Abstract
Some degenerations involving the peripheral retina can result in a rhegmatogenous retinal detachment. Currently, there are no clear guidelines for retinal screening and/or management of these peripheral retinal degenerations in patients with or without recent onset posterior vitreous detachment or in those prior to refractive surgery or intraocular procedures. This article aims to provide a set of recommendations for the screening and management of peripheral retinal degenerations based on a common consensus obtained from an expert panel of retinal specialists.
Keywords: Cataract surgery, laser capsulotomy, posterior vitreous detachment, refractive surgery, retinal degeneration, retinal detachment
Peripheral retinal degeneration (PRD) is a broad term which includes various lesions such as the lattice degeneration, snail-track degeneration, snowflake degeneration, atrophic or operculated retinal holes, peripheral retinal tears, senile retinoschisis, white and dark without pressure areas, paving stone degeneration and peripheral cystoid degeneration.[1] While most of them are clinically insignificant, a few of these degenerations such as lattice degeneration, degenerative retinoschisis, peripheral retinal tears, cystic retinal tufts and, rarely, zonular traction tufts. can result in rhegmatogenous retinal detachment (RD).[2]
Posterior vitreous detachment (PVD) plays a crucial role in precipitating RD in eyes with PRDs.[3] PVD is defined as the separation of the posterior cortical vitreous from the internal limiting membrane of the retina and is the most common cause of floaters. The development of PVD usually occurs either spontaneously in old age, as seen in most cases, or can be brought about by events such as cataract and refractive surgeries, intravitreal injections, retinal trauma, uveitis, pan-retinal photocoagulation, laser capsulotomy and syndromic diseases such as Marfan syndrome or Stickler syndrome.[4,5,6,7,8,9,10,11,12,13,14] Anomalous, acute, symptomatic PVD with recent-onset flashes and floaters (within 3 months duration), in the presence or absence of PRD, can lead to various deleterious effects on the retina as a result of abnormal traction at the vitreoretinal interface.[15] This can lead to the development of retinal tears and cause RD. Thus, it is important to properly evaluate the retinal periphery for the presence of PRDs. Their subsequent management plays an important role in reducing the risk of RD, especially under special circumstances such as prior to refractive surgery or any intraocular procedure or in patients with abnormal vitreous such as in Sticker syndrome or Marfan syndrome.
There are peripheral retinal lesions such as retinal breaks or lattice degeneration that can predispose to RD. Treatment of these lesions would be required to achieve prophylaxis against the development of RD. In a Cochrane systemic meta-analysis review published in 2014, Wilkinson attempted to assess the efficacy and need for preventive treatment for PRDs in the presence or absence of symptomatic PVD but failed to draw any conclusive evidence.[16] A randomized controlled trial would be ideal but impractical considering the long follow-up time needed and the rarity of the occurrence. The next best evidence is a consensus of expert opinion–based guidelines. The inherent demographic differences seen in the Indian population which shows increased prevalence of high myopia and myopia-related PRDs necessitates that we reconsider these guidelines as they apply to this population.[17,18,19,20] Since the past several decades, surgical correction of refractive errors, particularly myopia, is on the rise. Vitreous changes have been documented following refractive and cataract surgeries and other intraocular procedures.[5,7,13,14] Thus, these patients are predisposed to a higher risk of developing RD in the presence of PRD. Specific guidelines for treating PRD in such situations are lacking. Hence, it is imperative to provide a set of recommendations to the practicing retinal specialists in the Indian subcontinent for the management of PRD under different situations.
