Abstract
COVID-19 primarily affects the respiratory system. We aimed to evaluate how pulmonary outcomes develop after COVID-19 by assessing participants from the first pandemic wave prospectively 3 and 12 months following hospital discharge.
Pulmonary outcomes included self-reported dyspnoea assessed with the modified Medical Research Council dyspnoea scale, 6-min walk distance (6MWD), spirometry, diffusing capacity of the lung for carbon monoxide (DLCO), body plethysmography and chest computed tomography (CT). Chest CT was repeated at 12 months in participants with pathological findings at 3 months. The World Health Organization (WHO) ordinal scale for clinical improvement defined disease severity in the acute phase.
Of 262 included COVID-19 patients, 245 (94%) and 222 (90%) participants attended the 3- and 12-month follow-up, respectively. Self-reported dyspnoea and 6MWD remained unchanged between the two time points, while DLCO and total lung capacity improved (0.28 mmol·min−1·kPa−1, 95% CI 0.12–0.44, and 0.13 L, 95% CI 0.02–0.24, respectively). The prevalence of fibrotic-like findings on chest CT at 3 and 12 months in those with follow-up chest CT was unaltered. Those with more severe disease had worse dyspnoea, DLCO and total lung capacity values than those with mild disease.
There was an overall positive development of pulmonary outcomes from 3 to 12 months after hospital discharge. The discrepancy between the unaltered prevalence of self-reported dyspnoea and the improvement in pulmonary function underscores the complexity of dyspnoea as a prominent factor of long-COVID. The lack of increase in fibrotic-like findings from 3 to 12 months suggests that SARS-CoV-2 does not induce a progressive fibrotic process in the lungs.
Short abstract
Even though an overall positive development of lung function is observed between 3 and 12 months after hospitalisation for COVID-19, the prevalence of dyspnoea and fibrotic CT findings remains unaltered, independent of disease severity in the acute phase https://bit.ly/3gArGNh
Introduction
The COVID-19 pandemic quickly became a challenge for the global healthcare system. The first variants of the SARS-CoV-2 virus had a predilection for respiratory epithelium, and the high prevalence of respiratory failure in these patients has drawn attention to the trajectory of pulmonary recovery and sequelae in survivors of COVID-19.
Evidence emerging after viral epidemics like severe acute respiratory syndrome (SARS) and Middle-East respiratory syndrome (MERS) showed that a restrictive respiratory pattern and reduced diffusing capacity of the lung for carbon monoxide (DLCO) were prevalent beyond 6 months after the acute infection [1]. Early reports during the COVID-19 pandemic also reported impairment of DLCO up to 12–16 weeks after hospital admission, with persistent radiological findings of ground-glass opacities (GGO) and parenchymal bands as possible early signs of pulmonary fibrosis on chest computed tomography (CT) [2–4]. Recent Chinese follow-up studies on COVID-19 have reported that DLCO is the most commonly impaired lung function parameter at 12 months after discharge in hospitalised patients [5, 6].
Pulmonary fibrosis is considered the end-stage feature of several diseases of the lung. The exact pathophysiological mechanism for development of progressive pulmonary fibrosis is not clear, but a dysregulated relationship between micro-injuries and remodelling processes is thought to be of importance [7]. After the SARS and MERS epidemics, persistent radiological changes in lung parenchyma were observed [1, 8]. A report on COVID-19 survivors also describes radiological features typical of pulmonary fibrosis persisting for as long as 1 year after the initial infection [9]. Given that COVID-19 is a new infectious disease, little is known about the development of such findings over time. Specifically, whether the pulmonary pathology persists as permanent but stable findings, or whether SARS-CoV-2 may initiate a progressive fibrotic pulmonary disease, is not established.
The main objective of this prospective cohort study was to investigate changes in pulmonary outcomes between 3 and 12 months after hospitalisation for COVID-19, defined by self-reported dyspnoea, 6-min walk distance test (6MWD), pulmonary function tests (spirometry, DLCO, body plethysmography) and chest CT. Secondly, we aimed to assess how disease severity during the acute phase was associated with pulmonary outcomes during the first year after discharge. Finally, we aimed to study the association between self-reported dyspnoea, DLCO and persistent findings on chest CT 12 months after hospital discharge.
Material and methods
Study design and setting
Patient-reported outcomes and lung function after hospital admission for COVID-19 (PROLUN) is a multicentre, prospective cohort study performed at six Norwegian hospitals during the first pandemic wave. The regional ethics committee for South-Eastern Norway approved the study (no. 125384), along with data protection officers at each participating centre. The study is registered with ClinicalTrials.gov (NCT 04535154).
Participants
A detailed description of the inclusion process has previously been reported [3]. Patients discharged from participating hospitals prior to 1 June 2020 were considered for eligibility. All participants gave informed consent prior to inclusion. Exclusion criteria were inability to give informed consent, age <18 years, living outside the hospitals’ catchment areas and participation in the World Health Organization (WHO) Solidarity trial [10].
Data collection
Participants were invited to outpatient follow-up visits at two time points: 3 and 12 months after hospital discharge for COVID-19. The follow-up visits consisted of self-reported dyspnoea, 6MWD, spirometry, DLCO, body plethysmography, blood sampling and chest CT. Only participants with chest CT findings consistent with COVID-19 sequelae at the 3-month visit repeated the scan at the 12-month visit.
