Abstract
It has been demonstrated that after two doses, SARS-CoV-2 mRNA vaccine-induced neutralizing antibodies against Omicron subvariants are much lower than against wild type virus and a booster dose greatly increases Omicron neutralization. We compared Spike-binding IgG responses against wild type virus and four SARS-CoV-2 Omicron subvariants in infection-naïve and previously-infected (hybrid immunity) individuals after the second and the third (booster) dose of BNT162b2. In both groups of individuals, antibodies for all four Omicron subvariants were lower than wild type antibodies. Compared to infection-naïve individuals, hybrid immunity resulted in higher antibodies levels after 2 doses of vaccine but not after the booster. In both groups, antibodies for wild type and all Omicron subvariants waned over an 8-month period post second dose but rebounded after the booster. These results underscore the importance of boosters to restore diminishing antibody levels for both infection-naïve and previously-infected individuals.
Keywords: SARS-CoV-2, Vaccine, Booster, Omicron, Antibodies, Hybrid Immunity
Abbreviations: nAb, neutralizing anitbody; WT, wild type; RBD, receptor binding domain
1. Introduction
With the emergence of SARS-CoV-2 variants, vaccine efficacy decreased. Booster doses appear to mitigate decreased efficacy against variants. Omicron BA.1 Neutralizing antibody (nAb) titers are 35-fold lower than D614G nAb after 2 doses of mRNA-1273 vaccine but after a booster dose, increase 20-fold above post-2nd dose Omicron titers [1]. Two doses of BNT162b2 provide limited protection against Omicron infections but a booster dose substantially increases protection [2]. Vaccination induces antibodies with higher and longer lasting nAb titers against Omicron in previously-infected (hybrid immunity) compared to infection-naïve individuals [3]. Hybrid immunity is superior to vaccination alone in preventing symptomatic Omicron infections [4]. To evaluate the effects of a booster vaccine dose on hybrid immunity to Omicron subvariants, we measured Spike-binding IgG (S IgG) against Omicron BA.2, BA.3, BA.4 and BA.5 after the second and the third (booster) dose of BNT162b2 in infection-naïve and previously-infected individuals.
2. Methods
2.1. Regulatory approval and study subjects
This study was approved by the Rush University institutional review board. All participants provided written informed consent. From our previous longitudinal study [5] of SARS-CoV-2 vaccine-induced receptor binding domain (RBD) IgG responses in healthcare workers (HCW), we identified a group of 78 individuals infected with SARS-CoV-2 prior to the first dose of vaccine and matched them for age and sex with 77 infection-naïve individuals. We tested available stored plasma from these 155 individuals collected at 1 month (March-May 2021), 8 months (September-November 2021) and 11 months (December 2021-February 2022) after the 2nd vaccine dose for S IgG against Wuhan (WT) and 4 Omicron subvariants (BA.2, BA.3, BA.4, BA.5).
Previously-infected individuals had a history of a positive PCR test and/or positive nucleocapsid (N) IgG prior to the first dose of vaccine; 71 had positive PCR tests between March 2020 and January 2021, when D614G was predominant. Seven individuals had positive N IgG detected (with histories of negative PCR results or no PCR testing) when they participated in SARS-CoV-2 antibody seroconversion research studies between April and November 2020. The infection-naïve individuals had no history of positive PCR, RBD IgG, or N IgG tests prior to the first dose of vaccine; and had negative N IgG at 1 month after the second dose. In the previously-infected group, the mean (±SD) age was 42 ± 13 (range = 22 to 71) with 64/78 (82 %) women and 60 (77 %) white. In the infection-naïve group, the mean age was 42 ± 13 (range = 24 to 68) with 62/77 (81 %) women and 61 (78 %) white.
2.2. RBD IgG assay
RBD (D614G) IgG was measured on an Abbott ARCHITECT i2000SR as described in our previously published study [5]. Results are reported in arbitrary units (AU) per milliliter, with values ≥ 50 AU/mL considered positive.
2.3. Spike-Binding IgG assays
Spike-binding IgG (S IgG) was measured with a validated assay using commercial plates manufactured by Meso Scale Discovery (Rockville, Maryland) that detect SARS-CoV-2 prefusion stabilized Spike protein of variants including wild type (WT), and Omicron subvariants BA.2, BA.3, BA.4, and BA.5. Results were analyzed using MSD Discovery Workbench 4.0.12, with values ≥ 1960 AU/mL considered positive.
2.4. Statistical analysis
Statistical analysis was conducted with Prism Version 9.2.0 (GraphPad Software, Inc, CA) using 2-tailed nonparametric Mann-Whitney test for comparison between two groups. A p-value < 0.05 is considered significant.
