Abstract
From a panel of nine inbred mice strains intranasally infected with Streptococcus pneumoniae type 2 strain, BALB/c mice were resistant and CBA/Ca and SJL mice were susceptible to infection. Further investigation revealed that BALB/c mice were able to prevent proliferation of pneumococci in the lungs and blood, whereas CBA/Ca mice showed no bacterial clearance. Rapidly increasing numbers of bacteria in the blood was a feature of CBA/Ca but not BALB/c mice. In the lungs, BALB/c mice recruited significantly more neutrophils than CBA/Ca mice at 12 and 24 h postinfection. Inflammatory lesions in BALB/c mice were visible much earlier than in CBA/Ca mice, and there was a greater cellular infiltration into the lung tissue of BALB/c mice at the earlier time points. Our data suggest that resistance or susceptibility to intranasal pneumococci may have an association with recruitment and/or function of neutrophils.
Streptococcus pneumoniae is a commensal of the human nasopharynx and a major human pathogen. It causes life-threatening diseases including pneumonia, septicemia and meningitis. Attention has therefore been focused on the mechanisms of its pathogenesis, especially on the role of its virulence determinants. The pneumococcal capsule is known to be essential for virulence, and several proteins produced by the pneumococcus, such as its toxin pneumolysin, have been implicated as virulence factors (reviewed in reference 18). Less attention has been paid to host genetic factors that determine resistance to pneumococcal infection. Previous studies of humans and mice have suggested that genetic factors strongly influence the host's response to infection. Natural resistance to infection with the intracellular parasites Leishmania donovani, Mycobacterium spp., and Salmonella enterica serovar Typhimurium has been found to be controlled by a dominant genetic locus on mouse chromosome 1 (6, 14, 21, 22, 25). This locus, referred to as Lsh, Bcg, or Ity, encodes natural resistance macrophage protein 1, which affects the macrophage's ability to destroy ingested intracellular parasites early in the infection process (30).
It has been reported that mouse strains differ in susceptibility to the pneumococcus (8, 23, 31). Furthermore, the effect of the pneumococcal toxin, pneumolysin, during bacteremia has been shown to be dependent on the genetic background of the mice used (4). Attempts to identify associations with individual genes involved in murine resistance to pneumococcal infection have pointed to a statistical link with the Akp-1 locus (8). However, no genetic study has been performed to confirm this association. Susceptibility has also been connected with the xid locus, which produces an X-linked inability to mount a humoral antibody response to a group of thymus-independent carbohydrate antigens (2, 9).
The xid locus found in the mouse strain CBA/N produces a defect that makes these mice incapable of producing antibodies against polysaccharides and phosphocholine, which are located in the cell wall of pneumococci and the F antigen, and the capsule (7, 12, 28). These mice are highly susceptible to pneumococcal infection (9). These antibodies are presumably generated in response to normal flora colonization (8). Evidence for the existence and efficacy of phosphocholine antibodies indicates that they may play an important role in the innate immune response (11, 17, 32). However, a number of authors have indicated that the genetic background of CBA mice renders this murine strain susceptible to pneumococcal infection and that this finding cannot be accounted for by the xid locus only but must involve the abrogating effect of hitherto unknown genes (8, 10, 26).
We revisited the question of pneumococcal genetic resistance and susceptibility by using a strategy that would allow detailed genetic mapping and subsequently, identification of resistance loci. For the first stage, identifying resistant and susceptible mouse strains, we have used a mouse model of pneumococcal pneumonia to examine the susceptibility of a number of inbred mouse strains to infection with a type 2 pneumococcus. In previous studies, no attempt had been made to define resistance by means other than survival time. Therefore, we enhanced our study by evaluation of the development of invasive pneumococcal disease. We report here the identification of mouse strains resistant or susceptible to invasive pneumococcal disease. In an attempt to identify the point during the course of pneumococcal infection at which the genes conferring resistance or susceptibility exert their effect, we examined several aspects of the pathogenesis of pneumococcal disease in these mice.
MATERIALS AND METHODS
Bacterial strains.
The type 2 S. pneumoniae strain used was D39 (NCTC 7466), from the National Collection of Type Cultures, Central Public Health Laboratory, London, United Kingdom. Pneumococci were routinely cultured on blood agar base (BAB) plates containing 5% (vol/vol) horse blood or in brain heart infusion (BHI) broth (BHI; Oxoid, Basingstoke, United Kingdom) containing 20% (vol/vol) fetal bovine serum (FBS; Gibco, Paisley, United Kingdom).
Preparation of the challenge dose.
BHI broth (10 ml) was inoculated with four to five colonies taken from a fresh culture plate of mouse-passaged S. pneumoniae and incubated overnight at 37°C. Bacteria were harvested by centrifugation (18,000 × g) and resuspended in 1 ml of fresh serum broth (FBS plus BHI [1:5]), and the pellet suspension was diluted with fresh serum broth to give an optical density at 500 nm of 0.7. The culture was incubated at 37°C for 4 to 5 h (optical density at 500 nm 1.6). Aliquots of this culture were stored at −70°C. Viable colony counts of thawed aliquots were performed as described below in duplicate on BAB–5% (vol/vol) horse blood. Pneumococci could be stored for at least 3 months at −70°C with no significant loss of viability. When required, the suspension was thawed slowly at room temperature, and bacteria were harvested by centrifugation before resuspension and dilution as appropriate in sterile 1× phosphate-buffered saline.
Viable cell counting.
