Abstract
Background:
Many cancer survivors with co-morbid diabetes receive less diabetes management than their non-cancer counterparts. We sought to determine if racial/ethnic disparities exist in recommended diabetes care within 12 months of an incident breast, prostate, or colorectal cancer diagnosis. Because co-morbid diabetes decreases long-term survival, identifying predictors of guideline-concordant diabetes care is important.
Methods:
Using the Surveillance, Epidemiology, and End Results cancer registry linked to Medicare claims, we included beneficiaries aged 67+ years with diabetes and incident, non-metastatic breast, prostate, or colorectal cancer between 2008–2013. Primary outcomes were diabetes care services 12 months after diagnosis: 1) HbA1c test, 2) eye exam, and 3) low-density lipoprotein (LDL) test. Using modified Poisson models with robust standard errors, we examined each outcome separately.
Results:
We included 34,643 Medicare beneficiaries with both diabetes and cancer. Mean age at diagnosis was 76.1 (SD 6.2), 47.2% were women; 35% had breast, 24% colorectal, and 41% prostate cancer. In the 12 months after incident cancer diagnosis, 82.4% received an HbA1c test, 55.3% received an eye exam, 77.8% had an LDL test, and 42.0% received all three tests. Compared to Non-Hispanic Whites, Blacks were 3% (95% CI 0.95–0.98) less likely to receive a HbA1c test, 10% (95% CI 0.89–0.92) less likely to receive a LDL test, and 8% (95% 0.89–0.95) less likely to receive an exam eye. Blacks and Hispanics were 16% (95% CI 0.81–0.88) and 7% (0.88–0.98) less likely to receive all three tests, after accounting for confounders. Racial/ethnic differences persisted across cancer types.
Conclusion:
Blacks and Hispanics with breast, prostate, and colorectal cancer and diabetes received less diabetes care after cancer diagnosis compared to Non-Hispanic Whites. Differences were not explained by socio-economic factors or clinical need.
Keywords: racial disparities, diabetes, quality of care, cancer outcomes
Introduction
Employing the American Cancer Society’s definition of a cancer survivor (from date of cancer diagnosis)1 there are 17 million cancer survivors in the United States2, 20% of whom have type 2 diabetes.3,4 As screening and diagnosis strategies improve, we anticipate the number of cancer survivors with diabetes will increase. Survivors with cancer and diabetes have a 50% increased risk of mortality up to 10 years after a cancer diagnosis compared to non-diabetic cancer counterparts.5,6 Potential reasons for this difference include diabetes-related cancer treatment delays7 and receipt of less aggressive cancer treatments due to uncontrolled diabetes.8–11 Racial/ethnic minorities with cancer are especially vulnerable because they have a greater comorbidity burden and face higher risks for poor cancer outcomes.12–14
A recent systematic review found that diabetes self-management (medication adherence and self-management behaviors) decline after an individual is diagnosed with cancer.15 Similarly, a SEER-Medicare study found that diabetes care quality metrics (annual hemoglobin A1c [HbA1c] exams, low-density lipoprotein [LDL] cholesterol tests, and eye exams) declined in the year after cancer diagnosis compared to the year before cancer diagnosis.16 This is a problem because worse glycemic control may put cancer patients with diabetes at risk for worse cancer outcomes.17,18 To date, no studies have examined if racial/ethnic minorities with cancer and diabetes are less likely to receive recommended diabetes care than their White counterparts.
Continued diabetes management during cancer care is critical to optimizing outcomes. However, little is known about which cancer patients receive diabetes care in the year after diagnosis. Using the Surveillance, Epidemiology, and End Results cancer registry linked to Medicare fee-for-service claims (SEER-Medicare), the objective of this study was to determine if racial/ethnic disparities in receipt of diabetes processes of care (i.e., HbA1c testing, eye examination, and LDL testing) exist for breast, prostate, and colorectal cancer patients with type 2 diabetes in the 12 months after an incident cancer diagnosis. Understanding which patients are less likely to receive recommended diabetes care can inform efforts to support diabetic cancer survivors and reduce potential inequities.
Methods
Data Source:
We used SEER data (2008–2013) linked to Medicare claims from 2007 through 2015. The linked dataset includes Medicare beneficiaries 65+ years diagnosed in SEER registry regions and contains information on cancer-related characteristics, demographics, and health services reimbursed by Medicare fee-for-service (Parts A and B).19 SEER cancer registries capture 35% of the U.S cancer population, which assures generalizability of study findings.20 This study was deemed exempt by the Institutional Review Board at Weill Cornell Medicine.
Cohort:
Beneficiaries aged 67+ years with incident, non-metastatic breast, prostate, or colorectal cancer who were alive 14 months after cancer diagnosis (to capture diabetes services) were included. Sixty-seven years was selected so that eligible individuals would have continuous Medicare Parts A and B enrollment for 24 months before (to identify diabetes status). As SEER provides month and year of diagnosis, we set day of diagnosis to the 15th. To define the 24-month period before diagnosis, we used claims from January 2006 to December 2007 and included individuals diagnosed with cancer after January 2008. Eligible individuals had a diabetes diagnosis in the 24 months before their cancer diagnosis date. We followed an approach validated by Miller and colleagues21,22 using ICD-9-CM diagnoses codes 250.xx and 362.0x. A diagnosis code appeared on at least one inpatient stay or on two different outpatient claims (on two different days) in the 24-month pre-diagnosis period.21 Outpatient claims required a face-to-face contact with a physician.21
Exclusion criteria:
Medicare beneficiaries: 1) diagnosed with cancer from an autopsy or death certificate (<1%), 2) with stage 4 cancer, and 3) with a prior history of cancer (Supplemental Figure 1).21
Defining a racial disparity:
We used the Institute of Medicine’s (IOM) definition of a racial disparity as published in the report Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. The IOM report defined a disparity as a difference in health service use between two racial/ethnic groups that cannot be explained by health status (e.g., comorbidity burden), clinical appropriateness (e.g., cancer stage) and patient preferences.23 24 Given that all individuals were continuously enrolled in Medicare fee-for-service, we were able to determine if disparities exist among individuals with relatively uniform health insurance coverage, which serves as a proxy for healthcare access.
