Abstract
Purpose:
To evaluate visual field changes in primary congenital glaucoma (PCG) with retinal nerve fiber layer thickness on optical coherence tomography.
Methods:
In this cross-sectional, observational study, consecutive PCG children who underwent combined trabeculotomy with trabeculectomy and on regular follow-up were enrolled. All patients were aged over four years and co-operative for RNFL OCT and visual field examination. Perimetry was done on Humphrey visual field (HVF) analyzer using 30-2 and 10-2 SITA standard algorithms as appropriate. If a reliable automated perimetry was not feasible, kinetic perimetry was done. The following were noted at baseline and every follow-up: age, sex, visual acuity, intraocular pressure (IOP), cup–disc ratio (CDR), corneal diameters, refraction, any topical antiglaucoma medications, surgeries underwent, age at surgery and duration between surgery and final examination.
Results:
Forty-eight eyes of 34 children operated for PCG and 19 eyes of 17 controls were analyzed. A statistically significant thinner average RNFL thickness of 87.2 ± 28 μm was noted in PCG eyes as compared to controls with 100.6 ± 7.2 μm (P = 0.04). The mean cup–disc area ratio on OCT in PCG eyes was 0.43 ± 0.2 (0.02–0.93) and in control eyes was 0.23 ± 0.07 (0.1–0.4) (P < 0.001). On RNFL OCT, there was significant focal RNFL loss in temporal superior (P = 0.003), nasal inferior (P = 0.037) and temporal inferior (P < 0.001) quadrants compared to controls. Among PCG eyes, 20/48 eyes (41.7%), had definitive, reproducible glaucomatous VF defects. Mean baseline IOP in PCG eyes with VF defect was 28.7 ± 5.7 mmHg and in eyes with normal VF was 24.6 ± 5.9 mmHg (P = 0.03). On univariate regression analysis, higher baseline IOP was significantly associated with both RNFL loss (odds ratio (OR): −2.17) and VF defects (OR: 3.35). Fluctuation in follow-up IOP (OR: 3.33) was also significantly associated with the presence of VF defects. On multivariable regression analysis maximum, IOP was significantly associated with RNFL loss and VF defects.
Conclusion:
Peripapillary RNFL thickness could be used to identify PCG eyes having visual field loss and possibly poor visual function from PCG eyes without visual field defects. Baseline and follow-up IOP, significantly correlated with RNFL thickness in PCG eyes.
Keywords: RNFL OCT in Primary congenital glaucoma, RNFL thickness in primary congenital glaucoma, visual field loss in PCG
Primary congenital glaucoma (PCG) is a genetically associated, developmental disorder which results in significant visual morbidity in children with an incidence of 1 in 10,000 to 1 in 68,000 live births in Caucasians to 1 in 3300 live births in Indians.[1,2] It is the cause of 4.2% of all childhood blindness in a population-based study in southern India.[3] Due to the early age of presentation, clinical diagnosis is based on the presence of blepharospasm, photophobia, lacrimation, enlarged corneal diameters, glaucomatous optic atrophy and Haab’s striae. Glaucoma surgeries can now lower intraocular pressure (IOP) significantly; however, there are many factors that determine final visual function like baseline glaucomatous optic neuropathy, high myopia, corneal complications and amblyopia.[1,4,5] Gusson et al.[6] reported the occurrence of optic nerve damage, cup–disc ratio >0.5, to be 36.4% in neonatal glaucoma and 68.8% in infantile glaucoma eyes.
In childhood glaucoma, monitoring visual function and IOP is required from presentation, though therapy with surgery and or medications and lifelong follow-up are required. However, in young children, identifying the extent of visual field loss or continued progression of glaucomatous loss is currently not possible by perimetry, even though it is imperative for appropriately adjusting target IOP and for further management. Several studies have shown structural and functional abnormalities with time domain optical coherence tomography (TD-OCT),[7,8,9,10] spectral domain OCT[11,12,13] and visual field (VF)[11,14] in older children, some similar to adults.[15,16] The introduction of handheld OCT during examination under anesthesia or in younger children who can fix during an OCT examination could provide an objective evaluation, if parameters associated with visual field loss are known.[17]
To the best of our knowledge, no study in literature has compared the OCT parameters with visual field loss in young children with PCG.
