Abstract
We aimed to develop a novel and effective technique for creating a smooth deep lamellar dissection of the cornea using a femtosecond (FS) laser for deep anterior lamellar keratoplasty (DALK), we conducted a retrospective eye bank study. Thirteen fresh human corneas were mounted on an artificial anterior chamber, and deep lamellar cuts were made with a 500-kHz VisuMax FS laser at a level of 50–80 mm anterior to the Descemet’s membrane (DM). A posterior diameter of 8 mm with a side cut angle of 110° was used for the anterior penetrating side cut. The anterior lamellar tissue was bluntly dissected. The residual posterior stromal beds and side cuts were examined with microscopy and intraoperative optical coherence tomography (OCT) and post-cut endothelial cell evaluations. All corneas revealed a smooth residual posterior stromal bed without any visible irregularities or ridges by microscopy and OCT imaging. Six corneas were suitable for post-cut endothelial cell evaluation 2 days after laser cut, with no significant endothelial cell loss post-laser and blunt dissection of the posterior stroma. FS laser deep lamellar keratoplasty utilizing an ultrafast laser to produce a smooth deep stromal dissection followed by blunt dissection and removal of the anterior stromal tissue yields a consistent and smooth residual stromal bed. The creation of a smooth lamellar dissection in the deep posterior cornea may result in more consistent DALK without the need for air bubble or manual baring of DM that has the risk for DM perforation.
Keywords: Anterior lamellar keratoplasty (ALK), deep anterior lamellar keratoplasty (DALK), femtosecond (FS) laser, femtosecond laser-enabled keratoplasty (FLEK)
Evolution of corneal transplantation has led to anterior and posterior lamellar surgeries that selectively target diseased portions of the cornea. Lamellar keratoplasties such as Descemet’s stripping automated endothelial keratoplasty (DSAEK), Descemet’s membrane endothelial keratoplasty (DMEK), and deep anterior lamellar keratoplasty (DALK) are now widely used in place of traditional penetrating keratoplasty (PK) for dealing with a wide range of corneal pathology.
Initially described by Anwar and Teichmann in 2002,[1] the “big-bubble” technique for DALK has been the mainstay for separating the stroma from Descemet’s membrane. This technique has improved corneal tissue utilization globally due to less-stringent endothelial count requirements.[1,2] The main obstacle to universal acceptance and conversion has been the surgical difficulty and the unpredictable rate of Descemet’s membrane (DM) perforation during the big-bubble procedure.[3,4] Many surgeons have adopted a deep anterior hand dissection in cases where full baring of DM is unattainable. Visual outcomes of these cases have been suboptimal mainly, in part, due to the irregular lamellar interface quality produced by hand dissections.[5,6]
The advent of femtosecond (FS) laser technology in corneal surgery has allowed surgeons to produce cuts not possible with manual techniques. The refractive surgeons were the first to benefit from the precision of the FS lasers. The smooth surface of the lamellar flap cut in laser in situ keratomileusis (LASIK) has significantly improved the reproducibility and safety of the procedure.[7,8,9] Furthermore, the anterior lamellar cut results in a smooth stromal bed that does not limit the visual acuity and optical quality.[10,11] Thus, many corneal surgeons have adopted the FS laser anterior lamellar keratoplasty procedure with success and have obtained excellent optical outcomes.[12,13]
Earlier studies attempted to replicate the same smooth cut in the posterior lamella of the cornea using the FS laser.[14,15,16] However, the inherent loose lamellar collagen fibers of the posterior stroma present a significant challenge to producing acceptable smooth cuts that would mimic deep cuts made by a microkeratome. Scanning electron microscopy (SEM) images of deep stromal cuts made by the FS laser have shown an irregular and rougher appearing surface with the appearance of concentric peripheral rings.[17,18,19,20,21,22] Additionally, there are controversial findings regarding endothelial damage with higher energy settings at deeper layers.
The following eye bank study illustrates an FS laser deep posterior lamellar corneal cut that closely resembles the smooth cut made in the anterior cornea by using an ultrafast FS laser with lower energy and closer set spot size and separation. Continued studies of this technique may prove useful in both DALK as well as in donor tissue preparation for endothelial keratoplasty (EK).
