Abstract
Objective
C1q/TNF-related proteins 1 (CTRP1) is a recently identified adiponectin associated with obesity-linked disorders and adverse cardiovascular events. The effect of CTRP1 on cardiac fibrosis has not yet been fully elucidated; thus, we aimed to explore this association.
Materials and Methods
In this experimental study, a mouse model of cardiac fibrosis was established by administering isoproterenol (ISO) (subcutaneously injecting 10 mg/kg/day for 3 days and then 5 mg/kg/day for 11 days). Mice were also injected with recombinant CTRP1 protein (200 μg/kg) 14 days after the final ISO administration. Adult mouse fibroblasts were isolated and stimulated with transforming growth factor (TGF) β1, followed by treatment with recombinant CTRP1. Primary bone marrow-derived macrophages were isolated from C57BL/6J mice and treated with recombinant CTRP1 as well.
Results
CTRP1 level was increased in mouse plasma and heart tissue 2 weeks after ISO injection. Our findings indicated that recombinant CTRP1 injection aggravated ISO-induced cardiac fibrosis and dysfunction. However, recombinant CTRP1 did not alter TGFβ1-induced fibroblast proliferation and activation or collagen transcription. Recombinant CTRP1 exacerbated ISO-induced macrophage infiltration and inflammatory response. We determined that macrophages treated with recombinant CTRP1 showed increased pro-inflammatory cytokine release. Fibroblasts co-cultured with macrophages treated with recombinant CTRP1 showed increased proliferation and collagen transcription. We also found that CTRP1 upregulated the NADPH oxidase 2 (NOX2)/p38 pathway in macrophages. When we inhibited p38 signaling, the pro-inflammatory effect of CTRP1 on macrophages was counteracted. Fibroblasts co-cultured with macrophages treated with a p38 inhibitor also showed limited proliferation and collagen transcription.
Conclusion
Cardiac fibrosis was aggravated with the activation of the NOX2/p38 pathway in macrophages after CTRP1 treatment.
Keywords: Cardiac Fibrosis, CTRP1, Fibroblast, Macrophage, NOX2
Introduction
Cardiac fibrosis is a common pathological process in many cardiovascular diseases, including acute and chronic inflammation, myocardial ischemia, pressure overload (e.g., hypertension), aging, and genetic cardiomyopathies (1). Unlike other organs, the heart has a limited capacity to recover after injury because cardiomyocytes lack the ability to proliferate. After heart injury, fibrous tissue proliferates to maintain the integrity of heart structure and function (2). Nevertheless, many cells such as fibroblasts and macrophages play a critical role in this repair process (3). Despite this repair process which prevents myocardial dysfunction or even rupture, persistent fibrosis and inflammation hamper cardiomyocytes from accessing oxygen and nutrients, promoting adverse cardiac remodeling (1). This leads to reduced cardiac contraction and an increased arrhythmogenic risk (4). The currently available clinical drugs are not effective in treating cardiac fibrosis. Thus, finding new specific therapeutic targets for cardiac fibrosis is of great importance for improving cardiac function, reducing infarct size, and delaying incident heart failure.
Cardiac macrophages, derived from resident tissue macrophages and bone marrow progenitor cells, cooperate in the initiation and maintenance of fibrotic responses (3). In the first stage of tissue inflammation, macrophages undergo "M1-like" activation (5). M1- like macrophages aggravate the inflammatory response of the heart by releasing pro-inflammatory cytokines and promoting phagocytosis and proteolysis. At a later stage, macrophages switch to the "alternative activated" (M2-like) repair phenotype and produce anti-inflammatory cytokines, such as interleukin (IL)-10 and transforming growth factor (TGF)β1 (6). M2-like macrophages aggravate scar formation and fibrosis by regulating the expression of extracellular matrix components and activating cardiac fibroblasts (7). Thus, targeting this "sterile" inflammation may be a useful therapeutic approach to inhibit the onset and progression of cardiac fibrosis.
CTRP1 is a member of a conserved family of secreted C1q/TNF-related proteins. Secreted hormones control energy metabolism via inter-organ crosstalk (8). Studies have reported both a protective and a deleterious effect of CTRP1 on cardiovascular diseases (9-12). Clinical studies have demonstrated that CTRP1 is a potential aggravating factor leading to adverse events. It was found that CTRP1 level was elevated in the plasma of hypertensive patients and was associated with subclinical target organ damage (11) and that increased serum CTRP1 levels increased adverse cardiovascular events in patients with coronary artery disease (13). Increased plasma CTRP1 levels were also observed in critically ill patients with sepsis and type 2 diabetes (10). Conversely, in an experimental animal model, CTRP1 seemed to have a cardioprotective effect. It was reported that CTRP1-deficient mice showed impaired glucose and lipid metabolism (14) and that CTRP1 can prevent sepsis-induced cardiomyopathy (9). These contradictory results on the role of CTRP1 still serve to indicate the close association of CTRP1 with cardiovascular disease. In the present study, we aimed to explore the functional role of recombinant CTRP1 in the mechanisms underlying cardiac fibrosis.