Study outcomes:
Guided by the Healthcare Effectiveness Data and Information Set (HEDIS) on diabetes quality of care, we considered three diabetes care processes in the 12 months after a cancer diagnosis.25,26 Using an approach developed by Research ANd Development (RAND)27 and implemented in previous SEER-Medicare studies,16,28,29 we examined claims for any of the following over the 12 months after cancer diagnosis: 1) HbA1c test, 2) eye exam, and 3) LDL test.26 We created an overall utilization measure defined as receipt of all indicators over 12-months (i.e., “all three measures”).16 To avoid capturing routine testing for cancer diagnosis not intended for diabetes management, we implemented a 45-day “wash-out” period after the date of cancer diagnosis (set to the 15th of each month). Beginning 45 days after the cancer diagnosis date, we examined claims over the following 12-months. Each diabetes indicator was examined as a binary outcome (any vs. no receipt).
Key Independent Variable:
Race/ethnicity was operationalized using the SEER race recode and SEER ethnicity variables. Race was categorized as White, Black, American Indian/Alaskan Native, and Asian or Pacific Islander. In SEER, ethnicity is determined using the Hispanic Identification Algorithm (NHIA).30 This algorithm has been shown to have high sensitivity (93%) and specificity (98%).31 We used a combination of these two variables to classify individuals as 1) Non-Hispanic White, 2) Non-Hispanic Black, 3) Hispanic, 4) Non-Hispanic Asian, and 5) Non-Hispanic Other.
Covariates:
We selected potential confounders based on Andersen’s Behavioral Model of Health Services Use.32 Covariates included: 1) predisposing characteristics (age at cancer diagnosis; gender), 2) enabling resources (census-tract level income [quartiles], Medicaid eligibility, receipt of a low-income subsidy, SEER geographic region, and urban/rural status, 3) evaluated need (comorbid conditions in the year before cancer diagnosis, presence of diabetes complications in the two years before cancer diagnosis, cancer stage [stage 1–3]), and 4) health service use. Comorbid conditions were calculated using Klabunde’s version of the Charlson comorbidity index, excluding diabetes, solid tumors, leukemia, and lymphomas.33–36 Consistent with our prior work, diabetes complications were defined by ICD-9-CM diagnosis codes 250.4x-250.9x (renal, ophthalmic, neurological, peripheral circulatory, and other specified manifestations; and unspecified complications) or 362.0x (diabetic retinopathy).16 Diabetes without complications were defined with ICD-9-CM codes 250.0x-250.3x.16 Health services included cancer treatments received including cancer surgery, chemotherapy, hormone therapy, and radiation within 12 months of cancer diagnosis.
Statistical Analyses:
We compared differences in characteristics by cancer type (breast, prostate, colorectal) and race/ethnicity using chi-square tests, separately. Using the Variance Inflation Factor (VIF), we assessed if socioeconomic variables (census-tract income, Medicaid eligibility, and low-income subsidy) were collinear. With and without the 45-day wash-out period, we described receipt of diabetes processes after cancer diagnosis for patients overall, by cancer type, and race/ethnicity. We examined each diabetes indicator separately and the three-measure indicator. We estimated multivariable modified Poisson models for each diabetes indicator.37 We modeled the likelihood of receiving: 1) all three measures, 2) a HbA1c test, 3) a LDL test, and 4) an eye exam, separately. Models were run with and without the 45-day wash-out period. To examine differences by race/ethnicity, we estimated adjusted models with the IOM definition (i.e., not adjusting for socioeconomic factors), and fully-adjusted models that included socioeconomic factors as well as all other covariates described above. Adjusted risk ratios (aRR) with 95% confidence intervals (CI) were calculated. Statistical analyses were conducted in SAS Version 9.4 with two-sided statistical tests and α of 5%.
Sensitivity Analyses:
To see if predictors of diabetes care varied by cancer type, we estimated models stratified by cancer type. Additionally, as HEDIS definitions are for individuals <75 years, we conducted age-stratified (<75 years and 75+ years) sensitivity analyses overall and by cancer type.
Results
Cohort Characteristics:
We included 34,643 Medicare beneficiaries with incident cancer and pre-existing diabetes (Table 1). Of these, 12,051 (35%) had breast, 8,208 (24%) had colorectal, and 14,384 (41%) had prostate cancer. Mean age at diagnosis was 76.1 years (SD 6.2). Seventy-one percent of the sample was White, 13.8% were Black, 8.5% were Hispanic, 3.8% were Asian, and 3.3% were of another race. Twenty percent were dually eligible for Medicaid, 31% had diabetes with complications, 51% had stage 1 cancer, and 41.3% had at least one comorbidity in addition to diabetes and cancer.