Self-reported dyspnoea
The modified Medical Research Council (mMRC) dyspnoea scale was used to assess dyspnoea [11, 12]. The scale ranges from 0 (no dyspnoea) to 4 (severe dyspnoea). Dyspnoea was defined as mMRC ≥2.
Pulmonary function tests
6MWD, spirometry and DLCO were performed as previously described [3], with a Jaeger MS-PFT Analyzer Unit (Höechberg, Germany or CareFusion type MasterScreen PFT, Yorba Linda, CA, USA; software SentrySuite V03.0.5; Vyaire Medical, Höechberg, Germany), and according to the American Thoracic Society's and European Respiratory Society's guidelines, as was body plethysmography [13–15]. Reference values and lower limit of normal (LLN) for 6MWD were calculated using Enright's equations for healthy adults [16]. The reference values of the Global Lung Function Initiative (GLI) network were used to calculate the percentage of predicted and LLN for dynamic and static lung volumes [17, 18].
Chest CT
The chest CT protocol and the image analyses have been described in previous reports [3, 19, 20]. Any presence of parenchymal bands, consolidations, interlobular septal thickening and reticular pattern was grouped together and defined as fibrotic-like findings [4]. For the current study, GGO and fibrotic-like findings were considered important. The 3- and 12-month scans were evaluated simultaneously by experienced chest radiologists in consensus, blinded for the participants’ clinical history.
Clinical variables
Electronic patient records of the participants provided baseline clinical characteristics (sex, age, ethnicity, height, weight, Charlson comorbidity index and history of smoking) [21]. The records from the hospital admissions were also used to classify the participants into three COVID-19 severity groups according to the WHO Ordinal Scale for Clinical Improvement (WHO 8-point scale): mild disease (hospitalised, no oxygen therapy), moderate disease (need for supplemental oxygen by mask or nasal prongs) and severe disease (need for noninvasive ventilation support, mechanical ventilation and/or additional organ support) [22].
Outcomes
The main aim of the study was to observe the changes in pulmonary outcomes at 3 and 12 months after hospital discharge for COVID-19. Pulmonary outcomes were mMRC score ≥2, 6MWD, forced vital capacity (FVC), forced expiratory volume in 1 s, DLCO, total lung capacity (TLC), any GGO, GGO ≥10% and any fibrotic-like findings on chest CT.
Statistical methods
For continuous data, we report mean±sd. Variables from the two time points were compared for those with data on both occasions, using paired t-test, Wilcoxon signed-rank test or McNemar's test, as appropriate.
The use of mixed models enabled us to use all available data at the two time points. The odds of reporting a higher mMRC score (categorised as 0, 1, ≥2) at 12 months compared to 3 months were determined with ordinal mixed logistic models, with random intercept for patient and other variables as fixed effect. Results are presented with odds ratio (OR) and 95% confidence interval (CI). In accordance with the statistical analysis plan and the final sample size, we limited the covariates in the ordinal mixed model to adjustment for disease severity [22], sex and age. Brant's test was deemed acceptable for checking the proportional odds assumption of mMRC scores 0, 1 and ≥2.
Changes in 6MWD, FVC, DLCO and TLC (continuous variables) from 3 to 12 months were assessed using separate linear mixed models, with random intercept for patient and fixed effect of other variables. Results are presented with beta coefficients and 95% CI. In the linear multivariable analyses we allowed adjustment for additional covariates. Disease severity, sex, age, body mass index (BMI) and Charlson comorbidity index [21] were chosen prior to data analysis.
Predictors of persistent findings on chest CT after 12 months were evaluated using logistic regression analysis, adjusting for age per 1 year and disease severity during hospitalisation. The severity of DLCO impairment was defined by the American Thoracic Society/European Respiratory Society (ATS/ERS) [23]. Associations between CT findings at 12 months and level of DLCO impairment were investigated by univariate logistic regression, with the CT findings as dependent variables.
In exploratory analyses, we assessed associations between 12-month findings of any fibrotic-like findings on chest CT, self-reported dyspnoea (mMRC 0 as reference group, versus 1 and ≥2) and DLCO by logistic regression. In multivariable analyses we adjusted for age, sex and disease severity. All statistical analyses and figures were performed with Stata SE 17 (StataCorp LLC, College Station, TX, USA). A p-value <0.05 was considered statistically significant, using two-sided tests.
Results
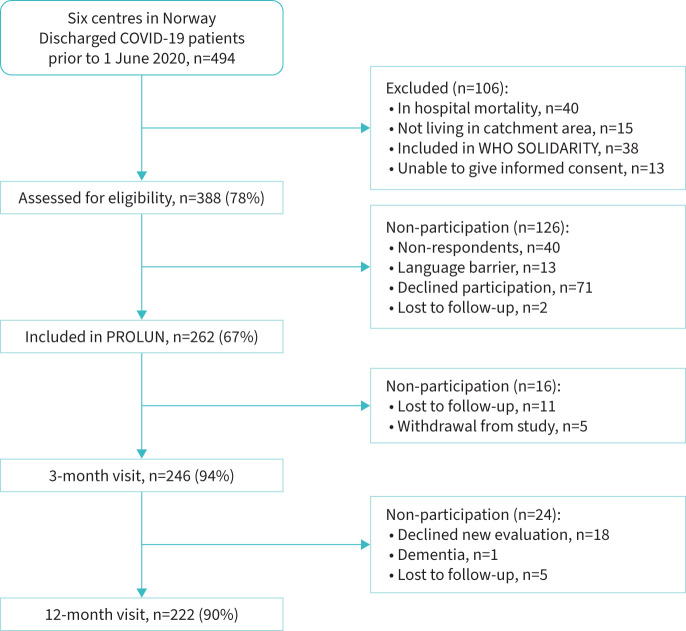
A total of 494 patients were discharged after hospitalisation for COVID-19 in this study's catchment area. Of 388 eligible patients, 262 were included (figure 1). 246 (94%) attended the 3-month visit, and 222 (90%) participants returned for the 12-month visit.