3. Results
By 11 months after the 2nd vaccine dose, 33 (42/3%) infection-naïve and 28 previously-infected (36.4 %) individuals had received a booster. In both the infection-naïve and the previously-infected group, a majority of individuals (75.7 % and 75.0 %, respectively) who received boosters had done so between the 8-month and 11-month blood draws, while the others received their boosters between the 5-month and 8-month timeframe. We did not test S IgG levels in individuals who did not receive a booster. Stored plasma samples available for testing decreased over time due to participant dropout in our previous longitudinal study [5]; and participants not having received boosters by 11 months. Between 5 and 8 months post 2nd vaccine dose, 1 infection-naïve individual and no previously-infected individuals had a breakthrough infection. Between 8 months and 11 months, 1 infection-naïve individual and 3 previously-infected individuals had a breakthrough infection.
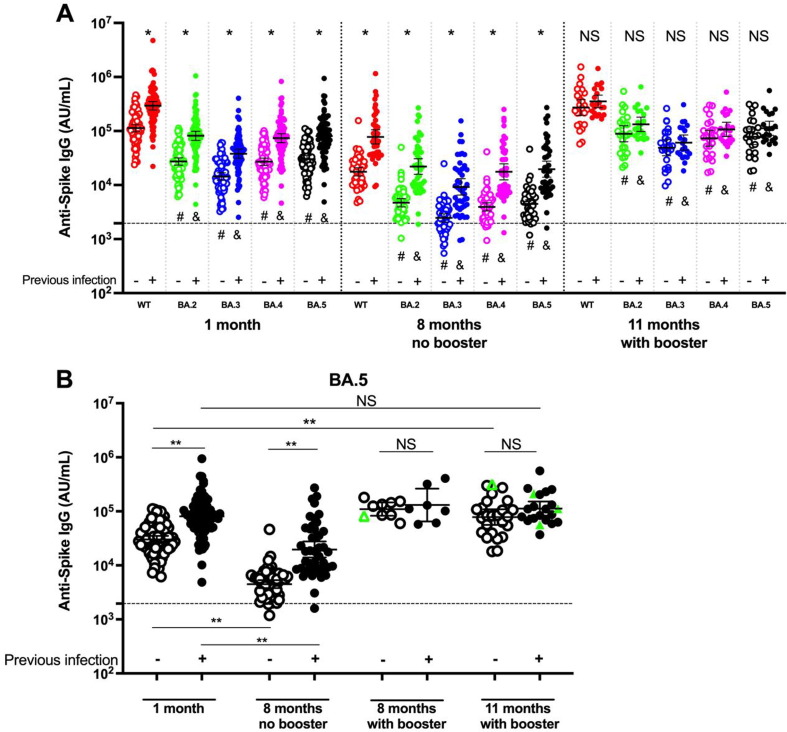
Our previous longitudinal study [5] found that RBD IgG levels had declined from 1 month to 5 months after the 2nd vaccine dose, were lowest at 8 months, and increased after a 3rd dose. Therefore, we measured Omicron variant-specific S IgG at peak (1 month) and trough (8 months) levels prior to booster, and after booster (11 months). Fig. 1 A (individuals boosted prior to 8 months or infected at any time between 1 month and 11 months are not included in panel A) shows that S IgG against all Omicron subvariants were lower (all p < 0.001) than WT (red circles) at all time points in both the infection-naïve and previously-infected groups; with BA.3 S IgG (blue) being the lowest (-5.5 to −8.5-fold) and BA.4 (purple) and BA.5 (black) the second lowest (-3.1 to −4.5-fold). For all subvariants, while antibody levels were higher (all p < 0.001) in the previously-infected (solid circles) group compared to the infection-naïve group (open circles), prior to booster (1 month and 8 months), there was no difference between the two groups after the booster (11 months).
Fig. 1.