This study compared retinal nerve fiber layer (RNFL) OCT and perimetry in previously operated PCG eyes with age and refraction matched controls, and further evaluated RNFL values in PCG eyes with glaucomatous visual field defects and those without defects. RNFL OCT was also correlated with pre- and post-filtration surgery clinical parameters.
Methods
This was a cross-sectional observational study at a tertiary ophthalmic hospital, after obtaining Institute Ethics Committee approval. It adhered to the tenets of the Declaration of Helsinki. Consecutive PCG patients aged over four years, being reviewed after undergoing combined trabeculotomy with trabeculectomy, were enrolled. PCG had been diagnosed based on clinical features proposed by the Childhood Glaucoma Research Network (CGRN). Clinical characteristics of presentation in infancy or early childhood (<3 years) with IOP >21 mmHg or with signs of IOP-driven ocular anatomical changes was noted on examination under anesthesia (Haab’s striae, corneal diameter >11 mm in newborn, >12 mm in infants, >13 mm at any age and glaucomatous optic disc) in the absence of other ocular and systemic abnormalities. Controls having a similar refractive status and age were recruited from among children presenting for routine ocular examinations. PCG children who were cooperative for OCT and visual field examination during the period June 2020 to July 2021 were included in the study. Patients uncooperative for spectral domain optical coherence tomography (SD-OCT) and Humphrey visual field imaging, those with poor fixation or unreliable visual fields, having other ocular diseases except refractive error or not willing to give consent for the study were excluded. Written informed consent was obtained from all parents or guardians, as applicable.
Age at surgery, duration between surgery and final examination, and the number of antiglaucoma medications and surgeries undertaken for PCG eyes were noted. On every visit, visual acuity, IOP, cup–disc ratio (CDR), corneal diameters, refraction and any topical antiglaucoma medications being used were noted. Age at examination, sex, baseline IOP, baseline corneal diameters, baseline CDR, maximum and minimum follow-up IOP, mean and fluctuation follow-up IOP (defined as 1 standard deviation of the IOP at all visits), final BCVA and final refraction were recorded. During examination under anesthesia, IOP was measured with Perkin’s handheld tonometer, and the horizontal corneal diameter was measured with Castroviejo calipers. Clinically optic nerve head evaluation (ONH) was done with the help of a direct or indirect ophthalmoscope. For older children, IOP was measured with Goldmann applanation tonometer on follow-up visits. Final BCVA was recorded using a Snellen chart and converted to log minimum angle of resolution (logMAR) units. Final refraction was noted as spherical equivalent.
All eyes underwent OCT using SPECTRALIS® SD-OCT (Heidelberg Engineering, Heidelberg, Germany) after dilating the eyes with 0.1% tropicamide eye drops. A minimum of two consecutive examinations with scan quality ≥20 was obtained. To obtain RNFL thickness measurements, a circular scan of 3.5 mm was centered on the ONH. An inbuilt software (version 1.10.2.0, Heidelberg Engineering, Heidelberg, Germany) divided the disc into four symmetrical 90° quadrants (superior S, nasal N, inferior I, temporal T) and averaged them to provide total average thickness. Additionally, superior and inferior quadrants were divided in 45° sectors (temporal superior or TS, nasal superior or NS, nasal inferior or NI and temporal inferior or TI, respectively).
Perimetry was done on Humphrey Visual Field analyzer (Carl Zeiss Meditec, Dublin, CA, USA) using 30-2 and 10-2 SITA standard algorithms as appropriate. Perimetry was considered reliable when false positives were <20%, false negatives <33% and fixation losses <20%. If a reliable automated perimetry was not obtained, kinetic perimetry (Goldmann perimeter, Haag- Streit model 940-K7, Bern, Switzerland) was done by a single experienced technician.
Statistical analysis
Numerical data was entered in Microsoft Excel version 16.6.5, and IBM Statistical Package for the Social Sciences (SPSS) version 23 (SPSS, Inc., Chicago, IL) was used for analysis. Normality of the data was tested with Kolmogorov–Smirnov test. Parametric numerical data were recorded as mean and standard deviation, while median and range were calculated for non-parametric data. As both eyes of the patients were taken for analysis, to eliminate bias, a generalized estimating equation (GEE) model was used to assess the difference of the baseline characteristics between PCG eyes and normal eyes, and in PCG eyes with VF defect and PCG eyes with normal VF. A P value <0.05 was considered significant.