FIGURE 1.
Flow chart of participant flow.
The majority of participants were male (n=151, 58%), and the mean±sd age at discharge was 58.6±14.2 years (table 1). Most participants had mild COVID-19 disease, with 91 (35%) not needing supplemental oxygen, and 126 (48%) receiving supplemental oxygen. 45 (17%) participants had severe disease. One participant received extracorporeal membrane oxygenation therapy.
TABLE 1.
Overview of demographic and clinical characteristics of the study population during hospital admission
| Total# | WHO ordinal scale 3 ¶ | WHO ordinal scale 4+ | WHO ordinal scale 5–7 § | |||||
| n | n | n | n | |||||
| Demographics | ||||||||
| Male sex | 262 | 151 (58) | 91 | 43 (47) | 126 | 74 (59) | 45 | 34 (76) |
| Age at discharge years | 262 | 58.6±14.2 | 91 | 55.8±14.8 | 126 | 60.0±14.8 | 45 | 60.3±10.2 |
| Education, two levels | 239 | 130 (54) | 83 | 55 (66) | 115 | 56 (49) | 41 | 19 (46) |
| Body mass index kg·m−2 | 249 | 28.3±4.7 | 85 | 27.2±4.6 | 121 | 28.8±4.8 | 43 | 29.1±4.2 |
| Smoking history | ||||||||
| Never | 229 | 133 (58) | 79 | 52 (66) | 109 | 61 (56) | 41 | 20 (49) |
| Former | 229 | 90 (39) | 79 | 25 (32) | 109 | 45 (41) | 41 | 20 (49) |
| Present | 229 | 6 (3) | 79 | 2 (2) | 109 | 3 (3) | 41 | 1 (2) |
| Comorbidities | ||||||||
| Diabetes mellitus | 262 | 22 (8) | 91 | 1 (1) | 126 | 14 (11) | 45 | 7 (16) |
| Hypertension | 239 | 80 (33) | 83 | 23 (28) | 115 | 45 (39) | 41 | 12 (29) |
| COPD | 262 | 13 (5) | 91 | 5 (5) | 126 | 6 (5) | 45 | 2 (4) |
| Obesity | 249 | 78 (31) | 85 | 19 (22) | 121 | 41 (34) | 43 | 18 (42) |
| Charlson comorbidity index | ||||||||
| 0 | 262 | 186 (71) | 91 | 72 (79) | 126 | 85 (67) | 45 | 29 (64) |
| 1 | 262 | 43 (16) | 91 | 10 (11) | 126 | 24 (19) | 45 | 9 (20) |
| ≥2 | 262 | 33 (13) | 91 | 9 (10) | 126 | 17 (13) | 45 | 7 (16) |
| Hospital stay | ||||||||
| Length of stay days, median (IQR) | 260 | 6 (3–12) | 90 | 3 (1–4) | 125 | 7 (5–11) | 45 | 19 (16–28) |
| ICU stay | 262 | 51 (19) | 91 | 1 (1) | 126 | 8 (6) | 45 | 42 (93) |
| No. of days, median (IQR) | 11 (5–15) | 4 (4–4) | 2.5 (3–1) | 12 (7–16) | ||||
| Invasive mechanical ventilation | 255 | 35 (14) | 91 | 0 | 125 | 0 | 45 | 35 (78) |
| No. of days, median (IQR) | 10 (8–15) | n/a | n/a | 10 (8–15) | ||||
| ECMO | 250 | 1 (0.4) | 91 | 0 | 126 | 0 | 45 | 1 (2) |
| WHO ordinal scale for clinical improvement | ||||||||
| 3: No supplemental oxygen | 262 | 91 (35) | 91 | 91 (100) | ||||
| 4: Supplemental oxygen | 262 | 126 (48) | 126 | 126 (100) | ||||
| 5: Noninvasive ventilation or high-flow O2 | 262 | 10 (4) | 45 | 10 (22) | ||||
| 6: Invasive mechanical ventilation | 262 | 34 (13) | 45 | 34 (76) | ||||
| 7: Invasive mechanical ventilation and additional organ support | 262 | 1 (0.4) | 45 | 1 (2) | ||||
| Chest radiograph | ||||||||
| Normal | 244 | 79 (32) | 79 | 39 (49) | 121 | 37 (31) | 44 | 3 (7) |
| One-sided opacities | 244 | 43 (18) | 79 | 17 (21) | 121 | 21 (17) | 44 | 5 (11) |
| Bilateral opacities | 244 | 122 (50) | 79 | 23 (29) | 121 | 63 (52) | 44 | 36 (82) |
| Blood sampling at admission | ||||||||
| pHƒ | 229 | 7.46±0.05 | 73 | 7.46±0.05 | 116 | 7.46±0.05 | 40 | 7.46±0.07 |
| PaO2ƒ kPa/mmHg | 231 | 9.8±2.2/73.5±16.5 | 73 | 11.0±2.4/82.5±18.0 | 117 | 9.4±1.9/70.5±14.2 | 41 | 8.8±2.1/66.0±15.7 |
| PaCO2ƒ kPa/mmHg | 231 | 4.4±0.9/33.0±6.7 | 73 | 4.5±0.7/33.7±5.2 | 117 | 4.3±0.7/32.2±5.2 | 41 | 4.4±1.4/33.0±10.5 |
| Base excessƒ mmol·L−1 | 223 | 2.1±1.9 | 70 | 1.7±1.1 | 113 | 2.3±2.1 | 40 | 2.1±2.1 |
| Lactate mmol·L−1ƒ | 218 | 1.3±1.5 | 70 | 1.1±0.5 | 109 | 1.2±0.7 | 39 | 1.8±3.2 |
| Haemoglobin g·dL−1## | 261 | 13.9±1.6 | 91 | 13.9±1.6 | 126 | 14.0±1.4 | 44 | 13.7±1.9 |