Table 1:
Cohort Characteristics for Cancer Patients with Diabetes, Over All and by Cancer Type
| All | Cancer type | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Breast | Colorectal | Prostate | |||||||
|
|
|||||||||
| N | % | N | % | N | % | N | % | ||
| All | 34,643 | 100 | 12,051 | 100 | 8,208 | 100 | 14,384 | 100 | |
|
|
|||||||||
| Age at diagnosis (years) | <.0001 | ||||||||
| 67–69 | 6311 | 18.2 | 2050 | 17.0 | 1112 | 13.6 | 3149 | 21.9 | |
| 70–74 | 10883 | 31.4 | 3497 | 29.0 | 2156 | 26.3 | 5230 | 36.4 | |
| 75–79 | 8491 | 24.5 | 2980 | 24.7 | 1959 | 23.9 | 3552 | 24.7 | |
| 80–84 | 5602 | 16.2 | 2117 | 17.6 | 1718 | 20.9 | 1767 | 12.3 | |
| 85+ | 3356 | 9.7 | 1407 | 11.7 | 1263 | 15.4 | 686 | 4.8 | |
| Gender | <.0001 | ||||||||
| Male | 18285 | 52.8 | 0 | 0.0 | 3901 | 47.5 | 14384 | 100.0 | |
| Female | 16358 | 47.2 | 12051 | 100.0 | 4307 | 52.5 | 0 | 0.0 | |
| Race | <.0001 | ||||||||
| Non-Hispanic White | 24461 | 70.6 | 8585 | 71.2 | 5905 | 71.9 | 9971 | 69.3 | |
| Non-Hispanic Black | 4790 | 13.8 | 1694 | 14.1 | 936 | 11.4 | 2160 | 15.0 | |
| Hispanic | 2936 | 8.5 | 953 | 7.9 | 663 | 8.1 | 1320 | 9.2 | |
| Asian | 1315 | 3.8 | 436 | 3.6 | 422 | 5.1 | 457 | 3.2 | |
| Other | 1141 | 3.3 | 383 | 3.2 | 282 | 3.4 | 476 | 3.3 | |
| Residence in a rural region | 3735 | 10.8 | 1274 | 10.6 | 973 | 11.9 | 1488 | 10.3 | 0.0001 |
| SEER geographic region | 0.0015 | ||||||||
| West | 13338 | 38.5 | 4676 | 38.8 | 3097 | 37.7 | 5565 | 38.7 | |
| South | 9476 | 27.4 | 3319 | 27.5 | 2161 | 26.3 | 3996 | 27.8 | |
| Northeast | 7530 | 21.7 | 2567 | 21.3 | 1929 | 23.5 | 3034 | 21.1 | |
| Midwest | 4299 | 12.4 | 1489 | 12.4 | 1021 | 12.4 | 1789 | 12.4 | |
| Income Quartile | <.0001 | ||||||||
| Quartile 1 | 8669 | 25.0 | 3087 | 25.6 | 2172 | 26.5 | 3410 | 23.7 | |
| Quartile 2 | 8662 | 25.0 | 3010 | 25.0 | 2121 | 25.8 | 3531 | 24.6 | |
| Quartile 3 | 8662 | 25.0 | 3043 | 25.3 | 2027 | 24.7 | 3592 | 25.0 | |
| Quartile 4 | 8650 | 25.0 | 2911 | 24.2 | 1888 | 23.0 | 3851 | 26.8 | |
| Medicaid eligibility | 7030 | 20.3 | 2912 | 24.2 | 2169 | 26.4 | 1949 | 13.6 | <.0001 |
| Low income subsidy | 1027 | 3.0 | 429 | 3.6 | 280 | 3.4 | 318 | 2.2 | <.0001 |
| Charlson Comorbidity Index | <.0001 | ||||||||
| 0 | 20326 | 58.7 | 7198 | 59.7 | 4180 | 50.9 | 8948 | 62.2 | |
| 1 | 6388 | 18.4 | 2294 | 19.0 | 1604 | 19.5 | 2490 | 17.3 | |
| 2+ | 7929 | 22.9 | 2559 | 21.2 | 2424 | 29.5 | 2946 | 20.5 | |
| Diabetes with complications | 10556 | 30.5 | 3685 | 30.6 | 2716 | 33.1 | 4155 | 28.9 | <.0001 |
| Cancer stage | <.0001 | ||||||||
| 1 | 17,554 | 50.7 | 8,091 | 67.1 | 2,067 | 25.2 | 7,396 | 51.4 | |
| 2 | 9,753 | 28.2 | 2,887 | 24.0 | 1,357 | 16.5 | 5,509 | 38.3 | |
| 3 | 5,804 | 16.8 | 722 | 6.0 | 4,385 | 53.4 | 697 | 4.9 | |
| Missing | 1,532 | 4.4 | 351 | 2.9 | 399 | 4.9 | 782 | 5.4 | |
| Cancer treatments | |||||||||
| Surgery | 22462 | 64.8 | 11343 | 94.1 | 7266 | 88.5 | 3853 | 26.8 | <.0001 |
| Chemotherapy/Hormone therapy | 9216 | 26.6 | 2211 | 18.4 | 1611 | 19.6 | 5394 | 37.5 | <.0001 |
| Radiation | 15191 | 43.9 | 6542 | 54.3 | 1013 | 12.3 | 7636 | 53.1 | <.0001 |
Unadjusted Diabetes Care Utilization:
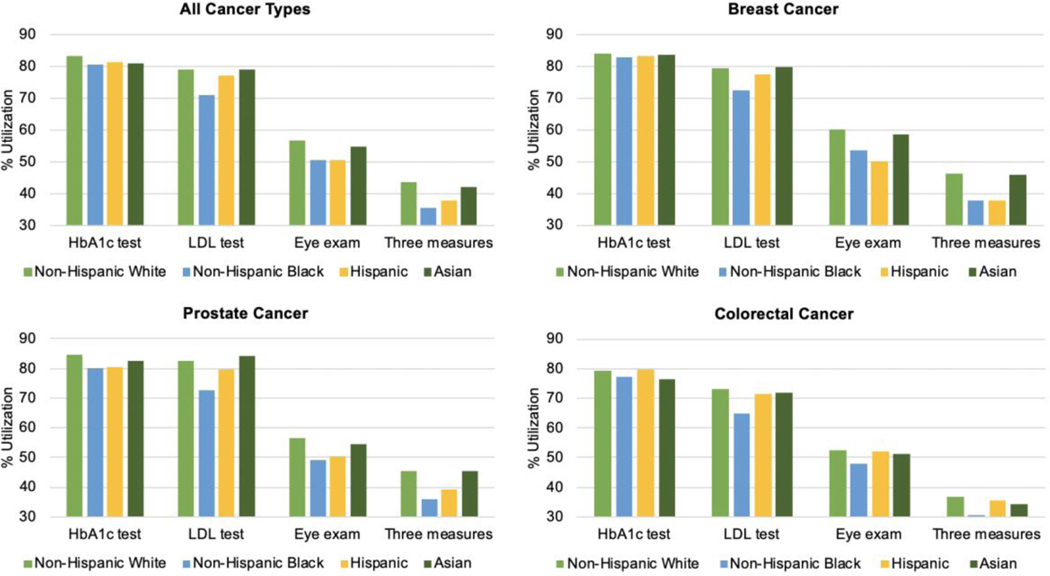
Results with and without the 45-day wash-out period were nearly identical; results with the wash-out period are presented in detail, while results without the wash-out period are shown in Supplemental Table 2. Overall, in the year after cancer diagnosis, 82% of beneficiaries received a HbA1c test, 78% received a LDL cholesterol test, 55% received an eye exam and 42% received all three tests (Table 2). Non-Hispanic Blacks had the lowest rates of utilization compared to other racial/ethnic groups (Table 3). In crude models, Blacks were 3% (0.95–0.98) less likely to receive a HbA1c test, 10% (95%CI 0.88–0.91) less likely to receive a LDL test, 11% (95% 0.96–0.92) less likely to receive an eye exam, and 19% (95% CI 0.78–0.85) less likely to receive all three indicators compared to Non-Hispanic Whites (Table 4). Hispanics also had lower rates of utilization and were 2% (95% CI 0.95–1.00 less likely to receive a HbA1c test, 3% (95% CI 0.95–0.99) less likely to receive a LDL test, 11% (95% CI 0.85–0.93) less likely to receive an eye exam, and 13% (95% CI 0.83–0.91) less likely to receive all three indicators compared to Non-Hispanic Whites.
