Abstract
Introduction:
Alzheimer disease (AD) is a complex neurodegenerative disorder with a progressive nature leading to neural damage and cognitive and memory deficit. The present study investigated the neuroprotective effects of Centella asiatica (CA) in Streptozotocin (STZ)-induced rat model of memory impairment and neuronal damage.
Methods:
The intracerebroventricular infusion of STZ (3 mg/rat) or saline (as the vehicle) was performed on days 1 and 3. CA (150 and 300 mg/kg/d) was administered through oral gavage for 21 days after model induction. We used the Y-maze test to assess the working memory-related performances of animals. Rats were then sacrificed, and their hippocampi were harvested for evaluation of neuronal density in the cornu ammonis (CA1, CA2, CA3) and Dentate Gyrus (DG) regions using stereology technique.
Results:
The intracerebroventricular infusion of STZ caused significant working memory impairment demonstrated in the Y-maze apparatus, with a significant decrease in alternative behavior compared to control animals (40.67±2.04 vs 73.00±1.88, P<0.0001). Oral administration of CA (150 and 300 mg/kg each day) for 21 days significantly improved STZ-induced working memory deficit (55.33±3.34 and 57.17±3.81 vs 40.67±2.04, P<0.013, P<0.004, respectively). Furthermore, 21 days of consecutive administration of CA significantly ameliorated STZ-induced neuronal loss in the CA1, CA2, and DG subfields of the hippocampus.
Conclusion:
Overall, these data demonstrate that CA increases neuronal density and improves cognitive impairment in the STZ-induced rat model of AD, thereby having promising therapeutic potential for neurodegenerative disorders. Accordingly, further studies are needed to determine the exact molecular mechanism of CA protective effects in brain disorders, particularly AD.
Highlights
Centella asiatica (CA) improved the STZ-induced working memory deficit.
CA could prevent hippocampal neural cell loss dose-dependent manner.
CA improved memory through mitigating neuronal loss in hippocampus.
Plain Language Summary
Memory loss is the first signs of dementia. It is well known that a healthy diet might be as good for your brain as it is for your heart. Numerous traditionally used medicinal herbs could significantly affect key events culminating in dementia and Alzheimer's disease. Centella asiatica, commonly known as Gotu Kola or Indian Pennywort, is a tropical, medicinal plant native to Southeast Asian countries. It is one of the becoming popular medicinal plants in the world. Centella asiatica (CA) is widely used in different traditional medicine systems for various purposes, such as reducing blood pressure, memory enhancement, and promoting longevity. In the present study, we tested the possible impact of CA leaf and stem extract in an animal model of memory damage. Memory impairment was induced in adult rats by intracerebral infusion of a neurotoxin chemical. Then, the memory-impaired animals were orally treated with 150–300 mg/kg of CA extract for 21 days. Finally, we tested their working memory by placing them in a Y-maze apparatus. Furthermore, their most involved brain part (hippocampus) was dissected, and its cell density was evaluated. Our findings exhibited that CA treatment considerably improved rats' memory performance, indicating by enhancing working memory score in the Y-maze task. In addition, CA treatment significantly prevented neuronal cell loss in the hippocampus of memory-impaired rats. This study shows that CA has beneficial effects on memory and cognitive function.
Keywords: Alzheimer disease, Working memory, Centella asiatica, Neuron Degeneration
1. Introduction
Alzheimer Disease (AD) is a progressive and age-related amyloid pathology associated with neural loss as well as learning and memory deficits ( Morgan et al., 2000). Intracerebroventricular (ICV) infusion of the low dose of Streptozotocin (STZ) is widely used to induce sporadic AD (sAD) model ( Rani, Deshmukh, Jaswal, Kumar, & Bariwal, 2016). The underlying mechanism is loading massive amyloid that commences inflammatory processes ( Bellenger et al., 2011) and causes neural damage and progressive cognitive decline (Hayate Javed et al., 2011; Shi, Zhang, Li, & Hölscher, 2017; Shingo, Kanabayashi, Kito, & Murase, 2013).
Memory is a process through which information is stored and retrieved ( Kandel et al., 2000). Several brain structures are responsible for learning and memory formation; among them, the hippocampus has a prominent role ( Hall, 2015; Robbins, Ersche, & Everitt, 2008; Ying et al., 2002). Accordingly, hippocampal lesions are frequently associated with memory loss and cognition impairment ( Broadbent, Squire, & Clark, 2004). Also, many investigations have mentioned the inflammatory cell death alongside a significant synaptic loss and reduced neural density in the hippocampus of human subjects with AD or animal models of cognition and memory impairments ( Bancher, Braak, Fischer, & Jellinger, 1993; Terry et al., 1991).
In recent decades, herbal medicine has gained prominence in the studies of human brain disorders. Many investigations addressed the preventive, protective, or ameliorative effects of herbal extracts in animal models of neurodegenerative diseases, including AD ( Dhanasekaran et al., 2009; Soumyanath et al., 2012; Veerendra Kumar & Gupta, 2003). Centella asiatica (CA) belongs to the family of Apiaceae (Umbelliferae) ( Kapoor, 2017) and shows several beneficial effects on the Central Nervous System (CNS), such as inducing mental calmness, reducing anxiety, and enhancing intelligence and memory ( Kumar & Gupta, 2002; Seevaratnam, Banumathi, Premalatha, Sundaram, & Arumugam, 2012). In ancient India, CA has considered a treatment for dementia ( Shinomol & Bharath, 2011). It has been shown that CA extract decreases oxidative stress markers ( Gray, Harris, Quinn, & Soumyanath, 2016; Haleagrahara & Ponnusamy, 2010; Jayashree, Muraleedhara, Sudarslal, & Jacob, 2003). Moreover, this herbal extract has been demonstrated to reduce amyloid-beta levels in the hippocampus ( Dhanasekaran et al., 2009).