Materials and Methods
Animals
In this experimental study, C57BL6J male mice were purchased from Beijing Huafukang Biological Co. Ltd. (Beijing, China). The mice were divided into four groups: vehicle-NS (normal saline), CTRP1-NS, vehicleisoproterenol (ISO), and CTRP1-ISO. Mice from the two ISO groups were injected subcutaneously with ISO (10 mg/kg/day for 3 days and then 5 mg/kg/day for 11 days). The mice in the two CTRP1 groups (n=12 for each group), following the last injection of ISO or NS, received 200 μg/kg recombinant CTRP1 full-length protein (ab151376, Abcam, Cambridge, UK) or vehicle solution for 2 weeks using an Alzet osmotic minipump (Durect, Cupertino, CA, USA). Animal experiments were performed per the guidelines of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised in 1996). All the animal experiments were reviewed and approved by Xiangyang Central Hospital's Animal Care and Use Committee.
Echocardiography measurements
Cardiac function was assessed using a Mylab30CV (ESAOTE) echocardiograph with an M-mode and pulseDoppler 15-MHz probe. The left ventricular ejection fraction (LVEF), LV shortening fraction (LVFS), and E/A ratio were calculated.
Picrosirius red and immunohistochemical staining
The LV collagen fraction of six heart samples from each group was evaluated using Picrosirius red (PSR) staining. The images obtained were processed and analyzed using Image-Pro Plus 6.0. Immunohistochemical staining was used to detect the number of CD68-positive macrophages in the heart tissue. Anti-CD68 (Abcam) was used as the primary antibody, whereas anti-rabbit horseradish peroxidase (Gene Tech, Shanghai, China) was used as the secondary antibody. The DAB substrate kit was used for colorimetric development (Gene Tech) for visualization.
Detection of inflammatory cytokines using the enzymelinked immunosorbent assay (ELISA)
The heart tissue and cell samples were lysed, and the levels of inflammatory cytokines, namely tumor necrosis factor α (TNFα), IL-1, IL-6, and TGFβ1, in the lysate were detected using ELISA kits (BioLegend). The ELISA kit for CTRP1 was purchased from Biovendor, Inc. (Czech Republic). After the color development using ELISA, the absorbance of the samples was measured using a microplate reader (BioTek, USA). The concentration of each inflammatory cytokine in the samples was estimated using the standard curve method.
Fibroblast isolation and culture
After removing the hearts of C57BL6J mice (4-6-weekold), the heart tissues were cut into small pieces and digested with 0.125% trypsin and collagenase five times. The samples were centrifuged and resuspended in a medium containing 10% fetal bovine serum (FBS). We used a 40-μm filter to remove large cell clumps. Then, we seeded the cells in a 10-cm dish for 90 minutes. Subsequently, non-adherent cells were removed, and α-SMA staining was used to verify that the isolated cells were fibroblasts, which were then stimulated with TGFβ1 (10 ng/μL). The control cells were treated with the same volume of phosphate-buffered saline (PBS) for 24 hours. Cells were then treated with 0, 1, 2, 4, or 8 μg/mL of CTRP1 diluted in PBS containing 0.1% bovine serum albumin (BSA) for 24 hours. The control group was treated with the same volume of PBS containing 0.1% BSA for 24 hours. A CCK-8 assay kit was used to detect cell proliferation.
Primary bone marrow-derived macrophage isolation and culture
Primary bone marrow-derived macrophages were extracted from the femur and tibia of C57BL6J male mice aged 6-8 weeks. The cells were cultured with DMEM-F12 containing 5% FBS, L-glutamine (5 mmol/L, Sigma), and recombinant macrophage colony-stimulating factor (MCSF, 25 ng/ml, Peprotech). MCSF and granulocyteMCSF (Peprotech) were used at 25 ng/ml and 50 ng/ml, respectively, to induce cell differentiation. Interferon (IFN)-γ (10 ng/ml, Peprotech) and lipopolysaccharides (LPS, 100 ng/ml, Sigma) were used to induce proinflammatory activation. Macrophages were then treated with CTRP1 (8 μg/mL) for 12 hours and co-cultured with fibroblasts. Macrophages were also treated with SB203580 (10 μM, MedChemExpress) for 12 hours to inhibit p38.
Western blot
Total proteins (50 μg/sample) were resolved using SDSPAGE. The proteins were transferred to the Immobilon membrane and incubated with primary antibodies specific for NOX2, total p38, and p-p38 GAPDH (Cell Signaling Technology). Following incubation with the secondary antibody, a color reaction was carried out using the enhanced chemiluminescence (ECL) reagent (Bio-Rad, USA). We used a ChemiDoc MP imaging system (BioRAD) for color rendering.
