Abstract
In the COVID-19 period, face masks increased exponentially. Several studies suggest that the rise in ocular discomfort symptoms during the pandemic is mostly part of dry eye disease and that these are due to the effect of face masks, resulting in the newly described term MADE, for “mask-associated dry eye”. The most commonly proposed mechanism states that wearing a face mask creates an unnatural upward airflow towards the ocular surface during expiration, although the increased temperature, humidity and levels of carbon dioxide of the exhaled air, stress, increased use of video display terminals, as well as changes in the ocular microbiota may contribute. Evidence supports that the use of face masks causes an increase in dry eye disease symptoms, a decreased tear break-up time, corneal epithelial trauma, periocular temperature changes and inflammatory markers secretion. Given that the use of masks may be frequent in some settings in the near future, it is important to establish its effects and consequences on the ocular surface.
Keywords: Face mask, Ocular surface, Dry eye, Tear film
1. Introduction
In the COVID-19 period, face masks took a front role in decreasing viral transmission and established as one of the main public health measures to prevent the spread of the virus. Although the world is currently in the process of moving away from wearing masks, its use has increased in hospital settings particularly, with health care workers and patients wearing them for long periods of time [1,2].
In addition, there is evidence that during the pandemic there has been an increase in the prevalence of ocular complaints, such as red eye, irritation, tearing and eye discomfort, being in some cases related to face masks. Several authors suggest that the rise in ocular discomfort symptoms is mostly due to dry eye disease associated to face masks, correlating with long periods of face mask wearing [3].
The effects of face masks on the ocular surface are an important public health issue because the ocular irritation and discomfort due to prolonged face mask use, along with fogging of glasses, could induce frequent removal of the mask and eye rubbing. In turn, this could be counterproductive to public health care measures [4].
Given that the use of masks may continue to be frequent in the near future, especially in the hospital environment or in future pandemics, it is important to establish its effects and consequences on the ocular surface. This may be useful to design strategies to minimize the possible impact on patients.
Hence, the present review deals with the effects of face masks on the ocular surface reported by published studies.
2. Methods
A literature search was conducted using the PubMed database on October 3rd of 2022. The search included any combination of the two keyword groups: (i) ‘dry eye’, ‘ocular’, ‘cornea’ or ‘tear’ and (ii) ‘mask’.
The initial PubMed search yielded 876 results. All published full-text articles in English were included, regardless of publication date. The exclusion criteria included lack of relevance and non-English language. The relevance of the articles was first determined based on title and abstract. Finally, the full text was appraised for inclusion based on relevance: studies regarding the use of face masks and outcome measures pertaining to the ocular surface (including symptoms, Schirmer test, tear film characteristics, ocular surface staining, tear osmolarity, inflammatory markers and periocular temperature). Letters to the editor, editorials, review articles and case reports were also included.
766 papers were excluded because they were not related to the current topic, with the majority of articles not investigating the use of face mask, but using “mask” as a verb, investigating the use of eye masks to treat dry eye or referring to the type of masking done in a clinical trial. Another 27 papers were excluded due to lack of relevance.
After the articles were filtered by relevance, 83 articles were obtained. Then, papers with duplicated data or that provided no additional information were excluded, while additional relevant studies were identified through manual search of the reference list of the already included articles and included. In total, 67 articles related to the use of face masks were considered for the present review.
3. Defining mask-associated dry eye
The first anecdotal observations of an increase in dry eye disease patients with the use of face masks date back to June 2020, when DE White, an American ophthalmologist, described this new condition on his blog and coined the acronym “MADE” for “mask-associated dry eye” [5]. In his early experience, this increase in dry eye disease symptoms did not seem to be more or less prevalent in any particular population. Patients with preexisting dry eye disease had more discomfort, while “new onset” dry eye disease tended to bring more visual symptoms.
Concurrently, Moshirfar et al. [3] described this increase in dry eye disease among face mask users as being more pronounced in subjects with occupations mandating prolonged face masks use, such as healthcare personnel, as well as in patients with pre-existing dry eye disease. The majority of individuals described an awareness of air blowing upward from the mask into their eyes. Based on this, the authors started to suggest possible mechanisms for this new condition.
However, there is no definition of MADE as such, with each author using different criteria. Laura Boccardo was one of the first authors to establish criteria for this condition and developed a specific 19-item questionnaire, the MADE-Q, which was later adopted and modified by other authors [6]. MADE-Q contained questions on age, sex, education, mask wearing time, symptoms and frequency of dry eye (i.e., burning sensation, foreign body sensation, itching sensation, dryness, eye pain, grittiness, or irritation), history of dry eye medicines, daily reading time, outdoor time, and visual display terminals time. For symptom frequency, participants could select never (0 time per day), sometimes (0–4 times per day), or often (>4 times per day). In Boccardo's study, MADE was defined as the condition in which dry eye symptoms are present at least sometimes and become worse using a face mask. By this definition, both people who had symptoms only while wearing the face mask, and people who had symptoms that became worse with the face mask were considered affected by MADE. Instead, people who had no symptoms, and those who had symptoms but no worsening, were considered not affected by MADE by Boccardo.
4. Possible underlying pathophysiological mechanisms
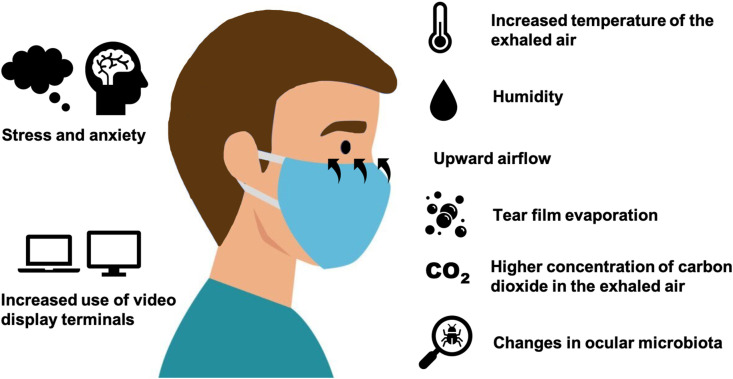
There are many theories about how face mask use affects the ocular surface (Fig. 1 ). The most common hypothesis states that wearing a face mask creates an unnatural upward airflow towards the ocular surface during expiration, which may increase in cases of an incorrectly or insufficiently fitted face mask.
Fig. 1.
Proposed mechanisms of the effect of the use of face masks on the ocular surface.
This upward airflow may influence the normal physiological conditions of the ocular and periocular surface by multiple mechanisms. Firstly, the exhaled air escaping upward from the edge of the mask may stimulate tear film evaporation, which would lead to inflammation, discomfort and dry eye symptoms [3]. This air convection might encourage aqueous tear evaporation through the disruption of the surface lipid layer or by maintaining a water vapor pressure gradient. Potentially, the aqueous-mucin layer may also be altered, thus reducing wettability. Similar to the use of facial masks, the use of continuous positive airway pressure (CPAP) treatment is known to be associated with ocular surface complications related to the increased air flow around the eye [7,8]. CPAP therapy has proven to cause increased ocular irritation, epiphora, tear evaporation, and conjunctival squamous metaplasia [[9], [10], [11]]. Other respirators also associate increased perceptions of eye dryness and epithelial punctate keratopathy, probably due to how air is directed upwards towards the ocular surface [12,13]. Therefore, there are many studies suggesting that increased air convection has a deleterous effect on the ocular surface.
A second possible mechanism is based on the higher concentration of carbon dioxide of the exhaled air (4–5%) compared to the inhaled air (0.4%). Carbon dioxide levels can be up to 10 times higher within a face mask [14,15]. An increase in carbon dioxide could result in changes in corneal nerve sensation, as it has been reported to increase corneal pain sensations in animal models [16]. Hypercapnia also is known to induce inflammation, although the effects on the ocular surface have not been thoroughly investigated [17]. Nevertheless, D'Souza et al. hypothesized that the increased carbon dioxide in the exhaled air flowing over the ocular surface may induce hypercapnia-related changes and alter the molecular profile [18]. The exhaled air is usually warmer than the environment and these changes in temperature might also promote tear evaporation [19].
Taping the masks to prevent air convection toward the eyes may interfere with the normal lower eyelid position by inducing mechanical ectropion and tear evaporation. Inducing a mechanical ectropion may be deleterious to ocular surface, causing lagophthalmos or reducing blinking and risking exposure keratopathy. Hence, taping of the upper mask edge should be performed in an appropriate manner so as not to disturb the normal lower lid position.