The present study aimed to explore the effect of chronic oral administration of CA aqueous extract against STZ-induced cognitive impairment and reduced neural density in the cornu ammonis (CA1, CA2, CA3) and Dentate Gyrus (DG) subfields of the hippocampus.
2. Methods
Animals
Adult male Wistar rats (weighing 200–250 g) were obtained from the Research Centre of Experimental Medicine, Birjand University of Medical Sciences. The rats were housed in polypropylene cages (3 per cage) under controlled condition (12 h light/12 h dark cycle, lights on 07:00) and temperature (21±2°C) with free access to food and water except for limited periods of experiments. Behavioral tests were performed during the light cycle between 8:00 to 14:00. Six animals were used in each experimental group, and each animal was tested once. All experiments and animal care were conducted according to guidelines of the Ethics Committee of Birjand University of Medical Sciences (Ethical code: IR.BUMS.REC.1387.298).
Chemicals and agents
Streptozotocin (STZ) was purchased (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in sterile and cold saline. The CA lyophilized powder was bought (TST Plant Medicines Co, Tehran, Iran) and dissolved in distilled water to obtain desired concentrations. Ketamine (Bremer Pharma GMBH, Bremerhaven, Germany) and xylazine (Alfasan Chemical Co., Woerden, Holland) were used for animal anesthesia. Toluidine blue was purchased from Merck Company (Germany). Other chemicals were bought from Merck or Sigma-Aldrich.
Surgery and drug treatment
For surgery, animals were anesthetized with Intraperitoneal (IP) injection of ketamine (80 mg/kg) and xylazine (15 mg/kg) and were placed in a stereotaxic device (Stoelting, Wood Dale, IL, USA). Ketamine and xylazine doses were chosen according to the previous research ( Adeli, Zahmatkesh, Tavoosidana, Karimian, & Hassanzadeh, 2017; BonakdarYazdi et al., 2017; Wang, Wang, Cheng, & Che, 2016). The 22-gauge guide cannula was fixed into the lateral ventricles according to the atlas of Paxinos and Watson (AP: 0.8 mm; ML: 1.8 mm; DV: 3.6 mm) ( Zilles, 2012). ICV infusion of STZ (3 mg, 1.5 mg/5 μL/side) was infused on days 1 and 3 through an injection needle (27 G) connected to a 50-μL Hamilton syringe (Hamilton, Reno, Nevada). Then after each injection, the needle was left for a further 30 s to facilitate the release of the drug solution. One week after recovery from stereotaxic surgery, normal rats were used for working memory assessment. The effective doses of CA were selected according to previous studies ( Chanana & Kumar, 2017; Sari et al., 2014a; Wanasuntron-wong, Tantisira, Tantisira, & Watanabe, 2012). CA (150 and 300 mg/kg/d) was administered with oral gavage for 21 days after model induction.
Experimental groups
In this experiment, 30 rats were randomly divided into 5 groups (n=6): 1) control group without surgery or drug intervention; 2) vehicle group received bilateral ICV infusion of saline; 3) STZ group received bilateral ICV infusion of STZ (3 mg, 1.5 mg/5 μL/side, on days 1 & 3); 4) STZ+CA150 animals received bilateral ICV infusion of STZ (3 mg, 1.5mg/5μL/side) on days 1 and 3 and oral gavage of CA (150 mg/kg, daily) from day 14 to day 35; and 5) STZ+CA300 animals received bilateral ICV infusion of STZ (3 mg, 1.5 mg/5 μL/side) on days 1 and 3 and oral gavage of CA (300 mg/kg, daily) from day 14 to day 35.
Y-maze test
Working memory of rats was evaluated using the Y-maze apparatus on day 14 for the STZ group and day 35 for STZ+CA groups. Y-maze test was done according to previous studies ( Aminyavari, Zahmatkesh, Farahmandfar, Khodagholi, Dargahi, & Zarrindast, 2019; Hughes, 2004). This device comprises three symmetrical arms (30 × 20 × 10 cm) named A, B, and C. The animals were placed at the end of one of the three arms and were allowed to explore. The procedure was conducted in an 8-min session with no interval. The alternative behavior, the overlapping triplet sets of consecutive entries into three different arms (A, B, and C) of the Y-maze apparatus of rats, was recorded. For instance, if the recorded sequence of arms entries is ABCACBBAC, we could consider four sets of correct alternative movement: 1) ABCACBBAC, 2) ABCACBBAC, 3) ABCACBBAC, and 4) ABCACBBA. Animals’ behavior was recorded by a camera placed above the Y-maze device. Surfaces of the apparatus were cleaned with alcohol between sessions. The percentage of alternative behavior was determined using the following calculation:
Tissue preparation
After completing the behavioral test, the animals were anesthetized with ketamine and xylazine mixture and then perfused with Phosphate-Buffered Saline (PBS) followed by 4% paraformaldehyde. The animal’s brain was harvested and subsequently immersed in a fixative solution (4% paraformaldehyde) for 48 h at 4 °C. After the dehydration using different degrees of alcohol, the samples were embedded in paraffin block and sectioned transversely (7 μm thick) using a microtome device (Leitz, Italy). The sections were then stained by toluidine blue for counting the number of neurons in different areas of the hippocampus. The sections were deparaffinized in xylene and rehydrated through a descending ethanol series. After that, the slides were rinsed in a staining dish containing 1% toluidine blue in 1% sodium borate solution (1 g of toluidine blue and 1 g of sodium borate in 100 mL distilled water) and allowed to react overnight at room temperature. The sections were then dehydrated through methanol (two times), cleared in xylene, and mounted in Entellan ( Hami, Hosseini, Shahi, Lotfi, Talebi, & Afshar, 2015).