Table 2:
Diabetes Care Management Utilization* One Year after Cancer Diagnosis
|
|
|||||
|---|---|---|---|---|---|
| Overall | Breast | Colorectal | Prostate | p-value | |
|
|
|||||
| # of patients | 34,643 | 12,051 | 8,208 | 14,384 | |
| HbA1c testa | 82.4% | 83.6% | 78.9% | 83.5% | <.0001 |
| LDL testb | 77.8% | 78.2% | 71.8% | 80.8% | <.0001 |
| Eye exam | 55.3% | 58.3% | 51.7% | 54.9% | <.0001 |
| Three measuresc | 42.0% | 44.3% | 35.7% | 43.6% | <.0001 |
Notes:
Utilization defined as the percentage of individuals with a claim for the specific service over 12-months
Includes a 45-day wash-out period which excluded claims within 45 day after cancer diagnosis date
HbA1c test: Hemoglobin A1C test
LDL test: Low-density lipoprotein cholesterol test
Three measures defined as receiving all three indicators (HbA1c, LDL, and eye exam)
Table 3:
Relative Risk Ratios Between Race/Ethnicity and Receipt of Diabetes Care Management
| HbA1c Testa | LDL Testb | Eye Exam | Three Measuresc | |
|---|---|---|---|---|
| Non-Hispanic Blacks | ||||
| Crude model | 0.97 (0.95–0.98)* | 0.90 (0.88–0.91)* | 0.89 (0.86–0.92)* | 0.81 (0.78–0.85)* |
| No SES Factors | 0.95 (0.94–0.97)* | 0.89 (0.87–0.90)* | 0.88 (0.85–0.91)* | 0.79 (0.76–0.82)* |
| Fully-adjusted | 0.97 (0.95–0.98)* | 0.90 (0.89–0.92)* | 0.92 (0.89–0.95)* | 0.84 (0.80–0.87)* |
|
| ||||
| Hispanics/Latinos | ||||
| Crude model | 0.98 (0.96–1.00)* | 0.97 (0.95–0.99)* | 0.89 (0.86–0.93)* | 0.87 (0.83–0.91)* |
| No SES Factors | 0.97 (0.95–0.99)* | 0.97 (0.95–0.99)* | 0.87 (0.84–0.91)* | 0.85 (0.80–0.89)* |
| Fully-adjusted | 0.99 (0.97–1.01) | 1.00 (0.98–1.02) | 0.93 (0.89–0.96)* | 0.92 (0.88–0.97)* |
|
| ||||
| Asians | ||||
| Crude model | 0.98 (0.95–1.00) | 1.00 (0.97–1.02) | 0.96 (0.92–1.01) | 0.96 (0.90–1.03) |
| No SES Factors | 0.99 (0.96–1.01) | 1.02 (0.99–1.06) | 0.96 (0.91–1.01) | 0.98 (0.92–1.05) |
| Fully-adjusted | 1.00 (0.97–1.02) | 1.05 (1.02–1.08)* | 1.00 (0.95–1.06) | 1.06 (0.99–1.14) |
|
| ||||
| Other | ||||
| Crude model | 1.00 (0.98–1.03) | 0.99 (0.96–1.02) | 1.01 (0.96–1.06) | 1.06 (1.00–1.13)* |
| No SES Factors | 1.00 (0.97–1.03) | 1.00 (0.97–1.03) | 1.01 (0.96–1.06) | 1.06 (0.99–1.13) |
| Fully-adjusted | 1.00 (0.98–1.03) | 1.00 (0.97–1.03) | 1.02 (0.97–1.07) | 1.07 (1.01–1.14)* |
Notes:
SES (Socio-economic factors). The “No SES Factors” and “Fully-adjusted” models adjust for age at diagnosis, gender, residence in a rural region, SEER geographic region, Charlson Comorbidity Index, diabetes with complications, cancer stage, and cancer treatments as described in Table 1.
SES factors include income quartile, Medicaid eligibility, and low income subsidy.