Reverse transcription-polymerase chain reaction (RTPCR)
The total mRNA of fibroblasts was extracted using the TRIzol reagent. A SmartSpec Plus spectrophotometer (Bio-Rad) was used to detect the ratio of OD260/OD280 to analyze mRNA purity. Two milligrams of mRNA was reverse transcribed to synthesize cDNA using a Roche Diagnostic Reverse Transcription Kit. The LightCycler 480 SYBR Green I Kit (Roche Diagnostics) was used for amplification. The PCR products were quantified using a LightCycler 480 SYBR® Green 1 Master Mix (04707516001, Roche Diagnostics). Following an initial 5 minutes denaturation step at 95˚C, a total of 42 primerextension cycles were carried out. Each cycle consisted of a 10 seconds denaturation step at 95˚C, at 20 seconds annealing step at 60˚C, and a 20 seconds incubation at 72˚C for extension. Then a final extension step was performed at 72˚C for 10 minutes. The double standard curve was used to quantify the PCR results. The target gene expression was normalized to that of GAPDH, as the housekeeping gene. Primers used for RT-PCR listed as follow:
Collagen I-
F: 5′-AGGCTTCAGTGGTTTGGATG-3′
R: 5′- CACCAACAGCACCATCGTTA-3′
Collagen III-
F: 5′- AAGGCTGCAAGATGGATGCT -3′
R: 5′- GTGCTTACGTGGGACAGTCA -3′
α-SMA-
F: 5′- AACACGGCATCATCACCAAC -3′
R: 5′- ACCAGTTGTACGTCCAGAGG -3′
GAPDH-
F: 5′- ACTCCACTCACGGCAAATTC -3′
R: 5′- TCTCCATGGTGGTGAAGACA -3′
Immunofluorescence staining
Paraformaldehyde (4%) and Triton™ X-100 (0.1%) were used to fix and permeabilize the cells, respectively. The cells were incubated with anti-PCNA and anti-α-SMA (Abcam, 1:100 dilution) at 37˚C for 1 hour and then with an Alexa Fluor 568 goat anti-rabbit immunoglobulin IgG (Invitrogen Life Technologies, CA, USA) as the secondary antibody. The nuclei were stained with DAPI. Images were obtained using a fluorescence microscope.
Statistical analyses
Data are expressed as the mean ± standard deviation. Two-way way ANOVA and Tukey’s post-hoc test were used to compare the four groups. An independent-sample t test was used for pairwise comparisons. Statistical significance was set at P<0.05.
Results
Recombinant CTRP1 aggravates ISO-induced cardiac fibrosis
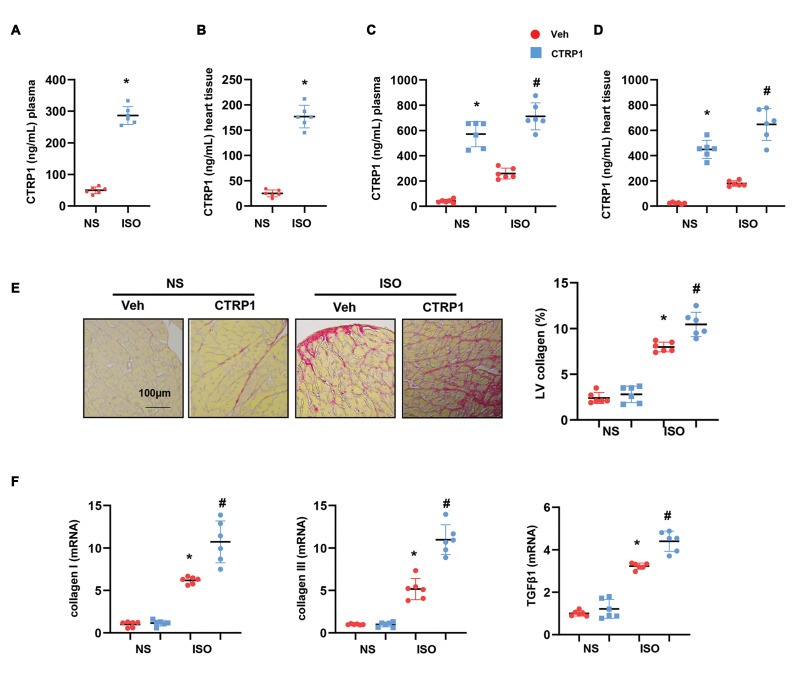
The expression of CTRP1 was evaluated in the mouse plasma and heart tissues after ISO injection. As shown in Figures 1A and B, the expression of CTRP1 was sharply elevated in the plasma and heart tissue from the ISO group as compared to that from the NS group. The mice were then injected with the recombinant CTRP1 protein. The concentration of CTRP1 in the mouse plasma and heart tissue was also evaluated using an ELISA. After 14 days of consecutive recombinant CTRP1 injection, the concentration of CTRP1 increased significantly in the plasma and heart tissue of both ISO-injected and NS-control mice (Fig.1C, D). The collagen volume was detected using PSR staining. Notably, CTRP1 increased LV collagen volume in mice after ISO administration (Fig.1E). The expression of fibrosis markers, such as collagen I, collagen III, and TGFβ1, was increased in CTRP1- injected mice after ISO stimulation, as detected by using RT-PCR (Fig.1F). These data suggest that the CTRP1 protein may aggravate cardiac fibrosis.
Fig 1.