Serial dilutions of 20 μl samples were done in sterile 96-well microtiter plates (GIBCO, Paisley, United Kingdom) containing 180 μl of sterile nanopure water per well. Serial dilutions to 10−7 were made with each sample. Dried BAB–5% (vol/vol) aerated horse blood plates were marked into six sectors, and three 20-μl aliquots of each dilution were plated into each sector; this procedure was repeated in duplicate. Once dry, plates were incubated at 37°C overnight. Colonies were counted in sectors containing a measurable number (approximately <300).
Inbred mouse strains.
Mice were obtained from Harlan Olac Ltd. (Shaw's Farm, Bicester, Oxon, United Kingdom). Female inbred mice and MF1 outbred mice were challenged at ∼9 weeks of age.
Measurement of antibodies to pneumococcal polysaccharide by enzyme-linked immunosorbent assay (ELISA).
Serum samples were taken from 10 inbred mice from each strain before challenge and stored at −20°C before use. Microtiter plates were coated with 23 serotypes of pneumococcal polysaccharide by the addition of a 1:100 dilution of Pneumovax II (Merck, Sharp & Dohme Ltd., Hoddesdon, United Kingdom) in 0.05 M carbonate buffer. Coated plates were left at 4°C overnight, washed four times with washing buffer (phosphate-buffered saline [PBS], 0.1% [vol/vol] Tween), and blocked for 2 h at room temperature with PBS–10% (vol/vol) FBS. Plates were then washed twice in washing buffer, and test sera diluted 1:100 in dilution buffer (PBS, 0.1% [vol/vol] Tween, 10% [vol/vol] FBS) were added. A positive control of anti-pneumococcal polysaccharide mouse whole immunoglobulin (Staten Serumsinstitut, Copenhagen, Denmark) diluted from 1:80 in dilution buffer was included. A negative control of normal mouse serum (Sigma, Poole, United Kingdom) diluted as for the test sera was also used. Loaded plates were then incubated at room temperature for 1 h and washed four times in washing buffer. Anti-mouse immunoglobulin-horseradish peroxidase conjugate (Amersham Life Sciences, Little Chalfont, United Kingdom) diluted 1:500 with dilution buffer was added, and the plate was incubated at room temperature for 1 h. Plates were then washed four times with washing buffer, the substrate o-phenylenediamine (Sigma) was used according to manufacturer's instructions, and the absorbance of the samples was measured at 492 nm.
Intranasal challenge of mice.
Mice were lightly anesthetized with 2.5 to 5.0% (vol/vol) fluothane (Zeneca Pharmaceuticals, Macclesfield, United Kingdom) over oxygen (1.5 to 2 liters/min), administered using a calibrated vaporizer, and challenged with 50 μl of PBS containing 106 CFU of type 2 S. pneumoniae administered into the nostrils. To look for invasive infection, the numbers of bacteria in the blood 24 h postinfection were determined; 100 μl of blood was taken from the tail vein, and viable counts were performed. Mice were monitored for visible clinical symptoms for 7 days, at which point the experiment was ended. Mice that were alive at this point were considered to have survived the pneumococcal challenge; mice that became moribund during the 7-day period were judged to have reached the endpoint of the assay (20). The time that the animal became moribund was recorded, and the animal was killed by cervical dislocation.
In experiments to examine the growth of bacteria in the lungs and blood, mice were infected intranasally as above. At prechosen intervals following infection, 100 μl of blood was taken from a tail vein from each mouse. Following this procedure, the mice were killed by cervical dislocation, and the lungs were removed into 10 ml of sterile distilled water. The tissues were then homogenized in a Stomacher-Lab blender (Seward Medical, London, United Kingdom). Viable counts in tissue homogenates and in blood samples were performed as described above.
Enumeration and differential analysis of lung leukocyte count.
The method used was modified from the methods of Curtis et al. (13) and Huffnagle et al. (15). Before removal of the lungs from sacrificed animals, vascular perfusion was performed by injection of 1 ml of Hanks balanced salt solution into the left ventricle of the heart. The tissue was then removed and placed into 10 ml of Hanks balanced salt solution. The lungs were then cut into small pieces and homogenized in 5 ml of digestion buffer (5% [vol/vol] FBS in RPMI 1640 with collagenase [0.5 mg/ml; 207 collagen digestion units; [Sigma] and DNase I [30 μg/ml; (87 U; [Sigma] from bovine pancreas) through a tea strainer (made of stainless steel with a wire grid with ∼256 holes/cm2) a total of three times. After homogenization, lung samples were incubated at 37°C for 30 min. Subsequently, digested tissue samples were pipetted to break up tissue fragments and passed through a column containing approximately 1 cm of nonabsorbent cotton wool in a glass Pasteur pipette to remove large pieces of debris. Collected cells in Falcon 2052 tubes (Becton Dickinson, Oxford, United Kingdom) were centrifuged at 332 × g for 5 min at 4°C. The supernatant was removed, and the cells were resuspended in 1 ml of 1× ammonium chloride-based lysing solution (PharMingen, San Diego, Calif.). After 5 min at room temperature to lyse the red blood cells, the remaining cells were brought to isotonicity by adding an excess volume of ice-cold 1× PBS. Following centrifugation at 322 × g for 5 min at 4°C, cells were washed with 1 ml of 1× PBS before a final resuspension in 1 ml of 5% FBS in RPMI 1640. The cells were then enumerated using hemocytometer (Improved Neubauer; Richardsons, Leicester, United Kingdom) with the addition of trypan blue in 1× PBS at a 1:1 volume ratio of stain to cell suspension. The total number of cells in each lung sample was diluted in 5% (vol/vol) FBS in RPMI 1640 to give between 7 × 104 and 1 × 105 cells per 50 μl.