Surgical Technique
Laser parameters
The 500-kHz VisuMax FS laser (Carl Zeiss Meditec AG, Jena, Germany) was set at the lamellar keratoplasty mode. The pachymetry was selected to create a posterior stromal bed with a residual stromal thickness (RST) of either 50 mm (n = 6) or 80 mm (n = 7). A diameter of 8 mm with a side cut angle of 110° was used for the anterior penetrating side cut. The lamellar cut was made in a spiral pattern with spot and track spacing at 3.0 and a spot energy of 300 nJ.
Operative technique
Fresh human corneas (n = 13) obtained from CorneaGen (Seattle, WA, USA) were mounted on artificial anterior chambers. A 500-kHz VisuMax FS laser was used to perform deep stromal lamellar dissection cuts. Ultrasound pachymetry (model 5100c; DGH Technology, Exton, PA, USA) was used to determine the pre-cut central corneal thickness, and the posterior lamellar cut was set for an RST of 50 or 80 mm. Following the deep lamellar cut, a straight side cut to the surface was made at a diameter of 8 mm which would then allow separation and removal of an anterior stromal cap, thereby leaving the deep posterior stromal bed exposed for evaluation. Following laser treatment, a blunt hook dissector was used to separate the side cut and find the posterior lamellar dissection plane. Gentle peeling and removal of the anterior corneal button was then performed to expose the posterior stromal dissection plane. Optical coherence tomography (OCT) imaging was taken throughout resection of the anterior button to ensure complete removal of loose tissue overlying the new stromal bed.
Posterior stromal bed evaluation
The posterior stromal dissection plane was assessed during and after corneal button removal by microscopy and intraoperative OCT (RESCAN), integrated to a Lumera 700 (Carl Zeiss Meditec, Inc, Dublin, CA, USA). Analysis of images was performed by an experienced cornea specialist to assess for aberrations, appropriate depth of incision, and integrity.
Endothelial cell evaluation
The corneas were sent back to the eye bank for post-laser and dissection endothelial cell evaluations. Due to a 2-day delay between the procedure and endothelial cell evaluation, only six of the 13 corneas were suitable for endothelial cell density (ECD) evaluation. The other seven corneas were too edematous to obtain ECD and scan. Pre- and post-laser-cut and dissected corneas were all evaluated for ECD using the CellChek D specular microscope (Konan Medical, Irvine, CA, USA).
Results
Corneas treated with 500 kHz VisuMax FS laser had an RST ranging from 50 to 80 mm. All corneas were treated with a spot energy of 300 nJ. The posterior diameter was set and remained at 8 mm, while the anterior diameter ranged from 8.28 to 9.12 mm due to a 110° side cut angle and it varied based on pachymetry. Treated corneas had an original pachymetry ranging from 503 to 734 mm. There was no significant difference in the demographic characteristics of the human donor corneas. After blunt dissection and removal of the anterior stromal button, microscopy of the residual stromal bed revealed a smooth dissection without posterior irregularities, ridges, or concentric peripheral rings [Fig. 1]. OCT imaging was taken throughout resection of the anterior button to ensure complete removal of the loose tissue overlying the new stromal bed. Still images of OCT were captured at various sites of the stromal bed. Analysis of OCT imaging showed smooth stromal beds with minimal aberrations, occasional wisps of torn stroma on the top of a smooth stromal bed, and clear-angled side cuts [Fig. 1].
Figure 1.
