Abstract
Introduction:
Today, humans live in a world surrounded by electromagnetic fields. Numerous studies have been conducted to discover the biological, physiological, and behavioral effects of electromagnetic fields on humans and animals. Given the biological similarities between monkeys and humans, The present research aimed to examine Visual Memory (VM), hormonal, genomic, and anatomic changes, in the male rhesus macaques exposed to an Extremely Low-Frequency Magnetic Field (ELF-MF).
Methods:
Four male rhesus macaques (Macaca mulatta) were used. For the behavioral tests, the animals should be fasting for 17 hours. For the tests such as visual memory, the animal’s cooperation was necessary. Using the radiation protocol, we exposed two monkeys to a 12-Hz electromagnetic field with a magnitude of 0.7 μT (electromagnetic radiation) four hours a day for a month. Before and after the exposure, a visual memory test was conducted using a coated device (visible reward) on a movable stand. Ten milliliters of blood was obtained from the femoral artery of each monkey, and half of it was used to examine cortisol serum levels using the MyBioSource kit (made in the USA). The other half of the blood was used to extract lymphocytes for assaying expressions of Glucocorticoid Receptor (GR) genes before and after radiation using the PCR method. Anatomic studies of the amygdala were carried out based on pre- and post-radiation Magnetic Resonance Imaging (MRI).
Results:
Research results indicated that visual memory in male primates increased significantly after exposure to the 12-Hz frequency. Hormonal analysis at the 12-Hz frequency showed a decrease in cortisol serum levels. However, visual memory and serum cortisol levels did not change considerably in male primates in the control group. There was no considerable amygdala volumetric difference after exposure to the 12-Hz frequency. The expression of the GR genes decreased in the 12-Hz group compared to the control group.
Conclusion:
In short, these results indicated that ELF might benefit memory enhancement because exposure to the 12-HZ ELF can enhance visual memory. This outcome may be due to a decrease in plasma cortisol and or expression of GR genes. Moreover, direct amygdala involvement in this regard cannot be recommended.
Highlights
The effects of Extremely Low-Frequency Electromagnetic Fields (ELF-EMF) of 12 Hz on monkeys were studied.
The results showed a reduction in the serum cortisol levels and the expression of GR genes.
The amygdala anatomical area changes were not significant in the experimental group.
In the experimental group, visual memory (delay of 30- and 60-s evaluation) improved after exposure to a frequency of 12 Hz.
Plain Language Summary
Extremely low-frequency electromagnetic fields are among the most important factors affecting humans. This study aimed to determine the fields of 12-Hz frequency on the visual memory changes of male monkeys. The importance of research is due to the cognitive similarity of monkeys to humans. The findings of the research can be attributed to humans. Behavioral, hormonal, genetic, and anatomical studies indicated improvement in visual memory (test monkeys versus control monkeys). This study demonstrates the effect of the 12-Hz frequency on the monkey’s visual memory. Researchers can study 12-Hz frequency in other cognitive indices.
Keywords: Electromagnetic Field, Cortisol, Glucocorticoid receptor, Rhesus monkey
1. Introduction
Electronic devices in industrial processes, research projects, energy generation and distribution, new transportation technologies, consumer goods, and medical applications have increased the exposure of living things to electromagnetic fields. Based on the research on electromagnetic and electric fields, high-pressure towers are very influential in this aspect. These fields have different effects on animals and humans ( Aliyari, Hosseinian, Sahraei, & Menhaj, 2018a; Kazemi et al., 2018). During the past decade, numerous studies have been carried out on the effects of low-frequency electromagnetic radiations on the function of different parts of the nervous system and memory in humans and animals. Although essential experiments have been conducted on humans to examine the effect of electromagnetic fields, more precise and comprehensive research has been undertaken on animal models. An important animal model for scientific research is Macaca mulatta, which is known as the rhesus macaque (monkey) ( Mitchell and Leopold, 2015; de Lorge and Grissett, 1977; Fabbri-Destro and Rizzolatti, 2008).
Cognitive-behavioral studies are among the modern studies on this animal model. Moreover, since 98% of human and rhesus macaque genes are similar, researchers used the animal model to conduct cognitive tests such as visual memory tests ( Fang et al., 2011; Kanthaswamy et al., 2013; Baharara and Zahedifar, 2012b). Electromagnetic fields might leave different effects on organisms, while Extremely Low-Frequency Eectro-magnetic Fields (ELF-EMF) lead to nerve stimulations. The effects of magnetic fields at low frequencies on the activity of the human brain have long been preoccupied in the minds of many researchers ( Marino and Becker, 1977; Kula, Sobczak, & Kuska, 2002; Sobczak, Kula, & Danch, 2002; Al-Akhras, Darmani, & Elbetieha, 2006; Zare, Hayatgeibi, Alivandi, & Ebadi, 2005).