It is less clear if prolonged face mask wear causes changes in the ocular microbiota. Extensive hygiene, face masks and greater disinfectant and antibiotic usage have the potential to disrupt normal composition of gut microbiota. Differences between pre and postpandemic gut microbiome have been noted, along with a decreasing trend of the facial microbiome diversity after wearing masks [[20], [21], [22]]. Bacterial hypercolonization may trigger the release of inflammatory mediators and bacterial lipase may alter tear film stability. However, we still do not understand the extent to which ocular microbiota will change nor its consequences. An increase in common cold and asthma has been noted among niqabs (facial cloth veils) wearers versus non-veil wearers [23]. Multiple questions have arisen in this regard, particularly about the microbial subpopulations along the inner and outer surfaces of a mask, as they may be critical in developing a dysbiotic microbiome [24]. Emerging findings suggest the existence of a gut-eye or a gut-eye-lacrimal gland microbiome axis, where gut dysbiosis may influence the onset and progression of multiple ocular diseases, including dry eye disease [25,26]. Therefore, dysbiosis and the overgrowth of atypical microflora in face mask users may have a role in the pathogenesis of ocular surface diseases.
An increased incidence of chalazion and blefaritis has been associated with the use of face mask, possibly due to its association with dry eye [[27], [28], [29]]. Dehydration could be a pathogenic factor for meibomian oil hardening and chalazion formation in healthcare workers wearing sealed goggles, as well as changes in the normal ocular surface flora. Further, mask wear may promote inflammation and increase the chances of transferring bacteria from the hands to the face [30].
On the other hand, online learning has increased and remote work from home has been implemented by many firms around the world to decrease the risk of COVID-19 transmission. This has resulted in an increased use of video display terminals, which may increase dry eye symptoms and ocular discomfort [[31], [32], [33]].
Also, an increase in the ventilation of closed spaces could aggravate these phenomena as well as lockdown in low humidity households with limited airflow. These conditions are known to promote worsening of ocular discomfort and it would be reasonable to expect that these modifications in lifestyle might result in worsening of dry eye disease [34].
Lastly, the COVID-19 pandemic has resulted in a substantial disruption of daily life, with high stress, anxiety levels and sleep impairments [35]. Previous research has shown that dry eye disease is associated with poorer self-perceived health status and greater self-reported psychological stress burden [36]. In the current pandemic, stress levels and sleep disturbance have been cited as reasons for worsening of dry eye symptoms, along with a reduction of pain threshold and inflammation [34]. Indeed, the term “quarantine dry eye” has been proposed by Napoli et al. referring to how lockdown lifestyle, including environmental and behavioral factors, diet, hydration, sleep deprivation and psychological stress is likely to affect the ocular surface health [37].
5. Effects of face mask use on the ocular surface of healthy individuals
5.1. Dry eye symptoms
Since the implementation of face masks in most settings, face mask wearers have described eye irritation, tearing, and red eye, with a subjective worsening in symptoms [3].
The largest survey on mask-associated symptoms was performed by Boccardo [6], who evaluated a total of 3605 patients, 18.3% of which experienced MADE, which was defined as a condition in which dry eye symptoms were present at least occasionally and became worse with the use of a face mask. About one-third of participants (32.1%) never experienced dry eye symptoms, 54.3% sometimes, and 13.6% often. There were no differences in ocular discomfort between participants who wore glasses or contact lenses, while those who wore no correction reported less symptoms.
Based on Boccardo's questionnaire, a cross-sectional survey to investigate the association between wearing face masks and dry eye susceptibility was conducted in Chinese daily face mask wearers [38]. Of 6925 participants, 419 participants sometimes or often experienced dry eye symptoms, and 128 participants experienced dry eye symptom aggravation, the overall rate of MADE incidence being 7.9% and increasing with longer mask wearing time. Longer time of face mask wearing, nonstandard wearing of face masks, reduced outdoor time, decreased daily reading time, shortened visual display terminals time, and dry environment were positively associated with MADE. Associations between perceived MADE and age, female sex, education, use of glasses and contact lenses, and pre-existing dry eye were made. Although those with pre-existing dry eyes were more prone to have MADE, 246 participants with no previous ocular treatment needed treatment for dry eye after wearing face masks.
Similarly, a self-administered 12-item survey was performed in 333 healthcare professionals working in a COVID-19 hospital [39]. The prevalence of self-reported MADE was found to be 70%. Having at least one dry eye symptom without wearing a mask and advanced age were possible risk factors for MADE. However, when the participants with self-reported MADE underwent an ophthalmological examination, only 30.7% had aqueous-type dryness with staining of the ocular surface. In another survey among 107 healthy students (mean age 28.5 years; 64.5% female), 72 (67.3%) reported to use face mask for more than 6 h per day. Eleven participants (10.3%) described appearance or worsening of ocular discomfort symptoms, and 21 (19.6%) reported the need for daily use of tear substitutes [7].
Other authors have also reported worsening of dry eye disease symptoms in large surveys among the general population [40,41] (see Table 1 ). The most commonly reported dry eye symptom was redness (29.3%), followed by burning (15.7%), pain (14.1%), tingling (10.9%), and rash (6.6%) [42]. When types of masks were analyzed, Erogul et al. found no correlation with symptoms, but with time of face mask use instead [42].
Table 1.
Pertinent studies assessing ocular surface symptoms with the use of face masks.
| Authors (Country) | Population | Examinations | Observations |
|---|---|---|---|
| Fan et al. (China) | General population (n = 6925) | Online questionnaire | 547 participants had MADE: 419 with new symptoms and 128 participants whose pre-existing dry eye symptoms had worsened with mask wearing. Perceived MADE associated with age, female sex, education, glasses, contact lenses, and pre-existing DED. |
| Boccardo (Italy) | General population (n = 3605) | Online questionnaire | 2447 had symptoms, 658 of which had exacerbated with masks. MADE was present in 18.3% of participants. No association between perceived MADE and age, refractive correction, and pre-existing ocular discomfort. A positive association was observed with female sex and retail work. |
| Al-Dolat et al. (Jordan) | Medical students (n = 1219) | Online questionnaire and OSDI | Symptomatic DED in 71.7%. Wearing a face mask was not associated with symptomatic DED. Female sex, allergy and >6 h looking at screens associated with symptomatic DED. |
| Neti et al. (Thailand) | General population (n = 535) | Online questionnaire | 37% had previous DED. During lockdown, the mean dry-eye symptom score dropped from 81.6 ± 15.9 to 79.8 ± 17.4 (P < 0.001). A negative correlation between age and visual display terminal usage. The female gender and increased visual display terminal usage were independently associated with worsening DED symptoms. |
| Erogul et al. (Turkey) | Health-care professionals (n = 396) | Online questionnaire | Redness (29.3%) was the most frequently encountered ocular surface symptom, followed by burning (15.7%), pain (14.1%), tingling (10.9%), and rash (6.6%). Significant relationship between face mask-wearing duration and ocular pain. |
| Dag et al. (Turkey) | Health-care professionals (n = 333) | Online questionnaire | Self-reported MADE prevalence was 70%. Having at least one DED symptom without a mask and advanced age were determined as possible risk factors for MADE. Examination of 195 participants with self-reported MADE revealed that 30.7% had aqueous-type dryness with ocular surface staining. |
| Giannaccare et al. (Italy) | Medicine students (n = 107) | Questionnaire and OSDI | 10.3% described appearance or worsening of ocular discomfort symptoms, and 19.6% needed tear substitutes daily. Mean OSDI score was 21, and 57% subjects scored ≥15. |
| Tangmonkongvoragul et al. (Thailand) | Medicine students (n = 528) | OSDI, PSS-10 and an interview | Prevalence of DED was 70.8%. Female sex, contact lens wear, and PSS-10 stress scores were significantly higher in the DED group. Contact lens use and PSS-10 score associated with DED severity. |
| Jahanbani-Ardakani et al. (Iran) | Health-care professionals (n = 215, >6 h) and healthy individuals (n = 149; <2 h of face mask use) | OSDI | OSDI scores were 27.20 ± 13.04 in the health-care group and 7.31 ± 3.9 in the control group (p < 0.001) |
| Krolo et al. (Croatia) | General population (n = 203) | OSDI | Group that used masks 3–6 h had significantly higher OSDI scores compared to <3 h (15.3 vs. 8.3). OSDI was significantly greater in prior DED (36.1 vs. 4.2). Participants with prior DED presented greater worsening, regardless of mask wear duration. |
| Shalaby et al. (Egypt) | Healthy subjects (n = 200: 100 surgical mask and 100 N95) | OSDI | OSDI scores were 22.53 ± 9.55 in the surgical mask group and 21.58 ± 9.6 in the N95 mask group, with no significant differences. The daily number of hours spent wearing a facemask correlated strongly with OSDI scores. |
| Bilici et al. (Turkey) | Health-care professionals (n = 74) | OSDI | Mean OSDI score was 28.6 ± 17.1 |
| Aksoy et al. (Turkey) | General population (n = 52) | OSDI at initial admission, after 8 h of face mask use and after 15 days of >8 h daily taped face mask use | There was a significant difference between all examination times in OSDI scores. |
