Abstract
A 13-year-old boy developed painless diminution of vision in left eye 15 days after taking first dose of coronavirus disease 2019 (COVID-19) vaccine (Corbevax). Fundus and fluorescein angiography revealed central retinal vein occlusion in the left eye. Blood investigations were noncontributory. He was administered three doses of pulse corticosteroids followed by a tapering dose of oral corticosteroids. Retinal vascular occlusion can occur following COVID-19 vaccination in children, and early and aggressive systemic anti-inflammatory therapy can be helpful.
Keywords: Corbevax vaccine, COVID-19, retinal vein occlusion, vascular occlusion
Vaccines have emerged as the greatest weapon for humanity to tackle the coronavirus disease 2019 (COVID-19) global pandemic. Several vaccines have been approved by various regulatory bodies following clinical trials that demonstrated safety and efficacy with a low complication rate.[1,2] However, a few reports of systemic side effects, especially thrombotic episodes, have been reported in patients receiving COVID-19 vaccines. Occurrence of new-onset uveitic entities and reactivation of previous intraocular inflammations have been reported following COVID-19 vaccinations. Retinal vascular occlusion has been reported widely in patients receiving COVID-19 vaccination, though a similar incidence has not been reported in children.[3] However, in India, vaccination of children aged 12–14 years has been started from March 2022, and only Corbevax has been approved for this age group.[4] Corbevax is a recombinant protein subunit COVID-19 vaccine. Here, we report a case of retinal vein occlusion in a 13-year-old boy following COVID-19 vaccination with Corbevax vaccine.
Case Report
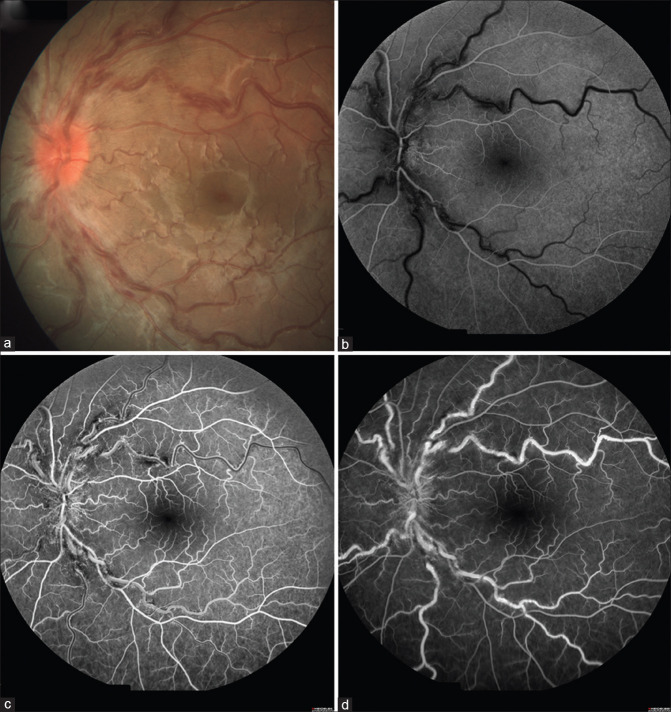
A 13-year-old boy presented to our outpatient department with a complaint of painless diminution of vision in the left eye for 2 weeks. He gave a history of taking the first dose of Corbevax COVID-19 vaccine 1 month ago. Best-corrected visual acuity (BCVA) in the right eye was 6/5, and BCVA in the left eye was 6/7.5. Anterior segment examination of both eyes and fundus examination of the right eye were unremarkable. Fundus examination of the left eye showed a hyperemic disk with blurred margins, dilated and tortuous retinal vessels, and scattered flame-shaped hemorrhages in all quadrants [Figs. 1a and 2a]. Fundus fluorescein angiography of the left eye showed a delay in venous filling in the early phase of angiogram and blocked fluorescence corresponding to the areas of hemorrhages [Fig. 2b–d]. Based on the above findings, a diagnosis of central retinal vein occlusion in the left eye was made. He was evaluated extensively, which revealed normal complete blood counts, complete lipid profile, coagulation profile, and liver and kidney function tests. His Mantoux test and QuantiFERON gold were negative, and serologies for syphilis and human immunodeficiency virus were negative. His antinuclear antibody and lupus anticoagulant antibody were negative, and systemic examination was noncontributory.
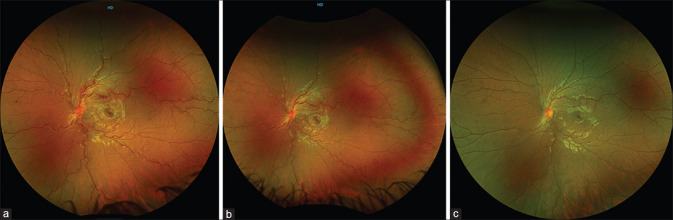
Figure 1.