| C-reactive protein mg·L−1¶¶, median (IQR) | 260 | 105 (33–190) | 90 | 31 (6–63) | 126 | 120 (59–174) | 44 | 264 (178–338) |
| Ferritin µg·L−1¶¶ | 223 | 1428±1750 | 72 | 660±1006 | 108 | 1346±1367 | 43 | 2918±2531 |
| Lymphocyte count## (×109·L−1) | 248 | 1.2±0.9 | 84 | 1.4±0.9 | 121 | 1.1±0.7 | 43 | 0.9±0.6 |
Data are presented as n (%) or mean±sd unless otherwise stated. WHO: World Health Organization; sd: standard deviation; COPD: chronic obstructive pulmonary disease; IQR: interquartile range; ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension. #: n=262; ¶: n=91; +: n=126; §: n=45; ƒ: arterial blood analysis at admission; ##: minimum levels during hospital stay; ¶¶: maximum levels during hospital stay.
For a detailed descriptive overview of the pulmonary outcomes at the two time points, see supplementary table S1.
Change in pulmonary outcomes between 3 and 12 months after hospital discharge for COVID-19
Between the 3- and 12-month follow-up visits there was no significant change in self-reported dyspnoea (table 2). 41 (21%) participants reported dyspnoea at the 3-month visit and 34 (16%) at the 12-month visit (p=0.25). Exercise capacity increased between the two time points, illustrated by an increase from mean±sd 542±123 m to 570±109 m (p=0.01) on the 6MWD.
TABLE 2.
Presentation of the pulmonary outcomes at the 3- and 12-month follow-up visits after hospital discharge for COVID-19
| 3-month visit | 12-month visit | ||||
| n | n | p-value | |||
| Self-reported dyspnoea | 204 | 211 | 0.25 | ||
| mMRC 0 | 99 (48) | 107 (51) | |||
| mMRC 1 | 64 (31) | 70 (33) | |||
| mMRC ≥2 | 41 (20) | 34 (16) | |||
| Exercise capacity | |||||
| 6MWD m | 190 | 542±123 | 174 | 570±109 | 0.01 |
| 6MWD % predicted | 185 | 99±21 | 174 | 114±33 | <0.001 |
| 6MWD <LLN | 190 | 5 (3) | 174 | 8 (5) | 0.02 |
| Spirometry | |||||
| FVC L | 244 | 3.8±1.1 | 221 | 3.9±1.1 | 0.30 |
| FVC % predicted | 244 | 95±14 | 221 | 96±14 | 0.06 |
| FVC <LLN | 244 | 21 (9) | 221 | 18 (8) | 0.81 |
| FEV1 L | 244 | 2.9±0.9 | 221 | 3.9±1.1 | 0.62 |
| FEV1 % predicted | 244 | 93±16 | 221 | 94±15 | 0.10 |
| FEV1 <LLN | 244 | 28 (11) | 221 | 17 (8) | 0.13 |
| Diffusion capacity of the lungs | |||||
| DLCO mmol min−1 kPa−1 | 242 | 7.3±2.2 | 218 | 7.6±2.1 | <0.001 |
| DLCO % predicted | 241 | 87±18 | 213 | 91±16 | <0.001 |
| DLCO <LLN | 242 | 61 (25) | 218 | 38 (17) | <0.001 |
| DLCO 60–79% predicted | 241 | 45 (19) | 216 | 24 (11) | <0.001 |
| DLCO 40–59% predicted | 15 (6) | 9 (4) | |||
| DLCO <40% predicted | 3 (1) | 0 | |||
| Body plethysmography | |||||
| TLC L | 214 | 5.9±1.5 | 177 | 6.1±1.5 | 0.03 |
| TLC % predicted | 202 | 92±15 | 166 | 94±15 | 0.02 |
| TLC <LLN | 214 | 51 (24) | 177 | 40 (23) | 0.64 |
| Chest CT | |||||
| Any GGO | 241 | 112 (46) | |||
| GGO >10% | 241 | 43 (18) | |||
| Fibrotic-like findings | 241 | 96 (40) | |||
| Chest CT at both time points | |||||
| Any GGO | 124 | 89 (72) | 124 | 62 (50) | <0.001 |
| GGO >10% | 124 | 39 (31) | 124 | 10 (8) | < 0.001 |
| Fibrotic-like findings | 124 | 81 (65) | 124 | 74 (60) | 0.16 |
Data are presented as n (%) or mean±sd unless otherwise stated. Paired t-test for continuous variables, Wilcoxon sign rank test for ordinal variables, and McNemar's test for categorical variables. mMRC: modified Medical Research Council dyspnoea scale; 6MWD: 6-min walk distance; LLN: lower limit of normal; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusion capacity of the lungs for carbon monoxide; TLC: total lung capacity; CT: computed tomography; GGO: ground-glass opacities; GGO >10%: >10% GGO in at least one lung zone; sd: standard deviation.