Methods
The objective of this article is to suggest nationally acceptable and preferred practice recommendations for the management of PRD. As a preamble to this study, a 15-point web-based questionnaire related to the understanding and management of PRD was circulated to the retinal specialists. The questionnaire was created on the Google Forms website[21] and the link was posted on different WhatsApp groups for the retinal specialists to participate in this anonymous survey. The questions were in a forced choice format and the questionnaire consisted of objective questions with multiple options to choose from. Once hosted on the website, there was no option of altering the questionnaire. The survey was open to responses for 2 weeks (February 2022) after which it was closed and data was analyzed. Over a period of 2 weeks, 171 responses were obtained. The analyses of these responses showed variations regarding the understanding and management of PRDs in different clinical scenarios. In order to provide a uniform screening and treatment pattern to be followed by all retinal specialists for the treatment of PRDs, an experienced expert panel of retinal specialists from high-volume premier eye institutes in the country was constituted. This panel of retinal specialists were presented with a set of relevant questions related to the management of PRDs in different clinical situations. The responses of the individual retinal specialist were assessed and a common consensus guideline for the management of PRD was created and recirculated to the expert panel for their final approval. Contentious points in the consensus guideline were discussed and a majority response from the expert panel was used to affirm the guideline. The document content below provides the recommendations for the management of PRDs based on the responses, inputs, discussions and approval from the expert panel.
Expert Panel Discussion
Need for having national guidelines for the treatment of PRDs
Currently, different practices are being followed by individual retinal specialists regarding the need for prophylactic barrage laser to the PRDs based on the understanding of disease pathogenesis, their experience and available scientific literature. There is no uniform treatment pattern being followed for treating PRDs. PRDs are more common in myopic patients; refractive surgeries are on the rise for correcting myopia, and with the increasing number of refractive surgeries, so are the number of medicolegal cases following them. In order to avoid medicolegal issues, there is a need for uniform practice to be followed by all retinal specialists for treating PRDs in myopic patients and for those undergoing refractive surgery. Additionally, having a guideline may help get rid of the doubt regarding treatment in the mind of a patient with PRD. The guidelines published by the American Academy of Ophthalmology in 2020 on PVD, retinal breaks and lattice degenerations provides guidelines that are useful for medical students and retina fellows but do not take into account the difficult case scenarios which a retinal specialist encounters in day-to-day clinical practice.[21] Thus, it becomes essential to provide a set of recommendations to all practicing retinal specialists regarding the treatment of PRDs.
Peripheral retinal degenerations that can predispose to develop RD
The expert panel identifies the following PRDs which can predispose to develop RD.
These include:
Lattice degeneration
Snail track degeneration
Degenerative retinoschisis
Cystic and zonular tractional retinal tuft
Retinal tear
Retinal hole
Peripheral retinal degenerations that need to be considered for treatment
The following lesions have been identified by the expert panel for treatment with laser therapy in order to prevent a possible RD:
Lesions which predispose to RD in a patient with recent-onset flashes and floaters
Lattice degeneration with a retinal hole
Retinoschisis with RD
Presence of horseshoe tear or retinal dialysis
Presence of retinal hole with a cuff of surrounding subretinal fluid
Traumatic retinal breaks
Lesions predisposed to RD in patients undergoing refractive surgery, cataract surgery, laser capsulotomy, or intravitreal injections
Lesions predisposed to RD in the fellow eye of an RD patient or patient with single-eyed status
Lesions predisposing to RD in syndromic patients such as Marfan syndrome or Stickler syndrome
Risk factors for considering treatment
The common risk factors identified by the expert panel prior to considering treatment include the following:
Recent onset flashes and sudden shower of floaters
Family history of RD
Single-eyed patient
Fellow eye affected by RD
Associated ocular syndromes like Marfan syndrome or Stickler syndrome
Patients planning to undergo refractive or cataract surgery, laser capsulotomy or intravitreal injections
Inability to access ophthalmic care by a retinal specialist in case of a retinal emergency
Time interval between treatment of peripheral retinal degeneration and follow-up examination or intraocular treatment
After laser photocoagulation of the retinal lesions, the adhesive force between the retina and choroid increases over a few days to 2 weeks.[22] Hence, the expert panel has recommended to have a follow-up examination after 14 days following treatment of PRD. Even for patients scheduled for intraocular or refractive surgery, laser capsulotomy or intravitreal injection, the interval for follow-up examination should be beyond 2 weeks.
