Abstract
Background:
Recently, attention has been paid to using plants with medicinal efficacy as alternates to antibiotics and growth promoters. Garlic is a traditional plant used as a spice and herb in broilers.
Aim:
To investigate the effect of garlic powder on broiler performance, carcass characteristics, blood hematology, and biochemistry.
Method:
A total of 240 1-day-old broiler chicks (Cobb500) were purchased from a local hatchery and randomly distributed into four dietary treatments. Each treatment comprised 5 replicates with 12 chicks each. Garlic powder (Allium sativum) was supplemented as follows: 0.0%, 0.1%, 0.2%, and 0.3%. The diets were formulated to be approximately isocaloric and cover all nutrient requirements for broilers throughout two stages of growth periods: starter diets (1–21 days) and finisher diets (22–42 days). At the end of the experiment, one bird was chosen from each replicate to measure the carcass characteristics and blood was collected.
Results:
The findings showed that birds fed a diet supplemented by 0.3% garlic powder was significantly (p < 0.05) better in terms of body weight, body weight gain, and feed conversion ratio compared to those birds fed 0%, 0.1%, or 0.2% garlic powder. In addition, feeding 0.1% or 0.2% garlic powder significantly increased (p < 0.05) packed cell volume, total white blood cells, neutrophil, eosinophil, monocyte, and lymphocyte compared to the control group. Furthermore, garlic supplementation led to a decrease in alanine aminotransferase, total cholesterol, triglycerides, and low density lipoprotein compared to the control. At the same time, high density lipoprotein was significantly increased (p < 0.05) by garlic supplementation.
Conclusion:
It can be concluded that the supplementation of 0.3% garlic powder as a growth promoter leads to reducing the cost of production through improving growth performance and enhancing birds’ health.
Keywords: Garlic, Broiler, Growth, Carcass characteristics, Hematology
Introduction
Poultry is an important source of animal protein, as it is considered an essential and effective sector in meeting the nutritional needs. The poultry industry has grown greatly in recent years and the poultry productivity has improved dramatically with high efficiency as a result of the advances and major efforts in applied research in several fields of science relevant to such industry. In recent years, plants with medicinal efficacy have been studied as alternatives to antibiotics and growth promoters (Kareem et al., 2018; Hameed, 2021). Recently, scientific articles have focused on employing natural feed supplements, such as organic acids and probiotics, to improve the gut health and intestinal microbiota of freshly hatched chicks (Gadde et al., 2017; Abo Ghanima et al., 2019; El-Hack et al., 2020; El-Moneim et al., 2020; Abou-Kassem et al., 2021).
Garlic supplementation has also been observed to contribute as a growth promoter and an immunological enhancer due to its biological capabilities, which include hepatoprotective, antioxidant, and antimicrobial actions (Karangiya et al., 2016; Sangilimadan et al., 2019).
Consumer demand for food that is believed to be fresh, wholesome, and free of hormones, antibiotics, dangerous chemicals, and produced in an environmentally friendly manner has risen in recent years. Furthermore, due to the residual effects of antibiotics as a growth promoter, a variety of feed additives have been utilized as an alternative to enhance the performance of birds. Phytogenic feed additives are plant-derived supplements used in animal nutrition to increase performance (Windisch et al., 2008). Garlic is one of the most traditional plants widely used as a spice and herb worldwide and it has been used for several purposes, including anti-atherosclerosis, antimicrobial, hypolipidemic, antithrombosis, antihypertension, antidiabetes, etc. (Mansoub, 2011). Garlic has a number of active ingredients, including ajoene, s-allyl cysteine, diallyl sulfide, and allicin (Rahmatnejad et al., 2009). Garlic, as a natural feed supplement, has been shown to enhance broiler performance and decrease mortality rate (Makwana et al., 2015; Karangiya et al., 2016; Puvača et al., 2019).
Garlic is classified as a wonder pharmaceutical in medicine because of its numerous health benefits. Garlic contains at least 33% of sulfur-containing substances, as well as a variety of enzymes, amino acids, and minerals. Garlic has been used as a significant feed supplement to enhance the growth performance and other biochemical characteristics of broiler chickens (Aarti and Khusro, 2020).