Visual memory is among the important functions of the nervous system, severely influenced by environmental factors. Two environmental factors affecting this function are stress and anxiety, leading to visual memory dysfunction. Magnetic fields affect brain cells and brain waves, as proved in extensive research on human and animal models. ELF-EMF with different frequencies and magnitudes reduce or increase the strength of different brain frequencies. However, the magnitude and frequency of magnetic fields that change brain waves are unknown. It is only found that people’s responses depend on the duration of exposure to the field and the effects of previous fields; thus, the sensitivity of humans to magnetic fields differs from person to person and requires extensive research ( Cvetkovic and Cosic, 2009; Cvetkovic, Fang, & Cosic, 2008; Cook, Thomas, & Prato, 2002).
The amygdala plays a key role in response to fear. Save amygdala and memory consolidation in other areas of the brain are affected. Glucocorticoid receptors in the hippocampus and amygdala play an important role in acquiring and consolidating spatial and emotional information ( Lynch, 2004; Mehrdad, Yousef, Ahmad, Naser, & Abbas, 2008; Otmakhova, Duzel, Deutch, & Lisman, 2013).
There are contradictory findings on the effects of ELFEMF on the cortisol hormone. For example, with an Extremely Low-Frequency (ELF) field of 0.13 μT, adrenal gland tissue secretion (serum cortisol) decreases in guinea pigs at a frequency of 5 Hz. Moreover, cortisol variations cause variations in sensory thresholds, memory, intelligence, and concentration. These effects manifest in the form of depression, fatigue, and rarely psychosis, as well as changes in hearing, taste, and smell due to low cortisol ( Hampton, Hampstead, & Murray, 2005; Phillips et al., 2006; Cook et al., 2002; Baharara and Zahedifar, 2012b).
Quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction) is a standard method used with microarray to measure the expression of genes. Beta-actin is used as a local standard for expressing target genes, including the Glucocorticoid Receptors (GRs) ( Al-Bader and Al-Sarraf, 2005; Gantasala, Papolu, Thakur, Kamaraju, Sreevathsa, & Rao, 2013). GR is a circulating active glucocorticoid transcription factor that affects different biological functions in the body. These receptors play an essential role in nerve cells and glands. The expression of GR receptors crucially affects learning and memory, especially in hippocampus cells. The stress system activates GR receptors and memory re-consolidation in rat hippocampus ( Conrad, 2005; Heijtz, Fuchs, E., Feldon, Pryce, & Forssberg, 2010; Patel, Katz, Karssen, & Lyons, 2008; Sandi, 1998). The monkey’s memory was examined by behavioral-cognitive, hormonal, and genomic analyses.
2. Methods
Study animals
Four adult male rhesus macaques (Macaca mulatta) 4–5 years old (average weight: 4 kg) were used. In this research, two monkeys were placed in the 12-Hz electromagnetic field. Two other monkeys were not in the field of electromagnetic fields as control. The animals received all needed vaccines (Hepatitis-B, HIV, and herpes). The monkeys were kept at the animal room laboratory of Neuroscience Research Center, Baqiyatallah University of Medical Sciences, for 150 days for adaptation. The animal room condition was as follows: 12/12 h dark and light with constant temperature (24±2°C) and adequate food and water (meal at 8, 12, and 16 o’clock, and water was provided with scaled water nipples container in a 1000-mL volume designed explicitly for monkeys). All experiments were conducted according to the Baqiyatallah Medical University Medical Ethics Committee number 112–1394.
ELF exposure procedure
The ELF equipment can generate different wave frequencies from 1 to 300 Hz (made by Dr Jafargholi Laboratory, Amir Kabir University of Technology, Tehran, Iran). This generator can produce a magnetic field of 0.7 μT in a 160 cm diameter circle field. The ELF exposure was conducted for each primate as follows: each primate was transferred to the shielded room in a 1×1×1 m Teflon cage. The cage was standing at a 50 cm distance from the ELF equipment, and the wave generator was turned on. The exposure lasted 4 h/day for four weeks for each animal. The results of the present study are descriptive.
Visual Memory (VM) response task
A device for recording (VM) behavior (visible) was designed for this test. The two coated dishes were on a movable stand ( Cook et al., 2002; Richter-Levin and Akirav, 2000; Kazemi et al., 2017 ). The animals have entered the test after 17 hours of fasting. The primates were transferred to the behavioral test room separately, and the test was carried out in 2 phases (four weeks, ten times a day).
Phase I: The visual memory behavior recorder was placed before the primates, and the primate’s favorite reward (peanut) was randomly put in one of the dishes. After 30 seconds, the dish was presented to the animal on a movable stand. The animal was allowed to make only one attempt to pick the reward from one of the two dishes. In other words, the animal had to remember the dish containing the reward, and if it opened the wrong container, it would be deprived of the reward. This test was carried out three times a day for 30 seconds delay before the eyes of the primate. This method was repeated in all four phases of the visual memory behavior function test.
