Abstract
A subunit or protein-based influenza vaccine can be a safer alternative to live attenuated vaccine (Flumist) and require fewer boosts than an inactivated vaccine (e.g. Fluzone). However, to form an effective subunit vaccine, an adjuvant is often needed. In this work we used electrospray to encapsulate the hydrophilic adjuvant CpG into microparticles made from the hydrophobic biodegradable polymer acetalated dextran. To understand the rate of particle degradation on CpG release, polymer that was slow (21 hrs at phagosomal pH 5) and fast (0.25 hrs at pH 5) degrading was used to encapsulate the adjuvant. The slow-degrading particles exhibited the greatest degree of innate immune stimulation of antigen-presenting cells in vitro. In mice, the broadly acting Computationally Optimized Broadly Reactive Antigen (COBRA) Y2 influenza hemagglutinin (HA) antigen was used with CpG particles, soluble CpG, or MF-59 like adjuvant Addavax. Particles and soluble CpG elicited similar induction of anti-HA antibodies and protection against lethal influenza challenge, but the sustained release particles elicited the highest levels antibody effector functions. These results demonstrate a suitable method for encapsulation of CpG oligonucleotide in a hydrophobic particle matrix, and suggest that sustained release of CpG from Ace-DEX microparticles could potentially be used to induce potent antibody effector functions.
Graphical Abstract

Introduction
For over 100 years, influenza has persisted as a significant source of morbidity and mortality worldwide with up to 5 million severe cases and one-half million deaths per a year, according to the World Health Organization (WHO). Additionally, its impacts are felt through a considerable economic burden on affected countries, which for the US alone can total $87.1 billion a year (Molinari et al., 2007). One of the most common Influenza type A (IAV) subtypes is H1N1. In the 2008–09 influenza season, 0.011% of the world’s population died from complications of the H1N1 pandemic, according to the Centers for Disease Control (CDC).
Seasonal influenza vaccines are most commonly used to help combat this respiratory infection. However, they do not provide adequate protection, with efficacy ranging from 19% to 60% in the years 2004 to 2015 (Belongia et al., 2016). Efficacy is hampered by antigenic drift as the virus evolves under selective pressure from immunity, as well as antigenic shifts; which occur when recombination between viruses from multiple species cause the emergence of influenza strains with very different antigenic characteristics, as during the 2009 H1N1 pandemic (Chowell et al., 2009; Gatherer, 2009). To increase influenza vaccine efficacy and overcome antigenic shift and drift, immense effort has been expended to generate a ‘universal’ influenza vaccine, targeting conserved epitopes present on a wide array of influenza isolated, such as the hemagglutinin stalk epitope (Impagliazzo et al., 2015; Sautto et al., 2018) or the matrix 2 ectodomain (M2e) (Tsybalova et al., 2018). However, antibodies targeting the stalk epitope are generally of relatively low affinity (Hoa et al., 2016), and a growing body of evidence indicates they have a high degree of polyreactivity and potential for autoreactivity (Bajic et al., 2019; Guthmiller et al., 2020; Khurana et al., 2020; Labombarde et al., 2022), while vaccination with M2e does not prevent infection, and demonstrated a rapidly waning humoral immune response (Deng et al., 2015; Tao et al., 2014).
The Computationally Optimized Broadly Reactive Antigen (COBRA) strategy represents an alternate approach to the elicitation of broadly reactive anti-influenza antibody responses, wherein multiple rounds of layered consensus building are employed to generate influenza HA antigens incorporating antigenic characteristics from multiple influenza antigens. Vaccination with these antigens can elicit broadly reactive HA-specific antibodies that can react with a wide swath of past and future influenza viruses (Allen et al., 2018; Carter et al., 2016; Huang et al., 2020; Sautto et al., 2020). This strategy was recently applied using sequences of H1N1 viral isolates from 2013–2019 to generate an H1N1 COBRA HA, Y2 (Huang et al., 2021a). Here we sought to develop an adjuvant formulation that would enhance the immune response to this antigen.
Influenza vaccine formulations most commonly incorporate squalene emulsion based adjuvants (e.g., MF59, AS03) that alone often fail to induce significant Th1-responses that drive protective immunity against the influenza virus (Bungener et al., 2008). Regarding squalene emulsion adjuvants, only MF59 (FLUAD™) is FDA-approved as an adjuvant for seasonal influenza vaccines. MF59 has been shown to increase anti-HA neutralizing antibody responses (Calabro et al., 2013), but studies have demonstrated that this is not necessarily correlative with protection against influenza infection (Impagliazzo et al., 2015). This suggests that cellular responses or antibody effector functions, which are not effectively activated by squalene emulsions (Calabro et al., 2013), are also involved in protection. Also, squalene emulsions have been linked to incidences of narcolepsy (Kim et al., 2015; Nohynek et al., 2012) and death (Europe, 28 November 2014).
An alternative to squalene based emulsions is to use a toll-like receptor (TLR) agonist that can stimulate a Th1 and cellular response. One such TLR agonist, CpG, has been recently FDA approved for use in a hepatitis B vaccine (Heplisav-B). However, delivery of soluble CpG is limited because it is an oligodeoxynucleotide and therefore liable to degradation by nucleases, and its hydrophilicity results in clearance from the injection site within 24 hours (Vicari et al., 2007). In addition, the negative charge of CpG limits its permeation through the cellular membrane to the endosome where its cognate receptor (TLR 9) is located. To overcome these issues, we have previously reported the encapsulation of CpG in acetalated dextran (Ace-DEX) microparticles (MPs) (Peine et al., 2013). Using emulsion, CpG was encapsulated in Ace-DEX MPs at a relatively poor encapsulation efficiency of 36.6% (Peine et al., 2013). We have previously improved the encapsulation of adjuvants in Ace-DEX MPs using electrospray, which limits diffusion into an external aqueous phase by generating particles in a continuous air phase (Duong et al., 2013; Junkins et al., 2018; Steipel et al., 2019).