| Al-Namaeh et al. (United States) | General population (n = 40) | OSDI (online) | Prevalence rates of mild, moderate and severe DED were 15%, 77.5%, and 7.5%, respectively. |
| Esen Baris et al. (Turkey) | Healthy health care professionals (n = 33) | OSDI | The mean OSDI score was 20.1 ± 8.3 (0–68.75) at 8 a.m. and 27.4 ± 10.4 (0–81.25) at 5 p.m. (p < 0.01). Use of a surgical mask for the entire work-day increased dry eye symptoms in healthy individuals. |
| Azzam et al. (Israel) | Health-care professionals (surgical masks n = 30 and N95 n = 30) | OSDI | Both masks caused dryness according to OSDI scores. DED was observed in 14 (46.7%) and 16 (53.3%) patients in groups 1 and 2, respectively. |
| Giannaccare et al. (Italy) | University students (n = 20) | OSDI questionnaire before and after 8 h of face mask use | With face masks, mean OSDI score worsened from 12.9 ± 12.6 to 19.4 ± 12.0. |
| D'Souza et al. (India) | Practicing ophthalmologists (n = 17) | OSDI | Significant increase in OSDI scores with face-masks. A significant increase in discomfort scale and vision scale contributed to this. |
| Alanazi et al. (Saudi Arabia) | Healthy subjects (n = 54) and controls (no mask; n = 50) | SPEED questionnaire | Median SPEED scores increased significantly before and after wearing a face mask (0.5 vs 0.1; p = 0.002) |
| Saldanha et al. (United States of America) | DED patients (n = 388) | Online questionnaire | Prevalences: 25% mild DED, 21% moderate DED and 54% severe DED. Reduced work-related efficiency was noted (moderate dry eye: 51%, mild: 39%, and severe: 38%). Respondents with moderate DED were more likely to note worsening symptoms: pain, headache and difficulty concentrating because of eye symptoms. |
| Scalinci et al. (Italy) | DED patients (n = 67) | OSDI | Median OSDI score increased from 2019 (18.75) to 2020 (20.83). |
| Mastropasqua et al. (Italy) | DED patients (n = 66) and healthy subjects (n = 62) | DEQS questionnaire at baseline and after 3 months | After 3 months, DEQS worsened in DED patients with >3 h of face mask use. DEQS significantly correlated with corneal dendritic cell density and HLA-DR at baseline and 3 months. |
MADE: mask-associated dry eye; DED: dry eye disease; OSDI: Ocular Surface Disease Index; PSS-10: Perceived Stress Scale-10; SPEED: Standardized Patient Evaluation of Eye Dryness; DEQS: Dry Eye-Related Quality-of-Life Score.
Large surveys have used non-standardized questionnaires, although other authors have observed an increase in Ocular Surface Disease Index (OSDI) scores with the use of face masks [18,[43], [44], [45], [46], [47]]. This increase was mainly due to an increase in the discomfort and the vision scales [18].
A large survey was performed among medical students in Jordan using the OSDI [48]. The questionnaire also contained 18 questions on sociodemography, ocular and medical history, face mask wear, the use of electronic devices and associations with ocular discomfort. A total of 1219 students completed the questionnaire, 546 (44.8%) of which reported wearing a face mask at least 3 h a day. A total of 874 (71.7%) students were considered to have dry eye disease according to the OSDI scores. New ocular symptoms of dryness caused by wearing the facemask were reported by 272 (22.3%) students and among those students already suffering from dry eye, 304 (32.7%) stated that wearing face masks worsened their condition.
In another large study, OSDI scores were 27.20 ± 13.04 in 215 health care professionals (face mask use >6 h) compared to 7.31 ± 3.9 in 149 healthy individuals (face mask use <2 h; p < 0.001). Krolo et al. [49] reached similar results in 203 participants, illustrating an increase in OSDI scores with the use of fase masks.
When comparing OSDI scores in 200 healthy individuals according to the type of face mask (surgical or N95), both groups experience an increase in scores, being this difference higher in those who had worn surgical masks compared to N95. This was probably due to differences in fitting and subsequent upward airflow [50].
Smaller studies have also reached similar conclusions. For example, Giannaccare et al. asked 20 healthy university students to complete the OSDI before and after 8 h of face mask use [51]. Initial OSDI scores were in the normal range, but worsened significantly after 8 h of continuous face mask use (from 12.9 ± 12.6 to 19.4 ± 12.0; P = 0.014). Similarly, in Esen Baris et al.‘s study, OSDI increased from 20.1 ± 8.3 (0–68.75) to 27.4 ± 10.4 at the end of the work-day (0–81.25) (p < 0.01) [43].
Although OSDI has been the most commonly used questionnaire to evaluate symptoms, Alanazi et al. [52] employed the SPEED questionnaire, which investigates the presence or absence, frequency, and severity of dry eye symptoms at three different timeframes (now, the last 72 h, and the last 3 months). Results showed that face mask use of 1 h decreased the scores, that is, symptoms increased.
5.2. Changes in tear film stability
Although surveys and questionnaires are useful for assessing self-reported discomfort in large populations, they lack the clinical confirmation of dry eye disease. This has been evaluated using tear break-up time (TBUT), which is the number of seconds that elapse between the last blink and the appearance of the first dry spot in the tear film (see Table 2 ).
Table 2.
Pertinent studies assessing ocular surface and tear film characteristics with the use of face masks.
| Authors (Country) | Population | Examinations | Observations |
|---|---|---|---|
| Marta et al. (Portugal) | General population (pre- and post-pandemic, n = 274) | NITBUT, lipid layer thickness, blink rate, Schirmer test, tear meniscus height, tear osmolarity and meibography | In the face mask use period, blink rate, tear menisucus height, tear osmolarity and loss area of the meibomian glands were worse; mean lipid layer thickness and Schirmer test were better and NITBUT was similar. |
| Tangmonkongvoragul et al. (Thailand) | Medicine students (n = 528) | Lipid layer thickness, meibography and blinking pattern. | Severe DED patients were likely to have higher meibomian gland tortuosity (not statistically significant) |
| Shalaby et al. (Egypt) | Healthy subjects (n = 200: 100 surgical mask and 100 N95) | TBUT, Schirmer test I, corneal fluorescein staining before and after 1-h face mask use | All tear film parameters worsened significantly in both groups. Changes were larger with surgical masks compared to N95. There was a strong positive correlation between the daily number of hours spent wearing a facemask and corneal staining. |
| Alanazi et al. (Saudi Arabia) | Healthy subjects (n = 54) and controls (no mask use; n = 50) | NITBUT, phenol red thread and tear ferning tests before wearing a face mask and immediately after its removal after 1 h use | Significant differences were found between the NITBUT measurements, before and after wearing a face mask. No differences were found in the phenol red thread and tear ferning tests scores. |
| Bilici et al. (Turkey) | Health-care professionals (n = 74) | NITBUT morning and afternoon | Mean NITBUT after 8 h was lower than baseline (p < 0.0001) |
| Azzam et al. (Israel) | Health-care professionals (surgical masks n = 30; N95 n = 30) | TBUT, corneal and conjunctival staining and meibography | Both masks caused dryness according to TBUT and Meibomian glandular loss. N95 mask caused significantly more dryness according to TBUT. |
| Aksoy et al. (Turkey) | General population (n = 52) | Schirmer test I, TBUT and corneal staining at initial admission (T1), after 8 h of face mask use (T2), after 15 days of >8 h daily wear of face masks with taping (T3) | Mean TBUT was 13.03 ± 2.18 at T1, 9.12 ± 1.85 at T2, and 12.78 ± 2.05 s at T3. There was a significant difference between T1 and T2, and between T2 and T3 in TBUT, Schirmer-1, and corneal staining. |
| Esen Baris et al. (Turkey) | Healthy health care professionals (n = 33) | NITBUT | Mean TBUT was 9.3 ± 1.0 (3–16) seconds at 8 a.m. and 8.3 ± 1.5 (3–14) seconds at 5 p.m. (p = 0.01). Use of a surgical mask for the entire work-day worsened TBUT |
| Giannaccare et al. (Italy) | Healthy subjects (university students; n = 20) | NITBUT, tear meniscus height, ocular redness and meibography before and after 8 h of face mask wearing | With face masks, tear meniscus height decreased significantly. Mean values of NITBUT, redness score and meibomian gland dropout did not change significantly. |
| Kapelushnik et al. (Israel) | Healthy subjects (n = 31) | Thermal images breathing normally with a surgical face mask | Ocular surface temperature was higher during expirium. The upper eyelid margin had the greatest temperature change. Sex, age, room temperature or body temperature had no effect. With taping, measurements were significantly lower in all regions compared to expirium without taping. No differences were observed in inspiration with and without taping. |
| D'Souza et al. (India) | Healthy subjects (practicing ophthalmologists; n = 17) | Schirmer test I, TBUT, tear film interferometry, OSI, corneal and conjunctival staining, concentration of proteins in tear samples and immune cell profile at two time-points (pre-face-mask-wearing period and post-face-mask-wearing period) | No significant changes in TBUT, Schirmer test I and OSI were observed post-face mask. Differences in some inflammatory markers. Higher proportions of leukocytes and natural killer T cells and a significant reduction in the proportions of eosinophils, B cells and plasma cells. |
| Arriola-Villalobos et al. (Spain) | Moderate-to-severe DED patients (n = 31) | NITBUT with a face-mask and after 10 min without a mask | First and average NITBUT increased without face masks |
| Mastropasqua et al. (Italy) | DED patients (n = 66) and healthy subjects (n = 62) | TBUT, Schirmer test I, corneal staining, confocal microscopy (corneal dendritic and goblet cell density), and impression citology (HLA-DR): baseline and 3 months. | After 3 months, Schirmer test worsened in DED patients. In controls, BUT and staining worsened only in >6 h face mask use. Dendritic cell density increased in DED patients and HLA-DR in controls with >3 h use |
DED: dry eye disease, NITBUT: non-invasive tear break-up time; TBUT: tear break-up time; OSI: objective scatter index.