Stereological analysis
The number of neurons in different hippocampus regions (CA1, CA2, CA3, and DG) were counted using the optical dissector method ( Gundersen, 1988). For counting, 8–10 sections in each hippocampus region were randomly selected. The sections were then observed under a light microscope (UPlan FI, Japan), connected to a computer by a high-resolution camera (BX51, Japan). Rectangular grids were placed on the examination area, and the number of neurons was calculated under 40× objectives. Finally, the neuronal density was calculated in different regions of the hippocampus by the following equation:
In this formula, ∑Q is the summation of counted neurons that appeared in sections, “a/f” is the area associated with each frame (10000 μm2), and “∑P” is the sum of frames associated points hitting the reference.
Statistical analysis
These analyses were performed using the GraphPad Prism V.6. One-way Analysis of Variance (ANOVA) followed by Tukey post hoc test was used for multiple comparisons of data gathered from the Y-maze device and the number of neurons in different hippocampus regions. P<0.05 was considered the level of significance. All results were presented as Mean±SEM.
3. Results
Effect of CA on memory performance in Y-maze
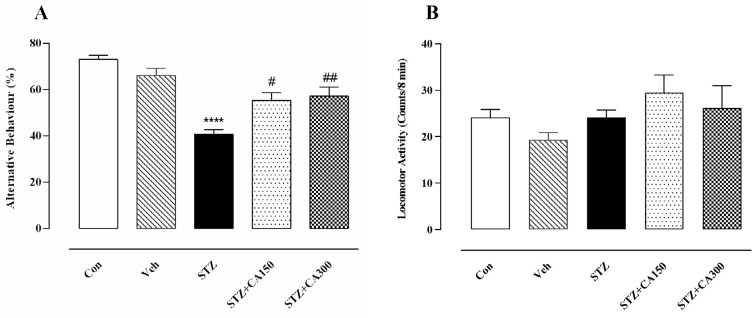
Behavioral findings showed a significant effect of CA on the working memory of rats in the Y-maze apparatus (F4, 25=17.05, P=0.0001). Bilateral ICV infusion of STZ diminished working memory, demonstrated by a significant decrease in alternative behavior than the control group (40.67±2.04 vs 73.00±1.88, P=0.001). Oral administration of CA for 21 days significantly improved STZ- induced memory dysfunction at doses of 150 mg/kg (55.33±3.43 vs 40.67±2.04, P=0.013) and 300 mg/kg (57.17±3.81 vs 40.67±2.04, P=0.004) (Figure 1A). Hence, CA showed an ameliorative effect in the STZ-induced rat model of AD. It is worth mentioning that no significant difference was observed in the total arm entries (F4, 25=1.51, P=0.22; Figure 1B) among groups, indicating that alterations in the behavior of animals were not related to musculoskeletal or motor dysfunction.
Figure 1.
Centella asiatica (CA) improved Streptozotocin (STZ)-induced working memory deficits
Animals received ICV infusion of saline/STZ alone or combined with CA (150 and 300 mg/kg). CA significantly increased the alternative behavior at doses of 150 and 300 mg/kg (A). No significant difference was observed between the groups regarding the total arms entries (B).
****P<0.0001 compared to the Control group. #P<0.01 and ##P<0.004 compared to the STZ -treated rats. Values are expressed as mean±SEM (n=6 in each group)
Effect of CA on neuronal density in different areas of the hippocampus
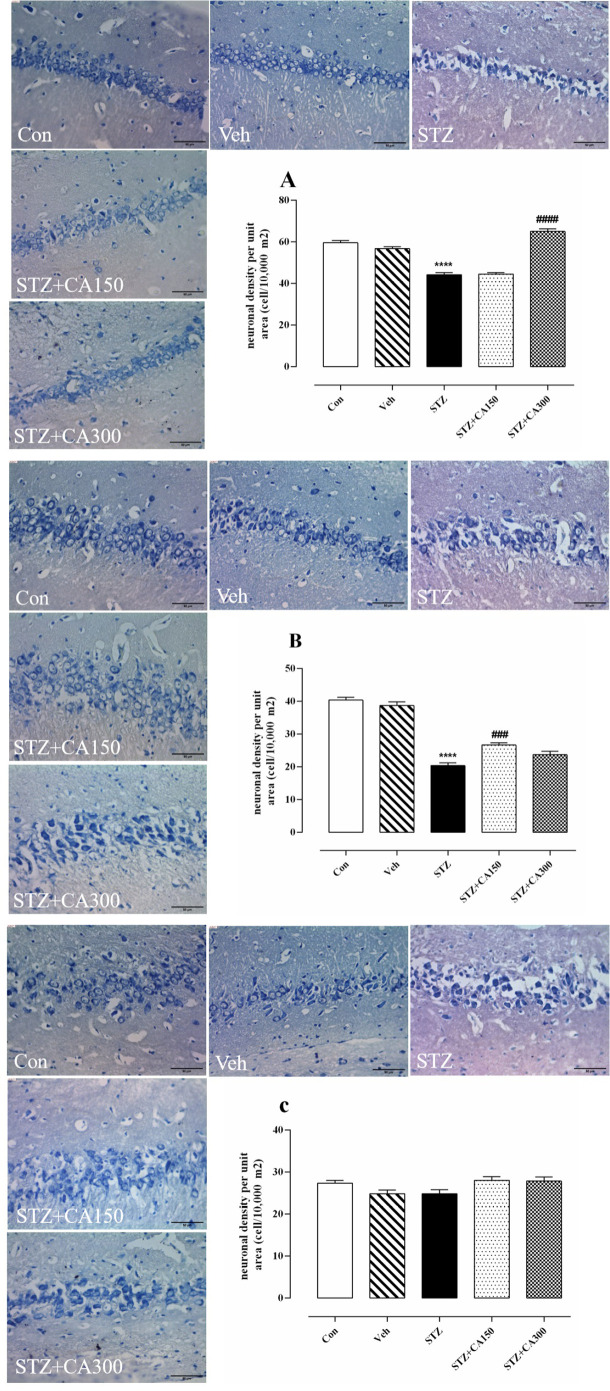
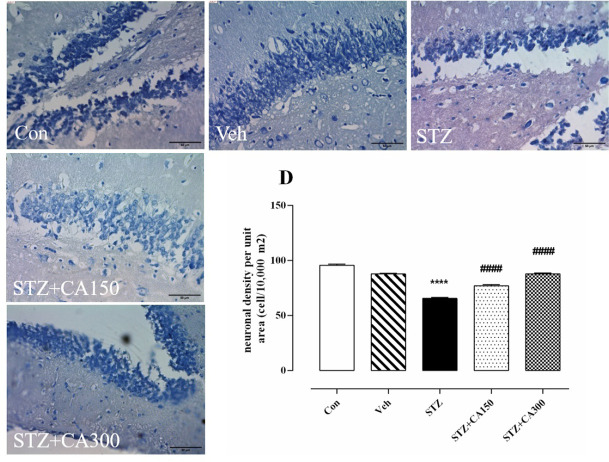
Histological analysis showed that CA had a significant effect on neural density in the CA1, CA2, and DG subfields of the hippocampus (CA1: F4, 25=81.35, P<0.001; CA2: F4, 25=92.30, P<0.001; DG: F4, 25=159.2, P<0.001). Neural density was significantly reduced in the CA1, CA2, and DG areas of the hippocampus of animals that received ICV infusion of STZ compared to the control group (CA1: 44.17±0.94 vs 59.50±1.14, P<0.001; CA2: 20.33±0.88 vs 40.33±0.84, P<0.001; DG: 65.50±0.84 vs 95.67±1.08, P<0.001). Oral administration of CA for 21 days increased neuronal density in STZ-treated animals in CA1 (300 mg/kg: 65.00±1.29 vs 44.17±0.94, P<0.001), CA2 (150 mg/kg: 26.67±0.66 vs 20.33±0.88, P=0.0006) and DG (150 mg/kg: 77.00±1.06 vs 65.50±0.84, P<0.001; 300 mg/kg: 87.83±0.87 vs. 65.50±0.84, P<0.001) compared to the control group (Figure 2A, B, and D). No significant difference was observed in the number of neurons in the CA3 subfield of the hippocampus among groups (P>0.05, Figure 2C).
Figure 2.