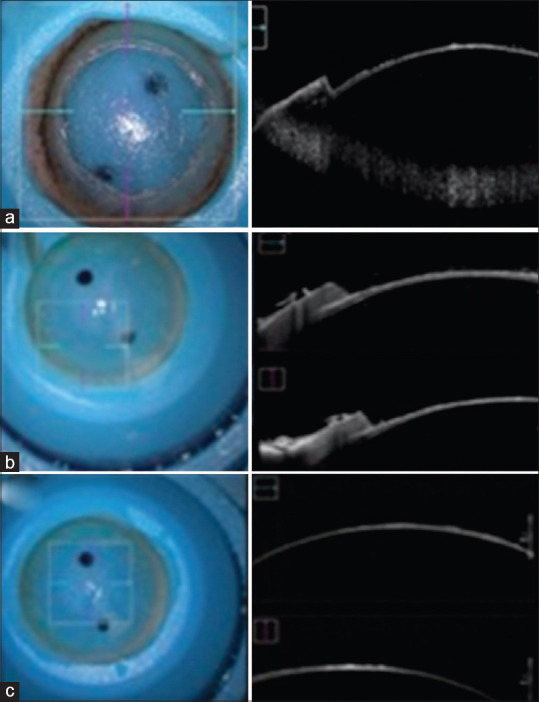
Microscopic and OCT imaging. Selected OCT images from three different corneas cut with VisuMax femtosecond laser. OCT = optical coherence tomography
Endothelial cell evaluation pre- and post-laser and blunt dissection revealed no significant endothelial cell loss (n = 6). Average pre-laser ECD was 2229 and post-laser ECD was 2313 (P > 0.05) [Table 1].
Table 1.
Cornea characteristics and pre- and post-ECD
| Cornea | Donor age/sex | Original pachymetry | Pre-ECD | Post-ECD |
|---|---|---|---|---|
| 1 | 62/F | 584 | 2045 | Unable |
| 2 | 69/M | 503 | 2096 | 1815 |
| 3 | 70/M | 620 | 2415 | 2778 |
| 4 | 63/M | 592 | 2882 | Unable |
| 5 | 57/M | 581 | 1616 | 1764 |
| 6 | 69/M | 574 | 2849 | 2506 |
| 7 | 60/M | 648 | 2646 | Unable |
| 8 | 63/M | 602 | 2740 | 3289 |
| 9 | 59/M | 585 | 2809 | Unable |
| 10 | 60/M | 655 | 2755 | Unable |
| 11 | 55/F | 542 | 2545 | Unable |
| 12 | 49/M | 734 | 3175 | Unable |
| 13 | 57/M | 569 | 1658 | 1730 |
ECD=endothelial cell density
Discussion
FS laser technology has made major contributions to the field of corneal keratoplasty. Customized trephination patterns such as the zigzag technique have been useful in improving visual recovery time, astigmatism, and higher-order aberrations.[23,24,25,26] Attempts to make a lamellar laser pass in the deep stroma to replace the big-bubble technique for DALK have posed challenges. The same gas bubbles that cause the harmless opaque bubble layer (OBL) in LASIK flap creation can more easily migrate vertically in the posterior cornea, and high-energy pulses, therefore, create an irregular dissection plane.[27] The ridged, irregular interface can scatter light, degrading optical performance and visual acuity. In this study, we demonstrate that the more modern ultrafast and lower-energy FS lasers can now be an effective method to produce smooth dissection by confining the gas expansion through low-energy pulses.
The results show the feasibility of utilizing FS laser for complete resection of the anterior portions of the cornea while maintaining a smooth stromal surface for better visual outcomes. The 13 corneas that underwent resection with VisuMax FS laser yielded no perforations in DM, which can significantly improve the perforation percentages found in classical DALK procedure.[28,29,30] Previous evidence of posterior stromal irregularities and concentric peripheral rings that were seen with the high-energy FS laser settings[17,18,19,20,21,22] are not seen with the faster, lower-pulse energy settings. It is likely that visual performance with these smoother interface beds will be better, although human clinical trials will need to further assess this potential benefit.
Recent studies have continued the expansion of FS laser applicability for multiple procedures including DALK. Buzzonetti et al.[31] utilized a 60-kHz IntraLase laser on 11 eyes to create initial lamellar cuts before DM splitting with the big-bubble technique. Similarly, Mosca et al.[32] performed laser-assisted DALK with a 500-kHz VisuMax laser on 21 eyes with a similar technique of splitting the stroma from DM using the big-bubble. FS laser application in DALK has shown reduced perforation of DM and improved standardization of the procedure.