Need for dilated retinal examination by a retinal specialist for screening peripheral retinal degenerations:
According to the expert panel, a dilated retinal examination is necessary as it is part of a comprehensive eye exam, similar to checking intraocular pressure, pupillary reaction, anterior segment findings and even optic disc status. Any ophthalmologist should be able to confidently perform a peripheral retinal evaluation without feeling the need to refer to a retinal specialist. Most myopic patients have a posteriorly migrated ora serrata which can be seen easily even without depression. In case the ophthalmologist is not confident about the indirect ophthalmoscopy findings, such patients can be referred to a retinal specialist. But all patients must undergo a dilated retinal examination for screening PRDs.
Replacement of dilated retinal examination with undilated, ultrawide-field retinal imaging using Optos®
The expert panel believes that the documentation of peripheral retinal lesions using non-mydriatic retinal imaging can be useful, provided good quality retina images are obtained in all quadrants. Good quality retinal images can be obtained by acquiring the retinal images using the steering imaging technique, i.e., one central image and four other images in four different gazes (superior, nasal, inferior and temporal). A few disadvantages with this imaging technique would be the presence of lid artifacts, parallax, pseudo color image, lack of stereopsis and inability to assess the vitreoretinal relationship. A recent study by Venkatesh et al.[23] noted that the reliability of the examination using Optos imaging for detecting peripheral lesions improved when the images were interpreted by a reader with prior retinal training. The tool is currently only good enough to detect the presence or absence of peripheral retinal lesions. In case the retinal lesions are identified on Optos imaging, such patients need to be referred to a retinal specialist to decide the need for treatment. With the advancement in technology, Optos retinal imaging does have the potential to substitute dilated retinal examination in the future. Currently, most retinal specialists do not have access to technology like Optos due to the high cost associated with it. It is a good alternative but cannot replace the cost-effective dilated fundus examination with scleral indentation.
Type of refractive surgery for correction of myopia and its effect on the treatment decision in eyes with peripheral retinal degeneration
The different refractive surgical techniques used in the treatment of myopia include surface ablation techniques like photorefractive keratotomy, laser in-situ keratomileusis (LASIK), femtosecond LASIK and intraocular procedures like intrastromal corneal ring segments, phakic intraocular lens (phakic IOL) and elective refractive lens exchange.[24,25,26,27,28,29] Other less commonly used refractive surgeries include radial keratotomy, thermal conductive keratoplasty, automated lamellar keratoplasty and epikeratoplasty. Newer procedures like small incision lenticule extraction (SMILE) are gaining popularity for the correction of myopia.[30]
During laser-assisted refractive surgeries like LASIK, there are significant ocular mechanical stressors like an increase in intraocular pressure (> 65 mmHg) during application of the microkeratome suction ring, an acoustic shock wave during the laser ablation, and a rapid lowering of intraocular pressure when the suction ring is decompressed.[31] These have the potential to cause changes in the vitreous, retina, and macula. Many patients complain of increased floaters (PVD) after LASIK.[32,33,34] Even in newer surgical techniques like the femtosecond LASIK, the incidence of PVD occurrence or progression is either higher or comparable to that seen in the microkeratome LASIK cohort of patients.[7,35] This may be due to a longer suction time during femtosecond LASIK despite a lower suction pressure.