Neuroprotective effect of Centella asiatica (CA) against Streptozotocin (STZ)-induced neurotoxicity Alteration of neuronal cell density in various subfields of the hippocampus. A: CA1, B: CA2, C: CA3, and D: DG.
*P<0.001 compared to the control group. #P<0.001 compared to STZ treated rats. Values are expressed as Mean±SEM (n=6 in each group).
4. Discussion
The present study examined the ameliorative effect of CA extract in the animal model of sporadic dementia of Alzheimer type developed by ICV infusion of STZ. Memory impairment is the most important symptom of AD; hence we engaged the Y-maze device to assess working memory deficit during the model induction. Bilateral ICV infusion of STZ (3 mg/kg) could impair the working memory of rats after 14 days, as indicated by a significant reduction in alternative behavior compared to control animals (Figure 1A). Histological assessments also showed a remarkable decrease in neuronal density in different hippocampus areas, which confirmed the behavioral outcome (Figure 2).
Based on a wealth of evidence, the hippocampus plays an essential role in different types of learning and memory. In this regard, hippocampal lesions contribute to significant memory impairment in human subjects or animal models ( De Haan, Mishkin, Baldeweg, & Vargha-Khadem, 2006). In line with our results, previous studies have demonstrated long-term and progressive memory impairment following ICV infusion of STZ, which is similar to the sporadic type of AD ( Lannert & Hoyer, 1998; Sharma & Gupta, 2001; Shi et al., 2017). Moreover, STZ produces a significant neuronal loss in the brain areas, including the hippocampus ( Dhull, D. K., Jindal, Dhull, Aggarwal, Bhateja, & Padi, 2012; Song et al., 2014; Veerendra Kumar & Gupta, 2003). STZ enhances glucose levels in the rat’s brain and, in turn, improves the conversion of amyloid precursor protein to amyloid-beta, which commences inflammatory processes ( Chu & Qian, 2005). These events decrease insulin expression and increase oxidative stress and the production of Reactive Oxygen Species (ROS) in the hippocampus, leading to neural apoptotic death and learning and memory deficit ( Ahmed et al., 2013; bAx; H Javed et al., 2012). Accordingly, ICV infusion of STZ could induce a proper animal model of sAD. Several studies demonstrate that the memory impairment following ICV infusions of STZ starts at day 14 and lasts more than 35 days ( Afshar, Shahidi, Rohani, Komaki, & Asl, 2018; Agrawal, Tyagi, Shukla, & Nath, 2009; Chen et al., 2012). Agrawal et al. demonstrated the STZ-induced spatial memory impairment and oxidative stress induction in rat brain after 14 days ( Agrawal et al., 2009). Furthermore, Afshar et al. reported the novel object recognition and passive avoidance learning impairment and a significant decrease in hippocampal BDNF levels 35 days after ICV STZ administration ( Afshar et al., 2018).
In addition, our results showed that oral administration of CA for 21 days after the development of the AD model improved working memory, as indicated by increased alternative behavior of rats (Figure 1A). We also observed that CA administration (150 and 300 mg/kg/d) increased neural density in the CA1, CA2, and DG subfields of the hippocampus in STZ-treated animals (Figure 2A, B, and D). Thereby, CA significantly ameliorates memory deficit and hippocampal damage in STZ-induced animal model of sAD.
Flavonoid enriched plants exert powerful action on the memory of mammals. These compounds maintain the normal functioning of neurons and even limit age-related neurodegeneration ( Vauzour, 2014). CA contains flavonoid compounds making it a potential herbal remedy for AD ( Zainol, Abd-Hamid, Yusof, & Muse, 2003). Moreover, asiatic acid, a triterpenoid, is present in CA extract and is a neuroprotective agent ( Rather, Justin-Thenmozhi, Manivasagam, Saravanababu, Guillemin, & Essa, 2019).