Of note, a study conducted by Lu et al.[33] in 10 eyes of patients with keratoconus and keratectasia utilized VisuMax FS laser to perform corneal cuts on both donor and recipient corneas without the use of the big-bubble technique. The study used a lower total energy (230 nJ spiral energy). This study showed good visual outcomes in patients, achieved RSTs of 74–105 mm, and had only one case of perforation in DM. This shows a promising future for a standardized FS laser dissection of the posterior cornea without reliance on the big-bubble technique.
Previous work with FS laser cuts in the deep posterior cornea has not shown to be toxic to the endothelial cells.[34] Our study similarly shows no significant difference in endothelial health pre- and post-laser and dissection on six of the 13 corneas that were suitable for post-cut ECD evaluation. As the energy used in these cases is very low, there is no significant observed endothelial damage.
While investigators were quick to make use of the FS laser’s ability to create lamellar cuts to prepare the donor buttons for DSAEK, initial results showed poorer visual outcomes in patients receiving laser-cut tissue in comparison to patients receiving standard penetrating keratoplasty (PKP).[35] Mootha et al.[20] showed a smoother stromal bed in corneas cut with either of the two tested microkeratomes compared to those cut with the FS laser. These studies, however, used early high-energy, slower FS lasers to prepare the DSAEK donor graft. Recent studies are showing more promise for FS laser-enabled posterior lamellar keratoplasty.[36]
Conclusion
Continued work with ultrafast FS laser technology will allow for smoother EK grafts with a more consistent thickness. Finally, translation of this technology into human clinical trials with continued refinement of laser parameters is necessary to assess the efficacy of this procedure in clinical practice for both anterior keratoplasties as well as in donor tissue preparation for posterior keratoplasties.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Anwar M, Teichmann KD. Deep lamellar keratoplasty: Surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet's membrane. Cornea. 2002;21:374–83. doi: 10.1097/00003226-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Heindl LM, Riss S, Bachmann BO, Laaser K, Kruse FE, Cursiefen C. Split cornea transplantation for 2 recipients: A new strategy to reduce corneal tissue cost and shortage. Ophthalmology. 2011;118:294–301. doi: 10.1016/j.ophtha.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Jhanji V, Sharma N, Vajpayee RB. Intraoperative perforation of Descemet's membrane during “big bubble” deep anterior lamellar keratoplasty. Int Ophthalmol. 2010;30:291–5. doi: 10.1007/s10792-009-9334-7. [DOI] [PubMed] [Google Scholar]
- 4.Unal M, Bilgin B, Yucel I, Akar Y, Apaydin C. Conversion to deep anterior lamellar keratoplasty (DALK): Learning curve with big-bubble technique. Ophthalmic Surg Lasers Imaging. 2010;41:642–50. doi: 10.3928/15428877-20100929-09. [DOI] [PubMed] [Google Scholar]
- 5.Borderie VM, Sandali O, Bullet J, Gaujoux T, Touzeau O, Laroche L. Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology. 2012;119:249–55. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Parente G, Sincich A, Tassinari G. Influence of graft-host interface on the quality of vision after deep anterior lamellar keratoplasty in patients with keratoconus. Cornea. 2011;30:497–502. doi: 10.1097/ico.0b013e3181d25e4d. [DOI] [PubMed] [Google Scholar]
- 7.Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004;30:26–32. doi: 10.1016/S0886-3350(03)00578-9. [DOI] [PubMed] [Google Scholar]