Furthermore, we have optimized adjuvant delivery and vaccine efficacy through varying the degradation rate of the Ace-DEX. Ace-DEX is unique in that its coverage of cyclic and acyclic acetals on the pendent groups of the dextran backbone varies with polymer reaction time. Using NMR, the cyclic acetal coverage (CAC) can be determined on the polymer, which translates to distinct degradation rates of the MP. Also, Ace-DEX is acid sensitive and degrades more rapidly at lower pH’s, such as found in the antigen presenting cell’s (APC’s) phagosome. We have previously reported that 20% CAC Ace-DEX particles comprised of 71K dextran have a degradation half-life of 28 and 0.25 hrs at pH 7.4 and 5.0, respectively. For 60% CAC particles, we have observed a degradation half-life of >336 and 21 hrs at pH 7.4 and 5.0, respectively (Chen et al., 2016). These differences in degradation rates translate to more effective T cell presentation and have been shown to impact influenza vaccine efficacy and immune response (Broaders et al., 2009; Chen et al., 2018; Chen et al., 2015). Here we applied these formulation strategies to the delivery of CpG to serve as an adjuvant to a Y2 COBRA HA-based vaccine. We evaluate innate immune responses to the formulated vaccine adjuvant with respect to APCs, in vitro. In a mouse model, we report generation of serum antibodies and the effectiveness of those antibodies through an antibody-dependent complement deposition (ADCD) assay and antibody-dependent cellular phagocytosis (ADCP) assay. Furthermore, we illustrate protection after a lethal influenza challenge in a mouse model of infection. Overall, we demonstrate the applicability of Ace-DEX encapsulate CpG as a broadly acting subunit influenza vaccine adjuvant.
Materials and Methods
Materials
Chemicals were purchased from MilliporeSigma (St. Louis, MO) unless otherwise indicated. Absolute ethanol was purchased from Decon Labs (King of Prussia, PA). 2-ethoxypropene was purchased from Matrix Scientific (Elgin, SC). Full length wild type (WT) influenza A (H1N1) HA protein amino acid sequences from 3,078 human H1N1 viruses collected from May 1, 2014 – September 30, 2016 were downloaded from the GISAID Epiflu database. These sequences were organized by their collection date, and put into the COBRA algorithm to generate the sequence of Y2 COBRA HA, which was produced and purified as previously described (Huang et al., 2021b).
Ace-DEX was synthesized from dextran from Leuconostoc mesenteroides with an average molecular weight of 60,000–76,000 Da as previously described (Broaders et al., 2009; Chen et al., 2016; Kauffman et al., 2012). To achieve 20 and 60 CAC, the reaction was carried out for four minutes or two hours, respectively. The polymer CAC was determined by degradation in 10% v/v deuterium chloride in deuterium oxide and quantification of the evolved acetone and ethanol using 1H NMR spectroscopy (Inova 400 MHz spectrometer) (Broaders et al., 2009).
Generation and Characterization of MPs
Electrospray of CpG and blank MPs was performed similarly to previous reports (Gallovic et al., 2022; Watkins-Schulz et al., 2019). Briefly, Ace-DEX was dissolved in absolute ethanol, and CpG was dissolved in molecular grade water. The two solutions were combined at a 9:1 v/v ratio of ethanol to water such that the resulting solution contained 20 mg/mL Ace-DEX and 0.2 mg/mL CpG, or no CpG for blank particles. The resulting solution loaded into a 5 mL glass gastight syringe (Hamilton) fitted with a 20-gauge stainless steel blunt needle and electrosprayed onto a stainless steel substrate at a rate of 0.2 mL/hr. with +2.5 kV potential on the collection substrate and −5.5 kV potential on the needle. The syringe and substrate were base bath and heat treated, respectively, to remove any potential endotoxin prior to spraying. The resulting powder was collected from the substrate and stored at −20 °C until use.
For visualization by SEM, electrosprayed MPs were suspended in Milli-Q grade water containing 0.04% v/v triethylamine and deposited on carbon tape (Electron Microscopy Sciences) and allowed to dry overnight. Dried particles were coated with 7–8 nm AuPd and imaged with a Hitachi S-4700 scanning electron microscope. Hydrodynamic diameter of MPs was determined using a Brookhaven NanoBrook 90Plus Zeta Particle Size Analyzer (Holtsville, NY). Particle suspensions were prepared immediately prior to analysis at 0.1 mg/mL in Milli-Q grade water containing 0.04% v/v triethylamine and sterile filtered through a 220 nm polyethersulfone syringe filter. Values are reported as the intensity-weighted average and standard deviation of 3 1-minute measurements.
To evaluate endotoxin, MPs were suspended in endotoxin-free water overnight at a concentration of 1 mg/mL. Particles were centrifuged at 21,000 x G for 5 min to pellet, and the supernatants were assessed for endotoxin content using a limulus amoebocyte lysate-based kit (Thermo Fisher A39552). All particle batches used for in vitro and in vivo evaluation had an endotoxin content of <0.1 EU/mL.
CpG loading in MPs was assessed using the Quant-iT™ OliGreen™ ssDNA Assay (Fisher O7582). Particles were suspended in 0.1M pH 5 acetate buffer and mixed in a heat block at 37 °C for 48 hours, and loading was assessed according to manufacturer instructions, but using a standard curve of CpG dissolved in the same 0.1M pH 5 acetate buffer. For release studies, MPs were suspended at 1 mg/mL in PBS or 0.1M pH 5 acetate buffer and set in a 37 °C heat block on a shaker. At the indicated time points, particle suspensions were centrifuged at 21,000 x G for 5 minutes to form a pellet, and the supernatant was transferred to a 96-well plate. The pellets were then lyophilized and analyzed for CpG content using the Oligreen assay detailed above. Percent release is reported as the percent decline in the CpG content of the pellet relative to its initial CpG content.
In vitro Experiments
DC2.4 cells (Sigma SCC142) or BMDCs generated from C57BL/6 mouse bone marrow cells cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (PeproTech 315–03) were seeded overnight in 96-well plates at 50,000 cells/well. Cells were given the indicated treatments the following day and incubated for 48 hr. Cells were then centrifuged at 500 x G for 5 minutes, and the supernatants analyzed for nitrite concentration using the Griess reagent (Promega G2930), LDH concentration to quantify cytotoxicity (Thermo 88954), and IL-6 and TNF-α concentration using mouse TNF-α (Fisher 88-7324-88) and IL-6 (Fisher 50-112-8863) sandwich ELISA kits. The remaining cells were then assessed for viability using an MTT assay as previously described (Chen et al., 2016).