Results
Forty-eight eyes of 34 patients (24 boys and 10 girls) operated for PCG and 19 eyes of 17 age and refraction matched controls, (9 boys and 8 girls), were analyzed. A combined trabeculotomy and trabeculectomy by a single surgeon (RS) was done for all PCG eyes at a mean age of 24.8 ± 69 (range 0.4–408) months, with a follow-up period of 143.2 ± 61.8, (36–324) months. Minimum and mean time period to enroll after surgery was 3 and 11 years, respectively. Baseline IOP in 8 PCG children and baseline CDR in 4 children could not be assessed because of corneal irregularities and haze during initial examination at the time of surgery. Baseline IOP, baseline CDR and baseline corneal diameters were statistically significantly different between PCG eyes and control eyes at review, (P < 0.001). Baseline IOP in PCG patients was 26.3 ± 6.17 (12–40) mmHg, while at review in the control group, it was15.26±0.3 (12-20) mmHg. The corneal diameter at last review in PCG patients was 13.5 ± 0.96 (10.5–15) mm and in controls was 11.7 ± 0.6 (11–13) mm. Maximum follow-up IOP in PCG eyes was 19.37 ± 6.7 (10–44) mmHg and mean follow-up IOP was 12 ± 3.4 (6.38–20.5) mmHg. One topical antiglaucoma medication was required in 11 PCG eyes, two medications in 6 eyes and three medications in 2 eyes. Two eyes needed a repeat trabeculectomy.
At the time of review, best corrected visual acuity in PCG eyes was 0.38 ± 0.3 (0.0–1.08), significantly lower than controls measuring 0.04 ± 0.09 (0.0–0.3) (P < 0.001).
On OCT, average RNFL thickness was 87.2 ± 28.1 (36–157) μm, in PCG eyes as compared to controls measuring 100.6 ± 7.2 (84–112) μm (P = 0.04). The average RNFL thickness was >100 μm in all control eyes. The mean cup–disc area ratio on OCT in PCG eyes was 0.43 ± 0.2 (0.02–0.93) and in control eyes was 0.23 ± 0.07 (0.1–0.4) (P < 0.001). There was a significant focal RNFL loss in temporal superior (P = 0.003), nasal inferior (P = 0.037) and temporal inferior (P < 0.001) quadrants between PCG and control eyes.
Among PCG eyes, 20/48 (41.7%) had definitive, reproducible glaucomatous visual field defects, while in the rest, perimetry was within normal limits. Out of 48 eyes, 23 eyes had produced reliable visual fields on automated perimetry with mean deviation (MD) and pattern standard deviation (PSD) of − 8.27 ± 10.8 and 3.6 ± 3.15, respectively. The minimum and mean age to produce reliable visual fields on automated perimetry was 7 and 15 years, respectively. The visual fields of the remaining 25 eyes were captured on kinetic perimetry. Tschopp et al.[18] showed us that seven- and eight-year-old children provided reliable visual fields. Mean baseline IOP in PCG eyes with visual field (VF) defect was 28.7 ± 5.7 mmHg and in eyes with normal VF was 24.6 ± 5.9 mmHg (P = 0.03). PCG eyes with VF defect had a lower final visual acuity than PCG eyes with normal fields (P < 0.001). PCG eyes with VF defects were also significantly more myopic than PCG eyes with normal VF (P = 0.03). Age at surgery, duration since surgery and age at review were comparable between PCG eyes with normal VF and PCG eyes with VF loss (P = 0.21, P = 0.28, and P = 0.13, respectively). Minimum and mean time period to enroll after surgery was three and eleven years, respectively. Baseline CDR and corneal diameters were comparable in PCG eyes with normal VF versus PCG eyes with VF defects (P = 0.31, P = 0.31, respectively). Also maximum, minimum, mean and fluctuation follow-up IOP were comparable between PCG eyes with normal VF and PCG eyes with VF defect (P = 0.15, P = 0.33, P = 0.81, and P = 0.16, respectively) [Table 1].