- 8.Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:804–11. doi: 10.1016/j.jcrs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Talamo JH, Meltzer J, Gardner J. Reproducibility of flap thickness with IntraLase FS and Moria LSK-1 and M2 microkeratomes. J Refract Surg. 2006;22:556–61. doi: 10.3928/1081-597X-20060601-07. [DOI] [PubMed] [Google Scholar]
- 10.Sarayba MA, Ignacio TS, Tran DB, Binder PS. A 60 kHz IntraLase femtosecond laser creates a smoother LASIK stromal bed surface compared to a Zyoptix XP mechanical microkeratome in human donor eyes. J Refract Surg. 2007;23:331–7. doi: 10.3928/1081-597X-20070401-04. [DOI] [PubMed] [Google Scholar]
- 11.Sarayba MA, Maguen E, Salz J, Rabinowitz Y, Ignacio TS. Femtosecond laser keratome creation of partial thickness donor corneal buttons for lamellar keratoplasty. J Refract Surg. 2007;23:58–65. doi: 10.3928/1081-597X-20070101-10. [DOI] [PubMed] [Google Scholar]
- 12.Almousa R, Samaras KE, Khan S, Lake DB, Daya SM. Femtosecond laser-assisted lamellar keratoplasty (FSLK) for anterior corneal stromal diseases. Int Ophthalmol. 2014;34:49–58. doi: 10.1007/s10792-013-9794-7. [DOI] [PubMed] [Google Scholar]
- 13.Bonfadini G, Moreira H, Jun AS, Campos M, Kim EC, Arana E. Modified femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Cornea. 2013;32:533–7. doi: 10.1097/ICO.0b013e31826e828c. [DOI] [PubMed] [Google Scholar]
- 14.Jones YJ, Goins KM, Sutphin JE, Mullins R, Skeie JM. Comparison of the femtosecond laser (IntraLase) versus manual microkeratome (Moria ALTK) in dissection of the donor in endothelial keratoplasty: Initial study in eye bank eyes. Cornea. 2008;27:88–93. doi: 10.1097/ICO.0b013e31815771f5. [DOI] [PubMed] [Google Scholar]
- 15.Mian SI, Soong HK, Patel SV, Ignacio T, Juhasz T. In vivo femtosecond laser-assisted posterior lamellar keratoplasty in rabbits. Cornea. 2006;25:1205–9. doi: 10.1097/01.ico.0000231491.95377.0b. [DOI] [PubMed] [Google Scholar]
- 16.Soong HK, Mian S, Abbasi O, Juhasz T. Femtosecond laser-assisted posterior lamellar keratoplasty: Initial studies of surgical technique in eye bank eyes. Ophthalmology. 2005;112:44–9. doi: 10.1016/j.ophtha.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Cleary C, Song JC, Tang M, Li Y, Liu Y, Yiu S, et al. Dual laser-assisted lamellar anterior keratoplasty with top hat graft: A laboratory study. Cornea. 2012;31:791–7. doi: 10.1097/ICO.0b013e318226da35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimakura M, Sakai O, Nakagawa S, Yoshida J, Shirakawa R, Toyono T, et al. Stromal bed quality and endothelial damage after femtosecond laser cuts into the deep corneal stroma. Br J Ophthalmol. 2013;97:1404–9. doi: 10.1136/bjophthalmol-2013-303328. [DOI] [PubMed] [Google Scholar]
- 19.Marian A, Nada O, Légaré F, Meunier J, Vidal F, Roy S, et al. Smoothness assessment of corneal stromal surfaces. J Cataract Refract Surg. 2013;39:118–27. doi: 10.1016/j.jcrs.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Mootha VV, Heck E, Verity SM, Petroll WM, Lakshman N, Muftuoglu O, et al. Comparative study of descemet stripping automated endothelial keratoplasty donor preparation by Moria CBm microkeratome, horizon microkeratome, and Intralase FS60. Cornea. 2011;30:320–4. doi: 10.1097/ICO.0b013e3181f22cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips PM, Phillips LJ, Saad HA, Terry MA, Stolz DB, Stoeger C, et al. “Ultrathin” DSAEK tissue prepared with a low-pulse energy, high-frequency femtosecond laser. Cornea. 2013;32:81–6. doi: 10.1097/ICO.0b013e31825c72dc. [DOI] [PubMed] [Google Scholar]
