Abstract
Perfluorooctanoic acid (PFOA) is an environmentally persistent perfluoroalkyl substance that is widely used in consumer products. Exposure to PFOA is associated with reproductive and developmental effects including endocrine disruption, delayed puberty in girls, and decreased fetal growth. In the United States, obesity affects 40% of women and 20% of girls, with higher rates in minority females. Obesity causes infertility, poor oocyte quality, miscarriage, and offspring defects. This study proposed that PFOA exposure would impact estrous cyclicity, ovarian steroid hormones, and the ovarian proteome and further hypothesized that obesity would impact PFOA-induced ovotoxicity. Female wild type (KK.Cg-a/a; lean) or KK.Cg-Ay/J mice (obese) received saline (CT) or PFOA (2.5 mg/kg) per os for 15 days beginning at 7 weeks of age. There were no effects on food intake, body weight, estrous cyclicity, serum progesterone, and heart, spleen, kidney, or uterus weight (p > .05). Ovary weight was decreased (p < .05) by PFOA exposure relative to vehicle control-treated mice in lean but not obese mice. Liquid chromatography-tandem mass spectrometry was performed on isolated ovarian protein and PFOA exposure altered the ovarian abundance of proteins involved in DNA damage sensing and repair pathways and reproduction pathways (p < .05) differentially in lean and obese mice. The data suggest that PFOA exposure alters ovary weight and differentially targets ovarian proteins in lean and obese females in ways that might reduce female fecundity.
Keywords: PFOA, obesity, ovary, DNA damage sensing and repair, reproduction, ovarian proteome
The female reproductive system performs important functions that support fertilization and pregnancy (Hoyer, 2010). The ovary has 2 major roles: production and secretion of 17β-estradiol (E2) and progesterone (P4), and development of oocytes (Hirshfield, 1991). Females are born with a finite number of oocytes (Hirshfield, 1991) that are ovulated or undergo atresia (Fortune et al., 2000). However, several factors including exposure to xenobiotics (Hoyer, 2005; Keating and Hoyer, 2009; Mattison, 1985) can affect proper ovarian functioning causing follicle loss (Keating et al., 2009; Klenov et al., 2021; Madden et al., 2014), DNA damage (Clark and Keating, 2020; Ganesan and Keating, 2016; Winship et al., 2020), and endocrine disruption (Hoyer, 2005) leading to temporary or permanent infertility (Hoyer and Keating, 2014).
The prevalence of obesity among adults in the United States is approximately 42% (Hales et al., 2020) with approximately 40% of women over 20 years and 20% of girls being obese (Hales et al., 2017, 2020). Minority women are affected by obesity at higher rates (Hales et al., 2020). Obesity is now commonplace in developed countries and has increased also in developing countries (OECD, 2017; Rössner, 2002; WHO, 2021). Obesity can lead to chronic diseases including cardiovascular disease (Rössner, 2002), type 2 diabetes (Rössner, 2002), dyslipidemia (Rössner, 2002), and hypertension (Rössner, 2002). In women, obesity has negative reproductive effects (Aune et al., 2014; Chu et al., 2007; Grodstein et al., 1994; Jungheim et al., 2010; McDonald et al., 2010; Pasquali et al., 2003; Pasquali and Casimirri, 1993; Rich-Edwards et al., 1994; Smith et al., 2007; Stothard et al., 2009; Watkins et al., 2003) including decreased fecundity (Grodstein et al., 1994; Rich-Edwards et al., 1994), poor oocyte quality (Jungheim et al., 2010), gestational diabetes (Chu et al., 2007), increased risk of birth defects (Stothard et al., 2009; Watkins et al., 2003), premature (McDonald et al., 2010; Smith et al., 2007), and still births (Aune et al., 2014) and is associated with polycystic ovarian syndrome (Pasquali and Casimirri, 1993). Obesity induces oxidative DNA damage and oxidative stress (Ganesan et al., 2017), induces basal DNA damage (Ganesan et al., 2014, 2017), alters phosphatidylinositol-3 kinase (PI3K) signaling (Nteeba et al., 2013, 2017), impairs the response of ovarian chemical metabolism proteins (Nteeba et al., 2014a, 2017), and depletes primordial follicles (Ganesan et al., 2017; Nteeba et al., 2014a,b). These findings suggest that the ovaries of obese females may be more sensitive to reproductive toxicants.
When DNA damage is detected, several mechanisms involved in the DNA damage repair (DDR) response are activated to prevent the DNA damage to be passed to daughter cells (Friedberg, 2003; Zhou and Zheng, 2013). Mammalian DNA repair pathways include base-excision repair (BER), homologous recombination (HR), nucleotide-excision repair (NER), and nonhomologous end joining (NHEJ) (Hoeijmakers, 2001). Single-strand DNA breaks (SSBs) are repaired by NER and BER mechanisms (Hoeijmakers, 2001). Transcription and normal replication can be affected mostly by exogenous sources that lead to helix-distorting lesions that are targeted and repaired by NER mechanisms (Hoeijmakers, 2001). Methylation, reactive oxygen species, hydroxylation, deamination, and chemical alterations of bases endogenously induced that alter transcription and replication are repaired by BER pathways (Hoeijmakers, 2001). Double-strand breaks (DSBs) are repaired by HR and NHEJ pathways (Hoeijmakers, 2001). When DNA replication is occurring to align the breaks in S and G2 phases, HR provides a second copy of the sequence, thus repairing the damage (Hoeijmakers, 2001). If a second copy is not available, NHEJ pathway intervenes on the G1 phase of the cell cycle to repair the damage (Hoeijmakers, 2001). DNA damage has been associated with aging (Finkel and Holbrook, 2000), genetic disorders (Finkel and Holbrook, 2000), and cancer (Hoeijmakers, 2001).
Per- and poly-fluoroalkylated substances (PFAS) are characterized by having strong bonds between carbon and fluorine groups (EFSA, 2008; Lau, 2012; USEPA, 2017) which confer both thermal and chemical stability (Bell et al., 2021) making PFAS resistant to degradation (EFSA, 2008) and persistent in the environment (Lau, 2012; ATSDR, 2021). Humans are exposed though ingestion, inhalation, and through dermal exposure (Post et al., 2012). Perfluorooctanoic acid (PFOA) is a fluorinated organic acid PFAS member found in consumer goods including nonstick cookware, water and stain-resistant carpet and fabric coatings, floor polish, fire-fighting foam, lubricants, and food packaging (EFSA, 2008; Post et al., 2012). Exposure to PFOA has been associated with hepatoxicity (EFSA, 2008; Post et al., 2012; ATSDR, 2021), carcinogenicity (Chang et al., 2014), developmental toxicity (EFSA, 2008; Post et al., 2012; ATSDR, 2021), and reproductive toxicity (EFSA, 2008; Post et al., 2012; ATSDR, 2021). In females, exposure to PFOA has been linked to delayed puberty in girls (Lopez-Espinosa et al., 2011), early menopause (Knox et al., 2011), endocrine disruption (Jensen and Leffers, 2008; Knox et al., 2011; Yang et al., 2022), reduced fertility (Fei et al., 2009; Vélez et al., 2015), and premature ovarian insufficiency (Zhang et al., 2018). It has been detected in follicular fluid of women (Heffernan et al., 2018) and is associated with decreased fetal growth (Gyllenhammar et al., 2018) and reduced childhood growth (Andersen et al., 2010). In mice, delayed puberty (Lau, 2012), changes in the female reproductive tract (Dixon et al., 2012), altered levels of steroid hormones (Chen et al., 2017; Lau, 2012), follicle loss (Yang et al., 2022), and reduced number and size of corpora lutea (Chen et al., 2017) have been observed due to exposure to PFOA.
Despite evidence indicating that PFOA causes reproductive toxicity, the mechanisms are not well understood. The purpose of this study was to investigate mechanisms of PFOA-induced ovarian toxicity including alterations to the levels of E2 and P4, and changes to the ovarian abundance of proteins involved in reproduction, and DNA damage sensing and repair. Additionally, an obese group of mice were included to ascertain if obesity would impact the ovarian impacts of PFOA exposure.
MATERIALS AND METHODS
Reagents
PFOA (CAS no. 335-67-1), phosphate-buffered saline (PBS), tris-HCl, tris-buffered saline (TBS), and nonfat dry milk were purchased from Sigma-Aldrich Inc. (St Louis, Missouri). Pierce bicinchoninic acid assay (BCA) Protein Assay Kit was obtained from Thermo Fisher Scientific (Rockford, Illinois). Glycine and Tween 20 were obtained from Fisher Bioreagents (Fair Lawn, New Jersey). E2 and P4 ELISA kits were purchased from DRG International, Inc. (Springfield, New Jersey).
Animal exposure
The Iowa State University Institutional Animal Care and Use Committee approved all the animal protocols for the study. Female wild-type normal non-agouti KK.Cg-a/a mice, designated lean hereafter (n = 19), and agouti lethal yellow KK.Cg-Ay/J mice, designated obese (n = 20), were obtained from Jackson Laboratories (Bar Harbor, Maine) at 5 weeks of age. Under identical controlled conditions: a light cycle of 12 h light/12 h darkness, temperature between 21°C and 22°C, and 20%–30% humidity, mice were housed in Innovive cages with 2 or 3 animals per cage. The mice were fed 2014 Teklad Global 14% Protein Rodent Diet and water ad libitum. Body weight gain and food intake were monitored twice per week. Food intake was calculated as food disappearance per cage/number of mice per cage. After 2 weeks of acclimation, at 7 weeks of age, mice were dosed with either saline solution as vehicle control (CT) or PFOA (2.5 mg/kg; 2.5 ppm) per os from a pipette tip for 15 days. There were 4 treatment groups—lean mice treated with vehicle control (LC); lean mice exposed to PFOA (LP); obese mice treated with vehicle control (OC), and obese mice exposed to PFOA (OP). The dose chosen was intermediate for the lowest observable adverse effect level (LOAEL) noted for PFOA in the testis (5 mg/kg; Li et al., 2011) and the liver (1 mg/kg; Loveless et al., 2008). The age of 7 weeks was chosen to avoid the decline in primordial and primary follicles in the obese mice which occurs from 12 weeks onwards (Ganesan et al., 2017; Nteeba et al., 2014a,b).
Monitoring of the estrous cycle
For 14 days, estrous cyclicity was monitored by performing vaginal cytology. The vagina was gently flushed with saline 3–5 times, using a sterile pipette tip (Caligioni, 2009). The final flush was collected and observed under the microscope (Caligioni, 2009). In the proestrus stage, most cells are nucleated epithelial, but some cornified and leucocytes are present (Byers et al., 2012; Caligioni, 2009). In the estrus stage, large cornified cells with irregular shape and no visible nucleus appear (Byers et al., 2012; Caligioni, 2009). During the metestrus stage, predominantly leukocytes appear, but some cornified epithelial and less nucleated epithelial cells are also apparent (Byers et al., 2012; Caligioni, 2009). Mostly polymorphonuclear leukocytes appear during the diestrus stage with few nucleated epithelial cells (Byers et al., 2012; Caligioni, 2009). Because of the similarity in cytology for the metestrus and diestrus stage, these 2 stages were combined. Statistical analysis was performed on the raw data and for the purpose of visualization, the percentage of time spent at each stage was calculated by the number of days per stage/14 days × 100.
Tissue collection
Mice were euthanized by CO2 asphyxiation followed by cervical dislocation on the second day of diestrus stage of the estrous cycle and tissues were collected. Cardiac puncture was performed to collect blood samples. The heart, spleen, kidneys, uterus, and ovaries were collected and weighed after trimming each of any excess fat. One ovary was flash frozen in liquid nitrogen and stored at −80°C for protein analysis.