Recombinant CTRP1 aggravates cardiac fibrosis induced by isoproterenol (ISO). A. The expression of CTRP1 in mouse plasma post-ISO injection (n=6) as detected by using enzyme-linked immunosorbent assay (ELISA). B. The expression of CTRP1 in mouse hearts post-ISO injection (n=6) detected by using ELISA. C. The expression of CTRP1 in mouse plasma post-recombinant CTRP1 injection (n=6) detected by using ELISA. D. The expression of CTRP1 in mouse hearts post-recombinant CTRP1 injection (n=6) detected by using ELISA. E. Representative image of picrosirius red (PSR) staining and quantification of left ventricular (LV) collagen volume (n=6). F. Transcription level of fibrosis markers in mouse hearts (n=6). *; P<0.05 vs. NS-Veh group, # ; P<0.05 vs. ISO-Veh group, NS; Normal saline, and Veh; Vehicle.
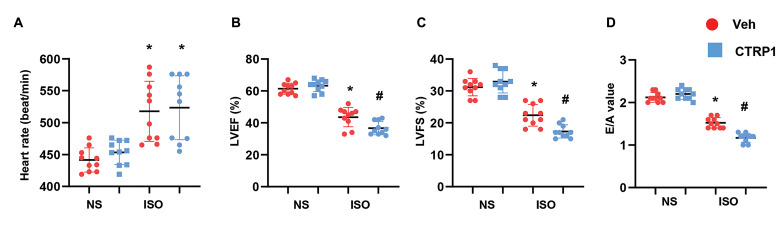
Recombinant CTRP1 aggravates ISO-induced cardiac dysfunction
Cardiac function was assessed by echocardiography to determine the effect of CTRP1 on ISO-induced cardiac dysfunction. As shown in Figure 2A, heart rate increased in the ISO group as compared to that of the NS control group. CTRP1 injection did not affect heart rate compared with vehicle NS or ISO administration. However, similar to the ISO injection, CTRP1 injection reduced LVEF and LVFS, suggesting deteriorated systolic cardiac function (Fig.2B). ISO injection also caused a decrease in E/A value, as did CTRP1 injection, suggesting deteriorated diastolic cardiac function (Fig.2C). Taken together, recombinant CTRP1 protein contributes to cardiac fibrosis and aggravates ISO-induced cardiac dysfunction.
Fig 2.
Recombinant CTRP1 aggravates cardiac dysfunction induced by ISO. Echocardiographic measurements in mouse hearts after recombinant CTRP1 injections (n=10). A. Heart rate, B. LV ejection fraction (LVEF) and LV fractional shortening (LVFS), and C. E/A ratio. ISO; Isoproterenol, LV; Left ventricular, and E/A; E value to A value.
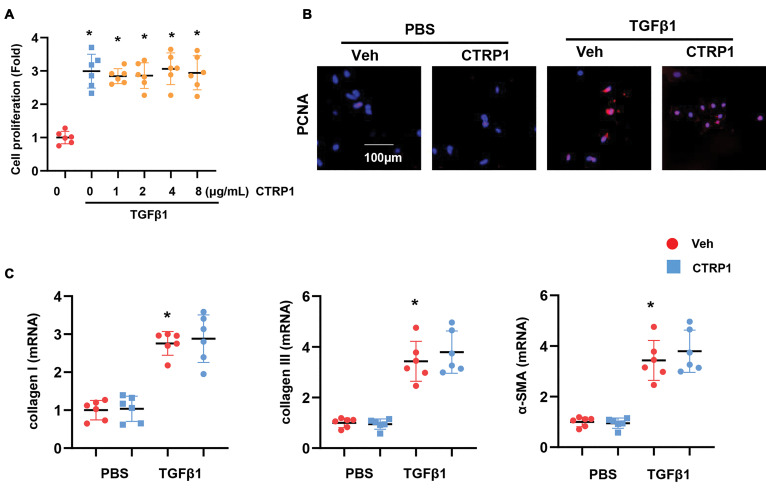
The effect of CTRP1 on fibroblasts
We hypothesized that CTRP1 regulates cardiac fibrosis by affecting fibroblast function. We isolated cardiac fibroblasts from adult mice and stimulated them with TGFβ1. These fibroblasts were treated with different concentrations of CTRP1. However, the CCK-8 assay showed no significant difference in fibroblast proliferation between the CTRP1 and TGFβ1 groups (Fig.3A). We then selected a higher dose of CTRP1 to treat the fibroblasts. Staining results for proliferating cell nuclear antigen (PCNA), a proliferation marker, showed no significant difference between fibroblasts treated with CTRP1+TGFβ1 and fibroblasts treated with TGFβ1 alone (Fig.3B). Fibroblasts were also stained with α-SMA to detect fibroblast activation levels. Consistently, the expression of α-SMA was not significantly different between the CTRP1+TGFβ1 and TGFβ1 groups (Fig.S1A, See Supplementary Online Information at www.celljournal.org). The expression levels of fibrosis markers, such as collagen I, collagen III, and α-SMA, detected by using RT-PCR, were not different in the CTRP1+TGFβ1 and TGFβ1 groups (Fig.3C). These data suggest that other cell types may account for the regulatory effect of recombinant CTRP1 on cardiac fibrosis.