SMILE is a relatively new refractive procedure designed to treat a range of refractive errors. In this flap-less procedure, a corneal lenticule is created using a femtosecond laser and then extracted through a small incision.[30] It is reported to achieve similar optical effects as femtosecond LASIK with excellent postoperative outcomes.[36] In the SMILE technique, there are no fluctuations in the intraocular pressure as in LASIK. Thus, one can assume that the incidence of PVD following SMILE would be lesser as compared to LASIK or femtosecond LASIK. On the other hand, phakic IOL is an intraocular surgery and often induces changes in the vitreous, leading to a higher risk of developing RD.[37]
The expert panel believes that, though it is good to know the type of refractive surgery being performed for correcting myopia, it may not be a criterion to decide the prophylactic treatment of PRDs. The refractive surgery being performed may help the retinal specialist to assess the risk of RD development in such eyes with PRDs which can be predisposed to RD. A retinal examination of myopic eyes, both pre-and postoperative, is therefore mandatory. Prophylactic treatment of retinal degenerations which can predispose the patient to retinal tears is advisable.
Presence or absence of PVD change the management practice in patient planning to undergo refractive surgery
Though PVD plays a significant role in causing RD in eyes with PRDs, its presence or absence plays a very limited role in deciding the management practice for prophylactic treatment to the retinal degenerations. As per the inputs from the expert panel, it is the recent onset PVD (flashes or floaters) that is more relevant. If the patient has symptoms because of acute PVD, a close follow-up is needed as the PVD is likely to be still progressing and this can lead to future retinal breaks or RD. It is, thus, preferable to postpone the refractive surgery for at least a period of 6-12 weeks in such situations. A complete PVD is also rather uncommon in a typical refractive surgery candidate, usually a young myope. More often, there is vitreoschisis posteriorly and unless there is an overlying operculum, one cannot assume that the vitreous has separated from that particular area of PRD even in the presence of a Weiss ring.
Validity of retinal examination findings
As of now, there are no studies in literature which propose a time frame regarding the validity of dilated retinal examination findings. The expert panel believes that the development of acute symptomatic PVD should be the most important criterion for repeating a dilated retinal examination, irrespective of whether the patient does or does not undergo a refractive surgery or intraocular surgery or procedure.
Follow-up retinal examination after refractive surgery or intraocular procedure
According to the expert panel, there is no need to follow-up with the patients after the refractive surgery or intraocular procedures either at 1-week or at 1-month. However, patients who develop recent-onset floaters or flashes should undergo retinal examination promptly.
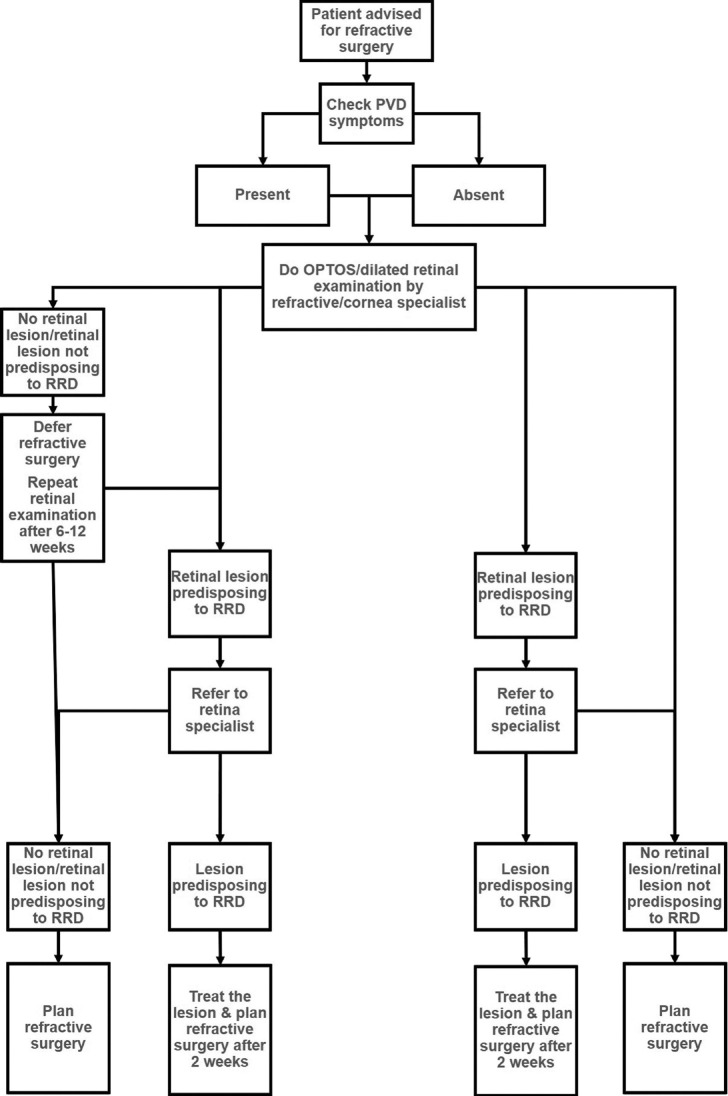
Figs. 1-3 provide guidelines for retinal examination, management of PRD and follow-up in a patient planning to undergo either refractive surgery or other intraocular procedures.