Vaccination and Lethal Challenge
All studies were conducted in accordance with National Institutes of Health’s guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee at UNC. Female C57BL/6J mice (n=10 per group) aged 6–8 weeks were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were immunized intramuscularly with the indicated treatments as intramuscular injections of 25 μL in each rear leg for a total volume of 50 μL per mouse. Each dose consisted of 1 µg COBRA and 10 µg CpG in the indicated formulation, where applicable. All antigen and adjuvant combinations were mixed in equal volumes and incubated for 20 minutes on ice prior to administration. Serum was collected by submandibular bleed on the indicated days, and anti-COBRA HA antibody titers were assessed. On day 35, mice were anesthetized with isoflurane and challenged intranasally with 600,000 pfu of Influenza A/California/07/2009 in 50 μL PBS. Mice were monitored daily for body weight and body condition score and euthanized upon losing 20 percent of their initial body mass.
Humoral Responses
Anti-Y2 HA titers were assessed as previously described (Batty et al., 2022). In brief, high-binding ELISA plates (Greiner 655061) were coated overnight with 100 ng/mL Y2 HA. Plates were washed, blocked, and incubated with serially diluted serum samples for one hour. Plates were washed and incubated with secondary antibodies (Goat Anti-Mouse IgG Fc-HRP 1033-05, Goat Anti-Mouse IgG2c-HRP 1078-05, or Goat Anti-Mouse IgG1-HRP 1071-05, Southern Biotech) for two hours, then washed and developed with TMB substrate (Surmodics Inc TMBW100001). Dilution curves were fit using Graphpad Prism 9 and used to calculate titers as described (Batty et al., 2022).
The hemagglutination inhibition (HAI) assay was used to assess functional antibodies to the HA that are able to inhibit agglutination of guinea pig erythrocytes for H3N2 viruses, and turkey erythrocytes for H1N1 viruses. HAI were adapted from the WHO laboratory influenza surveillance manual (Organization, 2011). Guinea pig red blood cells are frequently used to characterize contemporary A(H3N2) influenza strains that have developed a preferential binding to alpha (2,6) linked sialic acid receptors (Katz et al., 2011; Oh et al., 2008).
To inactivate nonspecific inhibitors, sera samples were treated with receptor-destroying enzyme (RDE) (Denka Seiken, Co., Japan) prior to being tested. Three parts of RDE was added to one part of sera and incubated overnight at 37°C. RDE was inactivated by incubation at 56°C for 30 min. RDE-treated sera were diluted in a series of two-fold serial dilutions in v-bottom microtiter plates. An equal volume of each A(H3N2) virus, adjusted to approximately eight hemagglutination units (HAU)/50μl in the presence of 20nM Oseltamivir carboxylate, was added to each well. The plates were covered and incubated at room temperature for 30 mins, and then 0.75% guinea pig erythrocytes (Lampire Biologicals, Pipersville, PA, USA) in PBS were added. Prior to use, the red blood cells (RBCs) were washed twice with PBS, stored at 4°C, and used within 24 h of preparation. The plates were mixed by gentle agitation, covered, and the RBCs were allowed to settle for 1 h at room temperature. The HAI titer was determined by the reciprocal dilution of the last well that contained non-agglutinated RBCs. Positive and negative serum controls were included for each plate.
In separate assays RDE-treated sera were diluted in a series of two-fold serial dilutions in v-bottom microtiter plates. An equal volume of each A(H1N1) virus, adjusted to approximately eight hemagglutination units (HAU)/50μl was added to each well. The plates were covered and incubated at room temperature for 20 mins, and then 0.8% turkey erythrocytes (Lampire Biologicals, Pipersville, PA, USA) in PBS were added. Prior to use, the RBCs were washed twice with PBS, stored at 4°C, and used within 24 h of preparation. The plates were mixed by gentle agitation, covered, and the RBCs were allowed to settle for 30 mins at room temperature. The HAI titer was determined by the reciprocal dilution of the last well that contained non-agglutinated RBCs. Positive and negative serum controls were included for each plate.
All mice were negative (HAI ≤ 1:10) for pre-existing antibodies to human influenza viruses prior to infection or vaccination, and for this study, a positive HAI reaction (HAI+), or “sero-protection”, is defined as an HAI titer ≥ 1:40, and “seroconversion” refers to a 4-fold increase in titer compared to baseline, as per the WHO and European Committee for Medicinal Products to evaluate influenza vaccines (EMA/CHMP/VWP/457259/, 2014).
The ADCP assay was adapted from previous methods (Butler et al., 2019; Earnest et al., 2019). Y2 COBRA HA was conjugated to 1.0 µm carboxylate-modified fluorescent microspheres (Fisher F8821) using sulfo-n-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), and remaining reactive sites were quenched with glycine. J774a.1 murine macrophages (ATCC TIB-67) were seeded overnight at 50,000 cells/well in a non-tissue culture treated 96-well plate. Antigen-coated beads were incubated with sera at the indicated dilutions for two hours, then added to the J774a cells for 1 hour. Cells were washed twice with 5 mM EDTA in PBS and fixed with 3% paraformaldehyde before analysis for particle uptake with an Attune NxT Flow Cytometer (Thermo Fisher). Phagocytic score is reported as the percent of particle positive cells times the geometric mean fluorescence intensity in the particle channel divided by ten thousand. The ADCD assay was performed as previously reported (Boudreau et al., 2020), using the same Y2 HA-conjugated beads as in the ADCP assay and substituting HEPES buffer for veronal buffer as previously reported (Zelek et al., 2018). Complement deposition score is reported as the percent of complement positive beads times the geometric mean fluorescence intensity in the complement channel divided by ten thousand.