DLCO improved between the two time points (table 2). 3 months after discharge 61 (25%) participants had DLCO <LLN, versus 38 (17%) after 12 months (p<0.001), indicating an improvement of the diffusion capacity. TLC increased in volume between 3 and 12 months, but the number of participants with TLC<LLN did not change significantly (table 2).
Of the participants, 126 (51%) presented with pathological findings on chest CT 3 months after hospitalisation for COVID-19, with 112 (46%) having any GGO, 43 (18%) GGO>10% and 96 (40%) showing possible signs of fibrosis (supplementary table S1). The 126 participants with pathological CT findings after 3 months repeated the CT scan 12 months after hospital discharge (table 2). Between the time points, a decrease in the number of participants with any GGO was observed, from 112 to 63 (p<0.001). 43 participants had GGO >10% at 3 months, versus 10 participants at 12 months (p<0.001). No change in the presence of fibrotic-like CT findings was observed between 3 (n=96) and 12 (n=75) months (p=0.16) (table 2).
There was no significant change in reported dyspnoea between the two time points in either univariate (OR 0.76, 95% CI 0.48–1.21) or multivariable analyses adjusted for disease severity, sex and age (OR 0.76, 95% CI 0.48–1.21) (table 3). Similarly, there was no difference in dyspnoea when comparing those with moderate and severe disease to those with mild disease.
TABLE 3.
Ordinal logistic mixed model analysis of self-reported dyspnoea by the modified Medical Research Council score at follow-up visits after discharge from hospital for COVID-19
| Univariate | Multivariable# | WHO disease severity group | ||||
| Pts./obs. | 12 months versus 3 months | Pts./obs. | 12 months versus 3 months | Moderate versus mild | Severe versus mild | |
| mMRC (0, 1, ≥2) | 239/415 | 0.76 (0.48–1.21) | 239/415 | 0.76 (0.48–1.21) | 0.63 (0.27–1.48) | 0.75 (0.25–2.30) |
The impact of change from 3 to 12 months and WHO disease severity are presented with odds ratio (95% CI) for being in a higher category of dyspnoea. Pts.: number of patients; Obs.: number of observations; WHO: World Health Organization; mMRC: modified Medical Research Council dyspnoea scale. #: adjusted for disease severity, sex and age.
In linear mixed model analysis there was a significant improvement of 6MWD (19 m, 95% CI 7–13), DLCO (0.30 mmol·min−1·kPa−1, 95% CI 0.16–0.44) and TLC (0.12 L, 95% CI 0.02–0.22) between the two time points after hospital discharge in univariate analysis (table 4). When adjusting for disease severity, sex, age, BMI and comorbidity, the improvement remained significant for DLCO (0.28 mmol·min−1·kPa−1, 95% CI 0.12–0.44) and TLC (0.13 L, 95% CI 0.02–0.24). Those with moderate and severe disease during hospitalisation showed less difference in DLCO and TLC between 3 and 12 months than those with mild disease (table 4). The full multivariate analyses with coefficients for all included covariates are presented in supplementary table 2a–e.
TABLE 4.
Linear mixed model analysis of exercise capacity and pulmonary function tests at follow-up visits after discharge from hospital for COVID-19
| Univariate | Multivariable# | WHO disease severity group | ||||
| Pts./Obs. | Change from 3 months to 12 months ¶ | Pts./Obs. | Change from 3 months to 12 months ¶ | Moderate versus mild | Severe versus mild | |
| 6MWD m | 206/364 | 19 (7–31) | 158/283 | 6 (−7–19) | −31 (−63–0) | −33 (−76–10) |
| FVC L | 249/465 | 0.03 (−0.02–0.07) | 197/369 | 0.02 (−0.03–0.08) | −0.18 (−0.41–0.05) | −0.23 (−0.54–0.08) |
| DLCO mmol·min−1·kPa−1 | 246/460 | 0.30 (0.16–0.44) | 194/363 | 0.28 (0.12–0.44) | −0.57 (−1.00– −0.14) | −1.18 (−1.75–−0.61) |
| TLC L | 224/391 | 0.12 (0.02–0.22) | 175/324 | 0.13 (0.02–0.24) | −0.57 (−0.96– −0.17) | −0.84 (−1.36– −032) |
The impact of change from 3 to 12 months and WHO disease severity are presented with beta coefficients (95% CI). Each row represents a separate model with a different dependent variable. Pts.: number of patients; Obs.: number of observations; 6MWD: 6-min walk distance; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; TLC: total lung capacity. #: all models are adjusted for WHO disease severity group, sex, age, BMI and Charlson comorbidity index (0, 1, ≥2); ¶: positive values represent increasing values.
Predictors of persistent chest CT findings at 12 months
Age was the only significant predictor of finding any GGO (OR 1.04, 95% CI 1.01–1.07) and any fibrotic-like finding (OR 1.04, 95% CI 1.01–1.07) 12 months after hospital discharge for COVID-19 (table 5).
TABLE 5.