Figure 1.
Guidelines for a patient planning to undergo refractive surgery
Figure 3.
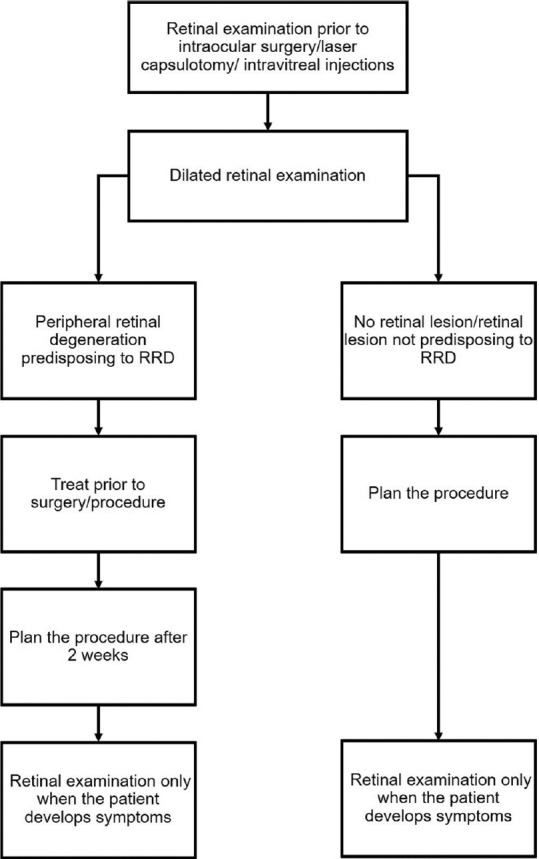
Guidelines for a patient planning to undergo cataract surgery, laser capsulotomy, or intravitreal injections
Figure 2.
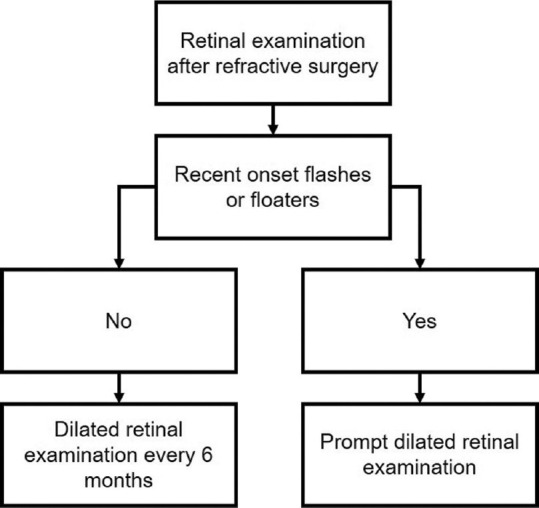
Follow-up guidelines for a patient who underwent refractive surgery
Based on the above clinical discussions with the expert panel, a few commonly encountered hypothetical clinical situations were carved and a decision to undergo prophylactic treatment of the PRD was made [Table 1].