Modified Poisson models used robust standard error and implemented a 45-day wash-out period, which excluded claims within 45 day after cancer diagnosis date. Estimates indicate relative risk ratios and 95% confidence intervals.HbA1c test: Hemoglobin A1C test; LDL test: Low-density lipoprotein cholesterol test; Three measures defined as receiving all three indicators within 12-months (HbA1c, LDL, and eye exam).
denote statistical significant p<0.05
HbA1c test: Hemoglobin A1C test
LDL test: Low-density lipoprotein cholesterol test
Three measures defined as receiving all three indicators (HbA1c, LDL, and eye exam)
Table 4:
Relative Risk Ratios Between Race/Ethnicity and Receipt of Diabetes Care Management by Cancer Type
| HbA1c Testa | LDL Testb | Eye Exam | Three Measuresc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostate | Breast | Colorectal | Prostate | Breast | Colorectal | Prostate | Breast | Colorectal | Prostate | Breast | Colorectal | |
| NHB | ||||||||||||
| Crude model | 0.94 (0.92–0.97) | 0.99 (0.96–1.01) | 0.97 (0.94–1.01) | 0.88 (0.86–0.90) | 0.91 (0.88–0.94) | 0.89 (0.84–0.93) | 0.87 (0.83–0.91) | 0.89 (0.85–0.94) | 0.91 (0.85–0.98) | 0.79 (0.74–0.84) | 0.81 (0.76–0.87) | 0.83 (0.75–0.92) |
| No SES | 0.93 (0.91–0.95) | 0.97 (0.95–1.00) | 0.97 (0.93–1.0) | 0.87 (0.85–0.90) | 0.91 (0.88–0.94) | 0.88 (0.84–0.93) | 0.86 (0.82–0.90) | 0.90 (0.86–0.94) | 0.91 (0.85–0.98) | 0.77 (0.73–0.82) | 0.81 (0.76–0.86) | 0.81 (0.73–0.9) |
| Fully-adjusted | 0.95 (0.93–0.97) | 0.98 (0.96–1.01) | 0.98 (0.94–1.02) | 0.89 (0.87–0.92) | 0.93 (0.90–0.95) | 0.90 (0.86–0.95) | 0.89 (0.85–0.94) | 0.93 (0.89–0.98) | 0.95 (0.88–1.02) | 0.82 (0.77–0.87) | 0.85 (0.80–0.91) | 0.86 (0.78–0.96) |
|
| ||||||||||||
| Hispanics/Latinos | ||||||||||||
| Crude model | 0.95 (0.93–0.98) | 0.99 (0.96–1.02) | 1.01 (0.97–1.05) | 0.96 (0.94–0.99) | 0.98 (0.94–1.01) | 0.98 (0.93–1.03) | 0.89 (0.84–0.94) | 0.83 (0.78–0.89) | 0.99 (0.92–1.07) | 0.87 (0.81–0.93) | 0.81 (0.75–0.88) | 0.97 (0.87–1.08) |
| No SES | 0.95 (0.92–0.97) | 0.99 (0.96–1.02) | 1.00 (0.96–1.04) | 0.97 (0.94–1.00) | 0.98 (0.94–1.02) | 0.98 (0.93–1.03) | 0.88 (0.83–0.93) | 0.82 (0.77–0.88) | 0.97 (0.89–1.05) | 0.85 (0.79–0.91) | 0.79 (0.73–0.86) | 0.94 (0.85–1.05) |
| Fully-adjusted | 0.97 (0.94–1.00) | 1.00 (0.97–1.03) | 1.01 (0.97–1.06) | 0.99 (0.96–1.02) | 1.00 (0.96–1.04) | 1.02 (0.97–1.08) | 0.93 (0.88–0.99) | 0.85 (0.80–0.91) | 1.03 (0.95–1.11) | 0.92 (0.86–1.00) | 0.85 (0.77–0.92) | 1.05 (0.94–1.17) |
|
| ||||||||||||
| Asians | ||||||||||||
| Crude model | 0.97 (0.93–1.02) | 1.00 (0.96–1.04) | 0.97 (0.92–1.02) | 1.02 (0.98–1.06) | 1.01 (0.96–1.06) | 0.98 (0.92–1.05) | 0.96 (0.88–1.05) | 0.98 (0.90–1.06) | 0.98 (0.89–1.08) | 1.00 (0.91–1.11) | 0.99 (0.89–1.10) | 0.93 (0.81–1.07) |
| No SES | 0.98 (0.94–1.02) | 1.01 (0.96–1.05) | 0.97 (0.92–1.03) | 1.04 (1.00–1.08) | 1.02 (0.98–1.08) | 1.00 (0.94–1.07) | 0.95 (0.87–1.04) | 0.96 (0.89–1.05) | 0.95 (0.86–1.05) | 1.01 (0.91–1.12) | 0.99 (0.89–1.10) | 0.92 (0.80–1.06) |
| Fully-adjusted | 0.99 (0.94–1.03) | 1.01 (0.97–1.06) | 0.98 (0.93–1.04) | 1.05 (1.01–1.10) | 1.04 (0.99–1.10) | 1.05 (0.98–1.12) | 0.99 (0.90–1.08) | 0.99 (0.91–1.08) | 1.02 (0.92–1.13) | 1.06 (0.95–1.18) | 1.05 (0.94–1.17) | 1.05 (0.91–1.21) |
|
| ||||||||||||
| Other | ||||||||||||
| Crude model | 1.00 (0.96–1.04) | 0.99 (0.95–1.04) | 1.03 (0.98–1.09) | 1.00 (0.96–1.04) | 1.00 (0.95–1.05) | 0.97 (0.90–1.05) | 1.08 (1.00–1.16) | 0.96 (0.88–1.05) | 0.95 (0.85–1.08) | 1.16 (1.07–1.27) | 0.99 (0.88–1.10) | 0.99 (0.84–1.16) |
| No SES | 0.99 (0.95–1.03) | 0.99 (0.95–1.04) | 1.03 (0.97–1.09) | 1.01 (0.96–1.05) | 1.00 (0.95–1.06) | 0.97 (0.90–1.05) | 1.09 (1.01–1.17) | 0.96 (0.88–1.05) | 0.96 (0.85–1.08) | 1.17 (1.07–1.27) | 0.99 (0.88–1.10) | 0.98 (0.84–1.15) |
| Fully-adjusted | 0.99 (0.96–1.03) | 0.99 (0.95–1.04) | 1.03 (0.98–1.09) | 1.01 (0.96–1.05) | 1.01 (0.95–1.06) | 0.98 (0.91–1.06) | 1.09 (1.02–1.18) | 0.96 (0.88–1.05) | 0.97 (0.86–1.09) | 1.18 (1.08–1.28) | 1.00 (0.89–1.11) | 1.00 (0.86–1.18) |
Notes: SES (Socio-economic factors). SES (Socio-economic factors). The “No SES Factors” and “Fully-adjusted” models adjust for age at diagnosis, gender, residence in a rural region, SEER geographic region, Charlson Comorbidity Index, diabetes with complications, cancer stage, and cancer treatments as described in Table 1. SES factors include income quartile, Medicaid eligibility, and low-income subsidy.