In general, results from previous studies of garlic and its derivatives in poultry showed increases in growth performance, hematological profile, product quality, immunological response, and antibacterial capabilities. Garlic, therefore, has a promising potential in both organic and traditional poultry processing (Singh et al., 2020). Consequently, the current feeding trial was conducted to investigate the effect of garlic powder on broiler performance, carcass characteristics, blood hematology, and biochemistry.
Materials and Methods
Birds and experimental diets
The current feeding trial was conducted at the Poultry Research Center, Faculty of Agriculture, Sebha University, Libya. The experiment was carried out at the broiler production unit. The garlic utilized in this study was purchased in raw form from a local market. It was then sliced into smaller pieces and allowed to dry in the sun. Fine grinding was used to dry the needed amount of garlic.
During the experiment, the in-house mean temperature ranged from 18.3°C to 32.8°C, with a relative humidity of 26%–81%. The research was conducted after approval by the Faculty of Agriculture for use of chicks and experimental methodologies.
A total of 240 1-day-old broiler chicks (Cobb500) were purchased from a local hatchery and distributed into four dietary groups at random. Each group was then split into 5 replicates with 12 birds in each group. Garlic powder was supplemented as follows: 0.0%, 0.1%, 0.2%, and 0.3%. The chicks were raised in ground pens (1.5 × 1.5 meters) with wood shavings mulch. The feeding program was separated into starter and finisher for 21 and 42 days, respectively (Table 1). According to the National Research Council (NRC, 1994), all mixes were formulated to meet the nutritional requirements. The feed and water were provided ad libitum. Vaccines against the prevalent infectious diseases were provided to all chicks. The body weight was weighed individually and feed consumption was documented weekly for each replicate. Body weight gain (BWG) and feed conversion ratio (FCR) were estimated accordingly.
Table 1. Ingredients and chemical composition of broiler diets (% as fed).
| Ingredients (kg) | Starter 1–21 days | Grower 21–42 days |
|---|---|---|
| Yellow corn | 553.00 | 601.00 |
| Soybean meal 44% | 310.00 | 262.00 |
| Corn gluten meal | 80.00 | 80.00 |
| Dicalcium phosphate | 15.00 | 15.00 |
| Lime stone | 13.00 | 13.00 |
| Salt (NaCl) | 3.5 | 3.5 |
| Veg. oil | 20.00 | 20.00 |
| L-lysine | 0.92 | 0.20 |
| DL-Methionine | 1.58 | 2.30 |
| Vitamin and mineral premixa | 3.00 | 3.00 |
| Total | 1,000 | 1,000 |
| Calculated analysis | ||
| Crude protein (%) | 23.46 | 21.3 |
| ME (kcal/ kg) | 3149 | 3285 |
| C/P | 134 | 154 |
| Fat | 5.8 | 6.4 |
| Crude fiber (%) | 2.44 | 2.63 |
| Calcium (%) | 1.02 | 0.98 |
| Phosphorus | 0.50 | 0.50 |
| Methionine (%) | 0.45 | 0.43 |
| Lysine (%) | 1.19 | 1.07 |
Premix each kg contains Vit A = 8,25,000 IU; Vit D3 = 1,20,000 IU; Vit K = 100 mg; riboflavin = 500 mg; thiamine = 80 mg; pyridoxine = 160 mg; Vit E = 800 mg; cyanocobalamine = 100 mcg; niacin = 1,200 mg; calcium pantothenate = 80 mg; manganese sulfate = 25 g; ferrous sulfate = 10 g; copper sulfate = 500 mg; zinc oxide = 8 g; potassium iodide = 100 mg; and coccidiostat = 60 g.
Blood samples
At the end of the experiment, blood samples were taken from five birds from each treatment (one bird per replicate). A sterile injector was used to draw blood samples from the wing vein. Then, samples were transferred into either vacuum or K3 EDTA vacuum tube. All samples were centrifuged at 3,000 g for 15 minutes and the plasma was decanted into vials and kept at −20°C until analysis (Alshelmani et al., 2017; Abdulla et al., 2019).