Results and Discussion
We sought to use electrospray to overcome the disadvantages of emulsion methods for encapsulating CpG in polymeric particles—namely poor encapsulation efficiency and a batch processing method (Peine et al., 2013). Electrospray demonstrated a very high encapsulation efficiency and generated MPs of a consistent size and flat or disc-shaped morphology (Fig. 1A-B, Table 1). Flat discs of this general morphology are often observed when spraying ethanol as the solvent for hydrophobic drugs, and the flat morphology most likely results from collapse of the sprayed droplet during rapid solvent evaporation while spraying (Collier et al., 2018; Junkins et al., 2018; Steipel et al., 2019). In addition, the use of fast-degrading 20 CAC Ace-DEX or slow-degrading 60 CAC Ace-DEX resulted in very different release profiles, allowing for the comparison of fast-releasing and slow-releasing adjuvant (Fig. 1C-D, S1A-B). While the rapid release from the 20 CAC particles prevented curve fitting of their release kinetics to estimate the 50% release time (t1/2), the 60 CAC MP release in acidic medium was well-fit by a pseudo first-order function (Fig S1A), while they exhibited essentially zero-order release kinetics in neutral medium (Fig. S1B). Zero order release kinetics are uncommon in polymeric particle drug delivery systems. It is possible that the porous nature of the electrosprayed Ace-DEX MPs eliminated the relationship between the amount of drug released and particle surface area, resulting in a constant release rate. Interestingly, the 60 CAC particles did not exhibit any burst release, contrasting with the substantial burst release observed from electrosprayed particles from another anionic DNA-derived adjuvant, cGAMP (Gallovic et al., 2022). This may be due to the substantially greater molecular weight of CpG, which could slow the diffusion of the molecule from the polymeric matrix. In the absence of significant diffusion, it is likely that the drug is primarily released by surface erosion, as these have been determined to be the primary two mechanisms of drug release from Ace-DEX particles (Stiepel et al., 2022).
Figure 1.

Representative scanning electron micrographs of electrosprayed 20 CAC (A) and 60 CAC (B) Ace-DEX MPs encapsulating CpG. Release kinetics of CpG MPs in neutral or acidic conditions over 48 hours (C) or 35 days (D) Data is presented as average ± standard deviation.
Table 1.
Yield, loading, size, and release characteristics of Ace-DEX MPs made with polymer of different CAC. Data is presented as average ± standard deviation.
| CAC | Yield (%) | Encapsulation Efficiency (%) | Final Weight Loading (μg CpG/mg MP) | Diameter (nm) | Polydispersity | t1/2 (hr) pH 7.4 | t1/2 (hr) pH 5.0 |
|---|---|---|---|---|---|---|---|
| 20 | 84.79 | 97.75 ± 5.81 | 9.77 ± 0.58 | 910 ± 95 | 0.33 ± 0.030 | <1 | <1 |
| 60 | 81.75 | 85.42 ± 4.70 | 8.54 ± 0.47 | 724 ± 6.1 | 0.07 ± 0.068 | 527.15 | 5.49 |
CpG-loaded 60 CAC Ace-DEX MPs induced expression of the proinflammatory cytokines TNF-α and IL-6 (Fig. 2) and the inflammatory signaling molecule nitric oxide (Fig. S2A) more potently than soluble CpG in both the DC2.4 murine dendritic cell line and ex vivo cultured bone marrow-derived dendritic cells (BMDCs). This indicates the potential for a more potent adjuvant effect or dose sparing of the adjuvant when delivered in MPs. The 60 CAC MPs were significantly more proinflammatory than the 20 CAC MPs. Considering their respective release profiles, this indicates the importance of particulate formulation of the adjuvant, where the 60 CAC MPs likely did not release substantial amounts of CpG until they were taken up by the cells and degraded in the endosome, colocalizing the release of the CpG with the endosomal TLR9 receptor. However, 20 CAC MPs still exhibited significant differences from soluble CpG in the induction of proinflammatory cytokines, indicating that even short-lived encapsulation of CpG in Ace-DEX is enough to alter its inflammatory signature, perhaps by biasing the method of adjuvant internalization toward phagocytosis. Blank particles exhibited minimal cytotoxicity or viability decreases in vitro at the tested concentrations, and CpG-loaded particles had similar toxicity to the soluble CpG, indicating most toxicity observed was driven by the adjuvant rather than the Ace-DEX (Fig. S3A-D).
Figure 2.

(A,C) TNF-α and (B,D) IL-6 production by (A,B) DC2.4 and (C,D) C57BL/6 bone-marrow derived dendritic cells (BMDCs) after culturing with CpG presented as soluble or encapsulated in Ace-DEX MPs for 48 hours. Cytokines were measured in cell supernatant by ELISA. Statistical significance between the values at the highest CpG treatment concentrations was evaluated using a one-way ANOVA with Tukey’s correction for multiple comparisons and is indicated with * P-value <0.05, *** P-value<0.001, and **** P-value <0.0001. Data is presented as average ± standard deviation.
Having established the increased potency of CpG-loaded electrosprayed Ace-DEX MPs in vitro, we compared the adjuvanticity of the fast- and slow-releasing MPs to soluble CpG or Addavax, an analog of the FDA approved adjuvant MF59, in vivo. C57/Bl6 mice were immunized with a prime-boost schedule (Fig. 3A). Addavax induced the highest total Y2 HA-specific total IgG titers, while there was no significant difference in total IgG titers between any of the CpG-containing groups (Fig. 3B). Addavax elicited significantly lower IgG2c (Fig. 3C) and significantly higher IgG1 (Fig. 3D) titers than the CpG-containing groups, consistent with the tendency for oil-in-water emulsions to provoke a largely Th2-skewed immune response (Beljanski et al., 2015; Lederer et al., 2020; Mitchell et al., 2016; Stark et al., 2019), while CpG induced greater Th1 skew. No significant differences in antibody titer were observed between any of the CpG-containing groups, but the 60 CAC CpG group exhibited significantly greater anti-Y2 HA antibody-dependent phagocytosis, a measure of antibody effector function (Fig. 4A). This was consistent with the higher mean titer of IgG2C elicited by the 60 CAC CpG MPs, as IgG2 antibodies are generally associated with effector function in mice (Clynes et al., 1998; Nimmerjahn et al., 2005). The 60 CAC CpG MPs also exhibited a high degree of antibody-dependent complement deposition (ADCD) (Fig. 4B), although this phenomenon was not statistically significant.