Spike (S) IgG against SARS-CoV-2 Omicron subvariants and WT in infection-naïve and previously-infected individuals at 1, 8 and 11 months after 2 doses or 3 doses of BNT162b2. A: Individuals with breakthrough infections or who received boosters prior to 8 months are not included in this panel. The data at 11 months consist only of individuals who received their booster between 8 and 11 months. S IgG was higher in the previously-infected (solid circles) group than in the infection-naïve (open circles) for all variants at 1 month and 8 months without booster but not at 11 months with booster. * p < 0.001 between infection-naïve and previously infected; NS = not significant between infection-naïve and previously-infected. All Omicron subvariant S IgG levels were lower than WT at all 3 time points. & p < 0.001 between WT (red circles) and each Omicron subvariant in the infection-naïve group. # p < 0.001 between WT and each Omicron subvariant in the previously-infected group. Horizontal black bars correspond to the geometric mean and the error bars indicate the 95 % CI. Horizontal dashed line denotes the assay limit of detection. The 2-tailed nonparametric Mann-Whitney test was used for comparison between two groups. A p value < 0.05 is considered significant. AU, arbitrary unit.B: BA.5 S IgG are plotted with the 8-month data separated into the group that had not (8 months no booster) or had (8 months with booster) received a booster prior to 8 months. We did not test plasma samples for S IgG from individuals who were not boosted by 11 months. Plasma samples available for testing decreased during this study due to participant dropout and participants not having received boosters by 11 months. Open symbols: infection-naïve; Closed symbols: previously-infected; Black circles: individuals without breakthrough infections; Green triangles: breakthrough infections. Between 5 and 8 months post 2nd vaccine dose, 8 infection-naïve individuals were boosted and 1 had a breakthrough infection (open green triangle); and 7 previously-infected individuals were boosted. Between 8 months and 11 months, 25 infection-naïve individuals were boosted and 1 had a breakthrough infection (open green triangle); and 21 previously-infected individuals were boosted and 3 had a breakthrough infection (solid green triangles). ** p < 0.001; NS = not significant. The 2-tailed nonparametric Mann-Whitney test was used for comparison between two groups. A p value < 0.05 is considered significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 1B shows BA.5 (the dominant variant at the time of this study) antibody kinetics before and after booster and is representative of results for the other subvariants. In both groups, BA.5 S IgG decreased from 1 month to 8 months without booster. After a booster, S IgG increased above 8-month levels in both groups. Whereas the booster increased levels above 1-month levels in the infection-naïve group (open circles), there was no significant increase beyond 1-month levels in the previously-infected group (solid circles). In the infection-naïve group, the geometric mean (95 % CI) of BA.5 S IgG at 1 month was 30,154 (25767–35288) AU/mL, decreased 6.72-fold at 8 months (p < 0.001), and after a booster (11 months) had a 2.24-fold increase vs 1 month (p < 0.001). In the previously-infected group, BA.5 S IgG was 80,834 (66760–97876) AU/mL at 1 month, decreased 4.13-fold at 8 months (p < 0.001), and after a booster (11 months) did not increase (1.40-fold) significantly vs 1 month. Regardless of booster timing, post-booster antibody levels seemed to reach similar levels. The boosted infection-naïve cohort S IgG levels were 114,231 (83577–156128) at 8 months-with booster, and 73,876 (53486–102037) at 11 months (p = 0.07). The boosted previously-infected cohort S IgG levels were 131,047 (65066–263937) at 8 months-with booster and 113,288 (80839–158761) at 11 months (p = 0.74). The individuals with breakthrough infections (green triangles) had similar antibody levels compared to the boosted levels.
4. Discussion
The Omicron subvariants BA.1/BA.2 and BA.4/BA.5 were responsible for 2 COVID-19 surges worldwide. By July 2022, BA.5 accounted for 78 % of cases in the United States and as of mid-October 2022 was still the predominant subvariant [6], [7]. It has been shown that BA.2.12.1, BA.4, and BA.5 substantially escape vaccine-induced nAb [8]. Our study is the first to compare vaccine-induced binding antibodies among 4 Omicron subvariants. Our results showing that vaccine-induced S IgG against all Omicron subvariants were significantly lower than WT S IgG parallels previous reports that vaccine-induced Omicron nAb are much lower than WT (D614G) nAb [8], [9]. This immune escape by Omicron might be one factor that explains the surge in infections with the Omicron BA.1, BA.2, BA.4 and BA.5 subvariants.
Interestingly, we found that S IgG against Omicron BA.3 was the lowest of the Omicron subvariants we tested. Whereas the BA.1, BA.2, BA.4 and BA.5 subvariants resulted in infection surges, BA.3 never became a widely-spreading subvariant. One reason for this discrepancy might be that we measured S IgG and BA.3 S IgG were lower because most of the mutations in BA.3 are in the spike protein and not the RBD [10]. It is possible that reduced numbers of mutations in the RBD of BA.3 resulted in BA.3 having reduced infectivity compared to the other Omicron subvariants [10].