TBUT and Schirmer I worsen with the use of face masks, supporting the theories on the possible mechanisms for mask-associated dry eye [46,47]. In 200 healthy subjects, TBUT and Schirmer values decreased after 1 h of face mask use and these differences were significantly larger in those with surgical masks compared to N95 [50].
Non-invasive TBUT (NITBUT) was evaluated by Alanazi et al. [52] before and after 1-h face mask use in healthy participants, showing a decrease of 5 s. Strong correlations were found between the SPEED score and the NITBUT measurements. Similarly, in 33 healthy health care professionals, Esen Baris et al. noted a decrease in NITBUT after 9 h of face mask use, which correlated with the OSDI scores [43].
Giannaccare et al. [51] investigated NITBUT, tear meniscus height, ocular redness and infrared meibography using Keratograph 5 M (Oculus, Wetzlar, Germany) before and after 8 h of continuous face mask use in 20 healthy controls. At baseline, mean values of all parameters were within normal range, but on the second examination, mean value of tear meniscus height had decreased around 20%. The remaining parameters did not change significantly.
When the effect of different types of masks on the ocular surface were compared, Azzam et al. found that N95 masks caused significantly more dryness according to decreasing TBUT and fluorescein staining [53].
However, other groups have obtained different results. For example, D'Souza et al. [18] observed an increase in Schirmer's test 1 and TBUT scores in healthy patients. This increase in tear quality parameters, did not seem to match the high OSDI scores obtained in that study. As for other tear quality parameters such as objective scatter index and lipid content, no significant differences were detected.
In a retrospective comparative study in Portugal, tear film properties of patients before lockdown, after lockdown but without mask mandate and after lockdown but with mask mandate were compared [54]. Lipid layer thickness, blink rate, Schirmer test, tear meniscus height, tear osmolarity, NITBUT, and meibomian glands loss were investigated. Tear osmolarity and meibomian glands loss were worse after lockdown, but mean lipid layer thickness increased. In the face mask use period, blink rate and tear meniscus height worsened, although Schirmer test was better and NITBUT was similar. Therefore, results seem to be contradictory and multiple mechanisms may be responsible for these changes. The authors postulate that the lipid layer thickness increase could be an adaptive response to the increase in NITBUT, an overestimation, since there was a significant decrease of tear film aqueous layer, or as a result of traumatic secretion of the meibomian gland with the face mask.
The detrimental effects of face mask may be more limited in the presence of a healthy ocular surface. Some authors have hypothesized that in healthy subjects the tear film homeostatic mechanisms may be capable to counteract the effects of face mask, at least in the short term [51].
5.3. Corneal epithelial trauma
Alterations in tear film stability may result in epithelial damage and corneal staining [46]. Face mask use might also increase the incidence of corneal epithelial damage due to direct trauma [55,56]. Their corners and the corrugated side edges are all potential sharp points that could lacerate the corneal surface [57]. For example, a 51-year-old man with no previous ocular history attended the emergency department for left eye immediate pain, tearing and foreign body sensation after face mask removal. The patient referred that his left eye had been scratched by the sharp edge of the face mask upon removal. Examination showed a corneal abrasion, without corneal infiltration which healed with hypromellose eye drops with full recovery [57]. In a single-center retrospective case series in China, nine patients had a unilateral ocular injury due to face masks [58]. The most frequently injured site was the cornea, which was affected in five patients. Two types of mechanisms were described: corneal lacerations due to its metal nose wires or other rigid sharp parts of the face masks and ocular contusions due to recoiling elastic mask straps snapping into the eyes.
Furthermore, corneal trauma with face masks could result in recurrent corneal erosion syndrome, as Tang et al. highlighted in a case report [59]. A 52-year-old man referred a painful red eye in the morning, which was associated with blurry vision and examination revealed an inferior corneal epithelial defect with loose epithelium extending up to the visual axis and a small overlying corneal infiltrate. The patient reported a history of a corneal abrasion with his face mask while adjusting it 5 months earlier. Based on the history of ocular trauma and the clinical findings, he was diagnosed with recurrent corneal erosion syndrome complicated by microbial keratitis.
5.4. Inflammatory markers
Alterations in inflammatory factors and proportion of immune cells in the tears of face mask users have suggested an imbalance in the ocular surface health [60].
Studies on this topic are scarce, the main work being D'Souza et al.‘s, which investigated the levels of different cytokines before and after face mask use [18]. A number of proinflammatory factors were significantly increased in the post face mask samples. However, IL-6 and IL-8, which are classic dry eye disease-related inflammatory factors were reduced after face mask use. Nevertheless, a subset of inflammatory factors and pain- or nociception-related factors were elevated, suggesting that there is an overall imbalance between proinflammatory and anti-nociceptive factors.
In addition, D'Souza et al. analyzed the ocular surface immune cell profile, obtaining significantly higher proportions of leukocytes and natural killer T cells in post face mask use samples, along with a significant reduction in the proportions of eosinophils, B cells and plasma cells [18]. This differs from the immune cell profile seen in dry eye disease patients, suggesting a different inflammatory mechanism [61].
Further, alterations in mucin levels can affect the tear film stability and have been reported to be decreased in dry eye disease patients [62]. D'Souza et al. obtained a mean increase in levels of mucins after face-mask use and hypothesized that this was due to hypercapnia. They also suggested that the increase in TBUT and Schirmer's values in their study could be attributable to the increase in mucin levels which would enhance tear film stability.
5.5. Periocular temperature changes
Changes in the ocular surface temperature can be associated with dry eye disease and tear film abnormalities [63,64]. Kapelushnik et al. [65] evaluated the effect of the changes induced by respiration while wearing a standard face mask. The use of a standard face mask resulted in significant changes in the ocular surface temperature during expiration, mostly due to the effects of air-jets towards the orbit. The change was most notable in the eyelid margins, with a mean rise of 0.5 °C during expiration. These differences were eliminated by taping the upper margin of the mask, preventing the airflow to the ocular region.
On the contrary, it is worth noting that applying heat to the eyelids has been recommended to treat dry eye disease secondary to meibomian gland dysfunction. In multiple studies, an increase in ocular surface temperature has resulted in an increase in TBUT [66,67]. The increase in the periocular temperature may facilitate the melting of the thickened secretions subsequently restoring the natural meibum in the tear film lipid layer and improving dry eye disease. However, it is unlikely that the small increase in periocular temperature due to the use of face masks (around 0.5 °C) may have an effect in meibomian gland dysfunction.