For differential analysis, 50-μl aliquots of the cell suspensions were centrifuged onto slides (Life Sciences, Basingstoke, United Kingdom) at 108 × g for 3 min using a Cytospin centrifuge (Life Sciences). Following centrifugation, slides were air dried briefly and then fixed in 100% methanol for 10 min. After fixation, differential staining was done with Giemsa stain (BDH, Poole, United Kingdom). Slides were examined under ×400 magnification, and mononuclear leukocytes, lymphocytes, and polymorphonuclear leukocytes (PMNs) identified. At least 200 cells were counted on each slide. Using the percentage of each type of leukocyte obtained from each slide, cell numbers of each leukocyte population were then calculated from the total number of cells counted per milliliter.
Histopathology.
At necropsy, tissue samples were immersed in 10% (vol/vol) formalin saline solution prior to conventional processing and embedding in paraffin wax. Histopathological assessment was performed on tissue sections stained with hematoxylin and eosin (BDH).
Scoring of the inflammatory process was performed on frozen sections from tissue samples processed and embedded in Tissue Tek OCT (Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands). Sections were then stained with hematoxylin and eosin, coded, and examined blind with regard to the following criteria: inflammatory cellular infiltration, bronchiolar hypertrophy, and the level of bronchiolar exudate and cellular infiltration. The severity of each was graded as 0 (none), 1 (slight), 2 (mild), 3 (moderate), 4 (marked), or 5 (severe). A total of four sections per animal were scored, and a median inflammatory lesion score was generated for numerical comparison.
Statistical analysis.
Data were analyzed by two-tailed Mann-Whitney U test, two-tailed t test, and one-way analysis of variance including multiple comparisons (Tukey).
RESULTS
Infection of inbred strains with type 2 pneumococci.
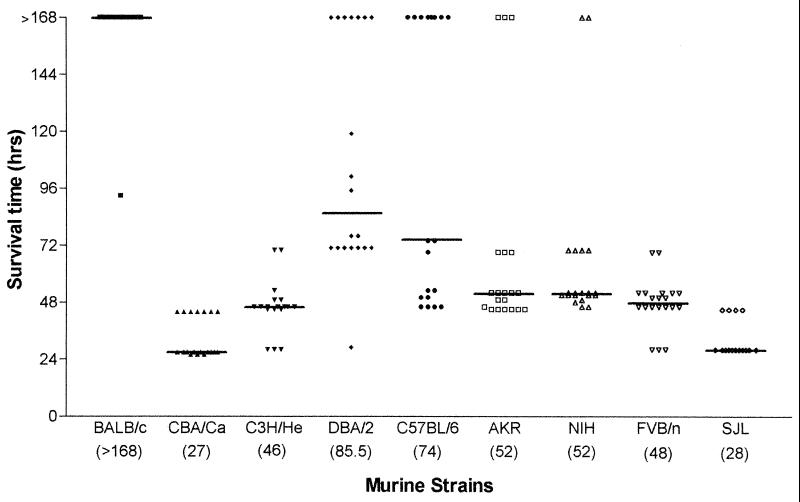
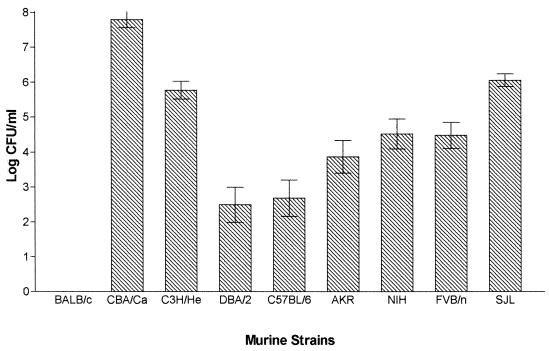
Groups of 20 female mice were infected with 106 S. pneumoniae strain D39 (type 2). Groups of five MF1 outbred mice were included with each challenge of inbred strains as a point of reference, because of the known susceptibility of this strain to pneumococcal intranasal challenge as reported previously (1). These mice have a (median survival time of 48 h. There was no statistical difference (P > 0.05) in the median survival times of the groups of MF1 mice that served as a point of reference for the other survival data presented here. Numbers of bacteria in the blood 24 h postinfection were determined, and survival times were recorded. Mouse strains differed considerably in their resistance to type 2 pneumococcal infection. A wide range of median survival times were seen (Fig. 1), correlating with the number of pneumococci in the blood 24 h postinfection (Fig. 2).
FIG. 1.
Median survival times of murine strains (n = 20 for each strain) following intranasal challenge with S. pneumoniae D39. Each datum point refers to one mouse. Figures in parentheses are median survival times for individual strains.
FIG. 2.
Number of pneumococci in the blood of murine strains (n = 20 for each strain) 24 h after intranasal challenge with S. pneumoniae D39. Error bars show the standard error of the mean.