Figure 3.

C57BL/6 mice were vaccinated intramuscularly on day 0 and 21 with PBS or Y2 COBRA HA (HA) with indicated adjuvant. Antibody endpoint titers were evaluated in day 28 serum for (B) total IgG, (C) IgG2C, and (D) IgG1 titers. Statistical significance is indicated with * P-value <0.05, ** P-value <0.005, *** P-value<0.001, and **** P-value <0.0001. Data is presented as average ± standard deviation.
Figure 4.

C57BL/6 mice (n=10) were vaccinated intramuscularly on day 0 and 21 with PBS or Y2 COBRA HA (HA) with indicated adjuvant and ADCP (A) and ADCD (B) were measured with day 28 sera. Data is presented as average ± standard deviation. Statistical significance is indicated with * P-value <0.05 between 15x dilutions.
Upon lethal challenge with the pandemic H1N1 influenza strain A/California/2009, all mice lost >10% of their total body weight, indicating significant infection and morbidity (Fig. 5A). This indicated that the immunity elicited by each vaccination was not sterilizing. None of the sera demonstrated detectable hemagglutination inhibition, indicating a poor capacity for neutralization (Fig. S4). However, the vaccinated mice still survived to a significantly greater extent than the unvaccinated, with nine of ten mice from the 60 CAC MP group, seven of ten from the 20 CAC MP and soluble CpG groups, and six out of ten from the Addavax-adjuvanted group surviving (Fig. 5B). While not statistically significant, the differences in these groups mirrored the differences in the effector function assays. Given the high level of antigen-binding titers in these groups and their poor neutralizing capacity, it is likely that protection this early in the course of vaccination was driven, at least in part, by their high degree of antibody effector function. While several studies have demonstrated that sustained delivery of antigen to the draining lymph node can promote affinity maturation and a greater neutralizing response (Cirelli et al., 2019; Roth et al., 2020; Tam et al., 2016), here we demonstrate that sustained adjuvant delivery may also be beneficial by promoting class switching and the development of greater effector functions of the humoral response, independent of induction of neutralizing antibodies. The benefits of this sustained release effect may not carry over to less stimulus-responsive adjuvant formulations. The increased adjuvanticity of the sustained release MPs may be driven in part by the passive targeting of the MP formulation to antigen-presenting cells such as dendritic cells, macrophages, and neutrophils, which preferentially take up particles in the 200 nm- ~5 um range. Thus, while the CpG in soluble or fast-degrading MP formulations can potentially diffuse away from the injection site to be metabolized or cause off-target inflammation, the sustained release formulation can release CpG on a very slow timescale until it is phagocytosed, where the acidic endosome environment will allow it to quickly release and promote proinflammatory signaling in the target cell.
Figure 5.
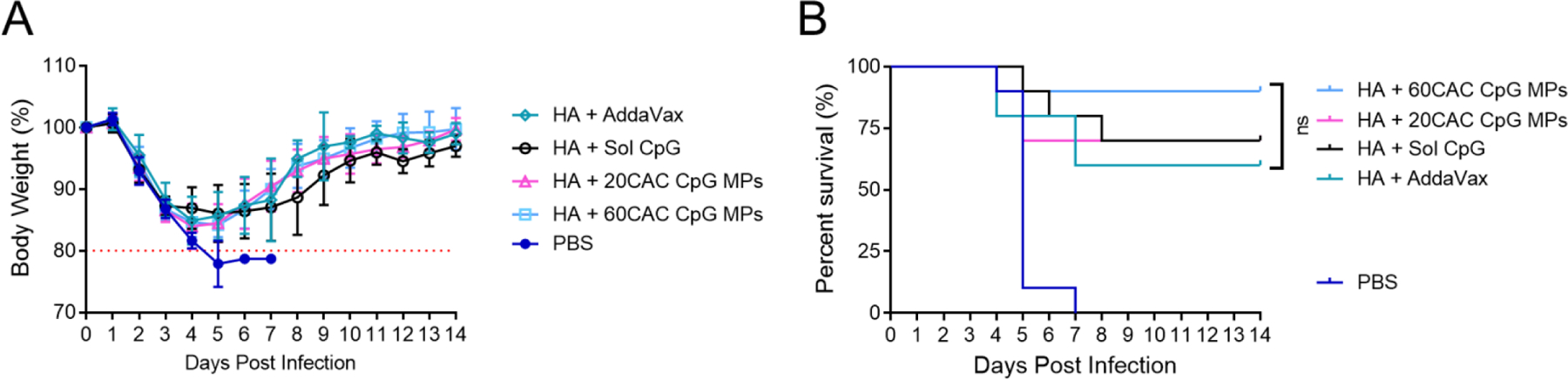
C57BL/6 mice (n=10) were vaccinated intramuscularly on day 0 and 21 with PBS or Y2 COBRA HA (HA) with indicated adjuvant and then challenged on day 35 with 600k pfu H1N1 A/California/2009 intranasally. (A) Body weight after challenge where a 20% loss of weight results in sacrifice of the infected mouse. Data is presented as average ± standard deviation. (B) Survival after challenge. Statistical comparison was performed with log-rank (Mantel-Cox) test with the Bonferroni correction for multiple comparisons.
Conclusion
We demonstrated that electrospraying is an efficient method for continuous encapsulation of the CpG oligonucleotide adjuvant into Ace-DEX MPs. The tunable degradation kinetics of Ace-DEX permitted variation of CpG release kinetics from less than one hour to greater than a month. We found that the sustained release form of CpG-loaded Ace-DEX MPs provoked the most potent inflammatory response in antigen-presenting cells in vitro. In an in vivo vaccination model, sustained release CpG induced similar levels of antigen-specific antibodies to soluble or rapidly-released CpG, but elicited significantly greater antibody effector functions that may serve an important role in the resolution of infection. Ongoing studies and future work will examine the adjuvant effect of 60 CAC CpG MPs over prime-only and longer vaccination schedule and compare sustained-release CpG MPs with other adjuvants formulated in Ace-DEX MPs for sustained release. We will also investigate the effects of formulating and co-administering sustained release forms of both COBRA antigen and adjuvant in Ace-DEX MPs, and investigate the role of T cells in the protective efficacy of sustained release CpG.