Modified Poisson models used robust standard error and implemented a 45-day wash-out period, which excluded claims within 45 day after cancer diagnosis date. Estimates indicate relative risk ratios and 95% confidence intervals.HbA1c test: Hemoglobin A1C test; LDL test: Low-density lipoprotein cholesterol test; Three measures defined as receiving all three indicators within 12-months (HbA1c, LDL, and eye exam).
denote statistical significant p<0.05
HbA1c test: Hemoglobin A1C test
LDL test: Low-density lipoprotein cholesterol test
Three measures defined as receiving all three indicators (HbA1c, LDL, and eye exam)
Adjusted Diabetes Care Utilization:
In adjusted models that did not include socioeconomic factors (i.e., income, Medicaid eligibility, and low income subsidy), compared to Non-Hispanic Whites, Blacks were 5% (95% CI 0.94–0.97) less likely to receive a HbA1c test, 11% (95% CI 0.87–0.90) less likely to receive an LDL cholesterol test, 12% (95% 0.85–0.91) less likely to receive an exam eye, and 21% (0.76–0.82) less likely to receive all three tests (Table 4). Hispanics were 3% (95% CI 0.95–0.99) less likely to receive a HbA1c test, 3% (95% CI 0.95–0.99) less likely to receive an LDL cholesterol test, 13% (95% CI 0.84–0.91) less likely to receive an exam eye, and 15% (95% CI 0.79–0.91) less likely to receive all three tests.
When socioeconomic factors were accounted for, estimates attenuated slightly but most remained statistically significant. Blacks were 3% (95% CI 0.95–0.98) less likely to receive a HbA1c test, 10% (95% CI 0.89–0.92) less likely to receive a LDL test, 8% (95% 0.89–0.95) less likely to receive an exam eye, and 16% (95% CI 0.80–0.87) less likely to receive all three tests compared to Non-Hispanic Whites, adjusting for demographic, socioeconomic, and clinical confounders (Table 4). Compared to Non-Hispanic Whites, Hispanics were 7% (95% CI 0.89–0.96) less likely to receive an eye exam and 8% (95% CI 0.88–0.97) less likely to receive all three tests.
Sensitivity Analyses (Stratified by Cancer Type):
In unadjusted models, breast and prostate cancer patients had similar rates of testing (Table 2). Compared to breast and prostate cancer patients, colorectal cancer patients had consistently lower rates of testing, which were most pronounced for LDL cholesterol tests and receipt of all three tests. Differences in testing between Whites and Blacks were largest among men with prostate cancer. That is, 45% of Non-Hispanic White prostate cancer survivors received all three tests compared to 36% of Black prostate cancer survivors (p<0.0001). Similarly, among breast cancer survivors, 46% of White women received all three tests compared to 38% of Blacks and 38% of Hispanics (p<0.0001). When demographic, socioeconomic, and clinical confounders were accounted for, Blacks and Hispanics with prostate cancer were 5% (0.93–0.98) and 3% (0.94–1.00) less likely to receive a HbA1c test compared to Non-Hispanics Whites (Table 4). For LDL cholesterol testing, Blacks were 7–11% less likely to receive a test across prostate, breast, and colorectal cancer types. For eye exams, Blacks and Hispanics with prostate and breast cancer were less likely to receive an exam. Finally, Blacks with prostate, breast, or colorectal were 15–18% less likely to receive all three tests compared to Non-Hispanic Whites. Hispanics with prostate or breast cancer were 8% (0.86–1.00) and 15% (0.88–0.92) less likely to receive all tests, respectively.
Sensitivity Analyses (Stratified by Age):
We observed similar rates of diabetes care between individuals <75 year and 75+ years old with 42% receiving all three measures in both groups (Appendix Table 2). When each measure was examined individually, we observed that compared to adults <75 years, adults 75+ years had slightly lower rates of HbA1c tests (84% vs. 81%) and LDL cholesterol tests (80% vs 76%) but higher rates of eye exams (53% vs. 58%). When differences by race/ethnicity were examined, we observed similar patterns with Blacks and Hispanics less likely to receive all indicators for individuals <75 and those 75+ years of age.
Discussion
Only 42% of Medicare beneficiaries with diabetes and breast, prostate, or colorectal cancer received at least one HbA1c test, LDL test, and eye exam in the year after their cancer diagnosis. Blacks and Hispanics were significantly less likely to receive all three tests than Non-Hispanic Whites after adjustment for comorbidity burden and cancer clinical characteristics. Racial/ethnic differences attenuated but persisted after adjustment for socioeconomic factors. Differences by race were not fully explained by health status and clinical need suggesting that observed differences are representative of disparities, as defined by the IOM.23,24 These disparities are concerning given that minorities are disproportionately impacted by diabetes and cancer.12–14 A 2005 study in breast cancer survivors found that comorbidity burden measured by the Charlson Comorbidity Index explained 50% of the all-cause survival disparity between Blacks and Whites.12 Another study in breast cancer survivors found that comorbidities accounted for 25% of survival disparities between Blacks and Whites. Both studies concluded that improved control of comorbidities such as diabetes could help reduce survival gaps between Black and White cancer survivors.12,38
Racial disparities in diabetes process of care measures in our study are comparable to disparities in the same process measures seen outside of cancer.39 A study among adults with diabetes but no cancer in the Veterans Affairs (VA) found that Blacks were significantly less likely to receive a LDL cholesterol test (72% vs. 80%) and eye exam (50% vs. 63%) compared to Whites.40 A study of Medicaid beneficiaries in California also reported that Blacks and Hispanics had lower rates of HbA1c testing compared to Non-Hispanics Whites (66% vs. 83%) and (77% vs. 83%), respectively.41 Similarly lower rates for Blacks and Hispanics were observed for LDL testing and eye exams. Outside of cancer, racial disparities in diabetes process measures extend to disparities in glycemic control.39 A meta-analysis of 11 studies reported significantly higher HbA1c values among Blacks with diabetes compared to Whites.42 Another study of 1.8 million Medicare Advantage beneficiaries found that Blacks with diabetes were 7% less likely to have controlled glucose compared to Whites.43 Similarly, a 2019 CMS report found that among Medicare Advantage beneficiaries, Blacks with diabetes were 5% and 12% less likely to have glucose and blood pressure, respectively, controlled compared to Whites with diabetes.44 Finally, Black VA patients with. diabetes were less likely to have controlled cholesterol and blood pressure compared to White patients.40 Given the additional complexities of managing cancer, Blacks with diabetes and cancer may benefit from additional support to ensure they receive recommended diabetes care in the year after a cancer diagnosis, which may help to reduce existing gaps in clinical control of diabetes.