The inbred mouse strains challenged with D39 could be divided into three groups, resistant, intermediate, and susceptible, on the basis of their survival times. BALB/c mice constituted the resistant group. Although one mouse succumbed to the infection (at 93 h), the remaining 19 mice survived to 7 days postchallenge, at which time the experiment was ended (median survival time, >168 h [Fig. 1]). Consistent with this median survival time, at 24 h postinfection no pneumococci were isolated from the blood of any of the mice challenged (Fig. 2). BALB/c mice were found to be statistically different from all other inbred strains in both median survival time (P < 0.01) and blood counts 24 h postinfection (P < 0.001). The majority of the strains tested (C3H/He, DBA/2, C57BL/6, AKR, NIH, and FVB/n) constituted an intermediate phenotype group for susceptibility to infection with D39 in both phenotypic parameters blood counts 24 h postinfection and median survival time (Fig. 1 and 2; Table 1).
TABLE 1.
Statistical analysis of median survival time and mean blood counts 24 h after infection of inbred strains challenged with S. pneumoniae D39
| Analysis | Strain | Approx calculated P
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AKR | BALB/c | C3H/He | C57BL/6 | CBA/Ca | DBA/2 | FVB/n | NIH | ||
| Mean survival time | BALB/c | <0.0001 | |||||||
| C3H/He | NSa | <0.0001 | |||||||
| C57BL/6 | <0.05 | <0.01 | <0.0001 | ||||||
| CBA/Ca | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| DBA/2 | <0.001 | <0.001 | <0.0001 | NS | <0.0001 | ||||
| FVB/n | NS | <0.0001 | NS | <0.01 | <0.0001 | <0.0001 | |||
| NIH | NS | <0.0001 | <0.001 | NS | <0.0001 | <0.0001 | <0.05 | ||
| SJL | <0.0001 | <0.0001 | <0.001 | <0.0001 | <0.05 | <0.0001 | <0.0001 | <0.0001 | |
| Mean blood counts | BALB/c | <0.001 | |||||||
| C3H/He | <0.05 | <0.001 | |||||||
| C57BL/6 | NS | <0.001 | <0.001 | ||||||
| CBA/Ca | <0.001 | <0.001 | <0.01 | <0.001 | |||||
| DBA/2 | NS | <0.001 | <0.001 | NS | <0.001 | ||||
| FVB/n | NS | <0.001 | NS | <0.05 | <0.001 | <0.01 | |||
| NIH | NS | <0.001 | NS | <0.05 | <0.001 | <0.01 | NS | ||
| SJL | <0.01 | <0.001 | NS | <0.001 | NS | <0.001 | NS | NS | |
NS, not significant (P > 0.05).
SJL and CBA/Ca mice were observed as susceptible to type 2 pneumococcal infection. The two murine strains were found to be similar to each other in blood counts 24 h postinfection (P > 0.05) but different in median survival time (P < 0.05). CBA/Ca mice were very sensitive to type 2 pneumococcal infection, quickly developing severe disease (median survival time, 27 h). Bacteremia also developed quickly, with high numbers (7.8 mean log CFU/ml) detected in the blood 24 h postinfection (Fig. 2). SJL mice also showed severe bacteremia, with a short survival time (median survival time, 28 h). Interestingly SJL mice showed a clinical pattern different from that of CBA/Ca mice. From the onset of symptoms at 21 h, they rapidly reached moribund state approximately 7 h later. In comparison, CBA/Ca were symptomatic at 12 h but did not reach the moribund state until approximately 15 h later, at 27 h. Both of these mouse strains exhibited statistically different survival times (SJL, P < 0.001; CBA/Ca, P < 0.0001) than the other inbred mice. CBA/Ca mice had significantly higher numbers of pneumococci in the blood 24 h postinfection (P < 0.01) than the other inbred mice tested.
Growth in the lungs and blood after intranasal challenge.
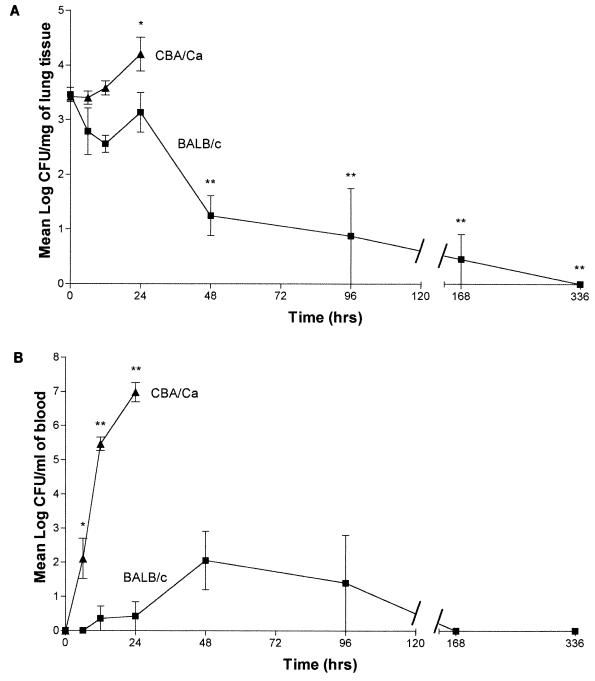
From the challenges of the inbred mice, BALB/c mice were selected as resistant and CBA/Ca mice were selected as susceptible. No preexisting anti-pneumococcal polysaccharide capsule antibodies were found in BALB/c and CBA/Ca mice by ELISA. Groups of 5 to 10 mice were taken at each time point after infection, and viable counts were performed on blood and lung tissue (Fig. 3). The pattern of growth in the lungs of BALB/c mice was different from that of CBA/Ca mice. In BALB/c mice, there was no significant change in pneumococcal numbers until after 24 h postinfection. By 48 h postinfection, the numbers of pneumococci in the lungs was significantly (P < 0.001) lower than at 0 h. After this point, bacterial numbers remained significantly lower than at 0 h (P < 0.001) until 336 h postinfection, when no bacteria were recovered. At this time, all five mice examined were found to have no detectable pneumococci in the lungs. In CBA/Ca mice, no fall in numbers was seen during the course of infection; rather, by 24 h postinfection numbers of pneumococci in the lungs increased, reaching a level of 4.2 mean log CFU/mg (P < 0.05 compared to 0 h) shortly before the animals became moribund.