Supplementary Material
Supplemental Figures
Figure S1. (A) Pseudo-first order curve fit of 60 CAC MP release in pH 5.0 media. (B) Zero order linear fit of 60 CAC MP release in pH 7.4 media.
Figure S2. Nitric oxide production by (A) DC2.4 and (B) C57BL/6 bone-marrow derived dendritic cells (BMDCs) measured by Griess assay after culturing with CpG presented as soluble or encapsulated in Ace-DEX microparticles (MPs) for 48 hours. Data is presented as average ± standard deviation.
Figure S3. (A,B) Viability and (C,D) cytotoxicity for (A,C) DC2.4 cells and (B,D) C57BL/6 Bone-Marrow Derived Dendritic cells (BMDCs) when cultured with increasing concentrations of CpG in Ace-DEX Microparticles (MPs). Data is presented as average ± standard deviation.
Figure S4. C57BL/6 mice (n=10) were vaccinated intramuscularly on day 0 and 21 with PBS or Y2 COBRA HA (HA) with indicated adjuvant and hemagglutination inhibition titers against Influenza A/California/07/2009 were measured with day 28 sera. Data is presented as average ± standard deviation.
Acknowledgements
This work was performed in part at the Chapel Hill Analytical and Nanofabrication Laboratory, CHANL, a member of the North Carolina Research Triangle Nanotechnology Network, RTNN, which is supported by the National Science Foundation, Grant ECCS-2025064, as part of the National Nanotechnology Coordinated Infrastructure, NNCI. Funding for this work was supported by NIH NIAID Collaborative Influenza Vaccine Innovation Centers (CIVICs) Contract #75N93019C00052 (PI: Ross) and NIH R01AI147497 (PI: Ainslie).
Kristy Ainslie reports financial support was provided by National Institutes of Health. Ted Ross reports financial support was provided by National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
Credit Author Statement
Writing was primarily CJB. Data collection was CJB and EAA except HAI. HAI and COBRA protein was provided by MAC and TMR. EMB and KMA designed experiments with CJB and EAA. EMB and KMA edited. KMA funded the project.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- Allen JD, Ray S, Ross TM, 2018. Split inactivated COBRA vaccine elicits protective antibodies against H1N1 and H3N2 influenza viruses. PLoS One 13, e0204284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic G, van der Poel CE, Kuraoka M, Schmidt AG, Carroll MC, Kelsoe G, Harrison SC, 2019. Autoreactivity profiles of influenza hemagglutinin broadly neutralizing antibodies. Sci Rep 9, 3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty CJ, Gallovic MD, Williams J, Ross TM, Bachelder EM, Ainslie KM, 2022. Multiplexed electrospray enables high throughput production of cGAMP microparticles to serve as an adjuvant for a broadly acting influenza vaccine. Int J Pharm 622, 121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Chiang C, Kirchenbaum GA, Olagnier D, Bloom CE, Wong T, Haddad EK, Trautmann L, Ross TM, Hiscott J, 2015. Enhanced Influenza Virus-Like Particle Vaccination with a Structurally Optimized RIG-I Agonist as Adjuvant. J Virol 89, 10612–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ, 2016. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 16, 942–951. [DOI] [PubMed] [Google Scholar]
- Boudreau CM, Yu WH, Suscovich TJ, Talbot HK, Edwards KM, Alter G, 2020. Selective induction of antibody effector functional responses using MF59-adjuvanted vaccination. J Clin Invest 130, 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JM, 2009. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc Natl Acad Sci U S A 106, 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungener L, Geeraedts F, Ter Veer W, Medema J, Wilschut J, Huckriede A, 2008. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine 26, 2350–2359. [DOI] [PubMed] [Google Scholar]
- Butler AL, Fallon JK, Alter G, 2019. A Sample-Sparing Multiplexed ADCP Assay. Front Immunol 10, 1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro S, Tritto E, Pezzotti A, Taccone M, Muzzi A, Bertholet S, De Gregorio E, O’Hagan DT, Baudner B, Seubert A, 2013. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 31, 3363–3369. [DOI] [PubMed] [Google Scholar]
- Carter DM, Darby CA, Lefoley BC, Crevar CJ, Alefantis T, Oomen R, Anderson SF, Strugnell T, Cortes-Garcia G, Vogel TU, Parrington M, Kleanthous H, Ross TM, 2016. Design and Characterization of a Computationally Optimized Broadly Reactive Hemagglutinin Vaccine for H1N1 Influenza Viruses. J Virol 90, 4720–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Collier MA, Gallovic MD, Collins GC, Sanchez CC, Fernandes EQ, Bachelder EM, Ainslie KM, 2016. Degradation of acetalated dextran can be broadly tuned based on cyclic acetal coverage and molecular weight. Int J Pharm 512, 147–157. [DOI] [PubMed] [Google Scholar]
- Chen N, Gallovic MD, Tiet P, Ting JP, Ainslie KM, Bachelder EM, 2018. Investigation of tunable acetalated dextran microparticle platform to optimize M2e-based influenza vaccine efficacy. Journal of controlled release : official journal of the Controlled Release Society 289, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Peine K, Bachelder E, Ainslie K, 2015. Micro- and Nano-particulate Strategies for Antigen Specific Immune Tolerance to Treat Autoimmune Diseases. Pharmaceutical Nanotechnology 3, 85–100. [Google Scholar]
- Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, Miller MA, 2009. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 361, 674–679. [DOI] [PubMed] [Google Scholar]