The duration of exposure to the high and steady temperature should be considered [65]. With face masks, the temperature rise is during a part of the breathing cycle, that is, during expiration. It is possible that there might be latent heat transfers due to the potentially higher humidity content of exhaled air, this might also not be enough to restore the meibum in the tear film and would result in a faster evaporation. On the contrary, warming devices or compresses would cause a constant and longer rise in temperature and this would explain the improvement in dry eye disease symptoms.
Therefore, the relationship between tear film properties and ocular surface temperature may be variable between patients and dry eye disease etiologies, so while certain changes in ocular surface temperature may be beneficial for some patients, others may suffer the opposite effect.
6. Effects of face mask use on the ocular surface of dry eye disease patients
Most studies have evaluated the effect of face-masks on the ocular surface of healthy patients, but few have investigated this in dry eye disease patients.
Large surveys agree that individuals with previous ocular discomfort are more likely to experience worsening of their symptoms, although dry eye disease diagnosis is specifically considered by few series [68]. In Boccardo's survey, participants who often had ocular discomfort were more likely to report a worsening of their condition while wearing a mask with an Odds Ratio of 1.28 (95% CI: 1.03–1.59) [6]. In Krolo et al.‘s study, participants with prior dry eye disease diagnosis presented greater worsening of their symptoms during mask use compared to those with no previous diagnosis, regardless of daily mask wear time.
In patients with previous dry eye disease diagnosis, an increase in OSDI scores of 2.09 (95% CI [1.05, 4.17]) (P < 0.0001) was noted between 2019 and 2020 [69]. A difference in OSDI scores was also observed when patients were stratified according to face mask use, prolonged and consistent face mask use being associated with higher scores.
Arriola-Villalobos et al. investigated tear film stability with and without a face mask in patients with moderate-to-severe dry eye disease [70]. In this study, 31 patients with moderate or severe dry eye disease according to the TFOS DEWS 2 report were included and NITBUT was investigated using Oculus Keratograph 5 M (Oculus, Wetzlar, Germany). All patients were on lubricating eye drops, 5 patients (16%) were on topical cyclosporine and 24 patients (77%) were on autologous serum. Mean first NITBUT with face mask was 6.2 ± 3.8 s, which increased to 7.8 ± 5.6 s without the use of mask (p = 0.029). The mean average NITBUT with face mask was 12.3 ± 4.8 s and it increased to 13.8 ± 5 s without the use of mask, being differences statistically significant (p = 0.006). Therefore, both measurements improved around 1.5 s without face mask.
7. Effects of face mask use on postoperative patients
Postsurgical ocular surface issues which could be attributed to face mask usage have been reported. Dry eye disease may worsen in postoperative patients, increasing the risk of secondary infections, keratitis and exposure keratopathy [71].
The development and exacerbation of postoperative dry eye after cataract surgery is common and could be worsened with the use of face masks. A deterioration in corneal staining and an increase in dryness has been reported in cataract patients on their first postoperative day [3,72]. Patients with previous corneal surgery may be at higher risk. For example, Chadwick and Lockington presented a 66-year-old female with previous LASIK, who developed corneal haze and superficial staining with topical fluorescein after cataract surgery [73]. The patient's face mask was very loose around her nose, which according to the authors, caused the patient's breath to be directed onto the anaesthetised postoperative ocular surface and resulted in a variant of exposure keratopathy.
Direct mask-mediated mechanical trauma to the cornea in postsurgical patients should be considered, especially in post-LASIK patients, where there is risk of flap dislocation. This was the case of a 26-year-old male patient who underwent femtosecond laser assisted in situ keratomileusis for a refractive error of −4 Dp in both eyes [74]. One day after an uneventful surgery, the patient experienced a decrease in his right eye vision, mild pain and watering, when his N95 mask hit the right eye while repositioning it. On examination, uncorrected distance visual acuity was 20/200 and slit-lamp examination revealed a superiorly displaced and edematous flap. The flap was repositioned and a bandage contact lens was placed. After 24 h, visual acuity was 20/20, with a well-centered flap and clear stroma.
Furthermore, concern has increased among ophthalmologists about a potential rise in the risk for postoperative infection [73]. The tear film plays a key role as a barrier against pathogens. Increased tear evaporation due to mask use, along with increase eye rubbing and face touching behaviors due to discomfort symptoms in an altered ocular surface due to surgery could increase the risk of postoperative infection [75].
Intraoperatively, the dispersion of oral micro-organisms towards ocular surface caused by upward flow of the patient's breath within the mask may be another potential risk factor for postoperative infections [76]. When evaluating air particles, Schultheis et al. reported a reduction in air particle counts directed toward the eye when subjects were speaking but had their upper edge of the face mask taped, compared to no taping [77]. Other studies have revealed that after wearing a face mask for more than 4 h, the periocular area becomes contaminated with the oral flora [78]. However, studies have shown no differences in endophthalmitis rate with the use of face masks [79,80].
8. Effects on ocular surface measurements
Accuracy and repeatability of ocular surface measurements, such as keratometric parameters, are essential and, although highly accurate, they are subject to variability. Tear film stability can influence the aberration structure of the anterior cornea and may affect the variability of measurements.
Based on this, Burgos-Blasco et al. designed a study to investigate whether the use of face masks in healthy individuals affected the reproducibility of the main keratometric parameters using Oculus Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany) [81]. Despite excellent reproducibility in most parameters and the absence of statistically significant differences between with an without face-mask, they seem to be more affected with the use of face-mask.
Other authors have reported correlations between tear pattern and the standard error of the mean of corneal topography parameters. As tear film instability and osmolarity increased, so did the error [82,83]. Some parameters other than keratometry may be more affected by ocular surface alterations with face mask use, although this has not been investigated [[84], [85], [86]]. Therefore, the use of artificial tears prior to ophthalmic evaluation might prove beneficial.
9. Preventive measures
Although face mask use should not be dissuaded despite MADE, some measures may be implemented to improve this condition. For example, in dry eye disease patients experiencing increase of symptoms, treatment should be intensified, as they may be more susceptible to the effects of face mask use on the ocular surface.
Patients diagnosed with MADE, or those who are experiencing a worsening of dry eye disease symptoms should try using a mask with a better superior fit, or even tape the superior area of the mask, to see if reducing the upward airflow improves the symptoms. Nair et al. evaluated the impact of taping the upper mask edge on ocular surface stability, dry eye symptoms, and tear osmolarity in N95 mask healthy users [87]. After taping the upper edge, an improvement in NIBUT, TBUT, tear lipid layer thickness, tear meniscus height, corneal staining and tear osmolarity was noted. There were no significant changes in visual acuity, Schirmer I, and OSDI score, although symptom improvement was reported by nearly 70% and this correlated with changes in the ocular surface stability parameters. However, the design-related specifics of a face mask may be an important determinant of the effectivity of the upper seal and may determine the severity of the changes in the ocular surface [88].
Patients undergoing ocular surgery should be advised to carefully tape the upper edge of their mask to their nose to prevent an inadvertent displacement of the mask which may cause mechanical trauma to the eye. Education on mask-wearing techniques can also help prevent mask-associated corneal abrasions while removing or adjusting the mask [59].
Other settings, such as ocular surgery have not been thoroughly evaluated and so, possible measures such as properly sealing the periocular skin so that air does not reach the surgical field during ocular surgery or the controversial use of face mask in these patients have to be further investigated. Also, patients undergoing examinations such as biometry or topography prior to surgery may benefit from artificial tear instillation before the examination, in order to improve tear film characteristics and the accuracy of such examinations. However, there are still many unanswered questions and these measures could still change in the future.
10. Future directions for research
Despite the emerging research in the effects of face mask wear on the ocular surface, there are still gaps in our understanding. There are millions of mask wearers in the world, but not everyone experiences dry eye symptoms, and even some people report an improvement in such symptoms when they wear a mask.
There are different types of masks, with different formats and upper fittings, which could have different consequences on the ocular surface. Face mask wearing time, the climate, previous ocular and general diseases, behavioral elements, occupation, and other sociodemographic factors should also be investigated in future studies. For example, quarantine related stress, depression and anxiety may also worsen dry eye disease and perhaps it should not be considered part of MADE. In this case, studies should be controlled for these factors in order to truly evaluate the prevalence and characteristics of MADE. More importantly, a clear definition of MADE with diagnostic criteria should be agreed on.