As prior studies have shown, minorities with and without cancer report poorly coordinated care45,46 and worse patient-physician communication,14 which may further complicate diabetes management in the year following a cancer diagnosis. A prior study among colorectal cancer survivors found that Blacks were more likely to experience problems with care coordination, access to care, and health information compared to Whites.47 However, to date, care coordination and patient-physician communication have not yet been studied in the context of diabetes management in the year following a cancer diagnosis. Future studies should employ quantitative and qualitative strategies to identify drivers of sub-optimal diabetes care among racial/ethnic minorities with cancer. By understanding why racial/ethnic minorities receive less diabetes processes of care despite being actively engaged in the healthcare system for their cancer treatment, we can develop targeted interventions to increase the receipt of recommended diabetes care processes during the year following a cancer diagnosis. For example, a possible intervention approach could be to connect patients with pre-existing diabetes to primary care physicians upon being diagnosed with cancer to ensure that their diabetes would be considered during the acute treatment phase.
Limitations:
We included stage I-III breast, colorectal, and prostate cancer patients aged 67+ years old with continuous Part A and B Medicare enrollment for 24 months before and 14 months after diagnosis, potentially limiting generalizability. Our cohort was chosen specifically to reflect cancer patients who would benefit most from diabetes management due to a non-metastatic cancer prognosis. Those with advanced disease or other cancer types may have different diabetes management patterns and needs. This study was unable to account for unmeasured confounders of racial disparities such as institutional racism, discrimination, and mistrust.48 Finally, our study did not capture glycemic or cholesterol control and reflects receipt of diabetes-related processes of care.
Conclusion
Racial/ethnic minorities with diabetes and breast, prostate, or colorectal cancer are less likely to receive three important processes integral to comprehensive diabetes care following their incident cancer diagnosis compared to Non-Hispanic White cancer survivors with diabetes. This observation is concerning given the high prevalence of diabetes and poor cancer outcomes among racial/ethnic minorities.39 Prior studies found that cancer patients de-prioritize diabetes management after cancer diagnosis regardless of race/ethnicity.15,16 However, decrements in diabetes care were larger among minorities. The next step in this line of inquiry is to determine why racial/ethnic minorities are less likely to receive comprehensive diabetes care after cancer diagnosis in order to develop targeted strategies to increase receipt of appropriate diabetes management for these vulnerable populations.
Supplementary Material
Supplemental Figure 1: Exclusion Cascade for Cohort
The cascade walks through exclusions made to construct the study cohort and with the number of individuals removed at each stage
Supplementary Table 1: Cohort Characteristics overall and by race/ethnicity
Supplementary Table 2: Diabetes Care Management Utilization* One Year after Cancer Diagnosis (no wash-out period)
Figure 1:
Diabetes Care Management Utilization* One Year after Cancer Diagnosis, by race
Notes:
*Utilization defined as the percentage of individuals with a claim for the specific service over 12-months
Includes a 45-day wash-out period which excluded claims within 45 day after cancer diagnosis date
aHbA1c test: Hemoglobin A1C test
bLDL test: Low-density lipoprotein cholesterol test
cThree measures defined as receiving all three indicators (HbA1c, LDL, and eye exam)
Funding:
K01 CA251645
Dr. Safford receives salary support for investigator-initiated research from Amgen, Inc. Dr. Leonard has served as a consultant for Sutro, Bayer, Gilead, AstraZeneca, Celgene, Merck, Morphosys, Beigene, Nordic Nanovector, Roche/Genentech, ADC Therapeutics, Sandoz, Karyopharm, Miltenyi, Akcea Therapeutics, and Epizyme.
Footnotes
Ethical approval: This study was deemed exempt by the Institutional Review Board at Weill Cornell Medicine.
My other co-authors and I have no conflicts of interest or financial disclosures. All authors have read and approved the manuscript for submission
Data availability:
The data that support the findings of this study are available from the National Cancer Institute.