- Cirelli KM, Carnathan DG, Nogal B, Martin JT, Rodriguez OL, Upadhyay AA, Enemuo CA, Gebru EH, Choe Y, Viviano F, Nakao C, Pauthner MG, Reiss S, Cottrell CA, Smith ML, Bastidas R, Gibson W, Wolabaugh AN, Melo MB, Cossette B, Kumar V, Patel NB, Tokatlian T, Menis S, Kulp DW, Burton DR, Murrell B, Schief WR, Bosinger SE, Ward AB, Watson CT, Silvestri G, Irvine DJ, Crotty S, 2019. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 177, 1153–1171 e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV, 1998. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci U S A 95, 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier MA, Junkins RD, Gallovic MD, Johnson BM, Johnson MM, Macintyre AN, Sempowski GD, Bachelder EM, Ting JP, Ainslie KM, 2018. Acetalated Dextran Microparticles for Codelivery of STING and TLR7/8 Agonists. Mol Pharm 15, 4933–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Cho KJ, Fiers W, Saelens X, 2015. M2e-Based Universal Influenza A Vaccines. Vaccines (Basel) 3, 105–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong AD, Sharma S, Peine KJ, Gupta G, Satoskar AR, Bachelder EM, Wyslouzil BE, Ainslie KM, 2013. Electrospray encapsulation of toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis. Mol Pharm 10, 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest JT, Basore K, Roy V, Bailey AL, Wang D, Alter G, Fremont DH, Diamond MS, 2019. Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J Exp Med 216, 2282–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA/CHMP/VWP/457259/, C.f.M.P.f.H.U.J.E.M.A., 2014. Guideline on influenza vaccines. Non-clinical and Clinical module 44, 1–31. [Google Scholar]
- Europe BN, 28 November 2014. Italy suspends Fluad flu vaccine from Novartis after deaths
- Gallovic MD, Junkins RD, Sandor AM, Pena ES, Sample CJ, Mason AK, Arwood LC, Sahm RA, Bachelder EM, Ainslie KM, Sempowski GD, Ting JP, 2022. STING agonist-containing microparticles improve seasonal influenza vaccine efficacy and durability in ferrets over standard adjuvant. Journal of controlled release : official journal of the Controlled Release Society 347, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatherer D, 2009. The 2009 H1N1 influenza outbreak in its historical context. J Clin Virol 45, 174–178. [DOI] [PubMed] [Google Scholar]
- Guthmiller JJ, Lan LY, Fernandez-Quintero ML, Han J, Utset HA, Bitar DJ, Hamel NJ, Stovicek O, Li L, Tepora M, Henry C, Neu KE, Dugan HL, Borowska MT, Chen YQ, Liu STH, Stamper CT, Zheng NY, Huang M, Palm AE, Garcia-Sastre A, Nachbagauer R, Palese P, Coughlan L, Krammer F, Ward AB, Liedl KR, Wilson PC, 2020. Polyreactive Broadly Neutralizing B cells Are Selected to Provide Defense against Pandemic Threat Influenza Viruses. Immunity 53, 1230–1244 e1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa LNM, Mai LQ, Bryant JE, Thai PQ, Hang NLK, Yen NTT, Duong TN, Thoang DD, Horby P, Werheim HFL, Fox A, 2016. Association between Hemagglutinin Stem-Reactive Antibodies and Influenza A/H1N1 Virus Infection during the 2009 Pandemic. J Virol 90, 6549–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Franca MS, Allen JD, Shi H, Ross TM, 2021a. Next Generation of Computationally Optimized Broadly Reactive HA Vaccines Elicited Cross-Reactive Immune Responses and Provided Protection against H1N1 Virus Infection. Vaccines (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Franca MS, Allen JD, Shi H, Ross TM, 2021b. Next Generation of Computationally Optimized Broadly Reactive HA Vaccines Elicited Cross-Reactive Immune Responses and Provided Protection against H1N1 Virus Infection. Vaccines (Basel) 9, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Owino SO, Crevar CJ, Carter DM, Ross TM, 2020. N-Linked Glycans and K147 Residue on Hemagglutinin Synergize To Elicit Broadly Reactive H1N1 Influenza Virus Antibodies. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, de Man M, Ding Z, Apetri A, Kukrer B, Sneekes-Vriese E, Tomkiewicz D, Laursen NS, Lee PS, Zakrzewska A, Dekking L, Tolboom J, Tettero L, van Meerten S, Yu W, Koudstaal W, Goudsmit J, Ward AB, Meijberg W, Wilson IA, Radosevic K, 2015. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science (New York, N.Y.) 349, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Junkins RD, Gallovic MD, Johnson BM, Collier MA, Watkins-Schulz R, Cheng N, David CN, McGee CE, Sempowski GD, Shterev I, McKinnon K, Bachelder EM, Ainslie KM, Ting JP, 2018. A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. Journal of controlled release : official journal of the Controlled Release Society 270, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JM, Hancock K, Xu XJE r.o.a.-i.t., 2011. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation 9, 669–683. [DOI] [PubMed] [Google Scholar]
- Kauffman KJ, Do C, Sharma S, Gallovic MD, Bachelder EM, Ainslie KM, 2012. Synthesis and characterization of acetalated dextran polymer and microparticles with ethanol as a degradation product. ACS Appl Mater Interfaces 4, 4149–4155. [DOI] [PubMed] [Google Scholar]
- Khurana S, Hahn M, Klenow L, Golding H, 2020. Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Lee SD, Lee E, Namkoong K, Choe KW, Song JY, Cheong HJ, Jeong HW, Heo JY, 2015. Incidence of narcolepsy before and after MF59-adjuvanted influenza A(H1N1)pdm09 vaccination in South Korean soldiers. Vaccine 33, 4868–4872. [DOI] [PubMed] [Google Scholar]
- Labombarde JG, Pillai MR, Wehenkel M, Lin CY, Keating R, Brown SA, Crawford JC, Brice DC, Castellaw AH, Mandarano AH, Guy CS, Mejia JR, Lewis CD, Chang TC, Oshansky CM, Wong SS, Webby RJ, Yan M, Li QZ, Marion TN, Thomas PG, McGargill MA, 2022. Induction of broadly reactive influenza antibodies increases susceptibility to autoimmunity. Cell Rep 38, 110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer K, Castano D, Gomez Atria D, Oguin TH 3rd, Wang S, Manzoni TB, Muramatsu H, Hogan MJ, Amanat F, Cherubin P, Lundgreen KA, Tam YK, Fan SHY, Eisenlohr LC, Maillard I, Weissman D, Bates P, Krammer F, Sempowski GD, Pardi N, Locci M, 2020. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 53, 1281–1295 e1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RA, Altszuler R, Frevert U, Nardin EH, 2016. Skin scarification with Plasmodium falciparum peptide vaccine using synthetic TLR agonists as adjuvants elicits malaria sporozoite neutralizing immunity. Sci Rep 6, 32575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB, 2007. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25, 5086–5096. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV, 2005. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23, 41–51. [DOI] [PubMed] [Google Scholar]
- Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsen P, Saarenpaa-Heikkila O, Kilpi T, 2012. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One 7, e33536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Barr IG, Mosse JA, Laurie KLJJ o.c.m., 2008. MDCK-SIAT1 cells show improved isolation rates for recent human influenza viruses compared to conventional MDCK cells 46, 2189–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH, 2011. WHO global influenza surveillance network: manual for the laboratory diagnosis and virological surveillance of influenza, Switzerland. [Google Scholar]
- Peine KJ, Bachelder EM, Vangundy Z, Papenfuss T, Brackman DJ, Gallovic MD, Schully K, Pesce J, Keane-Myers A, Ainslie KM, 2013. Efficient Delivery of the Toll-like Receptor Agonists Polyinosinic:Polycytidylic Acid and CpG to Macrophages by Acetalated Dextran Microparticles. Molecular Pharmaceutics 10, 2849–2857. [DOI] [PubMed] [Google Scholar]
- Roth GA, Gale EC, Alcantara-Hernandez M, Luo W, Axpe E, Verma R, Yin Q, Yu AC, Lopez Hernandez H, Maikawa CL, Smith AAA, Davis MM, Pulendran B, Idoyaga J, Appel EA, 2020. Injectable Hydrogels for Sustained Codelivery of Subunit Vaccines Enhance Humoral Immunity. ACS Cent Sci 6, 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautto GA, Kirchenbaum GA, Abreu RB, Ecker JW, Pierce SR, Kleanthous H, Ross TM, 2020. A Computationally Optimized Broadly Reactive Antigen Subtype-Specific Influenza Vaccine Strategy Elicits Unique Potent Broadly Neutralizing Antibodies against Hemagglutinin. J Immunol 204, 375–385. [DOI] [PubMed] [Google Scholar]
- Sautto GA, Kirchenbaum GA, Ross TM, 2018. Towards a universal influenza vaccine: different approaches for one goal. Virol J 15, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark FC, Akache B, Ponce A, Dudani R, Deschatelets L, Jia Y, Sauvageau J, Williams D, Jamshidi MP, Agbayani G, Wachholz K, Harrison BA, Li X, Krishnan L, Chen W, McCluskie MJ, 2019. Archaeal glycolipid adjuvanted vaccines induce strong influenza-specific immune responses through direct immunization in young and aged mice or through passive maternal immunization. Vaccine 37, 7108–7116. [DOI] [PubMed] [Google Scholar]
- Steipel RT, Gallovic MD, Batty CJ, Bachelder EM, Ainslie KM, 2019. Electrospray for generation of drug delivery and vaccine particles applied in vitro and in vivo. Mater Sci Eng C Mater Biol Appl 105, 110070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiepel RT, Pena ES, Ehrenzeller SA, Gallovic MD, Lifshits LM, Genito CJ, Bachelder EM, Ainslie KM, 2022. A predictive mechanistic model of drug release from surface eroding polymeric nanoparticles. Journal of controlled release : official journal of the Controlled Release Society 351, 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ, 2016. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci U S A 113, E6639–E6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Ziemer KS, Gill HS, 2014. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (Lond) 9, 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsybalova LM, Stepanova LA, Shuklina MA, Mardanova ES, Kotlyarov RY, Potapchuk MV, Petrov SA, Blokhina EA, Ravin NV, 2018. Combination of M2e peptide with stalk HA epitopes of influenza A virus enhances protective properties of recombinant vaccine. PLoS One 13, e0201429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari A, Schmalbach T, Lekstrom-Himes J, Morris M, Al-Adhami M, Laframboise C, Leese P, Krieg A, Efler S, Davis H, 2007. Safety, pharmacokinetics and immune effects in normal volunteers of CPG 10101 (ACTILON), an investigational synthetic Toll-like receptor 9 agonist. Antiviral therapy 12, 741–751. [PubMed] [Google Scholar]
- Watkins-Schulz R, Tiet P, Gallovic MD, Junkins RD, Batty C, Bachelder EM, Ainslie KM, Ting JPY, 2019. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8(+) T cell-mediated anti-tumor immunity. Biomaterials 205, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelek WM, Harris CL, Morgan BP, 2018. Extracting the barbs from complement assays: Identification and optimisation of a safe substitute for traditional buffers. Immunobiology 223, 744–749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures
Figure S1. (A) Pseudo-first order curve fit of 60 CAC MP release in pH 5.0 media. (B) Zero order linear fit of 60 CAC MP release in pH 7.4 media.
Figure S2. Nitric oxide production by (A) DC2.4 and (B) C57BL/6 bone-marrow derived dendritic cells (BMDCs) measured by Griess assay after culturing with CpG presented as soluble or encapsulated in Ace-DEX microparticles (MPs) for 48 hours. Data is presented as average ± standard deviation.
Figure S3. (A,B) Viability and (C,D) cytotoxicity for (A,C) DC2.4 cells and (B,D) C57BL/6 Bone-Marrow Derived Dendritic cells (BMDCs) when cultured with increasing concentrations of CpG in Ace-DEX Microparticles (MPs). Data is presented as average ± standard deviation.
Figure S4. C57BL/6 mice (n=10) were vaccinated intramuscularly on day 0 and 21 with PBS or Y2 COBRA HA (HA) with indicated adjuvant and hemagglutination inhibition titers against Influenza A/California/07/2009 were measured with day 28 sera. Data is presented as average ± standard deviation.