Measurement of blood hematology
An automatic blood analyzer (BC 3,200, Nanshan, Shenzhen 518,057, P.R. China) was used to determine the concentrations of white blood cells (WBC), red blood cells (RBC), packed cell volume (PCV), and lymphocytes in whole blood samples.
Measurement of blood biochemistry
The concentrations of total protein, albumin, globulin, glucose, and cholesterol in serum samples were determined by an automatic biochemical analyzer (Spectrophotometer V 1.0; Revision for Alpha-1,101, 1,102, 1,502; Laxco Inc.).
Slaughter characteristics
Five birds from each treatment were randomly chosen at the end of the feeding trial (42 days of age). The birds were fasted overnight and the pre-slaughter weights were recorded. They were slaughtered by halal neck cut (Alshelmani et al., 2016).
Statistical analysis
All data were subjected to statistical analysis using the general linear model procedure of statistical analysis system (SAS, 2003). Data were analyzed using one-way ANOVA and the experimental design was based on a completely randomized design. Tukey’s test was carried out to compare the means of treatment at a 5% probability (p < 0.05). The statistical model used for the feeding trial was Yijk = μ + Tij + Eijk, where Yijk = observation; μ = population mean; Tij = the effect of diet (garlic powder); and Eijk = experimental error.
Ethical approval
The feeding trial was approved by the Department of Animal Production, Faculty of Agriculture, Sebha University, Libya. All animal welfare protocols were followed.
Results
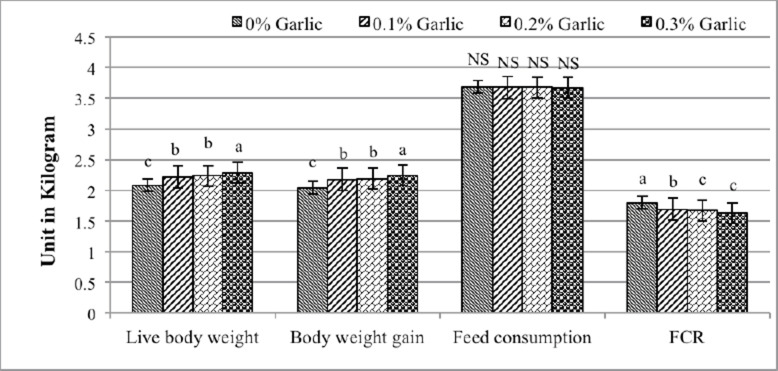
Figure 1 shows the effect of garlic supplementation on broiler performance. The findings show that the birds provided food supplemented with 0.3% garlic powder significantly (p < 0.05) improved in terms of live body weight, BWG, and FCR compared to those groups fed diets supplemented with 0%, 0.1%, and 0.2% garlic powder. In terms of feed consumption, there were no significant changes across the dietary treatments.
Fig. 1. Effect of garlic supplementation on broiler performance. a, b, and c = mean ± SE. Means with different letters in the same cluster are significantly different (p < 0.05).
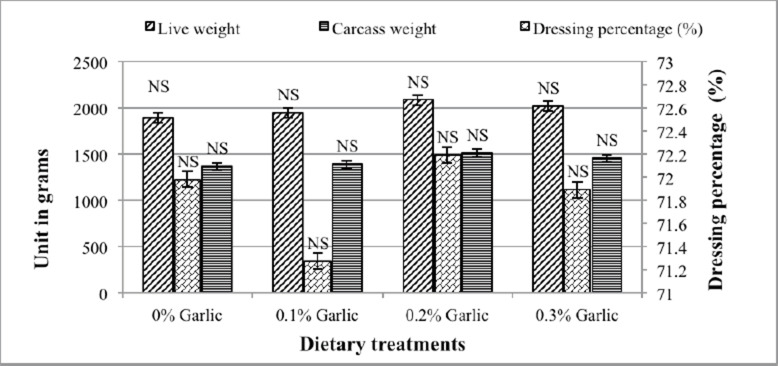
Figure 2 shows the effect of garlic supplementation on dressing percentage. The findings showed no significant effect (p > 0.05) on dress percentage among the dietary treatments.