Table 1.
Comparison of parameters between primary congenital glaucoma having normal visual field versus those with glaucomatous field loss
| Parameter | PCG eyes with normal VF | PCG eyes with VF loss | P |
|---|---|---|---|
| Age at examination (months) | 149.57±67 (60-348) | 194±97.2 (48-516) | 0.13 |
| Age at surgery (months) | 14.5±42 (0.4-228) | 39.3±94.4 (1-408) | 0.21 |
| Duration between surgery and examination (months) | 135±57.9 (54-324) | 154.7±66.6 (36-324) | 0.28 |
| Baseline IOP (mm Hg) | 24.6±5.9 (12-38) | 28.7±5.7 (16-40) | 0.03 |
| Baseline CDR | 0.54±0.2 (0.1-1) | 0.59±0.2 (0.1-0.9) | 0.319 |
| Baseline Corneal diameter (mm) | 13.2±1 (10.5-15) | 13.6±0.7 (12-15) | 0.317 |
| Maximum follow up IOP (mm Hg) | 18.2±5.8 (10-34) | 20.9±7.6 (12-44) | 0.15 |
| Minimum follow up IOP (mm Hg) | 6.64±2.9 (2-12) | 6.4±2 (2-10) | 0.33 |
| Mean follow up IOP (mm Hg) | 11.8±3.4 (6.38-18.9) | 12.4±3.4 (7.46-20.46) | 0.81 |
| Fluctuation IOP (mm Hg) | 3.2±1.5 (0.96-8.8) | 3.8±1.9 (1.38-9.83) | 0.16 |
| Final visual acuity (log MAR) | 0.25±0.3 (0.0-1.08) | 0.57±0.3 (0.0-1.08) | <0.001 |
| Final refraction (in Diopters) | −1.44±2 (−7.25 to+2.5) | −2.97±3 (−6.5 to+6.5) | 0.03 |
| RNFL average (µm) | 93.4±22 (45-146) | 78.5±33.6 (36-157) | <0.001 |
| TS RNFL (µm) | 123.5±30.4 (52-183) | 92.7±45 (30-165) | <0.001 |
| NS RNFL (µm) | 115.2±41.5 (0-186) | 86.2±49 (27-195) | 0.009 |
| NASAL (µm) | 72.3±22.6 (19-113) | 69.6±33.2 (25-156) | 0.73 |
| NI RNFL (µm) | 109±37.3 (10-119) | 81±38.5 (3-135) | 0.001 |
| TI RNFL (µm) | 121.7±27.4 (51-188) | 89±42.8 (15-146) | <0.001 |
| Temporal (µm) | 66.4±15.8 (36-119) | 58.7±11.2 (44-95) | 0.04 |
| Cup/disc ratio | 0.39±0.19 (0.06-0.76) | 0.49±0.3 (0.02-0.93) | 0.02 |
VF, Visual field; PCG, Primary congenital glaucoma; RNFL, Retinal nerve fiber layer; NI, Nasal inferior; TI, Temporal inferior; NS, Nasal superior; TS, Temporal superior. Significant P values shown in bold numbers
On OCT, there was a significantly greater RNFL loss in TS, TI and NI quadrants in PCG eyes with VF defect compared to PCG eyes with normal VF (P < 0.001). Average RNFL was also significantly thinner in PCG eyes with VF defect compared to PCG eyes with normal VF (P < 0.001).
On univariate regression analysis, this study found that higher baseline IOP was significantly associated with both RNFL loss on OCT (OR: −2.17, 95% CI: −1.67~(−0.08), P < 0.03) and VF defects on perimetry (OR: 3.35, 95% CI: 0.04 ~ 0.17, P < 0.001). Fluctuation in follow-up IOP (OR: 3.33, 95% CI: 0.17 ~ 0.69, P < 0.001) was also significantly associated with the presence of VF defects. On multivariable regression analysis maximum follow-up IOP, mean follow-up IOP and median follow-up IOP were significantly associated with RNFL loss and VF defects.