Association of World Health Organization COVID-19 disease severity and age with persistent findings on chest computed tomography 12 months after hospital discharge for COVID-19 (n=126)
| Odds ratio (95% CI) | p-value | |
| Any GGO# | ||
| Group 4 versus 3 | 0.63 (0.25–1.60) | 0.33 |
| Group 5–7 versus 3 | 1.89 (0.65–5.56) | 0.24 |
| Age | 1.04 (1.01–1.07) | 0.01** |
| GGO >10% in one lung zone# | ||
| Group 4 versus 3 | 0.38 (0.05–2.87) | 0.35 |
| Group 5–7 versus 3 | 2.97 (0.54–16.30) | 0.21 |
| Age | 1.01 (0.96–1.07) | 0.66 |
| Any fibrotic-like findings# | ||
| Group 4 versus 3 | 0.67 (0.26–1.72) | 0.41 |
| Group 5–7 versus 3 | 2.61 (0.82–8.35) | 0.10 |
| Age | 1.04 (1.01–1.07) | 0.05* |
Multivariable logistic regression. GGO: ground-glass opacities. #: dependent variable. *: p<0.05; **: p<0.01.
Association of self-reported dyspnoea, DLCO and fibrotic-like findings on chest CT
In univariate logistic regression, there was an association between any fibrotic-like findings on chest CT and DLCO 12 months after hospital discharge for COVID-19 (OR 0.80, 95% CI 0.67–0.95). This was true both for DLCO <LLN and the severity of impairment of DLCO. When adjusting for age, sex and disease severity, the association was no longer statistically significant (table 6).
TABLE 6.
Associations between any fibrotic-like findings on chest CT, patient-reported dyspnoea and diffusing capacity of the lung by logistic regression (n=115)
| Univariate | Multivariable | |||
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Any fibrotic-like finding on chest CT# | ||||
| mMRC 1 versus 0 | 0.62 (0.27–1.44) | 0.26 | 0.70 (0.28–1.75) | 0.45 |
| mMRC ≥2 versus 0 | 0.83 (0.29–2.35) | 0.72 | 0.74 (0.24–2.25) | 0.60 |
| DLCO mmol·min−1·kPa−1 | 0.80 (0.67–0.95) | 0.01 | 0.79 (0.58–1.06) | 0.12 |
Multivariable logistic regression analysis adjusted for age, sex and disease severity. CI: confidence interval; CT: computed tomography; mMRC: modified Medical Research Council dyspnoea scale; DLCO: diffusing capacity of the lung for carbon monoxide. #: dependent variable in logistic regression.
Discussion
In this prospective cohort study of COVID-19 survivors from the first wave of the COVID-19 pandemic in Norway, we investigated pulmonary outcomes 3 and 12 months following hospital discharge. The study population was non-vaccinated, had not previously been exposed to SARS-CoV-2, and were admitted to hospital before international guidelines for treatment of COVID-19 existed. No significant improvement of self-reported dyspnoea was observed, while there was an improvement in DLCO and TLC from 3 to 12 months after discharge. Participants with severe COVID-19 had worse outcomes in DLCO and FVC than those with mild disease, but there was no difference in dyspnoea or 6MWD. The amount of GGO on chest CT decreased during the study period. However, fibrotic-like findings on chest CT were still common and were not significantly altered during the first year after COVID-19.
Approximately one-sixth of the participants reported dyspnoea at both 3- and 12 months after hospital discharge for COVID-19. Interestingly, there was no decrease in reported dyspnoea between the two time points, despite an improvement observed in some of the other pulmonary outcomes like DLCO and TLC, along with a reduction in the amount of GGO on chest CT. Unaltered findings of fibrotic-like nature on chest CT were more in accordance with the finding of a stable level of self-reported dyspnoea, as was the minor improvement in walking distance, which was not significant in multivariable analysis and was not within what is considered to be of clinical relevance [24]. However, dyspnoea was not statistically associated with persistent fibrotic-like findings on chest CT in our cohort. When evaluating the change in dyspnoea across disease severity groups, no difference between those with moderate or severe disease and those with mild disease was observed. Dyspnoea could also be a symptom of cardiac dysfunction. However, this was not found in a recent study presenting echocardiographic findings from our cohort [25].
Recent reports point to hyperventilation, persistent deconditioning and dysfunctional breathing as explanatory factors for the reported discomfort related to breathing following COVID-19 [26–28]. Even though an exact definition and characterisation of the long-COVID syndrome is still lacking, dyspnoea is one of the most common complaints in the aftermath of the acute infection [6, 29]. Summarised, our findings indicate that dyspnoea in the setting of long-COVID is multifactorial and not solely related to changes in pulmonary function or the severity of the acute disease.
Several studies have emphasised diffusion capacity as the primarily affected pulmonary function parameter after COVID-19, along with evidence of a restrictive respiratory pattern, as illustrated by decreased TLC [2–4]. In the present study, there was a significant improvement of DLCO from 3 to 12 months, both in absolute values and the proportion of participants with values <LLN, suggesting spontaneous recovery over time. An increase in diffusion capacity over time has also been described in studies from prior viral epidemics and recent COVID-19 follow-up studies [5, 6, 30, 31]. Improvement of DLCO over time in our cohort could be explained by an attenuation of the initial inflammation caused by the virus. This could correspond to the observed reduction of GGO on chest CT. In contrast to GGO, there was a stable prevalence of fibrotic-like findings on chest CT, making these radiological findings unlikely to have a major impact on diffusion capacity. Accordingly, we did not find a significant association between fibrotic-like findings and DLCO.
Similar results were observed for TLC. Decreased TLC is found in patients with a restrictive ventilatory pattern, as seen in patients with pulmonary fibrosis or thoracic muscle weakness [32]. The stable level of fibrotic-like CT findings in our cohort indicates that the improvement in TLC during the first year after COVID-19 could be explained by improved general health status and muscle strength, rather than pulmonary fibrosis on chest CT.