Table 1.
Decision-making in different scenarios in a patient with peripheral retinal degeneration
| Clinical situation | Prophylactic treatment needed |
|---|---|
| Lattice degeneration in an eye and occasional floaters and flashes (Asymptomatic lattice) | No |
| Lattice degeneration without retinal hole in an eye with recent onset floaters and flashes (Symptomatic lattice) | Yes |
| Lattice degeneration in an eye with no flashes or floaters and is planning to undergo refractive surgery/laser capsulotomy/intravitreal injection/cataract surgery | Yes |
| Lattice degeneration in an eye with no symptoms and a past history of blunt trauma | No |
| Retinal break in an eye with no symptoms and a past history of blunt trauma | Yes |
| Lattice degeneration without retinal holes or tears in an eye with no symptoms and fellow eye showing RD | Yes |
| Lattice degeneration without retinal hole and no symptoms in a single-eyed patient | No |
| Lattice degeneration without retinal hole and recent onset flashes or floaters in a single-eyed patient | Yes |
| Lattice degeneration without retinal hole in a patient of Stickler syndrome or Marfan syndrome | Yes |
| Incidental atrophic/operculated retinal hole | No |
| Multiple rows of lattice degeneration involving 360° retinal periphery in an asymptomatic patient | No |
| Lattice degeneration in a patient planned for vitreoretinal surgery for a full-thickness macular hole | Yes |
RD, Retinal detachment
Conclusion
In conclusion, patients undergoing refractive surgery or intraocular procedure need a thorough retinal examination to look for peripheral lesions. Prophylactic barrage laser photocoagulation needs to be done for predisposing degenerative retinal lesions at least 2 weeks before surgery or procedure. A retinal examination should be done at regular intervals for long term to check for the development or progression of PVD, retinal tears, retinal detachment and macular pathologies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Campagnoli TR, Smiddy WE. Peripheral retinal abnormalities. In: Medina CA, Townsend JH, Singh AD, editors. Manual of Retinal Diseases. Cham: Springer International Publishing; 2016. pp. 243–7. [Google Scholar]
- 2.Lewis H. Peripheral retinal degenerations and the risk of retinal detachment. Am J Ophthalmol. 2003;136:155–60. doi: 10.1016/s0002-9394(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed F, Tripathy K. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; [Updated 2022 Feb 21]. 2022. Jan, Posterior Vitreous Detachment. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563273/ [PubMed] [Google Scholar]
- 4.Yonemoto J, Ideta H, Sasaki K, Tanaka S, Hirose A, Oka C. The age of onset of posterior vitreous detachment. Graefes Arch Clin Exp Ophthalmol. 1994;232:67–70. doi: 10.1007/BF00171665. [DOI] [PubMed] [Google Scholar]
- 5.Coppé AM, Lapucci G. Posterior vitreous detachment and retinal detachment following cataract extraction. Curr Opin Ophthalmol. 2008;19:239–42. doi: 10.1097/ICU.0b013e3282fc9c4a. [DOI] [PubMed] [Google Scholar]
- 6.Morita H, Funata M, Tokoro T. A clinical study of the development of posterior vitreous detachment in high myopia. Retina. 1995;15:117–24. doi: 10.1097/00006982-199515020-00005. [DOI] [PubMed] [Google Scholar]