We found that 8 months after vaccination, all Omicron S IgG levels were significantly lower (approximately 7-fold for BA.5) than at 1 month. After a booster, antibody levels were 2-fold higher than at 1 month in the infection-naïve group and equivalent to 1-month levels in the previously-infected group. Two doses of SARS-CoV-2 vaccine provide limited protection against Omicron infections but a booster dose substantially increases protection [2]. Additionally, BNT162b2 effectiveness against Omicron infections is highest in the first 3 months after the second dose, declines to 10 % or below thereafter, and rebounds after a booster to the same effectiveness seen in the first 3 months [11]. Our S IgG findings that antibody levels increase after a booster are aligned with the above clinical findings, and suggest that a rise in antibody levels after a booster dose could be related to the rebound in vaccine efficacy.
Our data demonstrate that prior infection and two doses of vaccine led to a more robust production of Omicron S IgG compared to 2 doses of the vaccine alone in infection-naïve individuals and is consistent with previous reports showing that prior infection confers increased neutralization to Omicron compared to infection-naïve individuals [3]. It has been shown that after receiving a third dose of vaccine, hybrid immunity provided better protection against Omicron BA.2 symptomatic infection compared to vaccine-induced immunity alone [4]. For all subvariants tested, we did not find that previously-infected individuals had significantly higher S IgG compared to the infection-naive cohort after receiving a booster. The lack of significantly higher S IgG in the previously-infected group may be a result of our small sample size or suggest that neutralizing antibodies, cellular immunity, or other factors might be the reasons that, clinically, hybrid immunity provides superior protection against symptomatic infections.
Our study was limited by small sample size and lack of demographic diversity of the participants (mostly white and female HCW) and therefore may not represent the general population. The small sample size did not allow us to find a difference in breakthrough infections between the previously-infected and infection-naïve groups. Although our antibody assays were binding assays and we did not perform neutralizing antibody assays, both binding antibodies and nAb have been shown to be good correlates of protection against SARS-CoV-2 infection [12]. Despite these limitations, our study supports published reports that vaccine-induced antibody activity wanes over time and reinforces the beneficial effects of booster doses in reversing falling antibody levels in both infection-naïve and previously-infected individuals against Omicron variants. For future SARS-CoV-2 variants of concern, variant-specific antibody studies will need to be performed to determine the humoral response to booster doses with the bivalent vaccines.
Funding
This work was partly funded by Abbott Diagnostics Division Research and Development funding. Spike-binding IgG (S IgG) assay plates were supplied by Meso Scale Discovery (Rockville, Maryland). The funders played no role in the design, analysis, or reporting of this research.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors M.A., M.S., and G.C. are employees and shareholders of Abbott Laboratories. A.L. has received consulting fees from Abbott Laboratories. The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O'Dell S., McDanal C., et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N Engl J Med. 2022 Mar 17;386(11):1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N., Stowe J., Kirsebom F., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S.Y., Park S., Kim J.Y., Kim S., Jee Y., Kim S.H. Comparison of Waning Neutralizing Antibody Responses Against the Omicron Variant 6 Months After Natural SARS-CoV-2 Infection (With/Without subsequent COVID-19 Vaccination) Versus 2-dose COVID-19 Vaccination. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac435. Jun 10:ciac435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N Engl J Med. 2022 Jul 7;387(1):21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson M, Stec M, Gosha A, Mohammad T, Boler M, Suarez RT, Behun D, Landay A, Cloherty G, Moy J. Longitudinal SARS-CoV-2 Vaccine Antibody Responses and Identification of Vaccine Breakthrough Infections Among Healthcare Workers Using Nucleocapsid IgG. J Infect Dis. 2022 Oct 20:jiac420. [DOI] [PMC free article] [PubMed]
- 6.https://www.rockefellerfoundation.org/case-study/variants-sublineages-and-recombinants-the-constantly-changing-genome-of-sars-cov-2/.
- 7.Mahase E. Covid-19: What we know about the BA.4 and BA.5 omicron variants. BMJ. 2022 Aug;9(378) doi: 10.1136/bmj.o1969. [DOI] [PubMed] [Google Scholar]
- 8.Hachmann NP, Miller J, Collier AY, Ventura JD, Yu J, Rowe M, Bondzie EA, Powers O, Surve N, Hall K, Barouch DH. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022 Jul 7;387(1):86-88. [DOI] [PMC free article] [PubMed]
- 9.Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022 Feb;602(7898):654-656. [DOI] [PMC free article] [PubMed]
- 10.Desingu P.A., Nagarajan K., Dhama K. Emergence of Omicron third lineage BA.3 and its importance. J Med Virol. 2022 May;94(5):1808–1810. doi: 10.1002/jmv.27601. Epub 2022 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun 2022;13:3082-3082. [DOI] [PMC free article] [PubMed]
- 12.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Team§; Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE) Team§; United States Government (USG)/CoVPN Biostatistics Team§. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022 Jan 7;375(6576):43-50. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.