Many studies have shown the safety borders of CA ( Chauhan & Singh, 2012; Chivapat, Chavalittumrong, & Tantisira, 2011; Roopesh, Salomi, Nagarjuna, & Reddy, 2011). The CA LD50 value was determined to be more than 10 g/kg suggesting a comparatively high margin of safety ( Chivapat et al., 2011). CA functions as an anti-oxidant agent and free radical scavenger that prevent lipid peroxidation and DNA damage ( Dhanasekaran et al., 2009; Rahman, Sayeed, Haque, Hassan, & Islam, 2012; Veerendra Kumar & Gupta, 2003). Brain oxidative damage and mitochondrial dysfunction, as well as programmed neurons death, occur in the STZ-induced animal model of sAD, which is responsible for cognitive and memory impairment ( Saxena, Patro, & Nath, 2011; Sharma & Gupta, 2001). Interestingly, CA improves mitochondrial enzymes activity and reduces caspase-3 and Bax/bcl-2 levels (anti-apoptotic factors) in the hippocampus ( Prakash & Kumar, 2013). Accordingly, CA can inhibit apoptotic neural death in the hippocampus of AD animal models via affecting AKT/GSK3β signaling pathway ( Rather et al., 2019).
Moreover, CA enhances phosphorylation of Cyclic AMP Response Element-Binding protein (CREB) as an essential transcription factor through ERK/RSK signaling pathway ( Xu, Cao, Khan, & Luo, 2008). CREB regulates the expression of critical genes, including Brain-Derived Neurotrophic Factor (BDNF) and cholinergic markers ( Xu et al., 2008). Nerve growth factors are crucial for hippocampal synaptic plasticity and nerve growth, as well as learning and memory formation ( Hsiao, Hung, Chen, & Gean, 2014). A significant reduction in BDNF has been observed in the hippocampus of the STZ-induced model of sAD ( Afshar et al., 2018). Strikingly, CA can improve BDNF level in rats’ hippocampus, which makes it an important candidate to relieve AD symptoms ( Handayani, Yolanda, & Kodariah, 2018). Cholinergic hypofunction is significantly involved in memory impairment induced by ICV infusion of STZ ( Agrawal et al., 2009). Due to the effect of CA on CREB activity, it is expected to enhance the transcription of cholinergic markers, choline acetyltransferase, and vesicular acetylcholine transporter. On the other hand, CA inhibits inducible nitric oxide synthase activity, thereby enhancing cholinergic neurotransmission, which is critical for learning and memory formation ( Sari et al., 2014b).
Furthermore, CA enhances dendritic arborization and synaptogenesis by modulating ERK1/2 and Akt signaling pathways ( Gray et al., 2018). It is suggested that asiatic acid enhances doublecortin and NOTCH1 protein levels in the hippocampus, which promote hippocampal neurogenesis ( Sirichoat et al., 2015). Strikingly, it has been demonstrated that CA increases synaptic markers in the hippocampus and frontal cortex through augmentation of the cAMP/PKA signaling pathway ( Gray et al., 2016). In addition, CA improves synaptic trafficking by increasing the expression of the AMPAR GluA1 subunit in the hippocampus ( Wong et al., 2019).
5. Conclusion
Taken together, we showed the ameliorative impact of CA on STZ-induced working memory impairment and hippocampal neural damage. The detection of the exact molecular mechanism of the effectiveness of CA requires further examination.
Ethical Considerations
Compliance with ethical guidelines
All experiments and animal care were conducted according to guidelines of the Ethics Committee of Birjand University of Medical Sciences (Ethical code: IR.BUMS.REC.1387.298).
Acknowledgments
Authors would like to thanks the Research Centre of Experimental Medicine of Birjand University of Medical Sciences for providing animal facilities.
Footnotes
Funding
This paper is based on the results of the MSc thesis of the first author which was financially supported by Birjand University of Medical Sciences (Grant No.:455561).
Authors' contributions
Conceptualization and Supervision: Mohammad Reza Saebipour and Mohammadmehdi Hassanzadeh-Taheri; Methodology: Mohsen Foadoddini and Samaneh Aminyavari; Investigation: Razyeh Sahraei and Mehran Hosseini; Data Analysis: Samaneh Aminyavari; Writing - original draft: Samaneh Aminyavari and Mehran Hosseini.
Conflict of interest
The authors declared no conflict of interest.