- 7.Osman MH, Khalil NM, El-Agha MS. Incidence of posterior vitreous detachment after femtosecond LASIK compared with microkeratome LASIK. Cornea. 2017;36:1036–9. doi: 10.1097/ICO.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 8.Williams DF, Mieler WF, Williams GA. Posterior segment manifestations of ocular trauma. Retina. 1990;10(Suppl 1):S35–44. doi: 10.1097/00006982-199010001-00006. [DOI] [PubMed] [Google Scholar]
- 9.Sebag J, Buzney SM, Belyea DA, Kado M, McMeel JW, Trempe CL. Posterior vitreous detachment following panretinal laser photocoagulation. Graefes Arch Clin Exp Ophthalmol. 1990;228:5–8. doi: 10.1007/BF02764282. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HT, Patterson R, Ryan SJ. Traumatic posterior vitreous detachment: Scanning electron microscopy of an experimental model in the monkey eye. Scan Electron Microsc. 1984;(Pt 3):1361–8. [PubMed] [Google Scholar]
- 11.Hogan MJ. Inflammation and its effect on the vitreous. Trans Ophthalmol Soc U K. 1975;95:378–81. [PubMed] [Google Scholar]
- 12.Alshahrani ST, Ghazi NG, Al-Rashaed S. Rhegmatogenous retinal detachments associated to Stickler syndrome in a tertiary eye care center in Saudi Arabia. Clin Ophthalmol. 2016;10:1–6. doi: 10.2147/OPTH.S91444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheard RM, Goodburn SF, Comer MB, Scott JD, Snead MP. Posterior vitreous detachment after neodymium: YAG laser posterior capsulotomy. J Cataract Refract Surg. 2003;29:930–4. doi: 10.1016/s0886-3350(02)01837-0. [DOI] [PubMed] [Google Scholar]
- 14.Geck U, Pustolla N, Baraki H, Atili A, Feltgen N, Hoerauf H. Posterior vitreous detachment following intravitreal drug injection. Graefes Arch Clin Exp Ophthalmol. 2013;251:1691–5. doi: 10.1007/s00417-013-2266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novak MA, Welch RB. Complications of acute symptomatic posterior vitreous detachment. Am J Ophthalmol. 1984;97:308–14. doi: 10.1016/0002-9394(84)90628-7. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson CP. Interventions for asymptomatic retinal breaks and lattice degeneration for preventing retinal detachment. Cochrane Database Syst Rev. 2014;9:CD003170. doi: 10.1002/14651858.CD003170.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam DSC, Fan DSP, Chan WM, Tam BSM, Kwok AKH, Leung ATS, et al. Prevalence and characteristics of peripheral retinal degeneration in Chinese adults with high myopia: A cross-sectional prevalence survey. Optom Vis Sci. 2005;82:235–8. doi: 10.1097/01.opx.0000159359.49457.b4. [DOI] [PubMed] [Google Scholar]
- 18.Pierro L, Camesasca FI, Mischi M, Brancato R. Peripheral retinal changes and axial myopia. Retina (Philadelphia, Pa) 1992;12:12–7. doi: 10.1097/00006982-199212010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Martín Sánchez MD, Roldán Pallarés M. [Myopia: frequency of lattice degeneration and axial length] Arch Soc Esp Oftalmol. 2001;76:291–6. [PubMed] [Google Scholar]
- 20.Gözüm N, Cakir M, Gücukoglu A, Sezen F. Relationship between retinal lesions and axial length, age and sex in high myopia. Eur J Ophthalmol. 1997;7:277–82. doi: 10.1177/112067219700700313. [DOI] [PubMed] [Google Scholar]
