1996;58(8):639‐654. 10.1016/s0024-3205(96)80003-8 [DOI] [PubMed] [Google Scholar]
- 30. Scott P, Trinchieri G. IL‐12 as an adjuvant for cell‐mediated immunity. Sem Immunol. 1997;9(5):285‐291. 10.1006/smim.1997.0084 [DOI] [PubMed] [Google Scholar]
- 31. Trinchieri G. Interleukin‐12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen‐specific adaptive immunity. Annu Rev Immunol. 1995;13:251‐276. 10.1146/annurev.iy.13.040195.001343 [DOI] [PubMed] [Google Scholar]
- 32. Zwirner NW, Ziblat A. Regulation of NK cell activation and effector functions by the IL‐12 family of cytokines: the case of IL‐27. Front Immunol. 2017;8:25. 10.3389/fimmu.2017.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trinchieri G. Immunobiology of lnterleukin‐12. Immunol Res. 1998;17(1‐2):269‐278. 10.1007/BF02786451 [DOI] [PubMed] [Google Scholar]
- 34. Lee KM, Kim YK. The role of IL‐12 and TGF‐β1 in the pathophysiology of major depressive disorder. Int Immunopharmacol. 2006;6(8):1298‐1304. 10.1016/j.intimp.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 35. Sutcigil L, Oktenli C, Musabak U, et al. Pro‐ and anti‐inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:1‐6. 10.1155/2007/76396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Köhler CA, Freitas TH, Stubbs B, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta‐analysis. Mol Neurobiol. 2017;55(5):4195‐4206. 10.1007/s12035-017-0632-1 [DOI] [PubMed] [Google Scholar]
- 37. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. curr top. Behav Neurosci. 2013;14:135‐151. 10.1007/7854_2012_211 [DOI] [PubMed] [Google Scholar]
- 38. Riya S, Sultana S, Daria S, et al. Evaluation of serum lysophosphatidic acid and lysophosphatidylcholine levels in major depressive disorder patients. Cureus. 2020;12(12):e12388. 10.7759/cureus.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kiecolt‐Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075‐1091. 10.1176/appi.ajp.2015.15020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Das R, Hasan MR, Daria S, Islam MR. Impact of COVID‐19 pandemic on mental health among general Bangladeshi population: a cross‐sectional study. BMJ Open. 2021;11(4):e045727. 10.1136/bmjopen-2020-045727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daria S, Islam MR. Increased suicidal behaviors among students during COVID‐19 lockdowns: a concern of student's mental health in Bangladesh. J Affect Disord Rep. 2022;8:100320. 10.1016/j.jadr.2022.100320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hossain MJ, Ahmmed F, Sarker MMR, et al. Factors associated with underprivileged E‐Learning, session jam phobia, and the subsequent mental distress among students following the extended university closure in Bangladesh. Front Public Health. 2022;9:807474. 10.3389/fpubh.2021.807474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Islam MR, Nahar Z, Hossain MS, et al. Prevalence and associated factors for elevated fear and depressive symptoms among the private service holders in Bangladesh during the Covid‐19 pandemic: a cross‐sectional study. Health Science Reports. 2022;5(5):e795. 10.1002/hsr2.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Islam MR, Quaiyum S, Pakhe SA, Repon MAU, Bhuiyan MA. Dataset concerning the mental health of healthcare professionals during COVID‐19 pandemic in Bangladesh. Data Brief. 2021;39:107506. 10.1016/j.dib.2021.107506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Islam MR, Daria S, Das R, Hasan MR. A nationwide dataset on the mental health of the Bangladeshi population due to the COVID‐19 pandemic. Data Brief. 2021;38:107347. 10.1016/j.dib.2021.107347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Repon MAU, Pakhe SA, Quaiyum S, Das R, Daria S, Islam MR. Effect of COVID‐19 pandemic on mental health among Bangladeshi healthcare professionals: a cross‐sectional study. Sci Prog. 2021;104(2):003685042110264. 10.1177/00368504211026409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cassano P, Fava M. Depression and public health. J Psychosom Res. 2002;53(4):849‐857. 10.1016/s0022-3999(02)00304-5 [DOI] [PubMed] [Google Scholar]
- 48. Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1044‐1053. 10.1016/j.pnpbp.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 49. Proma MA, Daria S, Nahar Z, Ashraful Islam SM, Bhuiyan MA, Islam MR. Monocyte chemoattractant protein‐1 levels are associated with major depressive disorder. J Basic Clin Physiol Pharmacol. Published online Jnauary 5, 2022. 10.1515/jbcpp-2021-0132 [DOI] [PubMed]
- 50. Anjum S, Qusar MMAS, Shahriar M, Islam SMA, Bhuiyan MA, Islam MR. Altered serum interleukin‐7 and interleukin‐10 are associated with drug‐free major depressive disorder. Ther Adv Psychopharmacol. 2020;10:204512532091665. 10.1177/2045125320916655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nishuty NL, Khandoker MMH, Karmoker JR, et al. Evaluation of serum Interleukin‐6 and c‐reactive protein levels in drug‐naïve major depressive disorder patients. Cureus. 2019;11(1):e3868. 10.7759/cureus.3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Islam MR, Islam MR, Ahmed I, et al. Elevated serum levels of malondialdehyde and cortisol are associated with major depressive disorder: a case‐control study. SAGE Open Med. 2018;6:205031211877395. 10.1177/2050312118773953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Das R, Emon MPZ, Chowdhury SF, Huque S, Zahan T, Islam MR. Evaluation of serum glial cell line‐derived neurotrophic factor in Bangladeshi major depressive disorder patients. Cureus. 2019;11(11):e6081. 10.7759/cureus.6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim YK, Suh IB, Kim H, et al. The plasma levels of interleukin‐12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry. 2002;7(10):1107‐1114. 10.1038/sj.mp.4001084 [DOI] [PubMed] [Google Scholar]
- 55. Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(1):11‐38. 10.1016/0278-5846(94)00101-m [DOI] [PubMed] [Google Scholar]
- 56. Kim JW, Lee YS, Han DH, Min KJ, Lee J, Lee K. Diagnostic utility of quantitative EEG in un‐medicated schizophrenia. Neurosci Lett. 2015;589:126‐131. 10.1016/j.neulet.2014.12.064 [DOI] [PubMed] [Google Scholar]
- 57. Islam MR, Islam MR, Shalahuddin Qusar MMA, et al. Alterations of serum macro‐minerals and trace elements are associated with major depressive disorder: a case‐control study. BMC Psychiatry. 2018;18(1):94. 10.1186/s12888-018-1685-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Islam S, Islam T, Nahar Z, et al. Altered serum adiponectin and interleukin‐8 levels are associated in the pathophysiology of major depressive disorder: a case‐control study. PLoS One. 2022;17(10):e0276619. 10.1371/journal.pone.0276619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials.