References
- Adeli S., Zahmatkesh M., Tavoosidana G., Karimian M., Hassanzadeh G. (2017). Simvastatin enhances the hippocampal klotho in a rat model of streptozotocin-induced cognitive decline. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 72, 87–94. [DOI: 10.1016/j.pnpbp.2016.09.009] [PMID ] [DOI] [PubMed] [Google Scholar]
- Afshar S., Shahidi S., Rohani A. H., Komaki A., Asl S. S. (2018). The effect of NAD-299 and TCB-2 on learning and memory, hippocampal BDNF levels and amyloid plaques in Streptozotocin-induced memory deficits in male rats. Psychopharmacology, 235(10), 2809–22. [DOI: 10.1007/s00213-018-4973-x] [PMID ] [DOI] [PubMed] [Google Scholar]
- Agrawal R., Tyagi E., Shukla R., Nath C. (2009). A study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology, 56(4), 779–87. [DOI: 10.1016/j.neuropharm.2009.01.005] [PMID ] [DOI] [PubMed] [Google Scholar]
- Ahmed M. E., Khan M. M., Javed H., Vaibhav K., Khan A., Tabassum R., et al. (2013). Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochemistry International, 62(4), 492–501. [DOI: 10.1016/j.neuint.2013.02.006] [PMID ] [DOI] [PubMed] [Google Scholar]
- Aminyavari S., Zahmatkesh M., Farahmandfar M., Khodagholi F., Dargahi L., Zarrindast M. R. (2019). Protective role of Apelin-13 on amyloid β25–35-induced memory deficit: Involvement of autophagy and apoptosis process. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89, 322–34. [DOI: 10.1016/j.pnpbp.2018.10.005] [PMID ] [DOI] [PubMed] [Google Scholar]
- Bancher C., Braak H., Fischer P., Jellinger K. A. (1993). Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neuroscience Letters, 162(1–2), 179–82. [DOI: 10.1016/0304-3940(93)90590-H] [PMID ] [DOI] [PubMed] [Google Scholar]
- Bellenger J., Bellenger S., Bataille A., Massey K. A., Nicolaou A., Rialland M., et al. (2011). High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: Inflammatory pathway inhibition. Diabetes, 60(4), 1090–9. [DOI: 10.2337/db10-0901] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonakdarYazdi B., Khodagholi F., Shaerzadeh F., Sharifzadeh A., Ahmadi R., Sanati M., et al. (2017). The effect of arsenite on spatial learning: Involvement of autophagy and apoptosis. European Journal of Pharmacology, 796, 54–61. [DOI: 10.1016/j.ejphar.2016.12.023] [PMID ] [DOI] [PubMed] [Google Scholar]
- Broadbent N. J., Squire L. R., Clark R. E. (2004). Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences, 101(40), 14515–20. [DOI: 10.1073/pnas.0406344101] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanana P., Kumar A. (2017). Further investigations on the neuroprotective potential of Centella asiatica against sleep deprivation induced anxiety like behaviour: Possible implications of mitoprotective and anti-stress pathways. Journal of Sleep Disorders: Treat Care, 6(2), 20–2. https://www.researchgate.net/profile/Priyanka-Chanana-2/publication/313510189 [Google Scholar]
- Chauhan P., Singh V. (2012). Acute and subacute toxicity study of the acetone leaf extract of Centella asiatica in experimental animal models. Asian Pacific Journal of Tropical Biomedicine, 2(2), S511–3. [DOI: 10.1016/S2221-1691(12)60263-9] [DOI] [Google Scholar]
- Chen Y., Tian Z., Liang Z., Sun S., Dai C. l., Lee M. H., et al. (2012). Brain gene expression of a sporadic (icv-STZ Mouse) and a familial mouse model (3xTg-AD mouse) of Alzheimer’s disease. PloS One, 7(12), e51432. [DOI: 10.1371/journal.pone.0051432] [PMID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivapat S., Chavalittumrong P., Tantisira M. H. (2011). Acute and sub-chronic toxicity studies of a standardized extract of Centella asiatica ECa 233. The Thai Journal of Pharmaceutical Sciences, 35(2011), 55–64. https://www.researchgate.net/profile/Mayuree-Tantisira/publica.pdf [Google Scholar]
- Chu W. Z., Qian C. Y. (2005). [Expressions of Abeta1-40, Abeta1-42, tau202, tau396 and tau404 after intracerebroventricular injection of streptozotocin in rats (Chinese)]. Di Yi Jun Yi Da Xue Xue Bao, 25(2), 168–70. [PMID ] [PubMed] [Google Scholar]
- De Haan M., Mishkin M., Baldeweg T., Vargha-Khadem F. (2006). Human memory development and its dysfunction after early hippocampal injury. Trends in Neurosciences, 29(7), 374–81. [DOI: 10.1016/j.tins.2006.05.008] [PMID ] [DOI] [PubMed] [Google Scholar]
- Dhanasekaran M., Holcomb L. A., Hitt A. R., Tharakan B., Porter J. W., Young K. A., et al. (2009). Centella asiatica extract selectively decreases amyloid β levels in hippocampus of Alzheimer’s disease animal model. Phytotherapy Research, 23(1), 14–9. [DOI: 10.1002/ptr.2405] [PMID ] [DOI] [PubMed] [Google Scholar]
- Dhull D. K., Jindal A., Dhull R. K., Aggarwal S., Bhateja D., Padi S. S. (2012). Neuroprotective effect of cyclooxygenase inhibitors in ICV-STZ induced sporadic Alzheimer’s disease in rats. Journal of Molecular Neuroscience, 46(1), 223–35. [DOI: 10.1007/s12031-011-9583-6] [PMID ] [DOI] [PubMed] [Google Scholar]
- Gray N. E., Harris C. J., Quinn J. F., Soumyanath A. (2016). Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. Journal of Ethnopharmacology, 180, 78–86. [DOI: 10.1016/j.jep.2016.01.013] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. E., Magana A. A., Lak P., Wright K. M., Quinn J., Stevens J. F., et al. (2018). Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochemistry Reviews, 17(1), 161–94. [DOI: 10.1007/s11101-017-9528-y] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H., Bagger P., Bendtsen T. F., Evans S. M., Korbo L., Marcussen N., et al. (1988). The new stereological tools: Disector, fractionator, nucleator, and intercepts and their use in pathological research and diagnosis. Acta Pathologica, Microbiologica, et Immunologica Scandinavica, 96(10), 857–81. [DOI: 10.1111/j.1699-0463.1988.tb00954.x] [PMID ] [DOI] [PubMed] [Google Scholar]