Furthermore, studies to characterize and quantify the microbial colonies on the face mask and determine the incidence of autoinoculation of such colonies are mandatory. The pathophysiologic mechanisms for the development of a dysbiotic microbiome should be elucidated [24]. Further, the role of mucins are to be investigated, as D'Souza et al. have presented the only results on the subject, without a clear pathophysiological explanation.
Long-term consequences of face mask use, especially in health care workers and patients with previous alterations of the ocular surface should be investigated, as up till now research has focused on short term effects. Preventive measures and possible treatments should be acknowledged. In this regard, supplementation with pre- and probiotics may present as a feasible and cost-effective approach to restore the microbiota and prevent ocular surface diseases.
11. Conclusions
As the current trends in the COVID-19 pandemic indicate the need for continued mask usage, at least in some settings or in cases of future pandemics, we are likely to observe an increase in ocular symptoms secondary to face mask use. Thus, greater knowledge of this condition and the possible underlying mechanisms will help us treat this condition more appropriately and design prevention strategies, particularly for more susceptible patients.
The rise in complaints of dry eye symptoms during the COVID-19 pandemic is most likely multifactorial. In addition, face masks may have different effects in different individuals. Multiple variables may be involved in the magnitude of the effect of face-masks on the ocular surface, such as dry eye disease severity, concomitant ocular pathologies, air humidity, hours with face-mask and type of face-mask [88]. Effects of long-term use of face mask on the ocular surface are still unknown as is the impact on visual, physical, and psychological functioning.
Despite common MADE symptoms in many patients, frequent wearing of face masks should not be dissuaded, as it is a crucial protective factor against COVID-19 and other respiratory viruses’ transmission. Instead, awareness among ophthalmologists of this condition and possible measures should be increased.
References
- 1.Leung C.C., Lam T.H., Cheng K.K. Mass masking in the COVID-19 epidemic: people need guidance. Lancet (London, England) 2020;395:945. doi: 10.1016/S0140-6736(20)30520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q., Yu C. The role of masks and respirator protection against SARS-CoV-2. Infect Control Hosp Epidemiol. 2020;41:746–747. doi: 10.1017/ice.2020.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moshirfar M., West W.B., Marx D.P. Face mask-associated ocular irritation and dryness. Ophthalmol Ther. 2020;9:397–400. doi: 10.1007/s40123-020-00282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey S.K., Sharma V. Mask-associated dry eye disease and dry eye due to prolonged screen time: are we heading towards a new dry eye epidemic during the COVID-19 era? Indian J Ophthalmol. 2021;69:448–449. doi: 10.4103/ijo.IJO_3250_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White D. BLOG: MADE: a new coronavirus-associated eye disease. 2020. https://www.healio.com/news/ophthalmology/20200622/blog-a-new-coronavirusassociated-eye-disease
- 6.Boccardo L. Self-reported symptoms of mask-associated dry eye: a survey study of 3,605 people. Contact Lens Anterior Eye. 2022;45 doi: 10.1016/j.clae.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannaccare G., Vaccaro S., Mancini A., Scorcia V. Dry eye in the COVID-19 era: how the measures for controlling pandemic might harm ocular surface. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:2567–2568. doi: 10.1007/s00417-020-04808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah P.V., Zhu L., Kazi A., Zhu A., Shalshin A. The correlation between non-invasive ventilation use and the development of dry eye disease. Cureus. 2021;13 doi: 10.7759/cureus.18280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayirci E., Yagci A., Palamar M., Basoglu O.K., Veral A. The effect of continuous positive airway pressure treatment for obstructive sleep apnea syndrome on the ocular surface. Cornea. 2012;31:604–608. doi: 10.1097/ICO.0b013e31824a2040. [DOI] [PubMed] [Google Scholar]
- 10.Harrison W., Pence N., Kovacich S. Anterior segment complications secondary to continuous positive airway pressure machine treatment in patients with obstructive sleep apnea. Optometry. 2007;78:352–355. doi: 10.1016/j.optm.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Singh N.P., Walker R.J.E., Cowan F., Davidson A.C., Roberts D.N. Retrograde air escape via the nasolacrimal system: a previously unrecognized complication of continuous positive airway pressure in the management of obstructive sleep apnea. Ann Otol Rhinol Laryngol. 2014;123:321–324. doi: 10.1177/0003489414525924. [DOI] [PubMed] [Google Scholar]
- 12.Powell J.B., Kim J.H., Roberge R.J. Powered air-purifying respirator use in healthcare: effects on thermal sensations and comfort. J Occup Environ Hyg. 2017;14:947–954. doi: 10.1080/15459624.2017.1358817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis R.J., Miller R.E., Peterson R.D., Jackson W.G. Contact lens wear with the USAF Protective Integrated Hood/Mask chemical defense ensemble. Aviat Space Environ Med. 1992;63:565–571. [PubMed] [Google Scholar]
- 14.Smith D., Pysanenko A., Spanel P. The quantification of carbon dioxide in humid air and exhaled breath by selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1419–1425. doi: 10.1002/rcm.4016. [DOI] [PubMed] [Google Scholar]
- 15.Rhee M.S.M., Lindquist C.D., Silvestrini M.T., Chan A.C., Ong J.J.Y., Sharma V.K. Carbon dioxide increases with face masks but remains below short-term NIOSH limits. BMC Infect Dis. 2021;21:354. doi: 10.1186/s12879-021-06056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X., Gallar J., Pozo M.A., Baeza M., Belmonte C. CO2 stimulation of the cornea: a comparison between human sensation and nerve activity in polymodal nociceptive afferents of the cat. Eur J Neurosci. 1995;7:1154–1163. doi: 10.1111/j.1460-9568.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Chacko B.K., Ricksecker A., Shingarev R., Andrews E., Patel R.P., et al. Modulatory effects of hypercapnia on in vitro and in vivo pulmonary endothelial-neutrophil adhesive responses during inflammation. Cytokine. 2008;44:108–117. doi: 10.1016/j.cyto.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Souza S., Vaidya T., Nair A.P., Shetty R., Kumar N.R., Bisht A., et al. Altered ocular surface health status and tear film immune profile due to prolonged daily mask wear in health care workers. Biomedicines. 2022;10 doi: 10.3390/biomedicines10051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borchman D., Foulks G.N., Yappert M.C., Mathews J., Leake K., Bell J. Factors affecting evaporation rates of tear film components measured in vitro. Eye Contact Lens. 2009;35:32–37. doi: 10.1097/ICL.0b013e318193f4fc. [DOI] [PubMed] [Google Scholar]
- 20.Peng Y., Zhang D., Chen T., Xia Y., Wu P., Seto W.-K., et al. Gut microbiome and resistome changes during the first wave of the COVID-19 pandemic in comparison with pre-pandemic travel-related changes. J Trav Med. 2021;28 doi: 10.1093/jtm/taab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Sun Q., Jiang D., Zhang X., Chen C., Yan D., et al. Characteristics of facial skin problems and microbiome variation during wearing masks for fighting against COVID-19. J Eur Acad Dermatol Venereol. 2021;35 doi: 10.1111/jdv.17580. e853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Nalluri H, Kaiser T, et al. Effect of COVID-19 precautions on the gut microbiota and nosocomial infections. Gut Microbes n.d.;13:1‐10. 10.1080/19490976.2021.1936378. [DOI] [PMC free article] [PubMed]
- 23.Ahmad E.F., Mohammed M., Al Rayes A.A., Al Qahtani A., Elzubier A.G., Suliman F.A. The effect of wearing the veil by Saudi ladies on the occurrence of respiratory diseases. J Asthma. 2001;38:423–426. doi: 10.1081/jas-100001497. [DOI] [PubMed] [Google Scholar]