Serum E2 and P4 hormone level quantification
Blood samples were centrifuged for 15 min at 10 621 rcf and 4°C, followed by discarding blood cells. Serum E2 (DRG Estradiol ELISA; EIA-2693; CV = 35.2) and P4 (DRG Progesterone ELISA; EIA-1561; CV = 27.8) were quantified by ELISA following the manufacturer’s instructions with 2 technical replicates per sample. For E2 quantification, there was sufficient serum for 39 samples (LC = 10; LP = 9; OC = 10; OP = 10) and adequate serum for measurement of 36 samples for P4 (LC = 10; LP = 9; OC = 9; OP = 8). Several samples were below the detectable range of the E2 assay (LC = 7; LP = 5; OC = 3; OP = 4) and for those samples, the limit of detection of the assay (10.6 pg/ml) was divided by √2 (Ogden, 2010) for inclusion in the statistical analysis. Additionally, E2 assay results were analyzed to include only samples that were within the detectable range of the assay: LC = 3; LP = 4; OC = 7; OP = 6. All samples were within the analytical range of the P4 assay.
Protein isolation
Total ovarian protein was isolated by homogenizing ovaries in lysis buffer (50 mM Tris-HCl and 1 mM EDTA; pH approximately 8.5). Samples (LC = 10; LP = 9; OC = 10; OP = 10) were centrifuged twice for 15 min at 10 621 rcf and the supernatant collected each time. A BCA assay was performed to measure protein concentration. Absorbance values were detected at 560 nm by an Eon Microplate Spectrophotometer (Bio-Tek Instruments Inc., Winooski, Vermont).
LC-MS/MS proteome analysis
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was performed for protein separation and identification as described previously (Clark et al., 2019). Briefly, total protein samples (LC = 5; LP = 5; OC = 5; OP = 5) were digested with trypsin/Lys-C for 16 h, dried down and reconstituted in buffer A (47.5 µl, 0.1% formic acid/water). An internal control, Peptide Retention Time Calibration (PRTC), was spiked into each sample. Protein samples and PRTC were injected onto a liquid chromatography column (Agilent Zorbax SB-C18, 0.5 mm × 150 mm, 5 µm) to be separated and analyzed with a mass spectrometer. Fragmentation patterns and intact results were compared with theoretical fragmentation patterns from MASCOT or Sequest HT to identify peptides.
Statistical analysis
Statistical analyses were performed on all endpoints with the exception of the LC-MS/MS data using GraphPad Prism 8.4.1 software. Two-way analysis of variance (2-way ANOVA) was performed to compare 2 independent variables (body composition and PFOA exposure and any interaction) using Tukey’s multiple comparison test. A p value ≤ .05 was defined as a statistically different result between treatments.
For the LC-MS/MS analysis, Metaboanalyst 3.0 (Xia et al., 2015; Xia and Wishart, 2016) was used for data analysis. Upon finding data integrity to be satisfactory (no peptide with more than 50% missing replicates, positive values for the area), missing value imputation was performed using a singular value decomposition method. Filtering, based on interquartile range, was performed to remove values that are unlikely to be of use when modeling the data, followed by generalized log transformation before data analysis. The relevant control and treatment samples were compared using a Student t test. Differences between groups were assessed by the Mann-Whitney rank sum test. All p values were 2 sided. To adjust for multiple comparisons, Bonferroni correction was applied and only p values less than .05 were considered significant. The PCA analysis was performed using the prcomp package and pairwise score plots providing an overview of the various separation patterns among the most significant components were accessed. The PLS regression was then performed using the plsr function provided by R pls package. The classification and cross-validation were also performed using the caret package. The Uniprot protein identifiers were used to retrieve biological pathway association of the proteins using DAVID 6.8 software.
RESULTS
Impact of PFOA Exposure on Food Intake and Body Weight Gain in Lean and Obese Mice
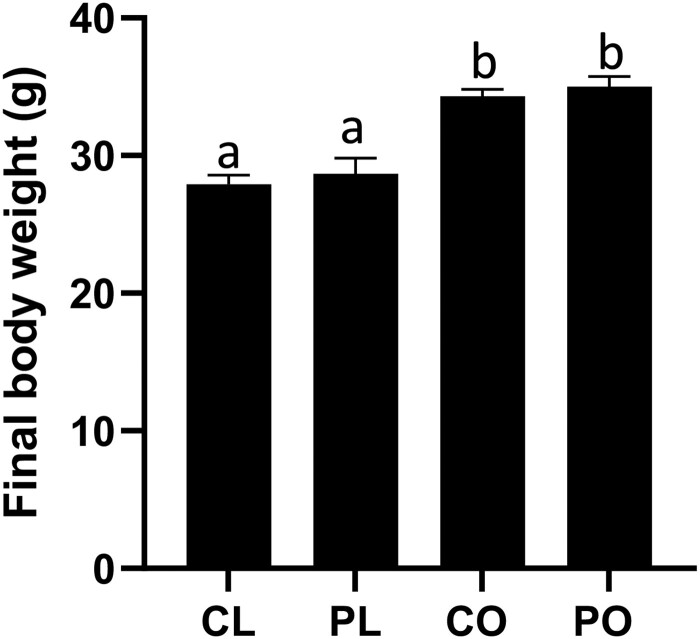
PFOA exposure did not affect food intake (p > .05) in lean or obese mice, however, as expected, mean food intake was higher in the obese compared with lean mice (LC = 62.45 ± 1.1 g; LP = 65.0 ± 1.7 g; OC = 82.0 ± 2.5 g; OP = 79.6 ± 3.1 g). At euthanasia, the obese mice were approximately 24% heavier (p < .05) than their lean counterparts (Figure 1). Body weight was not affected (p > .05) by PFOA exposure in lean or obese mice (Figure 1; treatment effect p = .36; obesity effect p ≤ .0001; interaction effect p = .97).
Figure 1.
Effects of PFOA exposure on body weight in lean and obese mice. Mice were weighed prior to euthanasia. Bars represent body weight (g) ± SEM. Superscript letters indicate significant differences; p < .05. LC, lean control-treated mice; LP, lean PFOA-exposed mice; OC, obese control-treated mice; OP, obese PFOA-exposed mice. n: LC = 10; LP = 9; OC = 10; OP = 10.
Effects of PFOA Exposure on Organ Weight in Lean and Obese Mice
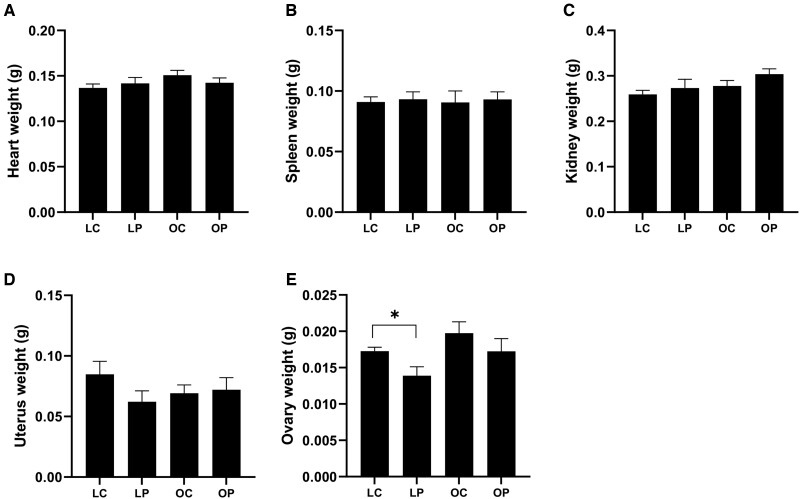
There was no effect of PFOA or obesity (p > .05) on (A) heart (treatment effect p = .76; obesity effect p = .18; interaction effect p = .22; Figure 2A), (B) spleen (treatment effect p = .72; obesity effect p = .97; interaction effect p = .98; Figure 2B), (C) kidney (treatment effect p = .14; obesity effect p = .07; interaction effect p = .65; Figure 2C), and (D) uterus (treatment effect p = .29; obesity effect p = .75; interaction effect p = .18; Figure 2D). Ovary weight was decreased (treatment effect p = .038; obesity effect p = .038; interaction effect p = .76; Figure 2E) due to PFOA exposure in lean, but not in obese mice (LC = 0.017 g ± 0.0005; LP = 0.014 g ± 0.001; OC = 0.02 g ± 0.001; OP = 0.017 g ± 0.001), relative to their respective vehicle control-treated counterparts.
Figure 2.
Effect of PFOA exposure on organ weight in lean and obese mice. After euthanasia, (A) heart, (B) spleen, (C) kidney, (D) uterus, and (E) ovary were collected and weighed (g). Bars represent mean weight ± SEM. Asterisks indicate statistical difference; p < .05. LC, lean control-treated mice; LP, lean PFOA-exposed mice; OC, obese control-treated mice; OP, obese PFOA-exposed mice. n: LC = 10; LP = 9; OC = 10; OP = 10.
Alterations to Ovarian Steroid Hormone Level and Estrous Cyclicity due to PFOA Exposure in Lean and Obese Mice
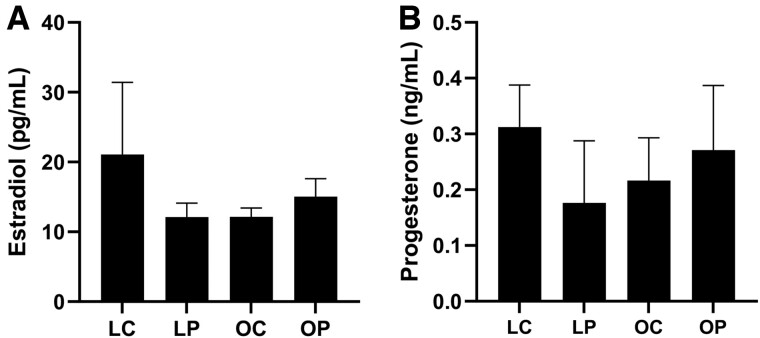
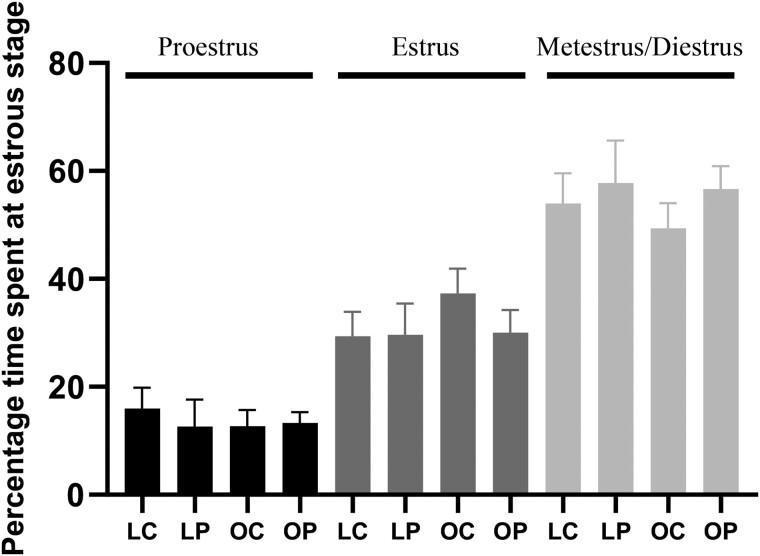
Detectable E2 levels were present in 37% of lean and 65% of obese mice regardless of PFOA exposure with a high level of variation in the lean vehicle control-treated mice. Circulating E2 was increased (p < .05) in obese but not lean mice by PFOA exposure when samples below the limit of detection were omitted (LC = 52.8 ± 29.7; LP = 17.9 ± 2.1; OC = 14.2 ± 1.1; OP = 20.1 ± 2.6). Inclusion of samples that fell below the limit of detection of the assay by dividing the lower detection limit by √2 indicated no effect of PFOA exposure on circulating E2 (treatment effect p = .59; obesity effect p = .60; interaction effect p = .29; Figure 3A) in lean or obese mice. The level of circulating P4 was not affected (treatment effect p = .67; obesity effect p = .99; interaction effect p = .32) by obesity or PFOA exposure in any of the groups (Figure 3B). Using ELISA as a quantification method, the level of circulating E2 was in the published range for mice in the diestrus phase of the estrous cycle, however P4 levels were lower than previously noted (Zenclussen, 2014). There was no effect of obesity, PFOA exposure, or additive impact of obesity and PFOA on the time spent at proestrus (treatment effect p = .70; obesity effect p = .72; interaction effect p = .57), estrus (treatment effect p = .47; obesity effect p = .39; interaction effect p = .43), and metestrus + diestrus (treatment effect p = .33; obesity effect p = .61; interaction effect p = .75; Figure 4).