FIG. 3.
(A) Time course of the growth of S. pneumoniae D39 in the lungs of BALB/c (n = 10 except for 96, 168, and 336 h for which n = 5) and CBA/Ca mice (n = 5). Data represent the mean of 5 or 10 mice per point, with error bars showing the standard error of the mean. ∗, P < 0.05 compared to time zero; ∗∗, P < 0.001 compared to time zero. (B) Time course of the growth of S. pneumoniae D39 in the blood of BALB/c (n = 10 except for 96, 168, and 336 h for which n = 5) and CBA/Ca mice (n = 5). Data represent the mean of 5 or 10 mice per point, with error bars showing the standard error of the mean. ∗, P < 0.01 compared to time zero; ∗∗, P < 0.001 compared to time zero.
Numbers of type 2 pneumococci in the blood of BALB/c and CBA/Ca mice were also significantly different. Until 12 h postinfection, no pneumococci were detected in the blood of BALB/c mice; at 12 h postinfection, pneumococci were detected in only 1 of 10 mice tested in this experiment. At 24 h postinfection, pneumococci were again detected in the blood from only 1 of 10 mice tested. At 48 h postinfection, pneumococci were isolated from 4 of the 10 mice challenged. At this point, the average number of pneumococci in the blood reached the peak level in BALB/c (2.1 mean log CFU/ml). Bacterial numbers declined after this time point, with no pneumococci being detected in the blood of any of the group after 168 h (7 days) or 336 h (2 weeks) postinfection. In contrast to BALB/c mice, pneumococci appeared in the blood of CBA/Ca mice by 6 h postinfection (P < 0.01 compared to 0 h), and their numbers increased rapidly to a mean blood count of 7 mean log CFU/ml at 24 h after infection (P < 0.001 compared to 0 h).
Cellular recruitment into the lungs following infection of BALB/c and CBA/Ca mice with D39 pneumococci.
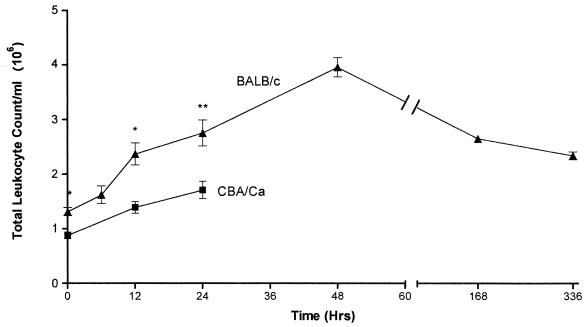
To examine the overall cellular response in the lungs of both susceptible and resistant inbred mice, groups of five mice were taken at each time point following intranasal infection. Lungs were removed, and the total number and differential analysis of leukocytes were evaluated. BALB/c mice were found to have a significantly different pattern of cellular recruitment in comparison to CBA/Ca mice (Fig. 4). BALB/c mice had statistically greater numbers of cells at 0 h (P < 0.005), 12 h (P < 0.005), and 24 h (P < 0.01). Both strains of mice recruited cells faster between 0 and 12 h than from 12 to 24 h. The endpoint for the CBA/Ca experiment was set at 24 h, but further analysis of BALB/c mice showed the peak of cellular recruitment at 48 h. After this point a reduction in the number of cells was observed until 336 h postinfection; the number of cells (mean total cell count 2.3 × 106) was at this point still statistically higher (P < 0.001) than the number at the start of the infection (mean total cell count 1.3 × 106).
FIG. 4.
Comparison of total leukocyte counts in digested lung samples from CBA/Ca (■) and BALB/c (▴) mice following infection with S. pneumoniae D39. Data represent the mean of five mice per time point except 168 h, which is from three mice. Error bars show the standard error of the mean. ∗, P < 0.005; ∗∗, P < 0.01 for BALB/c leukocyte levels to compared to CBA/Ca levels.
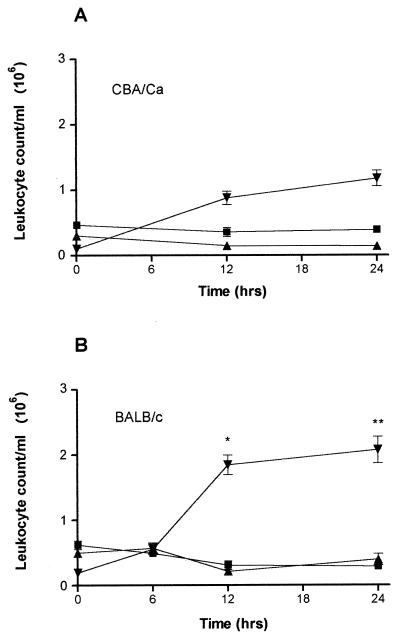
Differential cell analysis revealed that the main difference in the cellular response was the recruitment of PMNs; this leukocyte population was made up almost entirely of neutrophils (Fig. 5). Both CBA/Ca and BALB/c mice were shown to have a higher influx of these cells postinfection. However, BALB/c mice recruited significantly more neutrophils than CBA/Ca mice did at both 12 h (P < 0.001) and 24 h (P < 0.01). The maximum recruitment of these cells in BALB/c mice was between 6 and 12 h, as implicated by the total leukocyte recruitment numbers.