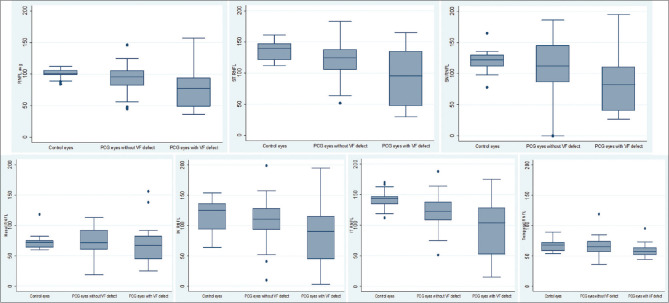
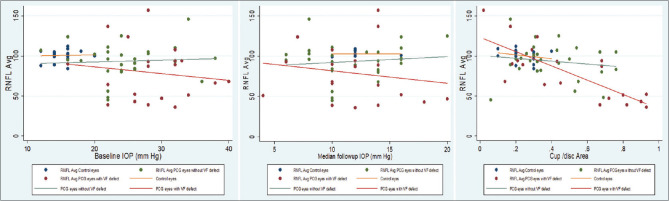
The mean RNFL thickness of each quadrant of OCT between control eyes, PCG eyes with normal VF and PCG eyes with VF defect were compared [Fig. 1]. A scatter plot of average RNFL thickness showed a negative relationship with baseline IOP, median follow-up IOP and cup–disc area among the three groups: controls, PCG eyes with normal VF, and PCG eyes with VF defect [Fig. 2].
Figure 1.
Box and whisker chart showing quadrant-wise comparison of mean RNFL thickness between control eyes, PCG eyes with normal VF and PCG eyes with VF defect
Figure 2.
Scatter plot showing the correlation between average RNFL thickness versus baseline IOP, median follow-up IOP and cup–disc diameter ratio between the three (control eyes, PCG eyes with normal VF and PCG eye with VF defect) groups
The association between pattern of visual field defect and mean RNFL thickness in corresponding quadrants was evaluated. Among 20 PCG eyes with VF defect, there were 6 eyes (30%) having a superior nasal step, 2 eyes (10%) a superior arcuate, 2 eyes (10%) an inferior nasal step, 1 eye (5%) an inferior arcuate, 4 (20%) eyes a double arcuate and 5 (25%) eyes an advanced VF defect. The mean RNFL thickness in corresponding quadrants is detailed in Table 2.
Table 2.
Association between pattern of visual field defect and RNFL thickness
| Pattern of VF defect | Number of patients | Polar RNFL loss (µm) in PCG group | Control RNFL thickness (µm) | ||
|---|---|---|---|---|---|
| Superior | |||||
| Nasal step | 6 | NI | TI | NI | TI |
| 96 (89-120) | 112 (87-127) | 119.26±24 (64-154) | 142±15.3 (112-170) | ||
| Arcuate | 2 | 89 (68,110) | 90 (66,114) | ||
| Inferior | |||||
| Nasal step | 2 | NS | TS | NS | TS |
| 32 (29,35) | 39 (39,39) | 120±17.2 (78-165) | 138.5±14.2 (112-161) | ||
| Arcuate | 1 | 100 | 103 | ||
| Double arcuate | 4 | NI+TI+NS+TS | NI+TI+NS+TS | ||
| 90±17.1 | 130±17.6 | ||||
| Advanced VF defect | 5 | Avg. RNFL | Avg. RNFL | ||
| 57.2±20.3 (43-93) | 100.63±7.2 (84-112) | ||||
VF, Visual field; RNFL, Retinal nerve fiber layer; NI, Nasal inferior; TI, Temporal inferior; NS, Nasal superior; TS, Temporal superior
Discussion
PCG is diagnosed clinically at an early age, when imaging of the posterior segment and functional evaluation of vision is generally not possible. This study compared RNFL OCT and perimetry in previously operated PCG eyes with age- and refraction-matched controls, and further evaluated RNFL values in PCG eyes with glaucomatous VF defects and those without defects.