Figure 3.
Ovarian steroid hormone impact of PFOA exposure in lean and obese mice. Circulating E2 and progesterone were measured by ELISA. LC, lean control-treated mice; LP, lean PFOA-exposed mice; OC, obese control-treated mice; OP, obese PFOA-exposed mice. Bars represent mean concentration ± SEM. E2n: LC = 10; LP = 9; OC = 10; OP = 10. P4n: LC = 10; LP = 9; OC = 9; OP = 8.
Figure 4.
Estrous cyclicity impact of PFOA exposure in lean and obese mice. The number of days at each stage of the estrous cycle were calculated over a 14-day period and presented as a percentage. Bars represent percentage of day at proestrus (black bars), estrus (dark gray bars), and metestrus + diestrus (light gray bars) ± SEM. Asterisk indicate differences between treatments; p < .05. LC, lean control-treated mice; LP, lean PFOA-exposed mice; OC, obese control-treated mice; OP, obese PFOA-exposed mice. n: LC = 10; LP = 9; OC = 10; OP = 10.
Effects of PFOA Exposure and Obesity on the Global Ovarian Proteome
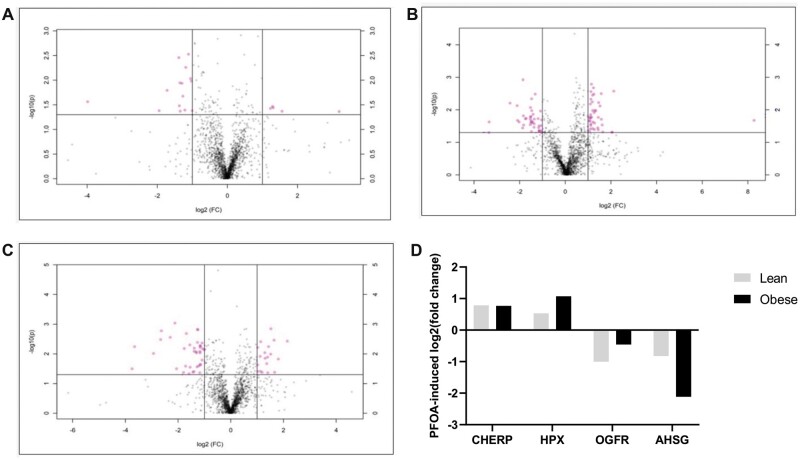
In lean mice, exposure to PFOA altered the abundance of 98 proteins (p < .05). Of these, the level of 36 were increased and 62 were decreased (Figure 5A). The biological or molecular functions of 16 (4 increased and 12 decreased) proteins are involved in DNA damage and repair (Table 1), and 7 (2 upregulated and 5 downregulated) in reproduction (Table 2).
Figure 5.
Impact of PFOA on the ovarian proteome in lean and obese mice. Total ovarian protein homogenates were analyzed by LC-MS/MS and bioinformatic comparison performed between peptides identified in (A) LC versus LP, (B) OC versus OP, and (C) LC versus OC. Dots above the solid horizontal line indicate increased (upper right corner) or decreased (upper left corner) proteins; n = 5/treatment; p < .05. D, The log2(fold-change) of proteins increased (CHERP and HPX) and decreased (OGFR and AHSG) by PFOA in both lean (gray bar) and obese (black bar) mice.
Table 1.
Altered Ovarian Abundance of Proteins Involved in DNA Damage Sensing and Repair in Lean PFOA-Exposed Relative to Lean Vehicle Control-Treated Mice
| UniProtID | Protein Name | Protein Abbreviation | p Value | Log2(Fold Change) |
|---|---|---|---|---|
| Q64522 | H2A clustered histone 21 | H2AC21 | .036 | 1.31 |
| P68433 | H3 clustered histone 1 | H3C1 | .037 | 1.23 |
| Q08943 | Structure-specific recognition protein 1 | SSRP1 | .036 | 0.73 |
| P43274 | H1.4 linker histone, cluster member | H1-4 | .016 | 0.34 |
| P07901 | Heat shock protein 90 alpha family class A member 1 | HSP90AA1 | .001 | −0.28 |
| P60335 | Poly(rC)-binding protein 1 | PCBP1 | .004 | −0.28 |
| Q921I1 | Transferrin | TF | .029 | −0.38 |
| O70456 | Stratifin | SFN | .026 | −0.42 |
| Q8VDP4 | Cell cycle and apoptosis regulator 2 | CCAR2 | .042 | −0.46 |
| Q3TCH7 | Cullin 4A | CUL4A | .037 | −0.6 |
| P34884 | Macrophage migration inhibitory factor | MIF | .044 | −0.6 |
| P26350 | Prothymosin alpha | PTMA | .007 | −0.70 |
| Q923G2 | RNA polymerase I, II, and III subunit H | POLR2H | .040 | −0.71 |
| P53996 | CCHC-type zinc finger nucleic acid-binding protein | CNBP | .003 | −0.83 |
| E9QAM5 | Helicase with zinc finger 2 | HELZ2 | .005 | −0.96 |
| Q3TKT4 | SWI/SNF related, matrix associated, actin-dependent regulator of chromatin, subfamily a, member 4 | SMARCA4 | .040 | −1.21 |
UniprotID refers to protein identifiers on Uniprot.
Protein abbreviation is the abbreviation for each altered protein.
Log2 refers to the log2 fold change in PFOA exposed relative to vehicle control-treated lean mice.
Table 2.
Altered Ovarian Abundance of Proteins Involved in Reproduction in Lean PFOA-Exposed Relative to Lean Vehicle Control-Treated Mice
| UniProtID | Protein Name | Protein Abbreviation | p Value | Log2(Fold Change) |
|---|---|---|---|---|
| P70274 | Selenoprotein P | SELENOP | .034 | 1.29 |
| P26361 | CF transmembrane conductance regulator | CFTR | .047 | 0.84 |
| P34884 | Macrophage migration inhibitory factor | MIF | .044 | −0.62 |
| Q9CYR6 | Phosphoglucomutase 3 | PGM3 | .032 | −0.67 |
| Q9JKV1 | ADRM1 26S proteasome ubiquitin receptor | ADRM1 | .038 | −0.78 |
| P29699 | Alpha-2-HS glycoprotein | AHSG | .014 | −0.82 |
| Q3TKT4 | SWI/SNF related, matrix associated, actin-dependent regulator of chromatin, subfamily a, member 4 | SMARCA4 | .040 | −1.21 |
UniprotID refers to protein identifiers on Uniprot.
Protein abbreviation is the abbreviation for each altered protein.
Log2 refers to the log2 fold change in PFOA exposed relative to vehicle control-treated lean mice.
A total of 129 proteins were altered in their level in the obese mice exposed to PFOA (p < .05). Eighty-eight proteins were increased and 41 were decreased (Figure 5B). The biological or molecular functions of 18 (12 increased and 6 decreased) proteins are involved in DNA damage and repair (Table 3), and 11 proteins (6 elevated and 5 reduced) in reproduction (Table 4).
Table 3.
Ovarian Proteins Involved in DNA Damage Sensing and Repair Altered in PFOA-Exposed Relative to Vehicle Control-Treated Obese Mice
| UniProtID | Protein Name | Protein Abbreviation | p Value | Log2(Fold Change) |
|---|---|---|---|---|
| P97431 | Interferon regulatory factor 6 | IRF6 | .030 | −1.01 |
| O55128 | Sin3A-associated protein 18 | SAP18 | .012 | −0.84 |
| Q8CH18 | Cell division cycle and apoptosis regulator 1 | CCAR1 | .022 | −0.79 |
| Q9D2M8 | Ubiquitin conjugating enzyme E2 V2 | UBE2V2 | .046 | −0.77 |
| P54728 | RAD23 homolog B, nucleotide excision repair protein | RAD23B | .032 | −0.58 |
| Q9JKB3 | Y-box-binding protein 3 | YBX3 | .016 | −0.45 |
| Q924A2 | Capicua transcriptional repressor | CIC | .050 | 0.38 |
| Q99MD9 | Nuclear autoantigenic sperm protein | NASP | .047 | 0.43 |
| P56546 | C-terminal-binding protein 2 | CTBP2 | .021 | 0.46 |
| P63085 | Mitogen-activated protein kinase 1 | MAPK1 | .022 | 0.64 |
| A2AWL7 | MAX gene-associated protein | MGA | .011 | 0.76 |
| Q8VCD5 | Mediator complex subunit 17 | MED17 | .033 | 0.98 |
| Q02780 | Nuclear factor I A | NFIA | .036 | 1.04 |
| P52479 | Ubiquitin-specific peptidase 10 | USP10 | .028 | 1.12 |
| Q9D1J3 | SAP domain containing ribonucleoprotein | SARNP | .034 | 1.17 |
| P52633 | Signal transducer and activator of transcription 6 | STAT6 | .002 | 1.21 |
| O08586 | Phosphatase and tensin homolog | PTEN | .019 | 1.25 |
| P42228 | Signal transducer and activator of transcription 4 | STAT4 | .031 | 1.39 |
UniprotID refers to protein identifiers on Uniprot.
Protein abbreviation is the abbreviation for each altered protein.
Log2 refers to the log2 fold change in PFOA exposed relative to vehicle control-treated obese mice.
Table 4.
Ovarian Proteins Involved in Reproduction Changed in PFOA-Exposed Relative to Vehicle Control-Exposed Obese Mice
| UniProtID | Protein Name | Protein Abbreviation | p Value | Log2(Fold Change) |
|---|---|---|---|---|
| P29699 | Alpha 2-HS-glycoprotein | AHSG | .009 | −2.12 |
| Q9CQK7 | RWD domain containing 1 | RWDD1 | .005 | −1.57 |
| Q99L45 | Eukaryotic translation initiation factor 2 subunit beta | EIF2S2 | .043 | −0.81 |
| P54728 | RAD23 homolog B, nucleotide excision repair protein | RAD23B | .032 | −0.58 |
| Q9JKB3 | Y-box-binding protein 3 | YBX3 | .016 | −0.45 |
| Q08879 | Fibulin 1 | FBLN1 | .010 | 0.49 |
| P30416 | FKBP prolyl isomerase 4 | FKBP4 | .017 | 0.49 |
| P19001 | Keratin 19 | KRT19 | .025 | 0.60 |
| P63085 | Mitogen-activated protein kinase 1 | MAPK1 | .022 | 0.64 |
| P06745 | Glucose-6-phosphate isomerase | GPI | .011 | 0.68 |
UniprotID refers to protein identifiers on Uniprot.
Protein abbreviation is the abbreviation for each altered protein.
Log2 refers to the log2 fold change in PFOA exposed relative to vehicle control-treated obese mice.
In the comparison between lean and obese controls, the abundance of 206 proteins were altered by obesity (p < .05). Of these, 93 were increased and 113 were decreased in their levels (Figure 5C). The biological or molecular functions of 39 (22 increased and 17 decreased) proteins are involved in DNA damage sensing and repair (Table 5), and 24 (10 increased and 14 decreased) proteins are involved in reproduction (Table 6).
Table 5.