Fig. 2. Effect of garlic supplementation on dressing percentage. NS: Nonsignificant.
The effect of garlic supplementation on hematological parameters is shown in Table 2. The results show that feeding diets supplemented with 0.1% and 0.2% garlic powder had significantly higher PCV (p < 0.05) than the control group. In addition, as compared to the control group, the broilers fed a diet supplemented with garlic powder exhibited a significant increase (p < 0.05) in total WBC, neutrophil, eosinophil, monocyte, and lymphocyte.
Table 2. Effect of garlic supplementation on hematological parameters.
| Parameters | Garlic level | p-value | |||
|---|---|---|---|---|---|
| 0% | 0.1% | 0.2% | 0.3% | ||
| Hemoglobin (g/dl) | 8.60 ± 0.32 | 9.12 ± 0.31 | 9.50 ± 0.39 | 8.74 ± 0.38 | NS |
| PCV (%) | 20.54a±0.63 | 23.60b ± 0.75 | 23.72bc ±1.24 | 22.20abc ±0.68 | * |
| Total RBC (million/ mm3) | 2.10 ± 0.05 | 2.11 ± 0.05 | 2.09 ± 0.06 | 2.02 ± 0.05 | NS |
| Total WBC (million/ mm3) | 70.12a ± 1.82 | 71.52ab ± 1.93 | 84.69c ± 2.18 | 84.11c ± 1.46 | ** |
| Neutrophil (million/ mm3) | 4.15a ± 0.14 | 4.31ab ± 0.13 | 4.51abc ± 0.11 | 4.68c ± 0.11 | * |
| Eosinophil (million/ mm3) | 0.51a ± 0.07 | 0.62ab ± 0.06 | 0.75c ± 0.09 | 0.88c ± 0.09 | * |
| Monocyte (million/ mm3) | 0.90a ± 0.03 | 0.98ab ± 0.03 | 1.07c ± 0.06 | 1.11c ± 0.03 | ** |
| Lymphocyte (million/mm3 | 63.19a ± 1.78 | 65.26ab ± 1.65 | 69.58c ± 1.00 | 70.89c ± 0.97 | ** |
a,b,cMean ± SE. Means with different superscripts in the same row are significantly different (p < 0.05).
Table 3 shows the effect of garlic supplementation on biochemical parameters. The findings show that feeding diets supplemented with garlic powder led to a significant decrease (p < 0.05) in alanine aminotransferase (ALT), total cholesterol, triglycerides, and low density lipoprotein (LDL) compared to the control group. On the contrary, the group of birds fed a diet supplemented with garlic powder exhibited a significant increase (p < 0.05) in high density lipoprotein (HDL) compared to the control group.
Table 3. Effect of garlic supplementation on biochemical parameters.
| Parameters | Garlic level | p-value | |||
|---|---|---|---|---|---|
| 0% | 0.1% | 0.2% | 0.3% | ||
| Serum glucose (mmol/l | 247.20 ±8.62 | 242.00 ±6.82 | 246.00 ± 1.09 | 243.20 ± 2.78 | NS |
| ALT (U/ml) | 25.29a ± 0.74 | 25.02ab ± 0.39 | 22.27c ± 0.55 | 24.32ab ± 0.31 | * |
| Total cholesterol (mg/dl) | 164.01a±2.66 | 145.48b ± 6.65 | 109.89c ± 2.25 | 108.48c ± 2.23 | ** |
| Triglycerides (mg/dl) | 102.74a±2.91 | 80.84b ± 2.18 | 52.74c ± 2.76 | 49.43c ± 3.11 | ** |
| HDL (mg/dl) | 46.42a ± 3.99 | 56.47ab ± 10.36 | 81.02c ± 3.57 | 82.13c ± 3.46 | ** |
| LDL (mg/dl) | 95.85a ± 4.88 | 60.59b ± 7.59 | 17.27c ± 3.85 | 15.21c ± 4.31 | ** |
a,b,c Mean ± SE. Means with different superscripts in the same row are significantly different (p < 0.05).