- 22.Ziebarth NM, Dias J, Hürmeriç V, Shousha MA, Yau CB, Moy VT, et al. Quality of corneal lamellar cuts quantified using atomic force microscopy. J Cataract Refract Surg. 2013;39:110–7. doi: 10.1016/j.jcrs.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farid M, Steinert RF, Gaster RN, Chamberlain W, Lin A. Comparison of penetrating keratoplasty performed with a femtosecond laser zig-zag incision versus conventional blade trephination. Ophthalmology. 2009;116:1638–43. doi: 10.1016/j.ophtha.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Levinger E, Trivizki O, Levinger S, Kremer I. Outcome of “mushroom” pattern femtosecond laser-assisted keratoplasty versus conventional penetrating keratoplasty in patients with keratoconus. Cornea. 2014;33:481–5. doi: 10.1097/ICO.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 25.Gaster RN, Dumitrascu O, Rabinowitz YS. Penetrating keratoplasty using femtosecond laser-enabled keratoplasty with zig-zag incisions versus a mechanical trephine in patients with keratoconus. Br J Ophthalmol. 2012;96:1195–9. doi: 10.1136/bjophthalmol-2012-301662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlain W, Omid N, Lin A, Farid M, Gaster RN, Steinert RF. Comparison of corneal surface higher-order aberrations after endothelial keratoplasty, femtosecond laser-assisted keratoplasty, and conventional penetrating keratoplasty. Cornea. 2012;31:6–13. doi: 10.1097/ICO.0b013e3182151df2. [DOI] [PubMed] [Google Scholar]
- 27.Kaiserman I, Maresky HS, Bahar I, Rootman DS. Incidence, possible risk factors, and potential effects of an opaque bubble layer created by a femtosecond laser. J Cataract Refract Surg. 2008;34:417–23. doi: 10.1016/j.jcrs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Fogla R, Padmanabhan P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2006;141:254–9. doi: 10.1016/j.ajo.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 29.Al-Torbak AA, Al-Motowa S, Al-Assiri A, Al-Kharashi S, Al-Shahwan S, Al-Mezaine H, et al. Deep anterior lamellar keratoplasty for keratoconus. Cornea. 2006;25:408–12. doi: 10.1097/01.ico.0000220777.70981.46. [DOI] [PubMed] [Google Scholar]
- 30.Michieletto P, Balestrazzi A, Balestrazzi A, Mazzotta C, Occhipinti I, Rossi T. Factors predicting unsuccessful big bubble deep lamellar anterior keratoplasty. Ophthalmologica. 2006;220:379–82. doi: 10.1159/000095864. [DOI] [PubMed] [Google Scholar]
- 31.Buzzonetti L, Laborante A, Petrocelli G. Refractive outcome of keratoconus treated by combined femtosecond laser and big-bubble deep anterior lamellar keratoplasty. J Refract Surg. 2011;27:189–94. doi: 10.3928/1081597X-20100520-01. [DOI] [PubMed] [Google Scholar]
- 32.Mosca L, Fasciani R, Tamburelli C, Buzzonetti L, Guccione L, Mandarà E, et al. Femtosecond laser-assisted lamellar keratoplasty: Early results. Cornea. 2008;27:668–72. doi: 10.1097/ICO.0b013e31816736b1. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Shi Y-H, Yang L-P, Ge YR, Chen XF, Wu Y, et al. Femtosecond laser-assisted deep anterior lamellar keratoplasty for keratoconus and keratectasia. Int J Ophthalmol. 2014;7:638–43. doi: 10.3980/j.issn.2222-3959.2014.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blériot A, Martin E, Lebranchu P, Zimmerman K, Libeau L, Weber M, et al. Comparison of 12-month anatomic and functional results between Z6 femtosecond laser-assisted and manual trephination in deep anterior lamellar keratoplasty for advanced keratoconus. J Fr Ophtalmol. 2017;40:e193–200. doi: 10.1016/j.jfo.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Cheng YY, Schouten JS, Tahzib NG, Wijdh RJ, Pels E, van Cleynenbreugel H, et al. Efficacy and safety of femtosecond laser-assisted corneal endothelial keratoplasty: A randomized multicenter clinical trial. Transplantation. 2009;88:1294–302. doi: 10.1097/TP.0b013e3181bc419c. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Tian L, Le Q, Zhao F, Zhao Y, Chen Y, et al. Femtosecond laser-assisted Descemet's stripping endothelial keratoplasty: A prospective study of 6-month visual outcomes, corneal thickness and endothelial cell loss. Int Ophthalmol. 2020;40:2065–75. doi: 10.1007/s10792-020-01383-8. [DOI] [PubMed] [Google Scholar]