Ovarian DNA Damage Sensing and Repair Proteins Altered in Obese Relative to Lean Mice
| UniProtID | Protein Name | Protein Abbreviation | p Value | Log2(Fold Change) |
|---|---|---|---|---|
| Q8CG72 | ADP-ribosylserine hydrolase | ADPRS | .032 | −3.745 |
| P62878 | Ring-box 1 | RBX1 | .042 | −1.7921 |
| O08586 | Phosphatase and tensin homolog | PTEN | .0066 | −1.7631 |
| O88291 | Zinc finger protein 326 | ZNF326 | .0064 | −1.3845 |
| Q8BZ21 | Lysine acetyltransferase 6A | KAT6A | .0092 | −1.2997 |
| Q8CIG3 | Lysine demethylase 1B | KDM1B | .0053 | −1.1687 |
| Q02248 | Catenin beta 1 | CTNNB1 | .0092 | −1.1661 |
| P10639 | Thioredoxin | TXN | .0052 | −0.98829 |
| P34884 | Macrophage migration inhibitory factor | MIF | .012 | −0.93639 |
| Q80US4 | Actin related protein 5 | ACTR5 | .046 | −0.93475 |
| P70288 | Histone deacetylase 2 | HDAC2 | .0055 | −0.92235 |
| Q8CCK0 | MacroH2A.2 histone | MACROH2A2 | .048 | −0.66778 |
| Q9WV02 | RNA-binding motif protein X-linked | RBMX | .016 | −0.65389 |
| Q8R081 | Heterogeneous nuclear ribonucleoprotein L | HNRNPL | .011 | −0.64919 |
| Q8BM75 | AT-rich interaction domain 5B | ARID5B | .0036 | −0.55808 |
| Q921I1 | Transferrin | TF | .0033 | −0.43368 |
| O35381 | Acidic nuclear phosphoprotein 32 family member A | ANP32A | .046 | −0.40138 |
| Q60973 | RB-binding protein 7, chromatin remodeling factor | RBBP7 | .032 | 0.27737 |
| Q9CQV8 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein beta | YWHAB | .046 | 0.32484 |
| Q61937 | Nucleophosmin 1 | NPM1 | .0053 | 0.34355 |
| P81117 | Nucleobindin 2 | NUCB2 | .019 | 0.35221 |
| Q9ERF3 | SKI8 subunit of superkiller complex | SKIC8 | .042 | 0.48708 |
| P63101 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta | YWHAZ | .043 | 0.51222 |
| Q6PDG5 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin subfamily c member 2 | SMARCC2 | .036 | 0.52058 |
| Q8CH18 | Cell division cycle and apoptosis regulator 1 | CCAR1 | .037 | 0.57973 |
| O08749 | Dihydrolipoamide dehydrogenase | DLD | .0014 | 0.58937 |
| O55128 | Sin3A-associated protein 18 | SAP18 | .022 | 0.59521 |
| Q91YE6 | Importin 9 | IPO9 | .024 | 0.59599 |
| O88543 | COP9 signalosome subunit 3 | COPS3 | .013 | 0.61813 |
| Q5RJI5 | BR serine/threonine kinase 1 | BRSK1 | .048 | 0.62241 |
| O88700 | BLM RecQ like helicase | BLM | .020 | 0.72346 |
| P68510 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein eta | YWHAH | .013 | 0.75795 |
| P61079 | Ubiquitin conjugating enzyme E2 D3 | UBE2D3 | .013 | 0.79235 |
| P42227 | Signal transducer and activator of transcription 3 | STAT3 | .037 | 0.91826 |
| P08775 | RNA polymerase II subunit A | POLR2A | .013 | 0.97614 |
| Q8CGB3 | Uveal autoantigen with coiled-coil domains and ankyrin repeats | UACA | .013 | 1.3975 |
| Q8CIG8 | Protein arginine methyltransferase 5 | PRMT5 | .043 | 1.4058 |
| Q02395 | Metal response element-binding transcription factor 2 | MTF2 | .0057 | 1.434 |
| Q2VPU4 | MLX interacting protein | MLXIP | .015 | 1.8047 |
UniprotID refers to protein identifiers on Uniprot.
Protein abbreviation is the abbreviation for each altered protein.
Log2 refers to the log2 fold change in obese relative to lean mice.
Table 6.
Ovarian Reproduction Proteins Altered by Obesity
| UniProtID | Protein Name | Protein Abbreviation | p Value | Log2(Fold Change) |
|---|---|---|---|---|
| Q06770 | Serpin family A member 6 | SERPINA6 | .0057 | −3.6512 |
| O08586 | Phosphatase and tensin homolog | PTEN | .0066 | −1.7631 |
| P26361 | CF transmembrane conductance regulator | CFTR | .041 | −1.4367 |
| Q02248 | Catenin beta 1 | CTNNB1 | .0092 | −1.1661 |
| O70167 | Phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2 gamma | PIK3C2G | .042 | −0.91511 |
| Q6NZC7 | SEC23 interacting protein | SEC23IP | .042 | −0.57556 |
| Q8BM75 | AT-rich interaction domain 5B | ARID5B | .0036 | −0.55808 |
| Q14AT2 | Testis expressed 11 | TEX11 | .0036 | −0.55572 |
| P48036 | Annexin A5 | ANXA5 | .036 | −0.51797 |
| P30416 | FKBP prolyl isomerase 4 | FKBP4 | .026 | −0.44588 |
| P54869 | 3-hydroxy-3-methylglutaryl-CoA synthase 2 | HMGCS2 | .040 | −0.37046 |
| O88844 | Isocitrate dehydrogenase (NADP(+)) 1 | IDH1 | .018 | −0.33729 |
| P06745 | Glucose-6-phosphate isomerase | GPI | .025 | −0.31511 |
| P24815 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 | HSD3B1 | .040 | −0.26552 |
| Q60973 | RB-binding protein 7, chromatin remodeling factor | RBBP7 | .032 | 0.27737 |
| P08228 | Superoxide dismutase 1 | SOD1 | .044 | 0.2967 |
| P81117 | Nucleobindin 2 | NUCB2 | .015 | 0.35221 |
| O08749 | Dihydrolipoamide dehydrogenase | DLD | .0014 | 0.58937 |
| P29699 | Alpha 2-HS glycoprotein | AHSG | .0059 | 0.68981 |
| Q99LD9 | Eukaryotic translation initiation factor 2B subunit beta | EIF2B2 | .016 | 0.69071 |
| P42227 | Signal transducer and activator of transcription 3 | STAT3 | .037 | 0.91826 |
| Q6R891 | Protein phosphatase 1 regulatory subunit 9B | PPP1R9B | .039 | 1.0235 |
| P16627 | Heat shock protein family A (Hsp70) member 1 like | HSPA1L | .042 | 1.1903 |
| B2RV46 | Spermatogenesis associated 6 like | SPATA6L | .0037 | 2.1449 |
UniprotID refers to protein identifiers on Uniprot.
Protein abbreviation is the abbreviation for each altered protein.
Log2 refers to the log2 fold change in obese relative to lean mice.
In both lean and obese mice, 4 proteins involved in processes relevant to both DNA damage sensing and reproduction that were changed in their level by PFOA exposure (log2(fold-change)) were: calcium homeostasis endoplasmic reticulum protein (CHERP) (lean: 0.78-fold increase; obese: 0.77-fold increase), hemopexin (HPX) (lean: 0.53-fold increase; obese: 1.1-fold increase), opioid growth factor receptor (OGFR) (lean: −1.0-fold decrease; obese: −0.45-fold decrease), and alpha 2-HS glycoprotein (AHSG) (lean: −0.82-fold decrease; obese: −2.1-fold decrease) (Figure 5D).
Of these proteins, 23, 28, and 63 proteins were identified that were unique to LC versus LP, OC versus OP, and LC versus OC, respectively. In addition, 1 protein was shared between LC versus LP and OC versus OP groups, 3 proteins were shared between LC versus LP and LC versus OC, 5 proteins were shared between OC versus OP and LC versus OC groups, and 1 protein was shared between the 3 comparisons (Figure 6).
Figure 6.
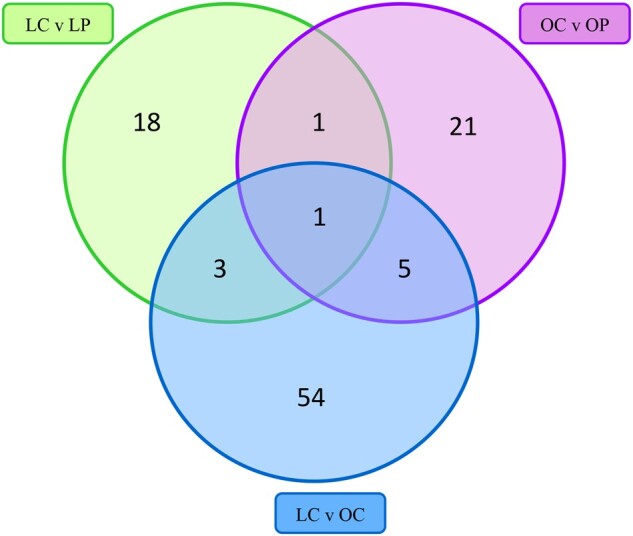
Proteins involved in DNA damage sensing and repair and reproduction that are in common or unique between treatments. The Venn diagram presents the number of ovarian proteins identified as being unique to treatment or altered in common by treatments involved in DNA damage sensing and repair and reproduction. The number of proteins in the green circle indicates the number of proteins identified in the comparison between LC and LP groups; the purple circle indicates the number of proteins identified in the comparison between OC and OP groups; and the blue circle indicates the number of proteins identified in the comparison between LC and OC groups. Overlapping areas of the circles illustrate the number of proteins that were altered by 2 or more groups.
DISCUSSION
Per- and polyfluoroalkylated substances have been used in industrial and commercial products since the 1940s (Lindstrom et al., 2011) because of their ability to repel water and oil (Bell et al., 2021). The U.S. Environmental Protection Agency (EPA) has not established maximum contaminant level of PFOA for drinking water (USEPA, 2017). However, newly issued advice from the EPA established drinking water health advisories of 0.004 ppt for PFOA and 0.02 ppt for PFOS. In the European Union, the Scientific Panel on Contaminants in the Food Chain (CONTAM) of the European Food Safety Authority (EFSA), established the Tolerable Daily Intake for PFOA of 1.5 µg/kg body weight per day (EFSA, 2008). From 1999 to 2016, the National Health and Nutrition Examination Survey (NHANES) demonstrated PFOA concentrations in the serum of the general U.S. population (Centers for Disease Control and Prevention, 2021a,b) and a study from the Netherlands estimated long-term intake median values for PFOA as 0.2 ng/kg/day and PFOS as 0.3 ng/kg/day (Noorlander et al., 2011). An arithmetic mean serum concentration of 691 ng/ml (range 72–5100 ng/ml) PFOA can occur through occupational exposure (Olsen et al., 2007). In humans, the PFOA half-life of elimination is approximately 2.7 and 3.8 years (Li et al., 2018; Olsen et al., 2007). In rats, the half-life is approximately 2–4 h in females (Vanden Heuvel et al., 1991), and from 4 to 9 days in males (EFSA, 2008), attributed to biological sex differences in renal excretion (Vanden Heuvel et al., 1991). Similarly, PFOA half-life is 15 days and 21 days in female and male mice, respectively (Lou et al., 2009).
The main routes of human exposure to PFOA are ingestion of contaminated food (both from animal exposure and food packaging) and drinking water followed by indoor air and dust inhalation, and house dust transfer from hand to mouth (Trudel et al., 2008). After exposure, PFOA is readily absorbed (EFSA, 2008) and PFAS are not considered to be biotransformed in vivo (ATSDR, 2021; Kemper and Nabb, 2005; Vanden Heuvel et al., 1991) before being eliminated mostly through urine, and bile (Vanden Heuvel et al., 1991). Accumulation of PFAS compounds occurs in blood, liver, kidney, testicles, brain (Jensen and Leffers, 2008), and ovaries (Vanden Heuvel et al., 1991). Human follicular fluid can contain PFOA (Heffernan et al., 2018) thus, PFAS could be a potential exposure for the oocyte (Ding et al., 2020). Although there are geographical as well as human ethnicity (Park et al., 2019) influences for PFAS exposure, the daily cumulative PFOA exposure remains unclear. To determine an ovarian impact of PFOA exposure, a relevant oral route of exposure and a dose of 2.5 mg/kg PFOA was examined which is intermediate for the LOAEL noted for PFOA in the testis (5 mg/kg; Li et al., 2011) and the liver (1 mg/kg; Loveless et al., 2008). A PFOA dose of 5 mg/kg was recently determined to cause ovarian follicle loss in mice (Yang et al., 2022), though the modes of action outside of steroidogenesis mRNA gene abundance were not examined.