Discussion
The findings are in line with Makwana et al. (2015), Makwana et al. (2019), Chitra (2020), and Hayat et al. (2022), who found that supplementing garlic powder to the basal diet of broilers improved growth performance. The improvement in broiler performance could be attributed to the antimicrobial and antioxidant properties of garlic (Chitra, 2020). Therefore, garlic was considered to be one of the significant alternatives to antibiotics as growth promoters in monogastric (Chitra, 2020). It is reported by Taufik and Maruddin (2019), Chitra (2020) that birds fed a diet supplemented with garlic powder improved villus height and goblet cells in the small intestines. Consequently, there was an efficient increase in nutrient absorption. Similar findings were reported by Kirubakaran et al. (2016) and Sheoran et al. (2017), who claimed that birds fed a diet supplemented with garlic improved BWG. Taufik and Maruddin (2019) mentioned that garlic contains active compounds capable of substituting the role of synthetic antibiotics used in poultry production. Thus, it positively reflects on their growth performance.
The findings are in agreement with Javandel et al. (2008), Amouzmehr et al. (2012), and Kharde and Soujanya (2014), who claimed that there was no significant effect on carcass characteristics for broiler chickens fed a diet supplemented with garlic. Similar results were reported by Taufik and Maruddin (2019), who reported no significant differences among dietary treatments supplemented with garlic in terms of carcass characteristics (Fig. 2).
The findings in Table 2 are consistent with those of Fadlalla et al. (2010), who reported that total WBC was higher in a group of birds fed a diet supplemented with garlic compared to the control group. Similar results were found by Hanieh et al. (2010), who observed that garlic supplementation led to an increase in WBC, lymphocytes, and immunoglobulin G in broilers. PCV is involved in the transport of oxygen and absorbed nutrients. Increased PCV shows better transportation and thus prevents anemia. In addition, PCV and hemoglobin were positively correlated with the nutritional status of the animals (Olumide and Odunowo, 2019). As shown in Table 3, the findings are consistent with Borgohain et al. (2019), who mentioned that garlic supplementation to commercial broiler diets led to a decrease in serum cholesterol, triglycerides, and fat deposition. Studies conducted by Prasadet al. (2009), Issa and Omar (2012) documented that a diet supplemented with garlic powder significantly decreased total cholesterol, LDL, and triglycerides, whereas HDL levels were increased in broilers. This could be attributed to the mechanisms of hypocholesterolemic and hypolipidemic action of garlic, which leads to a decrease in hepatic activities of cholesterogenic and lipogenic enzymes, such as malic enzyme, fatty acid synthase, and glucose-6-phosphate dehydrogenase.
Conclusion
In conclusion, a diet supplemented with 0.3% garlic powder improved broiler performance. In addition, the supplementation led to an increase in monocyte, lymphocyte, and HDL, and a decrease in LDL levels, which can be reflected on the health status of the birds. It can be concluded that supplementation of 0.3% garlic powder as a growth promoter leads to reducing the cost of production through improving growth performance and enhancing birds’ health.
Conflict of interest
The authors declare that there is no conflict of interest.
Authors’ contributions
MAK designed and applied the feeding trial. All authors were equal in terms of data analysis, writing, reading, revising, and approving the final manuscript.