FIG. 5.
Differential analysis of macrophages (■), lymphocytes (▴), and neutrophils (▾) in digested lung samples from CBA/Ca (A)and BALB/c (B) mice following infection with S. pneumoniae D39. Data represent the mean of five mice per point, with error bars showing the standard error of the mean. ∗, P < 0.001; ∗∗, P < 0.01 for BALB/c neutrophil levels as compared to CBA/Ca levels.
Histopathology of BALB/c and CBA/Ca lung tissue following infection with D39 pneumococci.
Histopathological assessment was performed on lung tissue samples taken from at least two mice sacrificed at each of various times following intranasal infection. These time points were between 0 to 24 h for CBA/Ca mice and 0 to 336 h for BALB/c mice. CBA/Ca mice showed no obvious lesions until 12 h postinfection (Fig. 6). At this time, the lungs of these mice showed mild, multifocal mixed inflammatory infiltration or a mild, multifocal acute peribronchial inflammatory cell infiltration. Extension of lesions into other areas of the lung was evident, with one animal out of two showing minimal diffuse, acute/subacute interstitial alveolitis. At 24 h postinfection, lesions in the lungs were more diffuse and involved peribronchial and perivascular regions through to interstitial alveolitis. The infection was also shown to extend to inflammation of the pleural membrane with a moderate, multifocally distributed, acute pleuritis.
FIG. 6.
Hematoxylin-and-eosin-stained paraffin wax cut lung tissue sections from BALB/c (A) and CBA/Ca (B) mice 12 h following infection with S. pneumoniae D39. PB and PV indicate areas of peribronchial and perivascular cellular infiltration. CBA/Ca mice showed some evidence of interstitial alveolitis (IA), but in BALB/c mice interstitial alveolitis was more severe.
Compared with CBA/Ca mice, BALB/c mice showed inflammatory lesions at a much earlier time postinfection. A minimal multifocal, acute peribronchial and perivascular, inflammatory cellular infiltration was seen at 6 h postinfection. By 12 h postinfection (Fig. 6) these lesions were more severe, and evidence of involvement of the lung parenchyma was observed as a minimal, multifocal acute interstitial alveolitis. At 24 h postinfection, these lesions were more severe and generally more diffuse. Some mice showed signs of a mild acute bronchitis. It was notable that CBA/Ca mice showed no bronchitis at this time point. At 48 h postinfection, peribronchial and perivascular, inflammatory cellular infiltration in the lungs of BALB/c mice was the same in severity as that seen at 24 h postinfection, although at this time point these lesions were more multifocal in distribution and the inflammatory infiltrate was acute/subacute in nature. Both interstitial alveolitis and bronchitis observed at 48 h postinfection were less severe than at 24 h postinfection. By 72 h postinfection, both perivascular infiltration and interstitial alveolitis were more severe than at 48 h postinfection. At this stage of the infection, inflammation was generally multifocal and had also extended to the pleural membrane as a minimal multifocal pleuritis. One week after initial infection (168 h), BALB/c mice showed signs of a mild, multifocal chronic interstitial alveolitis with a moderate multifocal lymphoid hyperplasia.
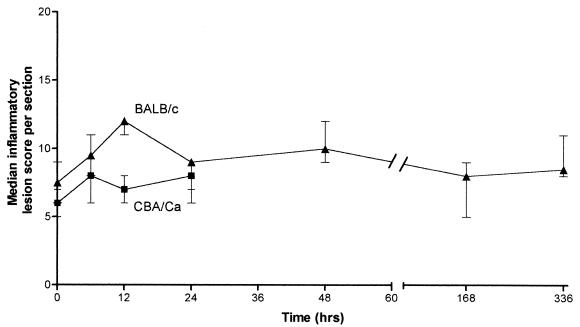
Cryostat frozen sections were given an inflammatory lesion score for a numerical comparison (Fig. 7). BALB/c mice had a statistically higher lesion score than CBA/Ca mice at all time points (P < 0.05). This result reflected the observation that BALB/c mice had a greater inflammatory cellular infiltrate than CBA/Ca mice. Differences between the strains were most evident at 12 h postinfection; lesions in BALB/c mice were more severe, with a greater median lesion score, than those in CBA/Ca mice by the criteria selected. BALB/c mice, with a peak median lesion score at 12 h, showed a higher level of perivascular, peribronchial, intrabronchial cellular infiltration and greater bronchiolar wall hypertrophy with increased bronchiolar exudate compared to that in CBA/Ca mice (Fig. 7). At 24 h (the endpoint of the experiment for CBA/Ca mice) the median lesion score was unchanged, whereas with BALB/c mice the lesion score fell to a median of 9. Further analysis of BALB/c mice until 336 h postinfection indicated a lower median inflammatory score than at 12 h. These lower scores were due to a difference in the general inflammatory state of the tissue compared to sections at 12 h.
FIG. 7.