At 7 weeks of age, lean and obese mice were orally exposed to 2.5 mg/kg body weight of PFOA for 15 days. A hyperphagia-induced model of obesity with a mutation of the agouti gene in the hypothalamus, which increases agouti expression decreasing melanocyte-stimulating hormone resulting in overeating (Duhl et al., 1994; Klebig et al., 1995; Lu et al., 1994; Michaud et al., 1993, 1994) was utilized. The lean counterparts are of the same genetic background and eat the same diet composition, but they consume less calories. This obesity model has altered circulating insulin (Yang et al., 2012), alterations to steroidogenesis (Nteeba et al., 2014b), decreased number of primordial follicles from 12 weeks of age onwards (Ganesan et al., 2017), and a diminished response to environmental toxicants (Ganesan et al., 2014, 2017; González-Alvarez et al., 2021). The obese mice were approximately 24% heavier than the lean mice at the end of dosing and this age was chosen to eliminate differences in ovarian follicle composition between the lean and obese mice at the start of PFOA exposure which has been noted from 12 weeks of age onward in this obese model (Nteeba et al., 2014b). It is recognized that this is likely at the moderate level of weight gain in women and greater effects may have been identified if a greater weight attainment was achieved, however, consideration of removing the confounding impact of obesity-induced follicle loss was important.
Conflicting results have been reported in animal studies and epidemiological studies that studied the relationship between exposure to PFOA and body weight. In humans, inverse associations between maternal blood and umbilical cord serum PFOA levels with birth weight and size are reported in some studies (Apelberg et al., 2007; Bach et al., 2015; Fei et al., 2007) but not others (Andersen et al., 2013; Høyer et al., 2015; Monroy et al., 2008; Nolan et al., 2009). Prenatal PFOA plasma levels have been associated with increased BMI in girls but not boys (Mora et al., 2017). Increased body weight due to PFOA exposure has been reported in rats (Du et al., 2019) but the opposite in PFOA-exposed mice (Wolf et al., 2007; Yang et al., 2009). In this study, the agouti overexpressing mice were heavier than their lean counterparts due to experimental design but there was no impact of PFOA exposure on body weight in either the lean or obese mice indicating lack of overt toxicity. In agreement with our findings, female BALB/c mice (6–8 weeks of age) had no effect of PFOA on body weight (Fairley et al., 2007), though a different route of exposure and dosage was used (Fairley et al., 2007). Most studies documenting PFOA-induced differences in body weight have used higher concentrations than in this study. Other potential reasons for differential impacts of PFOA on body weight include different strains of mice, differences between species, as well as the dosing duration and animal stage of development.
Food intake and the weight of heart, spleen, kidney, and uterus were not affected by PFOA exposure or obesity. Decreased uterine weight has been reported in PFOA exposed (1, 5, and 10 mg/kg) BALB/c and C57BL/6 mice (Yang et al., 2009) albeit in a dose- and strain-dependent manner (Yang et al., 2009). Lower and acute PFOA exposure (0.01 mg/kg/day) increased uterine weight (Dixon et al., 2012), supporting influence of the dosing paradigm. Dermal PFOA exposures decreased spleen weight (Fairley et al., 2007) and adverse impacts on spleen weight were also noted in male C57BL/6 mice (Yang et al., 2002). As with body weight, potential explanations for differences between the current study and others include animal model, dose, and duration of PFOA exposure.
Ovarian weight was increased in neonatal female rats (PND 1–5), and juvenile rats (PND 26–30) exposed to 1 mg/kg and 10 mg/kg of PFOA, respectively, for 5 days (Du et al., 2019). There was no effect on ovarian weight in pregnant female Kunming mice that received gestational exposure to 2.5, 5, or 10 mg/kg/day of PFOA (Chen et al., 2017). CD-1 female mice at 30 days of age exposed to 5, 10, and 20 mg/kg of PFOA for 10 days did not have altered ovarian and uterine weight (Yang et al., 2022). In the current study, ovarian weight was decreased in the lean but not obese mice exposed to PFOA. Lack of an effect of PFOA exposure on ovarian weight in the obese mice illustrates differences in the PFOA response in the obese relative to lean mouse ovary. The decrease in ovary weight might be explained by follicle loss caused by PFOA exposure, because PFOA was demonstrated to reduce the number of primordial and growing follicles (Du et al., 2019; Yang et al., 2022) as well as corpora lutea (Chen et al., 2017; Du et al., 2019). Furthermore, PFOA and PFOS can disrupt gap junction communication between granulosa cells and oocytes in mice and swine (Dominguez et al., 2016; Lopez-Arellano et al., 2019) potentially resulting in oocyte death and follicle loss. These differences might be also attributed to the dose level, dosing duration, mouse strain, and the developmental status.
Per- and poly-fluorinated compounds are potential endocrine disrupting chemicals (Jensen and Leffers, 2008). In this study, there was no difference between the lean and obese mouse response to PFOA exposure on E2 serum levels when samples lower than the level of detection of the assay were included, however omission of those below assay detection limit samples indicated that circulating E2 was increased in obese but not lean mice and is an avenue for further exploration. Perhaps due to blood being collected during diestrus, several samples were below the detectable range of the E2 assay resulting in a smaller sample size in the lean group compared with the obese group. In the future, ovaries at the estrus cycle stage could be collected for a more appropriate quantification of the effects of E2. Serum P4 concentration was not affected by PFOA exposure. A study in reproductive aged women discounted an association between PFOA exposure and E2 serum concentrations (Knox et al., 2011). An association between E2 and testosterone (T4) with serum PFOA level was demonstrated in men, but not in women (Steenland et al., 2010). No association between PFOA serum level and E2 levels in Taiwanese girls (Zhou et al., 2016) or between serum PFOA, E2, and P4 in women has been reported (Barrett et al., 2015). In animal models, serum E2 was increased on GD7 in pregnant female Kunming mice exposed to 10 mg/kg/day of PFOA (Chen et al., 2017). Additionally, on GD13, serum P4 was decreased in mice exposed to 5 and 10 mg/kg/day of PFOA (Chen et al., 2017). Neonatal exposure (PND 1–5) to 0.1 and 1 mg/kg/day PFOA for 5 days increased serum E2 in female rats (Du et al., 2019). Young female C57BL/6 mice exposed to 5 mg/kg of PFOA for 5 days per week for 4 weeks (Zhao et al., 2010) had no observed alteration in serum E2, but P4 was increased during the estrus and proestrus stages (Zhao et al., 2010). In mice exposed to 1, 5, 10, or 20 mg/kg PFOA, there was no impact on E2, but P4 was decreased at 5 mg/kg exposure (Yang et al., 2022). Secretion of P4 was not altered by 0.012–24 µM PFOA exposure in cultured porcine theca cells (Chaparro-Ortega et al., 2018). However, in granulosa cells, P4 and E2 secretion were decreased at 0.12 µM and 0.012 µM, respectively, indicating a concentration dependence (Chaparro-Ortega et al., 2018). Thus, in this study, an endocrine disrupting impact of PFOA cannot be discounted and is worthy of future investigation.
The time spent in proestrus, estrus, metestrus, and diestrus stages were not affected by PFOA exposure, obesity, nor was there any additive effect of obesity and PFOA. In women, irregular and longer menstrual cycles have been associated with higher levels of serum PFOA (Fei et al., 2009; Lyngsø et al., 2014; Zhou et al., 2017) although other studies reported no association between menstrual cycle length and serum PFOA level (Lum et al., 2017). Neonatal exposure of rats to 0.1 and 1 mg/kg/day PFOA for 5 days affected post-pubertal estrous cyclicity (Du et al., 2019), but exposure to 10 mg/kg/day of PFOA caused temporary post-pubertal acyclicity (Du et al., 2019). Juvenile mouse exposure (PND 26–30) to 1 mg/kg/day PFOA also resulted in irregular cyclicity (Du et al., 2019). Lack of a phenotypic impact of estrous cyclicity could translate to an insidious impact of PFOA exposure that may not be apparent to an affected female.
Based upon the previous studies of PFOA-induced follicle loss at similar exposure levels (Yang et al., 2022), this study focused instead on proteomic impacts within the ovary. Altered ovarian abundance of proteins involved in DNA damage sensing and repair and reproduction were examined in ovaries of lean and obese mice exposed to PFOA. Several DDR proteins were altered by PFOA exposure in ovaries from lean mice. A nuclear helicase protein (Li et al., 2016), HELZ interacts with and stimulates the peroxisome proliferator-activated receptor α (PPARα); (Surapureddi et al., 2002) and is involved in DNA repair, gene transcription, and RNA processing (Katano-Toki et al., 2013). In the ovary, PPAR α, β/δ, and γ are detectable in granulosa, theca, and stromal cells and in the corpus luteum (Ding et al., 2020). Members of the PPAR family are involved in oogenesis, folliculogenesis, and ovulation (Ding et al., 2020). Lean PFOA mice had decreased levels of ovarian HELZ2 which could disrupt PPAR signaling.
Linker histone (H1) family members, including the subtype H1.4 linker histone, cluster member (H1-4) stabilizes chromatin and is essential for chromatin fiber maintenance and assembly (Bednar et al., 2017; Hergeth and Schneider, 2015). Ovarian H1-4 is increased by PFOA exposure in lean mice and alterations in levels of H1 family members can upregulate or downregulate specific genes (Hergeth and Schneider, 2015). The level of H1 phosphorylation is indicative of cellular DNA damage level (Hergeth and Schneider, 2015) and expression of H1 subtypes are proposed as biomarkers for ovarian cancer (Medrzycki et al., 2012). The interaction between linker histones H1 with chromatin is regulated by Prothymosin alpha (PTMA; Gomez-Marquez and Rodríguez, 1998; Karetsou et al., 1998, 2004). Increased PTMA is observed in breast (Tsitsiloni et al., 1993), and prostate cancer (Suzuki et al., 2006) as well as other types of aggressive cancer (Tsitsiloni et al., 1993). Decreased PTMA slows intranuclear linker histone exchange between chromatin sites (George and Brown, 2010) altering cell proliferation and replication and in lean mice exposed to PFOA, ovarian PTMA was decreased potentially leading to ovotoxicity.
The switch/sucrose non-fermenting (SWI/SNF) complex includes SWI/SNF related, matrix associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4) (Hodges et al., 2016), and involved in chromatin remodeling to regulate DNA replication and repair (Hodges et al., 2016). Promotion of HR repair is facilitated by SMARCA4 (Kurashima et al., 2020) and in the ovary, alterations in SMARC4 have been associated with ovarian carcinomas (Agaimy et al., 2015; Moes-Sosnowska et al., 2015). Ovarian abundance of SMARCA4 is decreased by PFOA exposure in lean mice, potentially altering the capacity of the ovary to respond to DNA damage.
Several proteins associated with DDR were also noted to be altered by PFOA exposure in obese mice including MGA which is involved in meiosis in embryonic and germline stem cells (Suzuki et al., 2016). An Mga mRNA variant has been found in meiotic germ cells and round spermatids (Kitamura et al., 2021). During oogenesis, oocytes that arise from primordial germ cells become arrested in meiosis prophase I, and meiosis resumes in the oocyte at the time of ovulation (Bahr and Milich, 2013; Hirshfield, 1991). After meiosis I is completed, meiosis II starts; however, this is also arrested in the second meiotic metaphase until fertilization occurs (Bahr and Milich, 2013; Hirshfield, 1991). In obese mice exposed to PFOA, MGA ovarian abundance was increased potentially leading to negative meiotic effects.
The mediator complex which includes MED17 (Kikuchi et al., 2015) has essential roles in transcription factor regulation (Malik and Roeder, 2010). Transcription is positively regulated by MED17, including the NER pathway, functioning as a switch between transcription and DNA repair (Kikuchi et al., 2015). In obese mice, PFOA exposure increased ovarian abundance of MED17, potentially indicating sensing of DNA damage and activation of NER pathways.
RAD23 homolog B, NER protein (RAD23B) is part of the xeroderma pigmentosum protein C (XPC)-RAD23-Centrin 2 (CEN2) complex, which senses and recruits repair factors to sites with DNA lesions in the NER pathway (GG-NER) (Bergink et al., 2012; Ng et al., 2003). Expression of circular RNA Rad23b was high in ovarian cancer tissues (Yu et al., 2021). Relevant to reproduction, RAD23B has been associated with the proper embryonic development, placental formation, and spermatogenesis (Ng et al., 2002). In the obese mice, ovarian protein abundance of RAD23B was increased by PFOA exposure, which might indicate that there is an increase in DNA damage that might lead to ovarian cancer or other reproductive effects.