References
- Aarti C, Khusro A. Role of garlic (Allium sativum) as feed supplements in poultry industries: an overview. World. News. Nat. Sci. 2020;29(3):151–161. [Google Scholar]
- Abdulla N.R, Loh T.C, Foo H.L, Alshelmani M.I, Akit H. Influence of dietary ratios of n-6: n-3 fatty acid on gene expression, fatty acid profile in liver and breast muscle tissues, serum lipid profile, and immunoglobulin in broiler chickens. J. Appl. Poult. Res. 2019;28(2):454–469. [Google Scholar]
- Abo Ghanima M.M, Bin-Jumah M, Abdel-Moneim A.M.E, Khafaga A.F, Abd El-Hack M.E, Allam A.A, El-Kasrawy N.I. Impacts of strain variation on response to heat stress and boldo extract supplementation to broiler chickens. Animals. 2019;10(1):24. doi: 10.3390/ani10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E, Elsadek M.F, Abdel-Moneim A.E, Mahgoub S.A, Elaraby G.M, Taha A.E, Elshafie M.M, Alkhawtani D.M, Abd El-Hack M.E, Ashour E.A. Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum) Poult. Sci. 2021;100(1):84–93. doi: 10.1016/j.psj.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshelmani M.I, Loh T.C, Foo H.L, Sazili A.Q, Lau W.H. Effect of feeding different levels of palm kernel cake fermented by Paenibacillus polymyxa ATCC 842 on nutrient digestibility, intestinal morphology, and gut microflora in broiler chickens. Anim. Feed. Sci. Technol. 2016;216:216–224. [Google Scholar]
- Alshelmani M.I, Loh T.C, Foo H.L, Sazili A.Q, Lau W.H. Effect of feeding different levels of palm kernel cake fermented by Paenibacillus polymyxa ATCC 842 on broiler growth performance, blood biochemistry, carcass characteristics, and meat quality. Anim. Prod. Sci. 2017;57(5):839–848. [Google Scholar]
- Amouzmehr A, Dastar B, Nejad J.G, Sung K.I, Lohakare J, Forghani F. Effects of garlic and thyme extracts on growth performance and carcass characteristics of broiler chicks. J. Anim. Sci. Technol. 2012;54(3):185–190. [Google Scholar]
- Borgohain B, Mahanta J.D, Sapcota D, Handique B, Islam R. Effect of feeding garlic (Allium sativum) on haematological, serum biochemical profile and carcass characteristics in broiler chicken. Int. J. Curr. Microbiol. Appl. Sci. 2019;8(10):492–500. [Google Scholar]
- Chitra P. Study the effect of dietary supplementation of garlic (Allium sativum) tulsi (Ocimum sanctum) leaf powder on growth performance of broilers. Pharm. Innov. 2020;9(7):70–72. [Google Scholar]
- El-Hack M.E.A, Alagawany M, Abdel-Moneim A.M.E, Mohammed N.G, Khafaga A.F, Bin-Jumah M, Othman S.I, Allam A.A, Elnesr S.S. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics. 2020;9(5):210. doi: 10.3390/antibiotics9050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Moneim A.E.M.E.A, El-Wardany I, Abu-Taleb A.M, Wakwak M.M, Ebeid T.A, Saleh A.A. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiot. Antimicrob. Prot. 2020;12(2):439–450. doi: 10.1007/s12602-019-09549-2. [DOI] [PubMed] [Google Scholar]
- Fadlalla I, Mohammed B, Bakhiet A. Effect of feeding garlic on the performance and immunity of broilers. Asian. J. Poult. Sci. 2010;4(4):182–189. [Google Scholar]
- Gadde U, Kim W, Oh S.T, Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health. Res. Rev. 2017;18(1):26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Hameed H. Feed additives in poultry. Assiut. Vet. Med. J. 2021;67(168):87–100. [Google Scholar]
- Hanieh H, Narabara K, Piao M, Gerile C, Abe A, Kondo Y. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in white leghorn chickens. Anim. Sci. J. 2010;81(6):673–680. doi: 10.1111/j.1740-0929.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Hayat S.U, Ali R, Khan A.H, ud Din I, Khan S, Ullah F, Hussain W, Shahzadi F, Khan R.A. Effect of garlic (Allium Sativum) supplementation on growth performance and serum biochemistry of broiler chicks. Am. Acad. Scient. Res. J. Eng. Technol. Sci. 2022;85(1):287–300. [Google Scholar]
- Issa K.J, Omar J.A. Effect of garlic powder on performance and lipid profile of broilers. Open. J. Anim. Sci. 2012;2:62–68. [Google Scholar]