Optos ultra-wide field fundus photographs of the left eye of a 13-year-old boy (a) at presentation, (b) after three doses of pulse corticosteroid, and (c) at 6-week follow-up
Figure 2.
Color photograph of the left eye showing (a) hyperemic, swollen optic disk with tortuous and engorged retinal veins with scattered retinal hemorrhages. Fundus fluorescein angiography of the left eye (b, c, and d) showing a delay in venous filling in the early phase of the angiogram and blocked fluorescence corresponding to the areas of hemorrhages
He was administered pulse corticosteroid (intravenous methylprednisolone 500 mg daily for three consecutive days). His BCVA improved to 6/6 following three doses of pulse corticosteroid, and fundus evaluation of the left eye revealed resolving hemorrhages [Fig. 1b]. The child was started on a tapering course of oral corticosteroids 1 g/kg body weight and was asked to review after 6–8 weeks. At 6 weeks follow-up, the left eye BCVA was 6/6, and fundus examination of the left eye revealed complete resolution of the retinal hemorrhages and disk edema [Fig. 1c].
Discussion
Retinal vascular occlusion following COVID-19 vaccination has been reported by various authors.[3,5,6] Although a definite causal relationship could not be drawn in these patients, many of these patients were young, healthy, and without any systemic comorbidities. However, vascular occlusion following COVID-19 vaccination was reported in patients below the age of 40 years, the youngest patient being 27 years old.[7] To the best of our knowledge, retinal vascular occlusion following COVID-19 vaccination has not been reported in children, and ours is the first such report.
Corbevax is a recombinant protein CoV-2 receptor-binding domain (RBD) subunit vaccine formulated with aluminum hydroxide (alum) and CpG deoxynucleotides. In addition to being relatively inexpensive, the vaccine has the advantage that it can be produced on a much larger scale in a short period of time and can be stored and distributed easily. To date, there is no report of vascular occlusion reported with the use of this vaccine. Retinal vascular occlusion has been reported in patients receiving Pfizer-BioNTech, Moderna, and AstraZeneca vaccines.[3] Our patient was investigated extensively to rule out the other causes of retinal vascular occlusion and he did not have any systemic ailments. Previously, COVID-19 vaccination was believed to induce a proinflammatory or procoagulant response in some patients.[8] The association between COVID-19 vaccination and retinal vascular occlusion may be inflammatory or immune mediated, and the exact mechanism remains unknown.[3,9] Our patient probably developed an inflammatory response following vaccination, which responded well to systemic anti-inflammatory treatment. Earlier, we treated a similar case of retinal vascular occlusion following COVID-19 vaccination in a 28-year-old young, healthy male with pulse and oral corticosteroid.[3] Prompt response to systemic anti-inflammatory therapy in both cases supports the possibility of inflammation-induced thrombosis.
Conclusion
To conclude, early and aggressive systemic anti-inflammatory therapy can be helpful in retinal vascular occlusion following COVID-19 vaccination.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta Majumder P, Prakash VJ. Retinal venous occlusion following COVID-19 vaccination: Report of a case after third dose and review of the literature. Indian J Ophthalmol. 2022;70:2191–4. doi: 10.4103/ijo.IJO_592_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidelines for COVID-19 Vaccination of Children Between 12-14 Years of Age |COVID-19 Inter-Ministerial Notifications |India. [Last accessed on 2022 Aug 04]. Available from: https://covid19.india.gov.in/document/guidelines-for-covid-19-vaccination-of-children-between-12-14-years-of-age/
- 5.Lee S, Sankhala KK, Bose S, Gallemore RP. Combined central retinal artery and vein occlusion with ischemic optic neuropathy after COVID-19 vaccination. Int Med Case Rep J. 2022;15:7–14. doi: 10.2147/IMCRJ.S328931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonawane NJ, Yadav D, Kota AR, Singh HV. Central retinal vein occlusion post-COVID-19 vaccination. Indian J Ophthalmol. 2022;70:308–9. doi: 10.4103/ijo.IJO_1757_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah PP, Gelnick S, Jonisch J, Verma R. Central retinal vein occlusion following BNT162b2 (Pfizer-BioNTech) COVID-19 messenger RNA vaccine. Retin Cases Brief Rep. 2021 doi: 10.1097/ICB.0000000000001214. doi: 10.1097/ICB.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 8.Jampol LM, Tauscher R, Schwarz HP. COVID-19, COVID-19 Vaccinations, and Subsequent Abnormalities in the Retina: Causation or Coincidence? JAMA Ophthalmol. 2021;139:1135–6. doi: 10.1001/jamaophthalmol.2021.3483. [DOI] [PubMed] [Google Scholar]
- 9.Peters MC, Cheng SS, Sharma A, Moloney TP. Retinal vein occlusion following COVID-19 vaccination. Clin Exp Ophthalmol. 2022;50:459–61. doi: 10.1111/ceo.14056. [DOI] [PubMed] [Google Scholar]