AHSG is a glycoprotein that inhibits insulin receptor tyrosine kinase and is associated with insulin resistance, diabetes type 2, and metabolic syndrome (Dabrowska et al., 2015). Increased plasma AHSG was associated with inactive ovaries during early lactation in dairy cows (Zhao et al., 2019). In this study, exposure to PFOA decreased ovarian abundance of AHSG in both lean and obese mice. Decreased AHSG might increase insulin signaling activating PI3K signaling, which regulates both viability (Brown et al., 2010) and activation of primordial follicles (Jagarlamudi et al., 2009; Liu et al., 2006; Reddy et al., 2005). Induction of low-grade inflammation can be induced by AHSG (Dabrowska et al., 2015), and PFAS levels have been linked with inflammation (Salihovic et al., 2020). Thus, there is potentially increased ovarian inflammation due to PFOA exposure in the lean and obese mice. The action of PI3K is antagonized by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) (Cantley, 2002). In the ovary, PTEN (oocyte-specific) null female mice undergo global primordial follicle activation (Jagarlamudi et al., 2009) and PFOA exposure increased PTEN abundance in obese mice. Thus, altered PI3K via PTEN caused by PFOA exposure could alter primordial follicle viability contributing to primordial follicle loss as documented recently (Yang et al., 2022).
In both lean and obese mice, PFOA exposure increased the abundance of CHERP and HPX but decreased AHSG and OGFR. Increased CHEPR could indicate endoplasmic reticulum stress due to PFOA exposure and within the ovary, this is documented to have roles in apoptosis (Huang et al., 2017) and reproductive dysfunction (Guzel et al., 2017). Increased ovarian levels of HPX was caused by PFOA exposure in both lean and obese mice, and although there is little documented regarding functions of ovarian HPX, it is an acute-phase protein that is present in follicular fluid (Angelucci et al., 2006) and higher levels of HPX precursor were identified in the follicular fluid of older compared with young women (Hashemitabar et al., 2014), potentially indicating a role in ovarian aging. Exposure to PFOA decreased the abundance of the OGFR in both lean and obese mice. The opioid growth factor (OGF) and the OGFR axis have decreased abundance in ovarian cancer (Fanning et al., 2012). The OGF delays the cell cycle (Fanning et al., 2012), potentially facilitating DNA repair, and also has roles in ovarian steroidogenesis (Kaminski et al., 2004). Exposure to PFOA has been determined to negatively impact steroidogenesis, with the OGF-OGFR being a potential mediator of this effect.
In summary, PFOA exposure differentially altered several aspects of ovarian biology between lean and obese mice, indicating an influence of body composition on PFOA-induced reproductive toxicity as we have noted with other chemicals (Clark et al., 2020; Ganesan et al., 2014, 2017; González-Alvarez et al., 2021; Nteeba et al., 2014a). Exposure to PFOA did not impact feed intake, body weight, or estrous cyclicity in either the lean or obese mice. In lean mice, ovarian weight was lower than control-treated mice with lack of this impact in the obese mice. In obese mice, PFOA exposure increased E2 level, albeit from a lower control level than in the lean mice. The DNA damage sensing and repair as well as reproductive protein response to PFOA exposure also differed between lean and obese mice. Taken together, these data help to provide an insight in possible mechanisms in which PFOA may induce ovarian toxicity and that the difference in the physiological status causes differences in the ovotoxicity induced by PFOA. It is recognized that enhanced geographical and ingestion PFOA/PFAS surveillance is needed to establish human intake exposures in both nonoccupational and occupational situations to better design studies upon which to base reproductive risk assessment.
FUNDING
Bailey Career Development Grant from Iowa State University to A.F.K.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors do not have any conflicts of interest.
Contributor Information
Maria Estefanía González-Alvarez, Department of Animal Science and Interdepartmental Toxicology Graduate Program, Iowa State University, Ames, Iowa 50011, USA.
Andrew Severin, Department of Animal Science and Interdepartmental Toxicology Graduate Program, Iowa State University, Ames, Iowa 50011, USA.
Maryam Sayadi, Department of Animal Science and Interdepartmental Toxicology Graduate Program, Iowa State University, Ames, Iowa 50011, USA.
Aileen F Keating, Department of Animal Science and Interdepartmental Toxicology Graduate Program, Iowa State University, Ames, Iowa 50011, USA.
REFERENCES
- Agaimy A., Thiel F., Hartmann A., Fukunaga M. (2015). Smarca4-deficient undifferentiated carcinoma of the ovary (small cell carcinoma, hypercalcemic type): Clinicopathologic and immunohistochemical study of 3 cases. Ann. Diagn. Pathol. 19, 283–287. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). (2021). Toxicological profile for perfluoroalkyls. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [PubMed]
- Andersen C. S., Fei C., Gamborg M., Nohr E. A., Sørensen T. I. A., Olsen J. (2010). Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am. J. Epidemiol. 172, 1230–1237. [DOI] [PubMed] [Google Scholar]
- Andersen C. S., Fei C., Gamborg M., Nohr E. A., Sørensen T. I. A., Olsen J. (2013). Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am. J. Epidemiol. 178, 921–927. [DOI] [PubMed] [Google Scholar]
- Angelucci S., Ciavardelli D., Di Giuseppe F., Eleuterio E., Sulpizio M., Tiboni G. M., Giampietro F., Palumbo P., Di Ilio C. (2006). Proteome analysis of human follicular fluid. Biochim. Biophys. Acta. 1764, 1775–1785. [DOI] [PubMed] [Google Scholar]
- Apelberg B. J., Witter F. R., Herbstman J. B., Calafat A. M., Halden R. U., Needham L. L., Goldman L. R. (2007). Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect. 115, 1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D., Saugstad O. D., Henriksen T., Tonstad S. (2014). Maternal body mass index and the risk of fetal death, stillbirth, and infant death: A systematic review and meta-analysis. JAMA 311, 1536–1546. [DOI] [PubMed] [Google Scholar]
- Bach C. C., Bech B. H., Brix N., Nohr E. A., Bonde J. P. E., Henriksen T. B. (2015). Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Crit. Rev. Toxicol. 45, 53–67. [DOI] [PubMed] [Google Scholar]
- Bahr J. M., Milich K. M. (2013). Ovarian physiology. In Ovarian Toxicology, (P. B. Hoyer, Ed.), p. 313. CRC Press, Boca Raton. [Google Scholar]
- Barrett E. S., Chen C., Thurston S. W., Haug L. S., Sabaredzovic A., Fjeldheim F. N., Frydenberg H., Lipson S. F., Ellison P. T., Thune I. (2015). Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil. Steril. 103, 1261–1270.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar J., Garcia-Saez I., Boopathi R., Cutter A. R., Papai G., Reymer A., Syed S. H., Lone I. N., Tonchev O., Crucifix C., et al. (2017). Structure and dynamics of a 197 BP nucleosome in complex with linker histone h1. Mol. Cell. 66, 384–397.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. M., De Guise S., McCutcheon J. R., Lei Y., Levin M., Li B., Rusling J. F., Lawrence D. A., Cavallari J. M., O’Connell C., et al. (2021). Exposure, health effects, sensing, and remediation of the emerging pfas contaminants - scientific challenges and potential research directions. Sci. Total Environ. 780, 146399. [DOI] [PubMed] [Google Scholar]
- Bergink S., Toussaint W., Luijsterburg M. S., Dinant C., Alekseev S., Hoeijmakers J. H., Dantuma N. P., Houtsmuller A. B., Vermeulen W. (2012). Recognition of DNA damage by XPC coincides with disruption of the XPC-RAD23 complex. J. Cell Biol. 196, 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., LaRocca J., Pietruska J., Ota M., Anderson L., Smith S. D., Weston P., Rasoulpour T., Hixon M. L. (2010). Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKB alpha/Akt1. Biol. Reprod. 82, 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers S. L., Wiles M. V., Dunn S. L., Taft R. A. (2012). Mouse estrous cycle identification tool and images. PLoS One 7, e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C. S. (2009). Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C. (2002). The phosphoinositide 3-kinase pathway. Science 296, 1655–1657. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2021a). Fourth National Report on Human Exposure to Environmental Chemicals: Volume Two: NHANES 2011–2016. Atlanta, GA: CDC.
- Centers for Disease Control and Prevention. (2021b). Fourth National Report on Human Exposure to Environmental Chemicals: Volume One: NHANES 1999–2010. Atlanta, GA: CDC.
- Chang E. T., Adami H.-O., Boffetta P., Cole P., Starr T. B., Mandel J. S. (2014). A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit. Rev. Toxicol. 44, 1–81. [DOI] [PubMed] [Google Scholar]
- Chaparro-Ortega A., Betancourt M., Rosas P., Vázquez-Cuevas F. G., Chavira R., Bonilla E., Casas E., Ducolomb Y. (2018). Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicol. In Vitro 46, 86–93. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhou L., Xu J., Zhang L., Li M., Xie X., Xie Y., Luo D., Zhang D., Yu X., et al. (2017). Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod. Toxicol. 69, 159–166. [DOI] [PubMed] [Google Scholar]
- Chu S. Y., Callaghan W. M., Kim S. Y., Schmid C. H., Lau J., England L. J., Dietz P. M. (2007). Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 30, 2070–2076. [DOI] [PubMed] [Google Scholar]
- Clark K. L., Keating A. F. (2020). Ataxia-telangiectasia mutated coordinates the ovarian DNA repair and atresia-initiating response to phosphoramide mustard. Biol. Reprod. 102, 248–260. [DOI] [PubMed] [Google Scholar]
- Clark K. L., Roach C. M., Keating A. F. (2020). Obesity alters the ovarian DNA damage response and apoptotic proteins. Reproduction 160, 751–760. [DOI] [PubMed] [Google Scholar]
- Clark K. L., Talton O. O., Ganesan S., Schulz L. C., Keating A. F. (2019). Developmental origins of ovarian disorder: Impact of maternal lean gestational diabetes on the offspring ovarian proteome in mice. Biol. Reprod. 101, 771–781. [DOI] [PubMed] [Google Scholar]
- Dabrowska A. M., Tarach J. S., Wojtysiak-Duma B., Duma D. (2015). Fetuin-a (AHSG) and its usefulness in clinical practice. Review of the literature. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 159, 352–359. [DOI] [PubMed] [Google Scholar]
- Ding N., Harlow S. D., Randolph J. F. Jr, Loch-Caruso R., Park S. K. (2020). Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 26, 724–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D., Reed C. E., Moore A. B., Gibbs-Flournoy E. A., Hines E. P., Wallace E. A., Stanko J. P., Lu Y., Jefferson W. N., Newbold R. R., et al. (2012). Histopathologic changes in the uterus, cervix and vagina of immature CD-1 mice exposed to low doses of perfluorooctanoic acid (PFOA) in a uterotrophic assay. Reprod. Toxicol. 33, 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez A., Salazar Z., Arenas E., Betancourt M., Ducolomb Y., Gonzalez-Marquez H., Casas E., Teteltitla M., Bonilla E. (2016). Effect of perfluorooctane sulfonate on viability, maturation and gap junctional intercellular communication of porcine oocytes in vitro. Toxicol. In Vitro 35, 93–99. [DOI] [PubMed] [Google Scholar]
- Du G., Hu J., Huang Z., Yu M., Lu C., Wang X., Wu D. (2019). Neonatal and juvenile exposure to perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS): Advance puberty onset and kisspeptin system disturbance in female rats. Ecotoxicol. Environ. Saf. 167, 412–421. [DOI] [PubMed] [Google Scholar]
- Duhl D. M., Stevens M. E., Vrieling H., Saxon P. J., Miller M. W., Epstein C. J., Barsh G. S. (1994). Pleiotropic effects of the mouse lethal yellow (Ay) mutation explained by deletion of a maternally expressed gene and the simultaneous production of agouti fusion RNAs. Development 120, 1695–1708. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA). (2008). Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts scientific opinion of the panel on contaminants in the food chain. EFSA J. 6, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley K. J., Purdy R., Kearns S., Anderson S. E., Meade B. (2007). Exposure to the immunosuppresant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol. Sci. 97, 375–383. [DOI] [PubMed] [Google Scholar]