References
- 1.ACS. Life After Cancer: Survivorship by the Numbers. American Cancer Society;2014. [Google Scholar]
- 2.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA: a cancer journal for clinicians. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes care. 2010;33(7):1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barone BB, Yeh HC, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes care. 2010;33(4):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein KB, Snyder CF, Barone BB, et al. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Digestive diseases and sciences. 2010;55(7):1839–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman RA, He Y, Winer EP, Keating NL. Racial/Ethnic differences in receipt of timely adjuvant therapy for older women with breast cancer: are delays influenced by the hospitals where patients obtain surgical care? Health services research. 2013;48(5):1669–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfield S, Blanco DM, Elashoff RM, Ganz PA. Patterns of care related to age of breast cancer patients. Jama. 1987;257(20):2766–2770. [PubMed] [Google Scholar]
- 9.Guadagnoli E, Shapiro C, Gurwitz JH, et al. Age-related patterns of care: evidence against ageism in the treatment of early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(6):2338–2344. [DOI] [PubMed] [Google Scholar]
- 10.Bennett CL, Greenfield S, Aronow H, Ganz P, Vogelzang NJ, Elashoff RM. Patterns of care related to age of men with prostate cancer. Cancer. 1991;67(10):2633–2641. [DOI] [PubMed] [Google Scholar]
- 11.Gold HT, Makarem N, Nicholson JM, Parekh N. Treatment and outcomes in diabetic breast cancer patients. Breast cancer research and treatment. 2014;143(3):551–570. [DOI] [PubMed] [Google Scholar]
- 12.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. Jama. 2005;294(14):1765–1772. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Robbins AS, Lin CC, et al. Factors That Contributed to Black-White Disparities in Survival Among Nonelderly Women With Breast Cancer Between 2004 and 2013. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(1):14–24. [DOI] [PubMed] [Google Scholar]
- 14.Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2007;16(3):413–428. [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro LC, Kaur H, Nilo D, Safford MM, DeRosa AP, Kern LM. Determining the Impact of a Cancer Diagnosis on Diabetes Management: A Systematic Literature Review. American journal of clinical oncology. 2019;42(11):870–883. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro LC, Soroka O, Kern LM, Leonard JP, Safford MM. Diabetes care management patterns before and after a cancer diagnosis: A SEER-Medicare matched cohort study. Cancer. 2020. [DOI] [PubMed] [Google Scholar]
- 17.Hershey DS. Importance of Glycemic Control in Cancer Patients with Diabetes: Treatment through End of Life. Asia-Pacific journal of oncology nursing. 2017;4(4):313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hershey DS, Hession S. Chemotherapy and Glycemic Control in Patients with Type 2 Diabetes and Cancer: A Comparative Case Analysis. Asia-Pacific journal of oncology nursing. 2017;4(3):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002;40(8 Suppl):Iv-3–18. [DOI] [PubMed] [Google Scholar]
- 20.Howlader N NA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review 1974–2014. Bethesda, MD: National Cancer Institute;2015. [Google Scholar]
- 21.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes care. 2004;27 Suppl 2:B10–21. [DOI] [PubMed] [Google Scholar]
- 22.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. American journal of medical quality : the official journal of the American College of Medical Quality. 1999;14(6):270–277. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine Committee on U, Eliminating R, Ethnic Disparities in Health C. In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington (DC): National Academies Press (US) Copyright 2002 by the National Academy of Sciences. All rights reserved.; 2003. [PubMed] [Google Scholar]
- 24.McGuire TG, Alegria M, Cook BL, Wells KB, Zaslavsky AM. Implementing the Institute of Medicine definition of disparities: an application to mental health care. Health services research. 2006;41(5):1979–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Standards of medical care in diabetes−−2010. Diabetes care. 2010;33 Suppl 1:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HEDIS. HEDIS and Quality Measurement. The National Committee for Quality Assurance. https://www.ncqa.org/hedis/measures/comprehensive-diabetes-care/. Published 2009. Updated 10/1/2008. Accessed 2019.
- 27.Asch SM, Sloss EM, Hogan C, Brook RH, Kravitz RL. Measuring underuse of necessary care among elderly Medicare beneficiaries using inpatient and outpatient claims. Jama. 2000;284(18):2325–2333. [DOI] [PubMed] [Google Scholar]
- 28.Snyder CF, Frick KD, Herbert RJ, et al. Quality of Care for Comorbid Conditions During the Transition to Survivorship: Differences Between Cancer Survivors and Noncancer Controls. Journal of Clinical Oncology. 2013;31(9):1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–1719. [DOI] [PubMed] [Google Scholar]
- 30.Group NRaEW. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. Springfield, Illinois: North American Association of Central Cancer Registries;2011. [Google Scholar]
- 31.Boscoe FP, Schymura MJ, Zhang X, Kramer RA. Heuristic algorithms for assigning Hispanic ethnicity. PloS one. 2013;8(2):e55689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? Journal of health and social behavior. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 34.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 35.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 36.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. Journal of clinical epidemiology. 1993;46(10):1075–1079; discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 37.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 38.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. Jama. 1994;272(12):947–954. [DOI] [PubMed] [Google Scholar]
- 39.NCQA. What is the current state of quality of care in diabetes? : National Committee for Quality Assurance;2011. [Google Scholar]
- 40.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Medical care. 2003;41(11):1221–1232. [DOI] [PubMed] [Google Scholar]
- 41.Meng YY, Diamant A, Jones J, et al. Racial and Ethnic Disparities in Diabetes Care and Impact of Vendor-Based Disease Management Programs. Diabetes care. 2016;39(5):743–749. [DOI] [PubMed] [Google Scholar]
- 42.Kirk JK, D’Agostino RB Jr, Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes care. 2006;29(9):2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. The New England journal of medicine. 2005;353(7):692–700. [DOI] [PubMed] [Google Scholar]
- 44.Martino SC EM, Hambarsoomian K. Racial, Ethnic, and Gender Disparities in Health Care in Medicare Advantage. Baltimore, MD: CMS Office of Minority Health; April 2019 2019. [Google Scholar]
- 45.Martino SC, Elliott MN, Hambarsoomian K, et al. Racial/Ethnic Disparities in Medicare Beneficiaries’ Care Coordination Experiences. Medical care. 2016;54(8):765–771. [DOI] [PubMed] [Google Scholar]
- 46.Weech-Maldonado R, Elliott M, Pradhan R, Schiller C, Hall A, Hays RD. Can hospital cultural competency reduce disparities in patient experiences with care? Medical care. 2012;50 Suppl:S48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayanian JZ, Zaslavsky AM, Guadagnoli E, et al. Patients’ perceptions of quality of care for colorectal cancer by race, ethnicity, and language. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(27):6576–6586. [DOI] [PubMed] [Google Scholar]
- 48.Smedley BS AY; Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Exclusion Cascade for Cohort
The cascade walks through exclusions made to construct the study cohort and with the number of individuals removed at each stage
Supplementary Table 1: Cohort Characteristics overall and by race/ethnicity
Supplementary Table 2: Diabetes Care Management Utilization* One Year after Cancer Diagnosis (no wash-out period)
Data Availability Statement
The data that support the findings of this study are available from the National Cancer Institute.