- Javandel F, Navidshad B, Seifdavati J, Pourrahimi G, Baniyaghoub S. The favorite dosage of garlic meal as a feed additive in broiler chickens ratios. Pakistan. J. Biologic. Sci. 2008;11(13):1746–1749. doi: 10.3923/pjbs.2008.1746.1749. [DOI] [PubMed] [Google Scholar]
- Karangiya V, Savsani H, Patil S.S, Garg D, Murthy K, Ribadiya N, Vekariya S. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World. 2016;9(3):245–250. doi: 10.14202/vetworld.2016.245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem K.Y, Abdulla N.R, Foo H.L, Mohd A.N, Zamri N.S, Loh T.C, Alshelmani M.I. Effect of feeding larvae meal in the diets on growth performance, nutrient digestibility and meat quality in broiler chicken. Ind. J. Anim. Sci. 2018;88(10):1180–1185. [Google Scholar]
- Kharde K.R, Soujanya S. Effect of garlic and neem leaf powder supplementation on growth performance and carcass traits in broilers. Vet. World. 2014;7(10):799–802. [Google Scholar]
- Kirubakaran A, Moorthy M, Chitra R, Prabakar G. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers. Vet. World. 2016;9(5):470–474. doi: 10.14202/vetworld.2016.470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makwana R, Bhagwat S, Parikh S, Savaliya B, Jadav C. Effects of dietary supplementation of garlic (Allium sativum) powder on growth performance and carcass characteristics of broilers. Ind. J. Vet. Sci. Biotechnol. 2019;15(1):67–70. [Google Scholar]
- Makwana R.B, Raval A.P, Chauhan H.D, Kulkarni R.C, Srivastava A.K, Bhagwat S.R, Rajgor B.B. Effects of garlic (Allium sativum) supplementation on growth performance, carcass characteristics and economics of broilers. J. Anim. Rese. 2015;5(4):843–848. [Google Scholar]
- Mansoub N.H. Comparative effects of using garlic as probiotic on performance and serum composition of broiler chickens. Ann. Biol. Res. 2011;2(3):486–490. [Google Scholar]
- NRC. 9th. Washington, DC: National Academy Press; 1994. Nutrient requirements of poultry. [Google Scholar]
- Olumide M.D, Odunowo O.O. Blood profile of broiler chickens fed supplemented garlic-based diets. Nig. J. Anim. Prod. 2019;46(3):253–262. [Google Scholar]
- Prasad R, Rose M, Virmani M, Garg S, Puri J. Lipid profile of chicken (Gallus domesticus) in response to dietary supplementation of garlic (Allium sativum) Int. J. Poult. Sci. 2009;8(3):270–276. [Google Scholar]
- Puvača N, Ljubojević Pelić D, Čabarkapa I, Popović S, Tomičić Z, Nikolova N, Lević J. Quality of broiler chickens carcass fed dietary addition of garlic, black pepper and hot red pepper. Technol. Eng. Manag. 2019;2:218–227. [Google Scholar]
- Rahmatnejad E, Roshanfekr H, Ashayerizadeh O, Mamooee M, Ashayerizadeh A. Evaluation the effect of several non-antibiotic additives on growth performance of broiler chickens. J. Anim. Vet. Adv. 2009;8(9):1757–1760. [Google Scholar]
- Sangilimadan K, Richard Churchil R, Premavalli K, Omprakash A. Effect of garlic (Allium sativum) on production performances and carcass traits of Nandanam Broiler-2. Int. J. Curr. Microbiol. Appl.Sci. 2019;8(4):2531–2538. [Google Scholar]
- SAS. Cary, NC: SAS Institute Inc; 2003. Statistical analytical system. [Google Scholar]
- Sheoran N, Kumar R, Kumar A, Batra K, Sihag S, Maan S, Maan N. Nutrigenomic evaluation of garlic (Allium sativum) and holy basil (Ocimum sanctum) leaf powder supplementation on growth performance and immune characteristics in broilers. Vet. World. 2017;10(1):121–129. doi: 10.14202/vetworld.2017.121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Kumar G, Yadav D, Rajput M, Srivastava R. Dietary supplementation of garlic as feed additive in poultry: a. World. News. Nat. Sci. 2020;29(3):151–161. [Google Scholar]
- Taufik M, Maruddin F. The effect of garlic solution supplementation on performance, carcass weight and abdominal fat of broiler chickens. IOP conference series: earth and environmental science; Bristol, UK: IOP Publishing. 2019. [Google Scholar]
- Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86(suppl. 14):E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]