Median inflammatory lesion score comparison between histological sections from BALB/c (▴) and CBA/Ca (■) lungs (n = 2 or 3) following a time course infection with S. pneumoniae D39. Values represent median lesion scores generated from evaluating inflammatory lesion criteria for a total of four sections per mouse at each time point. Bars show the range of lesion scores.
DISCUSSION
The host genetic factors that control innate resistance to pneumococcal disease are not well understood. To begin to define host genetic factors that may play a role in susceptibility to invasive pneumococcal disease, we have used a murine model of pneumococcal disease. Murine models provide an important tool in examining both familial inheritance and the dissection of the biology behind genetic traits. The long-term objective of this study is to identify and map candidate genes of the mouse genome. This report describes the results of the first stage of this process, to select phenotypically distinct resistant and susceptible parental strains for further genetic analysis and investigate the nature of disease progression in these identified strains.
Following intranasal pneumococcal infection, mouse strains differed markedly in susceptibility as represented by survival time and numbers of pneumococci in the blood. After pneumococcal infection, a susceptible phenotype (CBA/Ca mice and SJL mice) and a resistant phenotype (BALB/c mice) were observed. By virtue of the variation in the distribution of phenotypes seen among all nine inbred strains, our data suggest that resistance to pneumococcal infection is a complex trait. However, the results do not rule out the possibility of a single gene effect with the different phenotypes observed generated from allelic variation in the inbred strains.
Resistant BALB/c and susceptible CBA/Ca mice now form the basis of continuing studies to identify the genes involved in host resistance. With a view to complementing future data from gene identification studies, these mouse strains were examined to investigate the biology associated their susceptibility/resistance phenotypes. Time course investigations into levels of pneumococci in the blood and lungs were performed to determine how the different phenotypic characteristics of the murine strains were related to the changes in pneumococcal numbers.
Differences between susceptible and resistant strains are seen clearly in the growth of pneumococci in the lungs and inflammation at that site. In CBA/Ca mice, numbers of pneumococci increased in the lungs over 24 h, whereas in BALB/c mice the numbers were unchanged during this time. By 48 h numbers of bacteria had declined in BALB/c mice, whereas by 27 h CBA/Ca mice were moribund and obviously could not enter this phase of bacterial decline. The events in the blood mirrored those seen in the lungs. CBA/Ca mice developed an early sepsis, extending to an uncontrolled bacterial increase, whereas in BALB/c mice only small numbers of bacteria were recovered from the blood, and then only occasionally. Maintenance of bacteremia appears to be dependent on the occurrence of high numbers of pulmonary bacteria, and BALB/c mice are able to manage the infection in the lungs.
Supportive evidence for a phenotypic pulmonary effect arises from analysis of the cellular response in the lungs of CBA/Ca and BALB/c mice. A better inflammatory response to infection is elicited in BALB/c mice than in CBA/Ca mice, with neutrophils ascertained as the majority cell type involved. The rate of influx of neutrophils in BALB/c mice was far greater than that in CBA/Ca mice. This recruitment difference may account for the different phenotypes observed between CBA/Ca and BALB/c when managing an invasive pneumococcal lung infection. However, from the data we cannot determine if, in addition to the recruitment difference, there is a difference in the antimicrobial activity of the two sets of neutrophils. That the ability to recruit inflammatory cells is important to the phenotypic differences between the two strains was supported by histopathological evidence. Inflammatory lesions in BALB/c lungs were visible much earlier than those in CBA/Ca lungs, with the difference in inflammatory score being accounted for primarily by the greater perivascular, peribronchial, and intrabronchial cellular infiltration.
Evidence that a difference in the recruitment of neutrophils is the basis of innate susceptibility to infection comes from other models of infection. For example, the differences in response of resistant BALB/c H-2k and susceptible CBA/CaH mice to Candida albicans lies in the short-lived cellular responses derived from the bone marrow, which was suggested as representing differences in differentiation and recruitment of PMNs between the strains (3). Furthermore, in an endobronchial inflammation model of infection with Pseudomonas aeruginosa, resistant BALB/c mice showed greater recruitment of PMNs into the bronchoalveolar spaces than susceptible DBA/2 mice (19). Morissette et al. (19) also found that DBA/2 mice lacked an adequate mechanism of bacterial clearance.
Recruitment and function of neutrophils has been reported to be controlled by genes associated with H-2 haplotype (16). Importantly, Marley et al. (16) also found that genes outside the major histocompatibility complex region were involved. In our experiments, the CBA/Ca strain with H-2k haplotype was significantly more susceptible than the H-2k AKR strain; BALB/c with H-2d haplotype was significantly more resistant than DBA/2, which is also H-2d. Although we are not in a position to identify the genes that confer resistance, the ongoing genotyping of generations from CBA/Ca × BALB/c crosses indicates that an association with H-2 has been excluded (data not shown).
In summary, this study has identified murine strains resistant and susceptible to intranasal infection with D39 (type 2) pneumococci. Further investigation of phenotypic characteristics of resistant BALB/c and susceptible CBA/Ca mice suggest an association between recruitment and/or function of neutrophils. The process of neutrophil recruitment is complex and an active area of research in pneumococcal pathogenesis. Mediators of inflammation such as tumor necrosis factor alpha, interleukin-10, and gamma interferon have been previously implicated in pneumococcal disease (5, 24, 27, 29). The molecules that influence the recruitment and function of neutrophils warrant further investigation. However, other components related to innate immunity, the inflammatory process, and receptors to which pneumococci bind should not be overlooked. For example, what is the role of adhesion molecules and complement? Studies with both BALB/c and CBA/Ca strains are in progress to elucidate the key components involved in the behavior of neutrophils in these animals in pneumococcal disease.