In contrast to what was observed for dyspnoea, there was an association between disease severity in the acute phase and both diffusion capacity and static lung volumes during the observation period. DLCO and TLC were lower for those with severe disease than those with mild disease.
Our cohort consists of patients from the first pandemic wave in Norway (March 2020 to June 2020). At that time, patient treatment was largely handled on a compassionate care basis and varied among the participating centres. In our study sample, there was no routine use of glucocorticoids and repurposed anti-viral drugs, but 12 participants received glucocorticoids during the hospital stay. The doses varied considerably, and the number of patients was too low to perform adequate statistical analyses regarding the effect on pulmonary outcomes. Dexamethasone, later implemented as standard of care for COVID-19 patients with respiratory failure to attenuate the host's inflammatory response to the virus, was not routinely administered to these patients at that time. Whether glucocorticoids augment the improvement in diffusion capacity in this patient group is currently not known.
Half of the participants who repeated the chest CT 1 year after discharge for COVID-19 still had findings of GGO in the lung parenchyma. GGO has been described as a common and reversible finding after lung infection [33]. Whether these findings represent reversible changes or are a sign of discrete pulmonary fibrosis of a more permanent character remains to be clarified. ∼60% of the participants who repeated the chest CT still had findings of fibrotic nature. However, the lack of association between dyspnoea, DLCO, TLC and persistent fibrotic-like findings on chest CT indicates that the persistent fibrotic-like findings were not prominent enough to hamper the improvement in these variables in our cohort. Contrary to what we observed for DLCO and TLC, we did not find that disease severity was a predictor for persistent fibrotic-like findings on chest CT 12 months after hospitalisation for COVID-19. The only significant predictor of persistent findings was older age. In addition, persistent fibrotic-like findings on chest CT were not associated with dyspnoea or reduced diffusion capacity 1 year after hospital admission for COVID-19.
Others have also found that fibrotic-like findings on chest CT persist up to 1 year after COVID-19 [9]. Thus, the stable proportion of fibrotic-like findings on chest CT in our cohort is promising and indicates that infection with SARS-CoV-2 does not induce a progressive pulmonary fibrosis. To confirm this assumption, a longer observation period of patients with radiological signs of interstitial fibrotic abnormalities after COVID-19 is needed. However, our results are in accordance with a recent Chinese report on the pulmonary consequences of SARS in a 15-year follow-up study, which reports that the primary amelioration of chest CT findings occurred during the first year following infection and remained stable in the following years [30].
Strengths and limitations
Non-participation is common in clinical follow-up studies and may influence the validity of the results. This is true regarding this report as well. However, the prospective cohort design, low rate of participants lost to follow-up and multicentre design are strengths of the current study. Participants were recruited from geographically different parts of Norway, making the study population representative of hospitalised COVID-19 patients in this country. There was no control group in this cohort study, and we did not have data on pulmonary function or chest CT of our participants prior to the SARS-CoV-2 infection. This study is not suited to evaluate various treatment modalities, including the use of glucocorticoids, which was later implemented as a standard of care for severe COVID-19. As the cohort consists of patients from the first pandemic wave of COVID-19, this report provides data on non-vaccinated COVID-19 patients who did not receive treatment guided by international guidelines. This can be seen as a strength of the study, providing future reference on pulmonary outcomes for the later SARS-CoV-2 variants.
Conclusion
In this prospective cohort study on COVID-19 patients discharged from hospital, there was an overall positive development of pulmonary outcomes between 3 and 12 months after discharge. Participants with severe disease had worse dyspnoea, DLCO and TLC values than those with mild disease. Persistent findings on chest CT were common 1 year after hospital discharge, but the lack of increase in fibrotic-like findings indicates that SARS-CoV-2 does not induce a progressive fibrotic process in the lungs. The discrepancy between the high and unaltered prevalence of self-reported dyspnoea and the improvement in pulmonary function tests underscores the complexity of dyspnoea as a prominent factor of long-COVID.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TABLE S1 00575-2022.tableS1 (83.7KB, pdf)
TABLE S2 00575-2022.tableS2 (83.3KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Authors' contributions: G. Einvik conceived the study. The protocol was designed by T.M. Aaløkken, E. Brønstad, M.T. Durheim, K. Stavem, O.H. Skjønsberg, H. Ashraf and G. Einvik in collaboration. Patient inclusion and data collection were performed by T.V. Lerum, E. Brønstad, M.T. Durheim, B.B. Aarli, K.M.A. Lund, K. Stavem, O.H. Skjønsberg and G. Einvik. T.M. Aaløkken, J.R. Rodriguez, C. Meltzer and H. Ashraf interpreted and analysed the CT findings. Statistical analyses were performed and analysed by T.V. Lerum and K. Stavem. The first draft of the manuscript was written by T.V. Lerum. All authors contributed with considerable critical review of the manuscript and approval of the final version.
Conflict of interest: T.V. Lerum has nothing to disclose.
Conflict of interest: C. Meltzer has nothing to disclose.
Conflict of interest: J.R. Rodriguez has nothing to disclose.
Conflict of interest: T.M. Aaløkken has nothing to disclose.
Conflict of interest: E. Brønstad has nothing to disclose.