- 24.Brooks J.K., Sultan A.S., Jabra-Rizk M.A. Prolonged facial mask wear is a concern for the development of dysbiotic microbiome. Respir Med Res. 2022;81 doi: 10.1016/j.resmer.2021.100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trujillo-Vargas C.M., Schaefer L., Alam J., Pflugfelder S.C., Britton R.A., de Paiva C.S. The gut-eye-lacrimal gland-microbiome axis in Sjögren Syndrome. Ocul Surf. 2020;18:335–344. doi: 10.1016/j.jtos.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitano P., Filippelli M., Davinelli S., Bartollino S., Dell'Omo R., Costagliola C. Influence of gut microbiota on eye diseases: an overview. Ann Med. 2021;53:750–761. doi: 10.1080/07853890.2021.1925150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silkiss R.Z., Paap M.K., Ugradar S. Increased incidence of chalazion associated with face mask wear during the COVID-19 pandemic. Am J Ophthalmol Case Reports. 2021;22 doi: 10.1016/j.ajoc.2021.101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mégarbane B., Tadayoni R. Cluster of chalazia in nurses using eye protection while caring for critically ill patients with COVID-19 in intensive care. Occup Environ Med. 2020;77:584–585. doi: 10.1136/oemed-2020-106677. [DOI] [PubMed] [Google Scholar]
- 29.Nemet A.Y., Vinker S., Kaiserman I. Associated morbidity of chalazia. Cornea. 2011;30:1376–1381. doi: 10.1097/ICO.0b013e31821de36f. [DOI] [PubMed] [Google Scholar]
- 30.Koshevarova V.A., Westenhaver Z.K., Schmitz-Brown M., McKinnon B.J., Merkley K.H., Gupta P.K. Blepharoconjunctivitis and otolaryngological disease trends in the context of mask wearing during the COVID-19 pandemic. Clin Pract. 2022;12:619–627. doi: 10.3390/clinpract12040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott C.R. Increased screen time and dry eye: another complication of COVID-19. Eye Contact Lens. 2021;47:433. doi: 10.1097/ICL.0000000000000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elhusseiny A.M., Eleiwa T.K., Yacoub M.S., George J., ElSheikh R.H., Haseeb A., et al. Relationship between screen time and dry eye symptoms in pediatric population during the COVID-19 pandemic. Ocul Surf. 2021;22:117–119. doi: 10.1016/j.jtos.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talens-Estarelles C., García-Marqués J.V., Cervino A., García-Lázaro S. Online vs in-person education: evaluating the potential influence of teaching modality on dry eye symptoms and risk factors during the COVID-19 pandemic. Eye Contact Lens. 2021;47:565–572. doi: 10.1097/ICL.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 34.Barabino S. A narrative review of current understanding and classification of dry eye disease with new insights on the impact of dry eye during the COVID-19 pandemic. Ophthalmol Ther. 2021;10:495–507. doi: 10.1007/s40123-021-00373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gargiulo A.T., Peterson L.M., Grafe L.A. Stress, coping, resilience, and sleep during the COVID-19 pandemic: a representative survey study of US adults. Brain Behav. 2021;11:e2384. doi: 10.1002/brb3.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M.T., Muntz A., Wolffsohn J.S., Craig J.P. Association between dry eye disease, self-perceived health status, and self-reported psychological stress burden. Clin Exp Optom. 2021;104:835–840. doi: 10.1080/08164622.2021.1887580. [DOI] [PubMed] [Google Scholar]
- 37.Napoli P.E., Nioi M., Fossarello M. The “quarantine dry eye”: the lockdown for coronavirus disease 2019 and its implications for ocular surface health. Risk Manag Healthc Pol. 2021;14:1629–1636. doi: 10.2147/RMHP.S277067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Q., Liang M., Kong W., Zhang W., Wang H., Chu J., et al. Wearing face masks and possibility for dry eye during the COVID-19 pandemic. Sci Rep. 2022;12:6214. doi: 10.1038/s41598-022-07724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dag U., Çaglayan M., Öncül H., Vardar S., Alaus M.F. Mask-associated dry eye syndrome in healthcare professionals as a new complication caused by the prolonged use of masks during covid-19 pandemic period. Ophthalmic Epidemiol. 2022:1–6. doi: 10.1080/09286586.2022.2053549. [DOI] [PubMed] [Google Scholar]
- 40.Neti N., Prabhasawat P., Chirapapaisan C., Ngowyutagon P. Provocation of dry eye disease symptoms during COVID-19 lockdown. Sci Rep. 2021;11 doi: 10.1038/s41598-021-03887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tangmonkongvoragul C., Chokesuwattanaskul S., Khankaeo C., Punyasevee R., Nakkara L., Moolsan S., et al. Prevalence of symptomatic dry eye disease with associated risk factors among medical students at Chiang Mai University due to increased screen time and stress during COVID-19 pandemic. PLoS One. 2022;17 doi: 10.1371/journal.pone.0265733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erogul O., Gobeka H.H., Kasikci M., Erogul L.E., Balci A. Impacts of protective face masks on ocular surface symptoms among healthcare professionals during the COVID-19 pandemic. Ir J Med Sci. 2022 doi: 10.1007/s11845-022-03059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esen Baris M., Guven Yilmaz S., Palamar M. Impact of prolonged face mask wearing on tear break-up time and dry eye symptoms in health care professionals. Int Ophthalmol. 2022;42:2141–2144. doi: 10.1007/s10792-022-02213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jahanbani-Ardakani H., Hosseini M., Almasi S., Khalili M.R. Letter to the editor: face mask-associated dry eye in health care professionals amid the COVID-19 pandemic. Optom Vis Sci. 2021;98:995–996. doi: 10.1097/OPX.0000000000001758. [DOI] [PubMed] [Google Scholar]
- 45.Al-Namaeh M. Comparing objective conjunctival hyperemia grading and the ocular surface disease index score in dry eye syndrome during COVID-19. JoVE. 2022 doi: 10.3791/63812. [DOI] [PubMed] [Google Scholar]
- 46.Aksoy M., Simsek M. Evaluation of ocular surface and dry eye symptoms in face mask users. Eye Contact Lens. 2021;47:555–558. doi: 10.1097/ICL.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 47.Bilici S., Toprak A., Buyukuysal C., Ugurbas S.H. The effect of day-long mask wearing on non-invasive break-up time. Graefes Arch Clin Exp Ophthalmol. 2022;260:3313–3319. doi: 10.1007/s00417-022-05709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Dolat W., Abu-Ismail L., Khamees A., Alqudah N., Abukawan M.M., Alrawashdeh H.M., et al. Is wearing a face mask associated with symptomatic dry eye disease among medical students during the COVID-19 era? An online survey. BMC Ophthalmol. 2022;22:159. doi: 10.1186/s12886-022-02377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krolo I., Blazeka M., Merdzo I., Vrtar I., Sabol I., Petric-Vickovic I. Mask-associated dry eye during COVID-19 pandemic-how face masks contribute to dry eye disease symptoms. Med Arch. 2021;75:144–148. doi: 10.5455/medarh.2021.75.144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shalaby H.S., Eldesouky M.E.E. Effect of facemasks on the tear film during the COVID-19 pandemic. Eur J Ophthalmol. 2022 doi: 10.1177/11206721221110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannaccare G., Pellegrini M., Borselli M., Senni C., Bruno A., Scorcia V. Diurnal changes of noninvasive parameters of ocular surface in healthy subjects before and after continuous face mask wearing during the COVID-19 pandemic. Sci Rep. 2022;12 doi: 10.1038/s41598-022-17486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alanazi M.A., El-Hiti G.A., Al-Tamimi R., Bawazir A.M., Almutleb E.S., Fagehi R., et al. Assessment of the effect of wearing a surgical face mask on tear film in normal eye subjects. J Ophthalmol. 2022;2022 doi: 10.1155/2022/2484997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azzam S.H., Nama A., Badarni H., Asael H., Dahoud W.A., Mimouni M., et al. Assessment of dry eye disease in N95 versus surgical face mask wearers during COVID-19. Indian J Ophthalmol. 2022;70:995–999. doi: 10.4103/ijo.IJO_1133_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marta A., Marques J.H., Almeida D., José D., Sousa P., Barbosa I. Impact of COVID-19 pandemic on the ocular surface. World J Clin Cases. 2022;10:9619–9627. doi: 10.12998/wjcc.v10.i27.9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang H., Zhang M., Chen M., Lin T.P.H., Lai M., Chen H. vol. 11. Asia-Pacific J Ophthalmol; Philadelphia, Pa: 2022. pp. 481–487. (Ocular trauma during COVID-19 pandemic: a systematic review and meta-analysis). [DOI] [PubMed] [Google Scholar]