Fig 3.
The effect of CTRP1 on fibroblasts. A. Cell proliferation was detected by using the CCK-8 assay for fibroblasts treated with recombinant CTRP1 (0, 1, 2, 4, 8 μg/mL) and TGFβ1. B. PCNA staining in fibroblasts treated with recombinant CTRP1 (8 μg/mL) and TGFβ1. C. Transcription level of fibrosis markers in fibroblasts (n=6). PBS; Phosphate balanced normal saline, TGFβ1; Transforming growth factor-β1, *; P<0.05 vs. PBS-Veh group, # ; P<0.05 vs. TGFβ1-Veh group, and Veh; Vehicle.
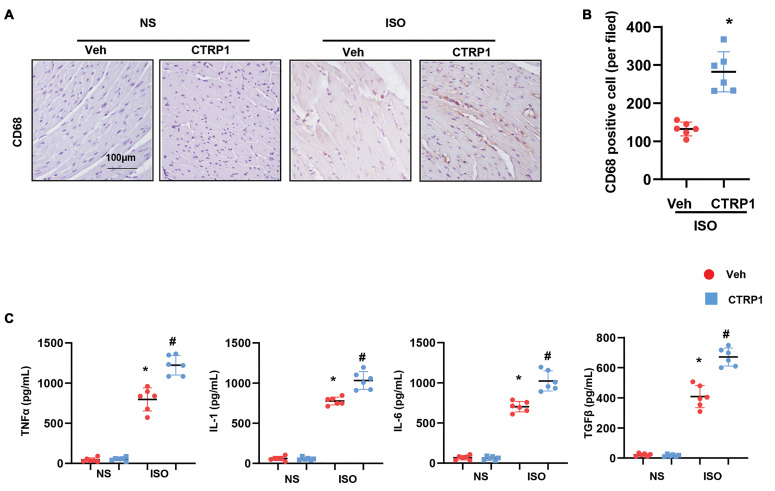
Recombinant CTRP1 increases macrophage-mediated inflammation in vivo
As immune cells, especially macrophages, play an essential role in cardiac fibrosis, we detected the effect of CTRP1 on macrophage infiltration and the inflammatory response in mice. Mouse hearts were stained with CD68 to label the cardiac macrophages. As shown in Figures 4A and B, the number of CD68-labeled macrophages increased remarkably in CTRP1-treated mice compared to that in the vehicletreated mice. The release of inflammatory cytokines, such as TNFα, IL-1, IL-6, and TGFβ1, was sharply increased in ISO-injected mice. A similar significant increase was seen in CTRP1-injected mice (Fig.4C). Thus, CTRP1 may regulate cardiac fibrosis by targeting cardiac macrophages.
Fig 4.
Recombinant CTRP1 increases macrophage-mediated inflammation in vivo. A. Representative image of CD68 staining, B. Quantification result of CD68-positive cell number (n=6). C. The release of pro-inflammatory cytokines in mouse hearts detected by using ELISA (n=6). ISO; Isoprenaline, *; P<0.05 vs. NS-Veh group, # ; P<0.05 vs. ISO-Veh group, NS; Normal saline, and Veh; Vehicle.
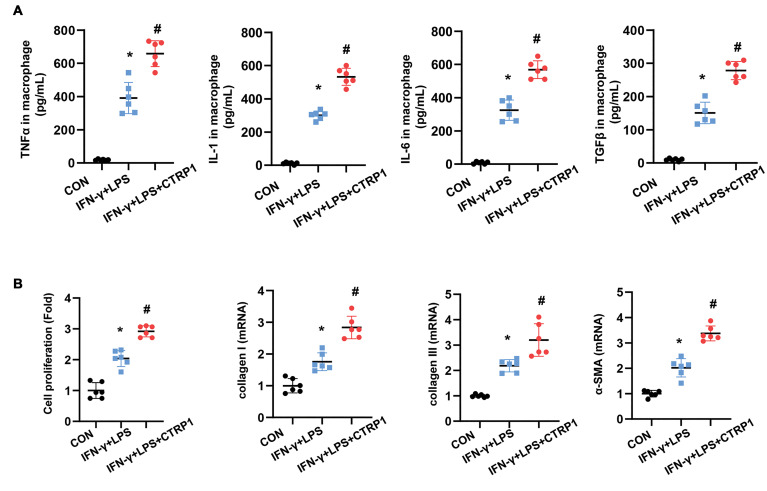
Recombinant CTRP1 enhances macrophage proinflammatory response and fibroblast activation
We evaluated the effects of CTRP1 on macrophages. CTRP1 treatment (8 μg/mL) of macrophages induced a remarkable increase in the release of inflammatory cytokines, such as TNFα, IL-1, IL-6, and TGFβ1 (Fig.5A). We co-cultured activated and CTRP1-treated macrophages with fibroblasts in a transwell dish and found that activated macrophages increased the proliferation and activation of fibroblasts. Similarly, CTRP1-treated macrophages increased fibroblast proliferation and activation, as evidenced by the results of the CCK-8 assay and the increased expression of collagen I, collagen III, and α-SMA (Fig.5B, C, Fig.S1B, See Supplementary Online Information at www.celljournal.org).