- Fanning J., Hossler C. A., Kesterson J. P., Donahue R. N., McLaughlin P. J., Zagon I. S. (2012). Expression of the opioid growth factor-opioid growth factor receptor axis in human ovarian cancer. Gynecol. Oncol. 124, 319–324. [DOI] [PubMed] [Google Scholar]
- Fei C., McLaughlin J. K., Lipworth L., Olsen J. (2009). Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 24, 1200–1205. [DOI] [PubMed] [Google Scholar]
- Fei C., McLaughlin J. K., Tarone R. E., Olsen J. (2007). Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ. Health Perspect. 115, 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Holbrook N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. [DOI] [PubMed] [Google Scholar]
- Fortune J. E., Cushman R. A., Wahl C. M., Kito S. (2000). The primordial to primary follicle transition. Mol. Cell. Endocrinol. 163, 53–60. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. (2003). DNA damage and repair. Nature 421, 436–440. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Keating A. F. (2016). The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol. Appl. Pharmacol. 292, 65–74. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Nteeba J., Keating A. F. (2014). Enhanced susceptibility of ovaries from obese mice to 7,12-dimethylbenz[a]anthracene-induced DNA damage. Toxicol. Appl. Pharmacol. 281, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S., Nteeba J., Madden J. A., Keating A. F. (2017). Obesity alters phosphoramide mustard-induced ovarian DNA repair in mice. Biol. Reprod. 96, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E. M., Brown D. T. (2010). Prothymosin alpha is a component of a linker histone chaperone. FEBS Lett. 584, 2833–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marquez J., Rodríguez P. (1998). Prothymosin alpha is a chromatin-remodelling protein in mammalian cells. Biochem. J. 333, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alvarez M. E., McGuire B. C., Keating A. F. (2021). Obesity alters the ovarian proteomic response to zearalenone exposure. Biol. Reprod. 105, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F., Goldman M. B., Cramer D. W. (1994). Body mass index and ovulatory infertility. Epidemiology 5, 247–250. [DOI] [PubMed] [Google Scholar]
- Guzel E., Arlier S., Guzeloglu-Kayisli O., Tabak M. S., Ekiz T., Semerci N., Larsen K., Schatz F., Lockwood C. J., Kayisli U. A. (2017). Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int. J. Mol. Sci. 18, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllenhammar I., Diderholm B., Gustafsson J., Berger U., Ridefelt P., Benskin J. P., Lignell S., Lampa E., Glynn A. (2018). Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environ. Int. 111, 191–199. [DOI] [PubMed] [Google Scholar]
- Hales C. M., Carroll M. D., Fryar C. D., Ogden C. L. (2017). Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS data brief, no 288. National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- Hales C. M., Carroll M. D., Fryar C. D., Ogden C. L. (2020). Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief, no 360. National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- Hashemitabar M., Bahmanzadeh M., Mostafaie A., Orazizadeh M., Farimani M., Nikbakht R. (2014). A proteomic analysis of human follicular fluid: Comparison between younger and older women with normal FSH levels. Int. J. Mol. Sci. 15, 17518–17540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan A. L., Cunningham T. K., Drage D. S., Aylward L. L., Thompson K., Vijayasarathy S., Mueller J. F., Atkin S. L., Sathyapalan T. (2018). Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int. J. Hyg. Environ. Health 221, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Hergeth S. P., Schneider R. (2015). The h1 linker histones: Multifunctional proteins beyond the nucleosomal core particle. EMBO Rep. 16, 1439–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield A. N. (1991). Development of follicles in the mammalian ovary. Int. Rev. Cytol. 124, 43–101. [DOI] [PubMed] [Google Scholar]
- Hodges C., Kirkland J. G., Crabtree G. R. (2016). The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb. Perspect. Med. 6, a026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H. (2001). Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374. [DOI] [PubMed] [Google Scholar]
- Høyer B. B., Ramlau-Hansen C. H., Vrijheid M., Valvi D., Pedersen H. S., Zviezdai V., Jönsson B. A. G., Lindh C. H., Bonde J. P., Toft G. (2015). Anthropometry in 5- to 9-year-old Greenlandic and Ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ. Health Perspect. 123, 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer P. B. (2005). Damage to ovarian development and function. Cell Tissue Res. 322, 99–106. [DOI] [PubMed] [Google Scholar]
- Hoyer P. B. 2010. 11.16—female reproductive toxicology. In Comprehensive Toxicology (McQueen C. A., Ed.), 2nd ed., pp. 339–345. Elsevier, Oxford. [Google Scholar]
- Hoyer P. B., Keating A. F. (2014). Xenobiotic effects in the ovary: Temporary versus permanent infertility. Expert Opin. Drug Metab. Toxicol. 10, 511–523. [DOI] [PubMed] [Google Scholar]
- Huang N., Yu Y., Qiao J. (2017). Dual role for the unfolded protein response in the ovary: Adaption and apoptosis. Protein Cell. 8, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagarlamudi K., Liu L., Adhikari D., Reddy P., Idahl A., Ottander U., Lundin E., Liu K. (2009). Oocyte-specific deletion of PTEN in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One 4, e6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. A., Leffers H. (2008). Emerging endocrine disrupters: Perfluoroalkylated substances. Int. J. Androl. 31, 161–169. [DOI] [PubMed] [Google Scholar]
- Jungheim E. S., Schoeller E. L., Marquard K. L., Louden E. D., Schaffer J. E., Moley K. H. (2010). Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 151, 4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski T., Siawrys C., Bogacka I., Okrasa S., Przala J. (2004). The influence of opioid peptides on steroidogenesis in porcine granulosa cells. Reprod. Domest. Anim. 39, 25–32. [DOI] [PubMed] [Google Scholar]
- Karetsou Z., Martic G., Tavoulari S., Christoforidis S., Wilm M., Gruss C., Papamarcaki T. (2004). Prothymosin α associates with the oncoprotein set and is involved in chromatin decondensation. FEBS Lett. 577, 496–500. [DOI] [PubMed] [Google Scholar]
- Karetsou Z., Sandaltzopoulos R., Frangou-Lazaridis M., Lai C. Y., Tsolas O., Becker P. B., Papamarcaki T. (1998). Prothymosin alpha modulates the interaction of histone H1 with chromatin. Nucleic Acids Res. 26, 3111–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano-Toki A., Satoh T., Tomaru T., Yoshino S., Ishizuka T., Ishii S., Ozawa A., Shibusawa N., Tsuchiya T., Saito T., et al. (2013). THRAP3 interacts with HELZ2 and plays a novel role in adipocyte differentiation. Mol. Endocrinol. 27, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating A. F., Hoyer P. B. (2009). Mechanisms of reproductive toxicity. In Chapter 24: Drug Metabolism in Pharmaceuticals; Concepts and Applications (A. F. Nassar, Ed.), pp. 697–734. John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- Keating A. F., J. Mark C., Sen N., Sipes I. G., Hoyer P. B. (2009). Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol. Appl. Pharmacol. 241, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper R. A., Nabb D. L. (2005). In vitro studies in microsomes from rat and human liver, kidney, and intestine suggest that perfluorooctanoic acid is not a substrate for microsomal UDP-glucuronosyltransferases. Drug Chem. Toxicol. 28, 281–287. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Umemura H., Nishitani S., Iida S., Fukasawa R., Hayashi H., Hirose Y., Tanaka A., Sugasawa K., Ohkuma Y. (2015). Human mediator med17 subunit plays essential roles in gene regulation by associating with the transcription and DNA repair machineries. Genes Cells 20, 191–202. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Uranishi K., Hirasaki M., Nishimoto M., Suzuki A., Okuda A. (2021). Identification of germ cell-specific Mga variant mRNA that promotes meiosis via impediment of a non-canonical PRC1. Sci. Rep. 11, 9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebig M. L., Wilkinson J. E., Geisler J. G., Woychik R. P. (1995). Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc. Natl. Acad. Sci. U.S.A. 92, 4728–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenov V., Flor S., Ganesan S., Adur M., Eti N., Iqbal K., Soares M. J., Ludewig G., Ross J. W., Robertson L. W., et al. (2021). The aryl hydrocarbon receptor mediates reproductive toxicity of polychlorinated biphenyl congener 126 in rats. Toxicol. Appl. Pharmacol. 426, 115639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox S. S., Jackson T., Javins B., Frisbee S. J., Shankar A., Ducatman A. M. (2011). Implications of early menopause in women exposed to perfluorocarbons. J. Clin. Endocrinol. Metab. 96, 1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima K., Kashiwagi H., Shimomura I., Suzuki A., Takeshita F., Mazevet M., Harata M., Yamashita T., Yamamoto Y., Kohno T., et al. (2020). SMARCA4 deficiency-associated heterochromatin induces intrinsic DNA replication stress and susceptibility to ATR inhibition in lung adenocarcinoma. NAR Cancer. 2, zcaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. (2012). Perfluoroalkyl acids: Recent research highlights. Reprod. Toxicol. 33, 405–409. [DOI] [PubMed] [Google Scholar]
- Li P., Lu G., Wang L., Cui Y., Wu Z., Chen S., Li J., Wen X., Zhang H., Mu S., et al. (2016). A rare nonsynonymous variant in the lipid metabolic gene HELZ2 related to primary biliary cirrhosis in Chinese Han. Allergy Asthma Clin. Immunol. 12, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fletcher T., Mucs D., Scott K., Lindh C. H., Tallving P., Jakobsson K. (2018). Half-lives of PFOS, PFHXS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 75, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Ramdhan D. H., Naito H., Yamagishi N., Ito Y., Hayashi Y., Yanagiba Y., Okamura A., Tamada H., Gonzalez F. J., et al. (2011). Ammonium perfluorooctanoate may cause testosterone reduction by adversely affecting testis in relation to PPARα. Toxicol. Lett. 205, 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom A. B., Strynar M. J., Libelo E. L. (2011). Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 45, 7954–7961. [DOI] [PubMed] [Google Scholar]
- Liu K., Rajareddy S., Liu L., Jagarlamudi K., Boman K., Selstam G., Reddy P. (2006). Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Dev. Biol. 299, 1–11. [DOI] [PubMed] [Google Scholar]
- Lopez-Arellano P., Lopez-Arellano K., Luna J., Flores D., Jimenez-Salazar J., Gavia G., Teteltitla M., Rodriguez J. J., Dominguez A., Casas E., et al. (2019). Perfluorooctanoic acid disrupts gap junction intercellular communication and induces reactive oxygen species formation and apoptosis in mouse ovaries. Environ. Toxicol. 34, 92–98. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M. J., Fletcher T., Armstrong B., Genser B., Dhatariya K., Mondal D., Ducatman A., Leonardi G. (2011). Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ. Sci. Technol. 45, 8160–8166. [DOI] [PubMed] [Google Scholar]
- Lou I., Wambaugh J. F., Lau C., Hanson R. G., Lindstrom A. B., Strynar M. J., Zehr R. D., Setzer R. W., Barton H. A. (2009). Modeling single and repeated dose pharmacokinetics of PFOA in mice. Toxicol. Sci. 107, 331–341. [DOI] [PubMed] [Google Scholar]
- Loveless S. E., Hoban D., Sykes G., Frame S. R., Everds N. E. (2008). Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol. Sci. 105, 86–96. [DOI] [PubMed] [Google Scholar]
- Lu D., Willard D., Patel I. R., Kadwell S., Overton L., Kost T., Luther M., Chen W., Woychik R. P., Wilkison W. O. (1994). Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371, 799–802. [DOI] [PubMed] [Google Scholar]
- Lum K. J., Sundaram R., Barr D. B., Louis T. A., Buck Louis G. M. (2017). Perfluoroalkyl chemicals, menstrual cycle length, and fecundity: Findings from a prospective pregnancy study. Epidemiology 28, 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngsø J., Ramlau-Hansen C. H., Høyer B. B., Støvring H., Bonde J. P., Jönsson B. A., Lindh C. H., Pedersen H. S., Ludwicki J. K., Zviezdai V., et al. (2014). Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: A cross-sectional study. Hum. Reprod. 29, 359–367. [DOI] [PubMed] [Google Scholar]