- 21.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Posterior vitreous detachment, retinal breaks, and lattice degeneration preferred practice pattern®. Ophthalmology. 2020;127:P146–81. doi: 10.1016/j.ophtha.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Narayanan R, Chandra KK. Commentary: Prophylactic laser barrage before laser-assisted in-situ keratomileusis. Indian J Ophthalmol. 2021;69:1861. doi: 10.4103/ijo.IJO_402_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesh R, Cherry JP, Reddy NG, Anilkumar A, Sridharan A, Sangai S, et al. Inter-observer agreement and sensitivity of Optomap images for screening peripheral retinal lesions in patients undergoing refractive surgery. Indian J Ophthalmol. 2020;68:2930–4. doi: 10.4103/ijo.IJO_2239_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swinger CA. Refractive surgery for the correction of myopia. Trans Ophthalmol Soc U K. 1981;101:434–9. [PubMed] [Google Scholar]
- 25.Binder PS. Radial keratotomy and excimer laser photorefractive keratectomy for the correction of myopia. J Refract Corneal Surg. 1994;10:443–64. [PubMed] [Google Scholar]
- 26.Kolahdouz-Isfahani AH, Wu FM, Salz JJ. Refractive keratotomy after photorefractive keratectomy. J Refract Surg. 1999;15:53–7. doi: 10.3928/1081-597X-19990101-09. [DOI] [PubMed] [Google Scholar]
- 27.Lee YC, Park CK, Sah WJ, Hahn TW, Kim MS, Kim JH. Photorefractive keratectomy for undercorrected myopia after radial keratotomy: Two-year follow up. J Refract Surg. 1995;11(3 Suppl):S274–9. doi: 10.3928/1081-597X-19950502-17. [DOI] [PubMed] [Google Scholar]
- 28.Renard P, Brochard-Caille B, Licha A, Couderc JL. [Correction of myopia by corneal surgery. Radial keratotomy, myopic keratomileusis] Bull Mem Soc Fr Ophtalmol. 1986;97:191–3. [PubMed] [Google Scholar]
- 29.Kim TI, AlióDel Barrio JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019;393:2085–98. doi: 10.1016/S0140-6736(18)33209-4. [DOI] [PubMed] [Google Scholar]
- 30.Reinstein DZ, Archer TJ, Gobbe M. Small incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomes. Eye Vis (Lond) 2014;1:3. doi: 10.1186/s40662-014-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaurasia SS, Gimeno FL, Tan K, Yu S, Tan DT, Beuerman RW, et al. In Vivo Real-time intraocular pressure variations during LASIK flap creation. Invest Ophthalmol Vis Sci. 2010;51:4641–5. doi: 10.1167/iovs.10-5228. [DOI] [PubMed] [Google Scholar]
- 32.Arevalo JF. Retinal complications after laser-assisted in situ keratomileusis (LASIK) Curr Opin Ophthalmol. 2004;15:184–91. doi: 10.1097/01.icu.0000120674.27548.9c. [DOI] [PubMed] [Google Scholar]
- 33.Arevalo JF. Posterior segment complications after laser-assisted in situ keratomileusis. Curr Opin Ophthalmol. 2008;19:177–84. doi: 10.1097/ICU.0b013e3282fb7c15. [DOI] [PubMed] [Google Scholar]
- 34.Mirshahi A, Schöpfer D, Gerhardt D, Terzi E, Kasper T, Kohnen T. Incidence of posterior vitreous detachment after laser in situ keratomileusis. Graefes Arch Clin Exp Ophthalmol. 2006;244:149–53. doi: 10.1007/s00417-005-0002-y. [DOI] [PubMed] [Google Scholar]
- 35.Gavrilov JC, Gaujoux T, Sellam M, Laroche L, Borderie V. Occurrence of posterior vitreous detachment after femtosecond laser in situ keratomileusis: Ultrasound evaluation. J Cataract Refract Surg. 2011;37:1300–4. doi: 10.1016/j.jcrs.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Jin Y, Wang Y, Xu L, Zuo T, Li H, Dou R, et al. Comparison of the optical quality between small incision lenticule extraction and femtosecond laser LASIK. J Ophthalmol. 2016;2016:2507973. doi: 10.1155/2016/2507973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rishi P, Attiku Y, Agarwal M, Narayanan R, Talwar D, Srinivasan B, et al. Retinal detachment after phakic intraocular lens implantation: A 10-year multicenter study. Ophthalmology. 2019;126:1198–200. doi: 10.1016/j.ophtha.2019.02.028. [DOI] [PubMed] [Google Scholar]