Fig 5.
Recombinant CTRP1 enhances macrophage pro-inflammatory response and increases fibroblasts activation. A. The release of pro-inflammatory cytokines in macrophages activated by IFN-γ+LPS and treated with recombinant CTRP1 (8 μg/mL) (n=6), B. Fibroblasts were co-cultured with activated or CTRP1-treated macrophages. Fibroblast proliferation detected by using the CCK-8 assay (n=6). C. Transcription level of fibrosis markers in fibroblasts (n=6). TNF-α; Tumor necrosis factor-α, CON; Control, IFN-γ; Interferonγ, LPS; Lipopolysaccharide, *; P<0.05 vs. CON group, and # ; P<0.05 vs. IFN-γ+LPS group.
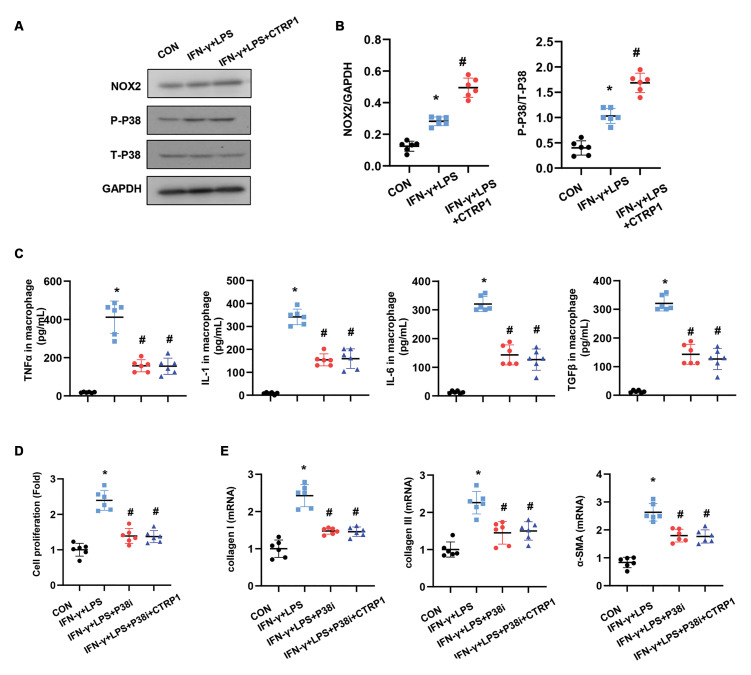
NOX2/p38 mediates the effect of CTRP1 on macrophages
Next, we explored the mechanism by which CTRP1 affects macrophage function. We found that NADPH oxidase 2 (NOX2) expression increased in activated macrophages, with CTRP1 driving the enhancement of this expression. Downstream molecular p38 activation was also increased in CTRP1-treated macrophages (Fig.6A, B). We then used SB203580 to inhibit p38 in the activated macrophages, which caused a reduction in the release of TNFα, IL-1, IL-6, and TGFβ1. CTRP1 treatment did not induce the release of these pro-inflammatory cytokines in p38-inhibited macrophages (Fig.6C). We also co-cultured these p38-inhibited macrophages with fibroblasts. As shown in Figure 6D, fibroblast proliferation and activation were observed in the p38 inhibition condition, whereas CTRP1-treated macrophages did not enhance fibroblast proliferation and activation under the p38 inhibition condition. Collectively, CTRP1 promotes cardiac fibrosis by regulating the NOX2/p38 pathway in macrophages.
Fig 6.
NOX2/p38 mediates the effect of CTRP1 on macrophages. A, B. Protein expression of NOX2, p-p38, and total p38 in activated or CTRP1-treated macrophages (n=6). C. Macrophages were activated by IFN-γ+LPS and treated with recombinant CTRP1 (8 μg/mL) and/or SB203580 (10 Μm). The release of pro-inflammatory cytokines in macrophages was detected using ELISA (n=6). D, E. Fibroblasts were co-cultured with activated CTRP1- and/ or SB203580-treated macrophages. D. Fibroblast proliferation detected by using the CCK-8 assay (n=6). E. Transcription of fibrosis markers in fibroblasts (n=6). TNF-α; Tumor necrosis factor-α, CON; Control, IFN-γ; Interferonγ, LPS; Lipopolysaccharide, *; P<0.05 vs. CON group, and # ; P<0.05 vs. IFN-γ+LPS group.
Discussion
CTRP1, a hormone that controls energy metabolism (8) has been reported to protect against cardiovascular diseases (9-12). However, we revealed the deleterious role of CTRP1 in cardiac fibrosis induced by ISO insult both in vivo and in vitro. We found that both recombinations of CTRP12 could aggravate ISO-induced cardiac fibrosis and dysfunction by exacerbating ISO-induced cardiac inflammation and macrophage dysfunction. These proinflammatory effects of CTRP1 lead to the proliferation and activation of myofibroblasts, resulting in aggravated fibrosis.