- Madden J. A., Hoyer P. B., Devine P. J., Keating A. F. (2014). Involvement of a volatile metabolite during phosphoramide mustard-induced ovotoxicity. Toxicol. Appl. Pharmacol. 277, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S., Roeder R. G. (2010). The metazoan mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 11, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison D. R. (1985). Clinical manifestations of ovarian toxicity. In Reproductive Toxicology (R. I. Dixon, Ed.), pp. 109–130. Raven Press, New York. [Google Scholar]
- McDonald S. D., Han Z., Mulla S., Beyene J.; on behalf of the Knowledge Synthesis Group (2010). Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: Systematic review and meta-analyses. BMJ 341, c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrzycki M., Zhang Y., McDonald J. F., Fan Y. (2012). Profiling of linker histone variants in ovarian cancer. Front. Biosci. 17, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud E. J., Bultman S. J., Klebig M. L., van Vugt M. J., Stubbs L. J., Russell L. B., Woychik R. P. (1994). A molecular model for the genetic and phenotypic characteristics of the mouse lethal yellow (Ay) mutation. Proc. Natl. Acad. Sci. U.S.A. 91, 2562–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud E. J., Bultman S. J., Stubbs L. J., Woychik R. P. (1993). The embryonic lethality of homozygous lethal yellow mice (Ay/Ay) is associated with the disruption of a novel RNA-binding protein. Genes Dev. 7, 1203–1213. [DOI] [PubMed] [Google Scholar]
- Moes-Sosnowska J., Szafron L., Nowakowska D., Dansonka-Mieszkowska A., Budzilowska A., Konopka B., Plisiecka-Halasa J., Podgorska A., Rzepecka I. K., Kupryjanczyk J. (2015). Germline SMARCA4 mutations in patients with ovarian small cell carcinoma of hypercalcemic type. Orphanet J. Rare Dis. 10, 32–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy R., Morrison K., Teo K., Atkinson S., Kubwabo C., Stewart B., Foster W. G. (2008). Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ. Res. 108, 56–62. [DOI] [PubMed] [Google Scholar]
- Mora A. M., Oken E., Rifas-Shiman S. L., Webster T. F., Gillman M. W., Calafat A. M., Ye X., Sagiv S. K. (2017). Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ. Health Perspect. 125, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J. M., Vermeulen W., van der Horst G. T., Bergink S., Sugasawa K., Vrieling H., Hoeijmakers J. H. (2003). A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev. 17, 1630–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J. M., Vrieling H., Sugasawa K., Ooms M. P., Grootegoed J. A., Vreeburg J. T., Visser P., Beems R. B., Gorgels T. G., Hanaoka F., et al. (2002). Developmental defects and male sterility in mice lacking the ubiquitin-like DNA repair gene mHR23B. Mol. Cell. Biol. 22, 1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan L. A., Nolan J. M., Shofer F. S., Rodway N. V., Emmett E. A. (2009). The relationship between birth weight, gestational age and perfluorooctanoic acid (PFOA)-contaminated public drinking water. Reprod. Toxicol. 27, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorlander C. W., van Leeuwen S. P., Te Biesebeek J. D., Mengelers M. J., Zeilmaker M. J. (2011). Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in The Netherlands. J. Agric. Food Chem. 59, 7496–7505. [DOI] [PubMed] [Google Scholar]
- Nteeba J., Ganesan S., Keating A. F. (2014a). Impact of obesity on ovotoxicity induced by 7,12-dimethylbenz[a]anthracene in mice. Biol. Reprod. 90, 68–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nteeba J., Ganesan S., Keating A. F. (2014b). Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol. Reprod. 91, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nteeba J., Ganesan S., Madden J. A., Dickson M. J., Keating A. F. (2017). Progressive obesity alters ovarian insulin, phosphatidylinositol-3 kinase, and chemical metabolism signaling pathways and potentiates ovotoxicity induced by phosphoramide mustard in mice. Biol. Reprod. 96, 478–490. [DOI] [PubMed] [Google Scholar]
- Nteeba J., Ross J. W., Perfield J. W. 2nd, Keating A. F. (2013). High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression. Reprod. Toxicol. 42, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden T. L. (2010). Handling results below the level of detection. Ann. Occup. Hyg. 54, 255–256. [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Burris J. M., Ehresman D. J., Froehlich J. W., Seacat A. M., Butenhoff J. L., Zobel L. R. (2007). Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). ( 2017). Obesity update 2017. Available at: https://www.oecd.org/health/obesity-update.html.
- Park S. K., Peng Q., Ding N., Mukherjee B., Harlow S. D. (2019). Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in pfas exposure. Environ. Res. 175, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R., Casimirri F. (1993). The impact of obesity on hyperandrogenism and polycystic ovary syndrome in premenopausal women. Clin. Endocrinol. 39, 1–16. [DOI] [PubMed] [Google Scholar]
- Pasquali R., Pelusi C., Genghini S., Cacciari M., Gambineri A. (2003). Obesity and reproductive disorders in women. Hum. Reprod. Update. 9, 359–372. [DOI] [PubMed] [Google Scholar]
- Post G. B., Cohn P. D., Cooper K. R. (2012). Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 116, 93–117. [DOI] [PubMed] [Google Scholar]
- Reddy P., Shen L., Ren C., Boman K., Lundin E., Ottander U., Lindgren P., Liu Y. X., Sun Q. Y., Liu K. (2005). Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev. Biol. 281, 160–170. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards J. W., Goldman M. B., Willett W. C., Hunter D. J., Stampfer M. J., Colditz G. A., Manson J. E. (1994). Adolescent body mass index and infertility caused by ovulatory disorder. Am. J. Obstet. Gynecol. 171, 171–177. [DOI] [PubMed] [Google Scholar]
- Rössner S. (2002). Obesity: The disease of the twenty-first century. Int. J. Obes. Relat. Metab. Disord. 26(Suppl 4), S2–4. [DOI] [PubMed] [Google Scholar]
- Salihovic S., Lind L., Larsson A., Lind P. M. (2020). Plasma perfluoroalkyls are associated with decreased levels of proteomic inflammatory markers in a cross-sectional study of an elderly population. Environ. Int. 145, 106099. [DOI] [PubMed] [Google Scholar]
- Smith G. C., Shah I., Pell J. P., Crossley J. A., Dobbie R. (2007). Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: A retrospective cohort study. Am. J. Public Health. 97, 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K., Fletcher T., Savitz D. A. (2010). Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect. 118, 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard K. J., Tennant P. W., Bell R., Rankin J. (2009). Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 301, 636–650. [DOI] [PubMed] [Google Scholar]
- Surapureddi S., Yu S., Bu H., Hashimoto T., Yeldandi A. V., Kashireddy P., Cherkaoui-Malki M., Qi C., Zhu Y.-J., Rao M. S., et al. (2002). Identification of a transcriptionally active peroxisome proliferator-activated receptor alpha-interacting cofactor complex in rat liver and characterization of PRIC285 as a coactivator. Proc. Natl. Acad. Sci. U.S.A. 99, 11836–11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Hirasaki M., Hishida T., Wu J., Okamura D., Ueda A., Nishimoto M., Nakachi Y., Mizuno Y., Okazaki Y., et al. (2016). Loss of max results in meiotic entry in mouse embryonic and germline stem cells. Nat. Commun. 7, 11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Takahashi S., Takahashi S., Takeshita K., Hikosaka A., Wakita T., Nishiyama N., Fujita T., Okamura T., Shirai T. (2006). Expression of prothymosin alpha is correlated with development and progression in human prostate cancers. Prostate 66, 463–469. [DOI] [PubMed] [Google Scholar]
- Trudel D., Horowitz L., Wormuth M., Scheringer M., Cousins I. T., Hungerbühler K. (2008). Estimating consumer exposure to PFOS and PFOA. Risk Anal. 28, 251–269. [DOI] [PubMed] [Google Scholar]
- Tsitsiloni O. E., Stiakakis J., Koutselinis A., Gogas J., Markopoulos C., Yialouris P., Bekris S., Panoussopoulos D., Kiortsis V., Voelter W. (1993). Expression of alpha-thymosins in human tissues in normal and abnormal growth. Proc. Natl. Acad. Sci. U.S.A. 90, 9504–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA). (2017). Technical fact sheet: Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Office of Land and Emergency Management.
- Vanden Heuvel J. P., Kuslikis B. I., Van Rafelghem M. J., Peterson R. E. (1991). Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J. Biochem. Toxicol. 6, 83–92. [DOI] [PubMed] [Google Scholar]
- Vélez M. P., Arbuckle T. E., Fraser W. D. (2015). Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Hum. Reprod. 30, 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins M. L., Rasmussen S. A., Honein M. A., Botto L. D., Moore C. A. (2003). Maternal obesity and risk for birth defects. Pediatrics 111, 1152–1158. [PubMed] [Google Scholar]
- Winship A. L., Griffiths M., Lliberos Requesens C., Sarma U., Phillips K. A., Hutt K. J. (2020). The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: Implications for fertility preservation. Hum. Reprod. 35, 1864–1874. [DOI] [PubMed] [Google Scholar]
- Wolf C. J., Fenton S. E., Schmid J. E., Calafat A. M., Kuklenyik Z., Bryant X. A., Thibodeaux J., Das K. P., White S. S., Lau C. S., et al. (2007). Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol. Sci. 95, 462–473. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2021). Obesity and overweight. Fact sheets. World Health Organization.
- Xia J., Sinelnikov I. V., Han B., Wishart D. S. (2015). Metaboanalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J., Wishart D. S. (2016). Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 55, 14.10.1–14.10.91. [DOI] [PubMed] [Google Scholar]
- Yang C., Tan Y. S., Harkema J. R., Haslam S. Z. (2009). Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57bl/6 and Balb/c mouse strains. Reprod. Toxicol. 27, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Lee Y., Gao L., Chiu K., Meling D. D., Flaws J. A., Warner G. R. (2022). Perfluorooctanoic acid (PFOA) disrupts ovarian steroidogenesis and folliculogenesis in adult mice. Toxicol. Sci. 186, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Xie Y., Alexson S. E. H., Dean Nelson B., DePierre J. W. (2002). Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem. Pharmacol. 63, 1893–1900. [DOI] [PubMed] [Google Scholar]
- Yang Z., Norwood K. A., Smith J. E., Kerl J. G., Wood J. R. (2012). Genes involved in the immediate early response and epithelial-mesenchymal transition are regulated by adipocytokines in the female reproductive tract. Mol. Reprod. Dev. 79, 128–137. [DOI] [PubMed] [Google Scholar]
- Yu J., An Y., Zou M., Li X., Dong X. (2021). Mechanism of circular RNA RAD23B for regulating glycolysis and proliferation of ovarian cancer cells by targeting microRNA-519b-3p. J. Biomater. Tissue Eng. 11, 1078–1083. [Google Scholar]
- Zenclussen M. L., Casalis P. A., Jensen F., Woidacki K., Zenclussen A. C. (2014). Hormonal fluctuations during the estrous cycle modulate heme oxygenase-1 expression in the uterus. Front. Endocrinol. (Lausanne) 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Tan R., Pan R., Xiong J., Tian Y., Wu J., Chen L. (2018). Association of perfluoroalkyl and polyfluoroalkyl substances with premature ovarian insufficiency in Chinese women. J. Clin. Endocrinol. Metab. 103, 2543–2551. [DOI] [PubMed] [Google Scholar]
- Zhao C., Shu S., Bai Y., Wang D., Xia C., Xu C. (2019). Plasma protein comparison between dairy cows with inactive ovaries and estrus. Sci. Rep. 9, 13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Tan Y. S., Haslam S. Z., Yang C. (2010). Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in c57bl/6 mice. Toxicol. Sci. 115, 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Zhang L., Tong C., Fang F., Zhao S., Tian Y., Tao Y., Zhang J.; Shanghai Birth Cohort Study (2017). Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ. Health Perspect. 125, 067012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zheng Y. (2013). Cell type-specific signaling function of RhoA GTPase: Lessons from mouse gene targeting. J. Biol. Chem. 288, 36179–36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hu L.-W., Qian Z., Chang J.-J., King C., Paul G., Lin S., Chen P.-C., Lee Y. L., Dong G.-H. (2016). Association of perfluoroalkyl substances exposure with reproductive hormone levels in adolescents: By sex status. Environ. Int. 94, 189–195. [DOI] [PubMed] [Google Scholar]