The average RNFL thickness was thinner (87.2 ± 28.1 μm) in PCG eyes as compared to 100.6 ± 7.2 μm in control eyes (P < 0.04). As similar demonstration by Parikh RS et al.[19] in a pilot study of 201 eyes, there was decrease in average RNFL thickness with increase in age. PCG children were significantly (P < 0.004) older (168.13 ± 83) than normal subjects (105.5 ± 22). They further stated that the RNFL thickness was about to decline after twenty years of age but here, in our study, average age of the PCG children was fourteen years only. Hence, this significantly reduced RNFL thickness in operated PCG eyes was associated with higher baseline IOP and inter-visit IOP fluctuation in this study. Hsia et al.[11] found these two factors responsible for structural progression on OCT in PCG eyes over time as well. A mean RNFL thickness of 100 μm, with the superior and inferior quadrant being thickest, was recorded by Srinivasan et al.[12] in south Indian normal children. Yanni et al.[20] noted a mean global RNFL thickness of 107.6 μm, and Hong et al.[21] 107.7 μm in normal children. The mean RNFL thickness of different quadrants of control eyes in our study was comparable with those of previously recorded values in normal children[22] and Indian adult eyes.[12,19] Our study found that the peripapillary RNFL thickness had a double-hump pattern following the inferior ≥ superior ≥ nasal ≥ temporal (ISNT) rule, while Leung et al.[23] observed a decrease of RNFL thickness from superior to inferior to temporal to nasal (SITN). Morales-Fernandez et al.[24] found that segmented macular layer analysis on SD-OCT shows a good capacity to discriminate between normal and glaucomatous eyes. Nieves-Moreno et al.[25] found that inner macular layers thickness and cpRNFL thickness show a good correlation with the mean deviation of the visual field in children with primary congenital glaucoma.
Superotemporal, inferotemporal and inferonasal quadrants had significantly reduced RNFL thickness in operated PCG eyes compared to normal healthy eyes in this study, with other studies also showing an asymmetrical loss of RNFL thickness in children.[7,8,9] Lever et al.[26] reported that parapapillary RNFL thickness was decreased in the superior, nasal, and inferior quadrants of childhood glaucoma patients compared to healthy controls. Srinivasan et al.[12] reported a concentric decrease in RNFL thickness and NRR, suggested to be because of diffuse loss in PCG eyes. Our study found that average RNFL thickness showed a negative relationship with cup–disc area among the three groups, namely, controls, PCG eyes with normal VF, and PCG eyes with VF defect. Amanda L Ely et al.[14] also recorded a negative relationship between increased ONH cupping and average postoperative RNFL thickness. Operated PCG eyes in our study had significantly reduced final visual acuity as compared to normal eyes, also noted by other studies.[7,11,12,27] Dahlman-Noor et al.[28] noted a reduced functional visual ability (FVA) in children with glaucoma.
Regarding the comparison of PCG eyes having VF defects and PCG eyes having a VF within normal limits in this study, there was a significantly higher baseline IOP noted in PCG eyes with VF defect than PCG eyes with normal VF. A higher baseline IOP could cause irreversible damage to the ONH, and such eyes were likely to have VF defects even when all follow-up IOP parameters were within target range. On regression analysis, this study found that higher baseline IOP, increased fluctuation of follow-up IOP, and higher follow-up IOP were significantly associated with RNFL loss and functional VF defects. On multivariable regression analysis, Hsia et al.[11] found that only inter-visit fluctuation IOP was significantly associated with OCT progression and higher average IOP was associated with VF progression. Our study found that RNFL loss was significantly different in all quadrants except nasal between PCG eyes with VF defects and PCG eyes with normal VFs. A similar pattern of RNFL loss was noted by Lever et al.[29] in a comparison of glaucoma patients and normal subjects, where they found the highest correlation of glaucoma with inferior quadrant and temporal inferior sector peripapillary RNFL loss, and found that SD-OCT-derived pRNFL and macular thickness measurements seem highly valuable for the diagnosis of pediatric glaucoma, but the discriminative ability was moderate.
Limitations of this study were the small number of eyes studied as cases and controls, and their variable baseline and follow-up clinical features. There were very few eyes with each pattern of VF loss, so that corresponding ranges of RNFL thickness at which a VF defect could be expected could not be statistically evaluated. Based on its cross-sectional nature, the study has failed to judge the progression of the disease in PCG eyes with RNFL OCT. Therefore, studies with larger sample size and longer follow-up are needed to judge the definite progression of the disease based on RNFL OCT, when visual fields are either unreliable or fails to show the progression.