Conflict of interest: B.B. Aarli reports personal fees for lectures and advisory board work from AstraZeneca, personal fees for lectures from GlaxoSmithKline, Novartis, Boehringer Ingelheim and Chiesi Pharma, outside the submitted work.
Conflict of interest: K.M. Aarberg-Lund has nothing to disclose.
Conflict of interest: M.T. Durheim reports grants to his institution and personal fees from Boehringer Ingelheim and Roche, and personal fees from AstraZeneca, outside the submitted work.
Conflict of interest: H. Ashraf reports grants from Boehringer Ingelheim, during the conduct of the study.
Conflict of interest: G. Einvik reports grants from Boehringer Ingelheim, during the conduct of the study; and personal fees for consultancy from AstraZeneca AB, outside the submitted work.
Conflicts of interest: O.H. Skjønsberg has nothing to disclose.
Conflict of interest: K. Stavem reports fees from UCB Pharma and MSD, outside the submitted work.
Support statement: This study was supported by Boehringer Ingelheim. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Ahmed H, Patel K, Greenwood DC, et al. . Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med 2020; 52: jrm00063. [DOI] [PubMed] [Google Scholar]
- 2.Guler SA, Ebner L, Aubry-Beigelman C, et al. . Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690. doi: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerum TV, Aalokken TM, Bronstad E, et al. . Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J 2020; 57: 2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lerum TV, Maltzahn NN, Aukrust P, et al. . Persistent pulmonary pathology after COVID-19 is associated with high viral load, weak antibody response, and high levels of matrix metalloproteinase-9. Sci Rep 2021; 11: 23205. doi: 10.1038/s41598-021-02547-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Liu X, Zhou Y, et al. . 3-month, 6–month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 2021; 9: 747–754. doi: 10.1016/S2213-2600(21)00174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Yao Q, Gu X, et al. . 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398: 747–758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thannickal VJ, Toews GB, White ES, et al. . Mechanisms of pulmonary fibrosis. Annu Rev Med 2004; 55: 395–417. doi: 10.1146/annurev.med.55.091902.103810 [DOI] [PubMed] [Google Scholar]
- 8.Huang WJ, Tang XX. Virus infection induced pulmonary fibrosis. J Transl Med 2021; 19: 496. doi: 10.1186/s12967-021-03159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luger AK, Sonnweber T, Gruber L, et al. . Chest CT of lung injury 1 year after COVID-19 pneumonia: the CovILD study. Radiology 2022; 304: 462–470. doi: 10.1148/radiol.211670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Solidarity Trial Consortium ; Pan H, Peto R, et al. . Repurposed antiviral drugs for covid-19 – interim WHO Solidarity Trial results. N Engl J Med 2021; 384: 497–511. doi: 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams N. The MRC breathlessness scale. Occup Med (Lond) 2017; 67: 496–497. doi: 10.1093/occmed/kqx086 [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CM, Elmes PC, Fairbairn AS, et al. . The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959; 2: 257–266. doi: 10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham BL, Steenbruggen I, Miller MR, et al. . Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019; 200: e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham BL, Brusasco V, Burgos F, et al. . 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49: 1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 15.Holland AE, Spruit MA, Troosters T, et al. . An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 16.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158: 1384–1387. doi: 10.1164/ajrccm.158.5.9710086 [DOI] [PubMed] [Google Scholar]
- 17.Stanojevic S, Graham BL, Cooper BG, et al. . Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50: 1700010. doi: 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 18.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansell DM, Bankier AA, MacMahon H, et al. . Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 20.Soyseth V, Aalokken TM, Mynarek G, et al. . Diagnosis of biopsy verified usual interstitial pneumonia by computed tomography. Respir Med 2015; 109: 897–903. doi: 10.1016/j.rmed.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20: e192–e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellegrino R, Viegi G, Brusasco V, et al. . Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 24.Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD 2005; 2: 125–129. doi: 10.1081/COPD-200050527 [DOI] [PubMed] [Google Scholar]
- 25.Ovrebotten T, Myhre P, Grimsmo J, et al. . Changes in cardiac structure and function from 3 to 12 months after hospitalization for COVID-19. Clin Cardiol 2022; 45: 1044–1052. doi: 10.1002/clc.23891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skjorten I, Ankerstjerne OAW, Trebinjac D, et al. . Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J 2021; 58: 2100996. doi: 10.1183/13993003.00996-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini DM, Brunjes DL, Lala A, et al. . Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail 2021; 9: 927–937. doi: 10.1016/j.jchf.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingul CB, Edvardsen A, Follestad T, et al. . Changes in cardiopulmonary exercise capacity and limitations 3 to 12 months after COVID-19. Eur Respir J 2022; in press [ 10.1183/13993003.00745-2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandal S, Barnett J, Brill SE, et al. . ‘Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021; 76: 396–398. doi: 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Li J, Liu H, et al. . Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020; 8: 8. doi: 10.1038/s41413-020-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorent N, Vande Weygaerde Y, Claeys E, et al. . Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res 2022; 8: 00004-2022. doi: 10.1183/23120541.00004-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Criee CP, Sorichter S, Smith HJ, et al. . Body plethysmography: its principles and clinical use. Respir Med 2011; 105: 959–971. doi: 10.1016/j.rmed.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 33.Koo HJ, Lim S, Choe J, et al. . Radiographic and CT features of viral pneumonia. Radiographics 2018; 38: 719–739. doi: 10.1148/rg.2018170048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
TABLE S1 00575-2022.tableS1 (83.7KB, pdf)
TABLE S2 00575-2022.tableS2 (83.3KB, pdf)