CTRP is a family of proteins similar to adiponectin, which plays an important role in immunity, development, and metabolism (8). As one of the members of CTRPs, CTRP1 has been reported to be associated with many diseases, including obesity, diabetes, renal fibrosis, and liver disease (8, 14-16). Recently, studies have found a close negative relationship between CTRP1 and cardiovascular diseases, such as coronary artery disease, hypertension, congestive heart failure, and atherosclerosis (11, 17, 18). CTRP1 recruits ERK1/2 and Jak-2 for aldosterone release and increases IL-6 release, thus acting in the pathogenesis of congestive heart failure (18). CTRP1 can increase the expression of adhesion molecules in endothelial cells and monocytes and aggravate atherosclerosis (17). However, some studies contradict these conclusions. CTRP1 has been reported to protect against sepsis-induced cardiomyopathy in mice (9) and attenuate doxorubicininduced cardiac injury in mice (19). Additionally, CTRP1 attenuates angiotensin II-induced cardiac hypertrophy in a mouse model (20). These contradictory results raise the question of whether human CTRP1 has a different function from that of murine CTRP1. In this study, we used recombinant full-length CTRP1 and found that it exacerbates ISO-induced cardiac fibrosis and dysfunction in mice.
Another important question is whether CTRP1 directly affects fibroblasts. While previous studies found that CTRP1 could directly affect cardiomyocytes (19, 20) and protect against renal fibrosis (21), none of these studies explored the direct effect of CTRP1 on fibroblasts. In our study, we stimulated fibroblasts with TGFβ1, the most pro-fibrotic factor. However, we did not observe any proor anti-fibrotic effects of CTRP1 on fibroblasts. Thus, other cell types may be the targets of CTRP1.
Macrophages are the source of pro-inflammatory and pro-fibrotic cytokines such as TGFβ1, IL-1, and IL-6. TGF-β and IL-6 induce fibroblast activation and produce collagen I, collagen III, and fibronectin. IL-1 induces increased synthesis and production of metalloproteinases (22). It was found that recombinant CTRP1 increased macrophage infiltration in atherosclerotic plaques and enhanced inflammatory responses in endothelial cells (17). Consistent with these results, we found that recombinant CTRP1 protein increased macrophage infiltration in mouse hearts and led to macrophage M1- like activation, which subsequently induced fibroblast activation and proliferation. NOX is an important source of reactive oxygen species (ROS), and activated macrophages produce considerable amounts of ROS (23, 24). This respiratory burst in macrophages results in the activation of specific signaling pathways, such as MAPKs, leading to the expression of pro-inflammatory mediators (24). In our study, we found that recombinant CTRP1 increased the expression of NOX2 in macrophages and enhanced the activation of p38. When we inhibited p38 in macrophages, the pro-inflammatory effect of recombinant CTRP1 was counteracted. The effect of CTRP1-treated macrophages on fibroblasts was also counteracted by p38 inhibition. These results suggest that CTRP1 promotes cardiac fibrosis by regulating cardiac macrophages.
Conclusion
Recombinant CTRP1 can promote ISO-induced cardiac fibrosis and dysfunction. Recombinant CTRP1 exerts these effects by directly acting on macrophages via the NOX2/p38 pathways. Thus, targeting CTRP1 expression during cardiac fibrosis may be a new treatment strategy
Supplementary PDF
Acknowledgements
This work was funded by The Foundation of National Natural Science Foundation of China (81560409). There is no conflict of interest in this study.