Conclusion
In conclusion, peripapillary RNFL thickness could be used to identify PCG eyes having VF loss and possibly poor visual function, from PCG eyes without visual field defects. Baseline IOP and IOP on follow-up significantly correlated with RNFL thickness in PCG eyes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Moore DB, Tomkins O, Ben-Zion I. A review of primary congenital glaucoma in the developing world. Surv Ophthalmol. 2013;58:278–85. doi: 10.1016/j.survophthal.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Senthil S, Badakere S, Ganesh J, Krishnamurthy R, Dikshit S, Choudhari N, et al. Profile of childhood glaucoma at a tertiary center in South India. Indian J Ophthalmol. 2019;67:358–65. doi: 10.4103/ijo.IJO_786_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandona L, Dandona R, Naduvilath TJ, McCarty CA, Nanda A, Srinivas M, et al. Is current eye-care-policy focus almost exclusively on cataract adequate to deal with blindness in India? Lancet Lond Engl. 1998;351:1312–6. doi: 10.1016/S0140-6736(97)09509-3. [DOI] [PubMed] [Google Scholar]
- 4.Zagora SL, Funnell CL, Martin FJ, Smith JEH, Hing S, Billson FA, et al. Primary congenital glaucoma outcomes:Lessons From 23 Years of Follow-up. Am J Ophthalmol. 2015;159:788–96.e2. doi: 10.1016/j.ajo.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Guo C, Wu Y, Xu L, Li M, Wang Z, Ni N, et al. Evaluation of preoperative speed of progression and its association with surgical outcomes in primary congenital glaucoma patients:A retrospective study. BMC Ophthalmol. 2017;17:170. doi: 10.1186/s12886-017-0565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gusson E, Chemello F, Longo R, Franzolin E, Vesentini R, Verlato G, et al. Primary congenital glaucoma surgery:Outcomes and visual function. Int Ophthalmol. 2021;41:3861–7. doi: 10.1007/s10792-021-01957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghasia FF, Freedman SF, Rajani A, Holgado S, Asrani S, El-dairi M. Optical coherence tomography in paediatric glaucoma:Time domain versus spectral domain. Br J Ophthalmol. 2013;97:837–42. doi: 10.1136/bjophthalmol-2012-302648. [DOI] [PubMed] [Google Scholar]
- 8.Hess DB, Asrani SG, Bhide MG, Enyedi LB, Stinnett SS, Freedman SF. Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am J Ophthalmol. 2005;139:509–17. doi: 10.1016/j.ajo.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 9.Nadeau S, Gire J, Coste R, Cornand E, Denis D. [Papillary retinal nerve fiber layer thickness measurement using optical coherence tomography in children with ocular hypertension and juvenile glaucoma] J Fr Ophtalmol. 2010;33:249–57. doi: 10.1016/j.jfo.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Mrugacz M, Bakunowicz-łazarczyk A. Optical coherence tomography measurement of the retinal nerve fiber layer in normal and juvenile glaucomatous eyes. Ophthalmologica. 2005;219:80–5. doi: 10.1159/000083265. [DOI] [PubMed] [Google Scholar]
- 11.Hsia Y, Lai TT, Su CC, Wang TH, Huang JY. Long-term structural and functional outcomes of primary congenital glaucoma. Graefes Arch Clin Exp Ophthalmol. 2021;259:2317–26. doi: 10.1007/s00417-021-05185-1. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan S, Addepalli UK, Rao HL, Garudadri CS, Mandal AK. Spectral domain optical coherence tomography in children operated for primary congenital glaucoma. Br J Ophthalmol. 2014;98:162–5. doi: 10.1136/bjophthalmol-2012-302486. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez FG, Sanders DS, Moon JJ, Gardiner SK, Reynaud J, Fortune B, et al. Effect of trabeculectomy on oct measurements of the optic nerve head neuroretinal rim tissue. Ophthalmol Glaucoma. 2020;3:32–9. doi: 10.1016/j.ogla.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ely AL, El-Dairi MA, Freedman SF. Cupping reversal in pediatric glaucoma--Evaluation of the retinal nerve fiber layer and visual field. Am J Ophthalmol. 2014;158:905–15. doi: 10.1016/j.ajo.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Guedes V, Schuman JS, Hertzmark E, Wollstein G, Correnti A, Mancini R, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology. 2003;110:177–89. doi: 10.1016/s0161-6420(02)01564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol Chic Ill 1960. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Proudlock FA, Gottlob I. Pediatric optical coherence tomography in clinical practice-recent progress. Invest Ophthalmol Vis Sci. 2016;57:OCT69–79. doi: 10.1167/iovs.15-18825. [DOI] [PubMed] [Google Scholar]