REFERENCES
- 1.Alexander J E, Berry A M, Paton J C, Rubins J B, Andrew P W, Mitchell T J. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb Pathog. 1998;24:167–174. doi: 10.1006/mpat.1997.0185. [DOI] [PubMed] [Google Scholar]
- 2.Amsbaugh D F, Hansen C T, Prescott B, Stashak P W, Barthold D R, Baker P J. Genetic control of the antibody response to type III pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972;136:931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashman R B, Papadimitriou J M. Genetic resistance to Candida albicans infection is conferred by cells derived from the bone marrow. J Infect Dis. 1992;166:947–948. doi: 10.1093/infdis/166.4.947. [DOI] [PubMed] [Google Scholar]
- 4.Benton K, Paton J P, Briles D E. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997;23:201–209. doi: 10.1006/mpat.1997.0150. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron Y, Ouellet N, Deslauriers A, Simard M, Olivier M, Bergeron M G. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley D J. Regulation of Leishmania populations within the host. II. Genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977;30:130–140. [PMC free article] [PubMed] [Google Scholar]
- 7.Briles D E, Horowitz J, McDaniel L S, Benjamin W H, Jr, Claflin J L, Booker C L, Scott G, Forman C. Genetic control of the susceptibility to pneumococcal infection. Curr Top Microbiol Immunol. 1986;124:103–120. doi: 10.1007/978-3-642-70986-9_7. [DOI] [PubMed] [Google Scholar]
- 8.Briles D E, Schroer K, Baker P, Davie J. Susceptibility of (CBA/N × DBA/2) F1 male mice to infection with type 3 (Streptococcus pneumoniae) In: Skamene E, editor. Perspectives in immunology: genetic control of natural resistance to infection and malignancy. London, England: Academic Press; 1980. pp. 173–177. [Google Scholar]
- 9.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles D E, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles E B, Tomasz A. Pneumococcal Forssman antigen: a choline-containing lipoteichoic acid. J Biol Chem. 1973;248:6394–6397. [PubMed] [Google Scholar]
- 12.Brundish D E, Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. J Biochem. 1968;110:573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis J L, Huffnagle G B, Chen G H, Warnock M L, Gyetko M R, McDonald R A, Scott P J, Toews G B. Experimental murine pulmonary cryptococcosis—differences in pulmonary inflammation and lymphocyte recruitment induced by encapsulated strains of Cryptococcus neoformans. Lab Investig. 1994;71:113–126. [PubMed] [Google Scholar]
- 14.Gros P, Skamene E, Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981;127:2417–2421. [PubMed] [Google Scholar]
- 15.Huffnagle G B, Strieter R M, Standiford T J, McDonald R A, Burdick M D, Kunkel S L, Toews G B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4(+) T-cells during a pulmonary Cryptococcus neoformans infection. J Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 16.Marley S B, Hadley C L, Wakelin D. Effect of genetic variation on induced neutrophilia in mice. Infect Immun. 1994;62:4304–4309. doi: 10.1128/iai.62.10.4304-4309.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDaniel L S, Benjamin W H, Jr, Forman C, Briles D E. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J Immunol. 1984;133:3308–3312. [PubMed] [Google Scholar]
- 18.Mitchell T J, Alexander J E, Morgan P J, Andrew P W. Molecular analysis of virulence factors of Streptococcus pneumoniae. J Appl Microbiol Suppl. 1997;83:62S–71S. doi: 10.1046/j.1365-2672.83.s1.7.x. [DOI] [PubMed] [Google Scholar]
- 19.Morissette C, Skamene E, Gervais F. Endobronchial inflammation following Pseudomonas aeruginosa infection in resistant and susceptible strains of mice. Infect Immun. 1995;63:1718–1724. doi: 10.1128/iai.63.5.1718-1724.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton D B, Griffiths P H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 21.Plant J E, Glynn A A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979;37:1–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Plant J E, Blackwell J M, O'Brien A D, Bradley D J, Glynn A A. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982;297:510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- 23.Rake G. Pathology of pneumococcus infection in mice following intranasal instillation. J Exp Med. 1936;63:17–31. doi: 10.1084/jem.63.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubins J B, Pomeroy C. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect Immun. 1997;65:2975–2977. doi: 10.1128/iai.65.7.2975-2977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skamene E, Gros P, Forget A, Kongshavn P A L, St. Charles C, Taylor B A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982;297:506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- 26.Takashima K, Tateda K, Matsumoto T, Ito T, Iizawa Y, Nakao M, Yamaguchi K. Establishment of a model of penicillin-resistant Streptococcus pneumoniae pneumonia in healthy CBA/J mice. J Med Microbiol. 1996;45:319–322. doi: 10.1099/00222615-45-5-319. [DOI] [PubMed] [Google Scholar]
- 27.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in the pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasz A. Choline in the cell wall of a bacterium: novel type of polymerlinked choline in pneumococcus. Science. 1967;157:694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]
- 29.van der Poll T, Marchant A, Keogh C V, Goldman M, Lowry S F. Interleukin-10 impairs host defence in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 30.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 31.Webster L T. Inherited and acquired factors in resistance to infection. J Exp Med. 1933;57:819–843. doi: 10.1084/jem.57.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yother J, Forman C, Gray B M, Briles D E. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982;36:184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]