Authors’ Contributions
C.L., H.H.; Participated in study design, data collection and evaluation, drafting and statistical analysis. C.L., Sh.Y., X.W.; Conducted molecular experiments and RT-qPCR. T.Zh., Q.Zh., Y.Zh.; Conducted animal experiments and extensively in interpretation of the data and the conclusion. Y.L., R.Zh., H.H.; Wrote and critically revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Spoladore R, Falasconi G, Fiore G, Di Maio S, Preda A, Slavich M, et al. Cardiac fibrosis: emerging agents in preclinical and clinical development. Expert Opin Investig Drugs. 2021;30(2):153–166. doi: 10.1080/13543784.2021.1868432. [DOI] [PubMed] [Google Scholar]
- 2.Reichardt IM, Robeson KZ, Regnier M, Davis J. Controlling cardiac fibrosis through fibroblast state space modulation. Cell Signal. 2021;79:109888–109888. doi: 10.1016/j.cellsig.2020.109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaidi Y, Aguilar EG, Troncoso M, Ilatovskaya DV, DeLeon-Pennell KY. Immune regulation of cardiac fibrosis post myocardial infarction. Cell Signal. 2021;77:109837–109837. doi: 10.1016/j.cellsig.2020.109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park S, Nguyen NB, Pezhouman A, Ardehali R. Cardiac fibrosis: potential therapeutic targets. Transl Res. 2019;209:121–137. doi: 10.1016/j.trsl.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara H, Takeda N, Komuro I. Pathophysiology and therapeutic potential of cardiac fibrosis. Inflamm Regen. 2017;37:13–13. doi: 10.1186/s41232-017-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baci D, Bosi A, Parisi L, Buono G, Mortara L, Ambrosio G, et al. Innate immunity effector cells as inflammatory drivers of cardiac fibrosis. Int J Mol Sci. 2020;21(19):7165–7165. doi: 10.3390/ijms21197165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Bai B, Ban B, Liu Z, Zhang MM, Tan BK, Chen J. Circulating C1q complement/TNF-related protein (CTRP) 1, CTRP9, CTRP12 and CTRP13 concentrations in Type 2 diabetes mellitus: in vivo regulation by glucose. PLoS One. 2017;12(2):e0172271–e0172271. doi: 10.1371/journal.pone.0172271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang W, Li W, Hu X, Hu R, Li B, Lan L. CTRP1 prevents sepsisinduced cardiomyopathy via Sirt1-dependent pathways. Free Radic Biol Med. 2020;152:810–820. doi: 10.1016/j.freeradbiomed.2020.01.178. [DOI] [PubMed] [Google Scholar]
- 10.Yagmur E, Buergerhausen D, Koek GH, Weiskirchen R, Trautwein C, Koch A, et al. Elevated CTRP1 plasma concentration is associated with sepsis and pre-existing type 2 diabetes mellitus in critically Ill patients. J Clin Med. 2019;8(5):661–661. doi: 10.3390/jcm8050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Z, Tian S, Liang W. Circulating CTRP1 levels are increased and associated with the STOD in essential hypertension in Chinese patients. Cardiovasc Ther. 2019;2019:4183781–4183781. doi: 10.1155/2019/4183781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen L, Wang S, Ling Y, Liang W. Association of C1q/TNF-related protein-1 (CTRP1) serum levels with coronary artery disease. J Int Med Res. 2019;47(6):2571–2579. doi: 10.1177/0300060519847372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muendlein A, Leiherer A, Saely C, Ebner J, Geiger K, Brandtner EM, et al. The novel adipokine ctrp1 is significantly associated with the incidence of major adverse cardiovascular events. Atherosclerosis. 2019;286:1–6. doi: 10.1016/j.atherosclerosis.2019.04.222. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez S, Lei X, Petersen PS, Tan SY, Little HC, Wong GW. Loss of CTRP1 disrupts glucose and lipid homeostasis. Am J Physiol Endocrinol Metab. 2016;311(4):E678–E697. doi: 10.1152/ajpendo.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Kim JD, Lee S, Jeong AL, Park JS, Yong HJ, et al. Circulating CTRP1 levels in type 2 diabetes and their association with FGF21. Int J Endocrinol. 2016;2016:5479627–5479627. doi: 10.1155/2016/5479627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei H, Wu D, Wang JY, Li L, Zhang CL, Feng H, et al. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res Cardiol. 2015;110(4):35–35. doi: 10.1007/s00395-015-0492-7. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Zhang RY, Wang XQ, Liu ZH, Shen Y, Ding FH, et al. C1q/ TNF-related protein-1: an adipokine marking and promoting atherosclerosis. Eur Heart J. 2016;37(22):1762–1771. doi: 10.1093/eurheartj/ehv649. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Liu S, Zhang RY, Luo H, Chen L, He WF, et al. Association between C1q/TNF-related protein-1 levels in human plasma and epicardial adipose tissues and congestive heart failure. Cell Physiol Biochem. 2017;42(5):2130–2143. doi: 10.1159/000479915. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Gao L, Huang Z, Liu Y, Guo S, Xing J, et al. C1qTNFrelated protein 1 attenuates doxorubicin-induced cardiac injury via activation of AKT. Life Sci. 2018;207:492–498. doi: 10.1016/j.lfs.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Gao L, Zhang D, Yao R, Huang Z, Du B, et al. C1QTNF1 attenuates angiotensin II-induced cardiac hypertrophy via activation of the AMPKa pathway. Free Radic Biol Med. 2018;121:215–230. doi: 10.1016/j.freeradbiomed.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Cheng F, Songyang YY, Wei J, Ruan Y. CTRP1 attenuates UUO-induced renal fibrosis via AMPK/NOX4 pathway in mice. Curr Med Sci. 2020;40(1):48–54. doi: 10.1007/s11596-020-2145-9. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Chang L, Du Q, Huang Y, Zhang X, Wu X, et al. Arctigenin induces an activation response in porcine alveolar macrophage through TLR6-NOX2-MAPKs signaling pathway. Front Pharmacol. 2018;9:475–475. doi: 10.3389/fphar.2018.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleniewska P, Piechota A, Skibska B, Gorąca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 2012;60(4):277–294. doi: 10.1007/s00005-012-0176-z. [DOI] [PubMed] [Google Scholar]
- 24.Youn GS, Lee KW, Choi SY, Park J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPKNF- kappaB/AP-1 signaling pathways in macrophages. Free Radic Biol Med. 2016;97:14–23. doi: 10.1016/j.freeradbiomed.2016.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.