- 18.Tschopp C, Safran AB, Viviani P, Bullinger A, Reicherts M, Mermoud C. Automated visual field examination in children aged 5-8 years. Part I:Experimental validation of a testing procedure. Vision Res. 1998;38:2203–10. doi: 10.1016/s0042-6989(97)00368-4. [DOI] [PubMed] [Google Scholar]
- 19.Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007;114:921–6. doi: 10.1016/j.ophtha.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Yanni SE, Wang J, Cheng CS, Locke KI, Wen Y, Birch DG, et al. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am J Ophthalmol. 2013;155:354–60.e1. doi: 10.1016/j.ajo.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong SW, Ahn YJ, Kang NY. Relationship between age and retinal nerve fiber layer thickness in normal children. Semin Ophthalmol. 2017;32:655–60. doi: 10.3109/08820538.2016.1157613. [DOI] [PubMed] [Google Scholar]
- 22.Turk A, Ceylan OM, Arici C, Keskin S, Erdurman C, Durukan AH, et al. Evaluation of the nerve fiber layer and macula in the eyes of healthy children using spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153:552–9.e1. doi: 10.1016/j.ajo.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Leung MMP, Huang RYC, Lam AKC. Retinal nerve fiber layer thickness in normal Hong Kong chinese children measured with optical coherence tomography. J Glaucoma. 2010;19:95–9. doi: 10.1097/IJG.0b013e3181a98cfa. [DOI] [PubMed] [Google Scholar]
- 24.Morales-Fernandez L, Jimenez-Santos M, Martinez-de-la-Casa JM, Sanchez-Jean R, Nieves M, Saenz-Frances F, et al. Diagnostic capacity of SD-OCT segmented ganglion cell complex versus retinal nerve fiber layer analysis for congenital glaucoma. Eye Lond Engl. 2018;32:1338–44. doi: 10.1038/s41433-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieves-Moreno M, García-Caride S, Morales-Fernandez L, Martínez-de-la-Casa JM, Sáenz-Francés F, Sánchez-Jean R, et al. The correlation between the thickness of the inner macular layers and the mean deviation of the visual field in children with primary congenital glaucoma. Arch Soc Espanola Oftalmol. 2019;94:536–9. doi: 10.1016/j.oftal.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Lever M, Halfwassen C, Unterlauft J, Bechrakis N, Manthey A, Böhm M. Retinal nerve fibre layer thickness measurements in childhood glaucoma:The role of scanning laser polarimetry and optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2021;259:3777–86. doi: 10.1007/s00417-021-05276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EJ, Han JC, Park DY, Kee C. Long-term morphologic fundus and optic nerve head pattern of progressive myopia in congenital glaucoma distinguished by age at first surgery. Sci Rep. 2020;10:10041. doi: 10.1038/s41598-020-67051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahlmann-Noor A, Tailor V, Abou-Rayyah Y, Adams G, Brookes J, Khaw PT, et al. Functional vision and quality of life in children with microphthalmia/anophthalmia/coloboma-a cross-sectional study. J AAPOS. 2018;22:281–5.e1. doi: 10.1016/j.jaapos.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Lever M, Halfwassen C, Unterlauft JD, Bechrakis NE, Manthey A, Böhm MRR. The paediatric glaucoma diagnostic ability of optical coherence tomography:A comparison of macular segmentation and peripapillary retinal nerve fibre layer thickness. Biology. 2021;10:260. doi: 10.3390/biology10040260. [DOI] [PMC free article] [PubMed] [Google Scholar]