- 56.Ramani S., Anusha A., Sundaresh D.D., Shetty S. Collateral damage: corneal injury due to mask use during the COVID-19 pandemic - a case series. Indian J Ophthalmol. 2022;70:306–307. doi: 10.4103/ijo.IJO_1861_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Au Sunny CL., Ko Callie KL. Corneal abrasion from removing face mask during the COVID-19 pandemic. Vis J Emerg Med. 2021;22 doi: 10.1016/j.visj.2020.100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou P., Jiang X., Li X.-M. Case series: ocular trauma secondary to masks during the COVID-19 pandemic. Optom Vis Sci. 2021;98:1299–1303. doi: 10.1097/OPX.0000000000001803. [DOI] [PubMed] [Google Scholar]
- 59.Tang Y.F., Chong E.W.T. Face mask-associated recurrent corneal erosion syndrome and corneal infection. Eye Contact Lens. 2021;47:573–574. doi: 10.1097/ICL.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 60.Mastropasqua L., Lanzini M., Brescia L., D'Aloisio R., Nubile M., Ciancaglini M., et al. Face mask-related ocular surface modifications during COVID-19 pandemic: a clinical, in vivo confocal microscopy, and immune-cytology study. Transl Vis Sci Technol. 2021;10:22. doi: 10.1167/tvst.10.3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair A.P., D'Souza S., Shetty R., Ahuja P., Kundu G., Khamar P., et al. Altered ocular surface immune cell profile in patients with dry eye disease. Ocul Surf. 2021;21:96–106. doi: 10.1016/j.jtos.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Portal C., Gouyer V., Gottrand F., Desseyn J.-L. Ocular mucins in dry eye disease. Exp Eye Res. 2019;186 doi: 10.1016/j.exer.2019.107724. [DOI] [PubMed] [Google Scholar]
- 63.Morgan P.B., Tullo A.B., Efron N. Infrared thermography of the tear film in dry eye. Eye. 1995;9(Pt 5):615–618. doi: 10.1038/eye.1995.149. [DOI] [PubMed] [Google Scholar]
- 64.Purslow C., Wolffsohn J. The relation between physical properties of the anterior eye and ocular surface temperature. Optom Vis Sci. 2007;84:197–201. doi: 10.1097/OPX.0b013e3180339f6e. [DOI] [PubMed] [Google Scholar]
- 65.Kapelushnik N., Benyosef S., Skaat A., Abdelkader A., Landau Prat D., Blum-Meirovitch S., et al. The effect of face masks during COVID-19 pandemic on ocular surface temperature-A clinical thermographic analysis. Diagnostics. 2022;12 doi: 10.3390/diagnostics12061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M.T.M., Liu L.J., McPherson R.D., Fuller J.R., Craig J.P. Therapeutic profile of a latent heat eyelid warming device with temperature setting variation. Contact Lens Anterior Eye. 2020;43:173–177. doi: 10.1016/j.clae.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Arita R., Morishige N., Shirakawa R., Sato Y., Amano S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf. 2015;13:321–330. doi: 10.1016/j.jtos.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Saldanha I.J., Petris R., Makara M., Channa P., Akpek E.K. Impact of the COVID-19 pandemic on eye strain and dry eye symptoms. Ocul Surf. 2021;22:38–46. doi: 10.1016/j.jtos.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scalinci S.Z., Pacella E., Battagliola E.T. Prolonged face mask use might worsen dry eye symptoms. Indian J Ophthalmol. 2021;69:1508–1510. doi: 10.4103/ijo.IJO_2641_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arriola-Villalobos P., Burgos-Blasco B., Vidal-Villegas B., Oribio-Quinto C., Ariño-Gutiérrez M., Diaz-Valle D., et al. Effect of face mask on tear film stability in eyes with moderate-to-severe dry eye disease. Cornea. 2021;40:1336–1339. doi: 10.1097/ICO.0000000000002734. [DOI] [PubMed] [Google Scholar]
- 71.Narayanan S., Redfern R.L., Miller W.L., Nichols K.K., McDermott A.M. Dry eye disease and microbial keratitis: is there a connection? Ocul Surf. 2013;11:75–92. doi: 10.1016/j.jtos.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ali Momin S.N., Siddiqui R. Mask-associated dry-eye in COVID-19 pandemic: a case report and review of the literature. J Pakistan Med Assoc. 2022;72:981–982. doi: 10.47391/JPMA.4157. [DOI] [PubMed] [Google Scholar]
- 73.Chadwick O., Lockington D. Addressing post-operative mask-associated dry eye (MADE) Eye. 2021;35:1543–1544. doi: 10.1038/s41433-020-01280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nair S., Kaur M., Titiyal J.S. LASIK flap dislocation following direct face mask-induced mechanical trauma. BMJ Case Rep. 2022;15 doi: 10.1136/bcr-2021-247824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lazzarino A.I., Steptoe A., Hamer M., Michie S. Covid-19: important potential side effects of wearing face masks that we should bear in mind. BMJ. 2020;369:m2003. doi: 10.1136/bmj.m2003. [DOI] [PubMed] [Google Scholar]
- 76.Anguita R., Brennan N., Ramsden C.M., Mehat M., Keegan D., Cahill R., et al. Patient generated aerosol in the context of ophthalmic surgery. Eur J Ophthalmol. 2022;32:2445–2451. doi: 10.1177/11206721211037823. [DOI] [PubMed] [Google Scholar]
- 77.Schultheis W.G., Sharpe J.E., Zhang Q., Patel S.N., Kuriyan A.E., Chiang A., et al. Effect of taping face masks on quantitative particle counts near the eye: implications for intravitreal injections in the COVID-19 era. Am J Ophthalmol. 2021;225:166–171. doi: 10.1016/j.ajo.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marín-Nieto J., Reino-Perez C., Santillana-Cernuda G., Díaz-Bernal J.M., Luque-Aranda R., García-Basterra I. Face mask contamination during COVID-19 PANDEMIA. A study on patients receiving intravitreal injections. Retina. 2021;41:2215–2220. doi: 10.1097/IAE.0000000000003202. [DOI] [PubMed] [Google Scholar]
- 79.Writing committee for the Post-Injection Endophthalmitis Study Group. Patel S.N., Tang P.H., Storey P.P., Wolfe J.D., Fein J., et al. The influence of universal face mask use on endophthalmitis risk after intravitreal anti-vascular endothelial growth factor injections. Ophthalmology. 2021;128:1620–1626. doi: 10.1016/j.ophtha.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka K., Shimada H., Mori R., Kitagawa Y., Onoe H., Tamura K., et al. Safety measures for maintaining low endophthalmitis rate after intravitreal anti-vascular endothelial growth factor injection before and during the COVID-19 pandemic. J Clin Med. 2022;11 doi: 10.3390/jcm11030876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgos-Blasco B., Oribio-Quinto C., Vidal-Villegas B., Ariño-Gutierrez M., Benítez-Del-Castillo J.M., Arriola-Villalobos P. Effect of face mask on topographic corneal parameters in healthy individuals. Eur J Ophthalmol. 2022 doi: 10.1177/11206721221106143. [DOI] [PubMed] [Google Scholar]
- 82.González-Méijome J.M., Queirós A., Jorge J., Fernandes P., Cerviño A., de Almeida J.B. External factors affecting data acquisition during corneal topography examination. Eye Contact Lens. 2007;33:91–97. doi: 10.1097/01.icl.0000240501.07041.f8. [DOI] [PubMed] [Google Scholar]
- 83.Epitropoulos A.T., Matossian C., Berdy G.J., Malhotra R.P., Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672–1677. doi: 10.1016/j.jcrs.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Meyer L.M., Kronschläger M., Wegener A.R. [Schleimpflug photography detects alterations in corneal density and thickness in patients with dry eye disease] Ophthalmologe. 2014;111:914–919. doi: 10.1007/s00347-013-2964-1. [DOI] [PubMed] [Google Scholar]
- 85.Wegener A.R., Meyer L.M., Schönfeld C.-L. Effect of viscous agents on corneal density in dry eye disease. J Ocul Pharmacol Therapeut. 2015;31:504–508. doi: 10.1089/jop.2014.0157. [DOI] [PubMed] [Google Scholar]
- 86.Asena L., Altınörs D.D., Ş Cezairlioğlu, Bölük S.O. Effect of dry eye on Scheimpflug imaging of the cornea and elevation data. Can J Ophthalmol. 2017;52:313–317. doi: 10.1016/j.jcjo.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Nair S., Kaur M., Sah R., Titiyal J.S. Impact of taping the upper mask edge on ocular surface stability and dry eye symptoms. Am J Ophthalmol. 2022;238:128–133. doi: 10.1016/j.ajo.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Arriola-Villalobos P., Burgos-Blasco B., Vidal-Villegas B., Oribio-Quinto C., Ariño-Gutiérrez M., Diaz-Valle D., et al. Comment on: tear stability with masks in dry eye disease. Cornea. 2022;41:e7. doi: 10.1097/ICO.0000000000002950. [DOI] [PubMed] [Google Scholar]