- Haleagrahara N., Ponnusamy K. (2010). Neuroprotective effect of Centella asiatica Extract (CAE) on experimentally induced parkinsonism in aged Sprague-Dawley rats. The Journal of Toxicological Sciences, 35(1), 41–7. [DOI: 10.2131/jts.35.41] [PMID ] [DOI] [PubMed] [Google Scholar]
- Hall J. E. (2015). Guyton and Hall textbook of medical physiology. Philadelphia: Elsevier Health Sciences. https://www.google.com/books/edition/Guyton_and_Hall_=frontcover [Google Scholar]
- Hami J., Hosseini M., Shahi S., Lotfi N., Talebi A., Afshar M. (2015). Effects of L-arginine pre-treatment in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s diseases in Balb/c mice. Iranian Journal of Neurology, 14(4), 195–203. [PMID ] [PMCID ] [PMC free article] [PubMed] [Google Scholar]
- Handayani A., Yolanda S., Kodariah R. (2018). Centella asiatica ethanol extract increases hippocampal brain derived neurotrophic factor in male Wistar rats. Universa Medicina, 37(2), 143–9. [DOI: 10.18051/UnivMed.2018.v37.143-149] [DOI] [Google Scholar]
- Hsiao Y. H., Hung H. C., Chen S. H., Gean P. W. (2014). Social interaction rescues memory deficit in an animal model of Alzheimer’s disease by increasing BDNF-dependent hippocampal neurogenesis. Journal of Neuroscience, 34(49), 16207–19. [DOI: 10.1523/JNEUROSCI.0747-14.2014] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. N. (2004). The value of spontaneous Alternation Behavior (SAB) as a test of retention in pharmacological investigations of memory. Neuroscience & Biobehavioral Reviews, 28(5), 497–505. [DOI: 10.1016/j.neubiorev.2004.06.006] [PMID ] [DOI] [PubMed] [Google Scholar]
- Javed H., Khan M., Ahmad A., Vaibhav K., Ahmad M., Khan A., et al. (2012). Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience, 210, 340–52. [DOI: 10.1016/j.neuroscience.2012.02.046] [PMID ] [DOI] [PubMed] [Google Scholar]
- Javed H., Khan M. M., Khan A., Vaibhav K., Ahmad A., Khuwaja G., et al. (2011). S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Research, 1389, 133–42. [DOI: 10.1016/j.brainres.2011.02.072] [PMID ] [DOI] [PubMed] [Google Scholar]
- Jayashree G., Muraleedhara G. K., Sudarslal S., Jacob V. (2003). Anti-oxidant activity of Centella asiatica on lymphoma-bearing mice. Fitoterapia, 74(5), 431–4. [DOI: 10.1016/S0367-326X(03)00121-7] [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H., Jessell T. M., Biochemistry D. O., Jessell M. B. T., Siegelbaum S., et al. (2000). Principles of neural science (Vol. 4): New York: McGraw-Hill Companies, Incorporated. https://www.google.com/books/edition/Principles_of_Neural_Science_Fourth_Edit/l=en [Google Scholar]
- Kapoor L. (2017). Handbook of Ayurvedic medicinal plants. Boca Raton: Routledge. [DOI: 10.1201/9780203719473] [DOI] [Google Scholar]
- Kumar M. V., Gupta Y. (2002). Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. Journal of Ethnopharmacology, 79(2), 253–60. [DOI: 10.1016/S0378-8741(01)00394-4] [PMID ] [DOI] [PubMed] [Google Scholar]
- Lannert H., Hoyer S. (1998). Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behavioral Neuroscience, 112(5), 1199–208. [DOI: 10.1037/0735-7044.112.5.1199] [PMID ] [DOI] [PubMed] [Google Scholar]
- Morgan D., Diamond D. M., Gottschall P. E., Ugen K. E., Dickey C., Hardy J., et al. (2000). Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature, 408(6815), 982–5. [DOI: 10.1038/35050116] [PMID ] [DOI] [PubMed] [Google Scholar]
- Prakash A., Kumar A. (2013). Mitoprotective effect of Centella asiatica against aluminum-induced neurotoxicity in rats: Possible relevance to its anti-oxidant and anti-apoptosis mechanism. Neurological Sciences, 34(8), 1403–9. [DOI: 10.1007/s10072-012-1252-1] [PMID ] [DOI] [PubMed] [Google Scholar]
- Rahman M. M., Sayeed M. S. B., Haque M. A., Hassan M. M., Islam S. A. (2012). Phytochemical screening, antioxidant, anti-Alzheimer and anti-diabetic activities of Centella asiatica. Journal of Natural Product and Plant Resources, 2(4), 504–11. https://d1wqtxts1xzle7.cloudfront.net/41004101/2 [Google Scholar]
- Rani V., Deshmukh R., Jaswal P., Kumar P., Bariwal J. (2016). Alzheimer’s disease: Is this a brain specific diabetic condition? Physiology & Behavior, 64(Pt A), 259–67. [DOI: 10.1016/j.physbeh.2016.05.041] [PMID ] [DOI] [PubMed] [Google Scholar]
- Rather M. A., Justin-Thenmozhi A., Manivasagam T., Sara-vanababu C., Guillemin G. J., Essa M. M. (2019). Asiatic acid attenuated aluminum chloride-induced tau pathology, oxidative stress and apoptosis via AKT/GSK-3β signaling pathway in Wistar rats. Neurotoxicity Research, 35(4), 955–68. [DOI: 10.1007/s12640-019-9999-2] [PMID ] [DOI] [PubMed] [Google Scholar]
- Robbins T., Ersche K., Everitt B. (2008). Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences, 1141(1), 1–21. [DOI: 10.1196/annals.1441.020] [PMID ] [DOI] [PubMed] [Google Scholar]
- Roopesh C., Salomi K. R., Nagarjuna S., Reddy Y. P. (2011). Diuretic activity of methanolic and ethanolic extracts of Centella asiatica leaves in rats. International Research Journal of Pharmacy, 2(11), 163–5. https://www.researchgate.net/publication/288261787_Diuretic_activity_of_methanolic_and_ [Google Scholar]
- Sari D. C. R., Aswin S., Susilowati R., Ar-Rochmah M., Prakosa D., Romi M., et al. (2014a). Ethanol extracts of Centella asiatica leaf improves memory performance in rats after chronic stress via reducing nitric oxide and increasing Brain-Derived Neurotrophic Factor (BDNF) concentration. GSTF Journal of Psychology, 1(1), 61–7 [DOI: 10.7603/s40790-014-0009-0] [DOI] [Google Scholar]
- Saxena G., Patro I. K., Nath C. (2011). ICV STZ induced impairment in memory and neuronal mitochondrial function: A protective role of nicotinic receptor. Behavioural Brain Research, 224(1), 50–7. [DOI: 10.1016/j.bbr.2011.04.039] [PMID ] [DOI] [PubMed] [Google Scholar]
- Seevaratnam V., Banumathi P., Premalatha M., Sundaram S., Arumugam T. (2012). Functional properties of Centella asiatica (L.): A review. International Journal of Pharmacy and Pharmaceutical Sciences, 4(5), 8–14. https://anunaki.com/wp-content/uploads/2021/06/FUNCTIONAL-PROR.pdf [Google Scholar]
- Sharma M., Gupta Y. (2001). Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sciences, 68(9), 1021–9. [DOI: 10.1016/S0024-3205(00)01005-5] [PMID ] [DOI] [PubMed] [Google Scholar]
- Shi L., Zhang Z., Li L., Hölscher C. (2017). A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signaling in the Alzheimer icv. STZ rat model. Behavioural Brain Research, 327, 65–74. [DOI: 10.1016/j.bbr.2017.03.032] [PMID ] [DOI] [PubMed] [Google Scholar]
- Shingo A. S., Kanabayashi T., Kito S., Murase T. (2013). Intracerebroventricular administration of an insulin analogue recovers STZ-induced cognitive decline in rats. Behavioural Brain Research, 241, 105–11. [DOI: 10.1016/j.bbr.2012.12.005] [PMID ] [DOI] [PubMed] [Google Scholar]
- Shinomol G., Bharath M. (2011). Exploring the role of “Brahmi” (Bacopa monnieri and Centella asiatica) in brain function and therapy. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery, 5(1), 33–49. [DOI: 10.2174/187221411794351833] [PMID ] [DOI] [PubMed] [Google Scholar]
- Sirichoat A., Chaijaroonkhanarak W., Prachaney P., Pannangrong W., Leksomboon R., Chaichun A., et al. (2015). Effects of asiatic acid on spatial working memory and cell proliferation in the adult rat hippocampus. Nutrients, 7(10), 8413–23. [DOI: 10.3390/nu7105401] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Hur B. E., Bokara K. K., Yang W., Cho H. J., Park K. A., et al. (2014). Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocin-induced Alzheimer rat model. Yonsei Medical Journal, 55(3), 689–99. [DOI: 10.3349/ymj.2014.55.3.689] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumyanath A., Zhong Y. P., Henson E., Wadsworth T., Bishop J., Gold B. G., et al. (2012). Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer’s disease: investigation of a possible mechanism of action. International Journal of Alzheimer’s Disease, 2012, 381974. [DOI: 10.1155/2012/381974] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., et al. (1991). Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology, 30(4), 572–80. [DOI: 10.1002/ana.410300410] [PMID ] [DOI] [PubMed] [Google Scholar]
- Vauzour D. (2014). Effect of flavonoids on learning, memory and neurocognitive performance: Relevance and potential implications for Alzheimer’s disease pathophysiology. Journal of the Science of Food and Agriculture, 94(6), 1042–56. [DOI: 10.1002/jsfa.6473] [PMID ] [DOI] [PubMed] [Google Scholar]
- Veerendra Kumar M., Gupta Y. (2003). Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clinical and Experimental Pharmacology and Physiology, 30(5–6), 336–42. [DOI: 10.1046/j.1440-1681.2003.03842.x] [PMID ] [DOI] [PubMed] [Google Scholar]
- Wanasuntronwong A., Tantisira M. H., Tantisira B., Watanabe H. (2012). Anxiolytic effects of standardized extract of Centella asiatica (ECa 233) after chronic immobilization stress in mice. Journal of Ethnopharmacology, 143(2), 579–85. [DOI: 10.1016/j.jep.2012.07.010] [PMID ] [DOI] [PubMed] [Google Scholar]
- Wang H., Wang H., Cheng H., Che Z. (2016). Ameliorating effect of luteolin on memory impairment in an Alzheimer’s disease model. Molecular Medicine Reports, 13(5), 4215–20. [DOI: 10.3892/mmr.2016.5052] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. H., Muthuraju S., Reza F., Senik M. H., Zhang J., Yeo N. A. B. M. Y., et al. (2019). Differential expression of entorhinal cortex and hippocampal subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors enhanced learning and memory of rats following administration of Centella asiatica. Biomedicine & Pharmacotherapy, 110, 168–80. [DOI: 10.1016/j.biopha.2018.11.044] [PMID ] [DOI] [PubMed] [Google Scholar]
- Xu Y., Cao Z., Khan I., Luo Y. (2008). Gotu Kola (Centella asiatica) extract enhances phosphorylation of cyclic AMP response element binding protein in neuroblastoma cells expressing amyloid beta peptide. Journal of Alzheimer’s Disease, 13(3), 341–9. [DOI: 10.3233/JAD-2008-13311] [PMID ] [DOI] [PubMed] [Google Scholar]
- Ying S. W., Futter M., Rosenblum K., Webber M. J., Hunt S. P., Bliss T. V., et al. (2002). Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. Journal of Neuroscience, 22(5), 1532–40. [DOI: 10.1523/JNEUROSCI.22-05-01532.2002] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainol M., Abd-Hamid A., Yusof S., Muse R. (2003). Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chemistry, 81(4), 575–81. [DOI: 10.1016/S0308-8146(02)00498-3] [DOI] [Google Scholar]
- Zilles K. (2012). The cortex of the rat: A stereotaxic atlas. Berlin: Springer Science & Business Media. https://www.google.com/books/edition/The_Cortex_of_the_Rat/c=frontcover [Google Scholar]