Phase II: To record visual memory behavior with a visible reward, the peanut was put in one of the coated dishes before the eyes of the animal, and the dish was presented to the animal following a delay of 60 seconds with a movable stand. The second phase was repeated three times a day ( Constantinidis and Procyk, 2004; Kazemi et al., 2018).
Magnetic Resonance Imaging (MRI)
The last phase of the tests consisted of Magnetic Resonance Imaging (MRI) of primates’ brains. To this end, the first arrangements were made with Imam Khomeini MRI Center. The primates had to be fasting for 9 hours before the MRI. Moreover, the primates were anesthetized with ketamine (10–20 mg/kg) before the MRI, and their brains were imaged using a 3T MRI device in the T1 and T2 phases with 3-mm sections of the axial, sagittal, and coronal regions (for better anatomic interpretation of the regions of concern). In the image interpretation phase, volumetric assessments of a special part of the memory, including the right amygdala, were analyzed in the ImageJ application (Shanthi and Singh). In interpreting MRI images of the primates, the T2 technique was used.
Hormonal assays
To obtain the blood samples, the primates were put in a stabilized state of consciousness, and 10 mL of blood was obtained from the femoral region (popliteal artery). Blood samples were obtained to measure neurotransmitter, hormonal and genomic factors before and after radiation and recovery phases. The blood samples were divided into two parts. The first part was centrifuged at 3000 RPM for 5 minutes at a temperature of 4°C, and the serum content was isolated and assayed to measure and compare variations of serum hormones using diagnostic kits for cortisol (purchased from MyBioSource, USA, with the 0.04–2000 mL/ng standard and unit range). Various hormone concentrations were measured in the pre-radiation, post-radiation, and recovery phases.
Glucocorticoid receptor assays
The second part of the blood samples was used for cellular and molecular assays. To this end, after collecting the blood samples, blood lymphocytes were isolated using the Ficoll solution in a centrifuge that was used for 5 minutes at 1500 rpm at the beginning, followed by another 15 minutes at 2500 rpm. The isolated lymphocytes were tested to determine the expression of GR genes using the PCR technique. Using peripheral blood lymphocytes, the expressed GR receptors were analyzed by PCR. To assess the impact of the expression of visual learning and visual memory of GR genes involved in mature and immature monkeys, we used the semi-quantitative Reverse Transcriptase-Polymerase Chain Reaction (semi-RT-PCR). The peripheral blood sample was collected from each animal at a related time, and the total mRNA was purified by the RNX-Plus kit (CinnaGen, Iran) under the manufacturer’s guidelines. The quantity and quality of each isolated RNA were evaluated using the NanoDrop spectrophotometer (Thermos, USA) and agarose gel electrophoresis, respectively. After that, to synthesize cDNA from each sample, the Bioneer kit (Takara, Japan) was applied. Briefly, 100 ng of each RNA sample was converted to cDNA by the master mix containing M-MLV (Moloney murine leukemia virus) reverse transcriptase, random hexamers, oligo(dT), and related buffer. Finally, the GR-2A gene expression was detected using PCR and the related specific primer set (Table 1). The mRNA expression of β-actin was surveyed as an internal control. All PCR reactions were performed in a thermocycler (Techne, UK) containing 1.5 μL cDNA, 0.2 mM of the deoxynucleic ide triphosphates (dNTPs), 2.5 mM MgCl2, 10 pmol of each primer and 1.5 U of Taq DNA Polymerase (CinnaGen, Iran). PCR program included 6 min initial denaturation at 94°C, 35 cycles of 45 s at 95°C, 45 s at 58°C for GR. To measure the density of amplicons, each PCR product was run on 2% agarose gel electrophoresis, stained by ethidium bromide, and visualized under a UV gel document. Finally, the density of each product band was measured by ImageJ software ( Hillmann, Ramdas, Multanen, Norman, & Harmon, 2000; Hosseini, Soleimanirad, Aghdam, Amin, & Fooladi, 2015).
Table 1.
Amygdala area sagittal section, before and after radiation (control and experiment)
| Monkey Codes | Amygdala Area (mm3) | |
|---|---|---|
|
| ||
| Pre-Radiation | Post-Radiation | |
| (12-Hz) B–F | 10.998 | 10.128 |
| (control) D–E | 13.581 | 13.683 |
The coronal sections show the D–E and B–F monkeys’ amygdala area, with the sagittal sections resulting from type T2 MRI before and after radiation.
3. Results
Results on the percentage of correct answers after irradiation the effects of ELF waves on visual memory and visual memory function of the primates revealed that in the B–F represents the experimental group who irradiated experimental monkeys (which were exposed to the 0.7 μT field at 12 Hz), visual memory (with a visible reward) changed considerably following the radiation. However, in the D–E group of control monkeys, visual memory did not change considerably (with a visible reward) (Figures 1, 2, 3).
Figure 1.
Visual memory response task
It shows the design of the two Plexiglas coated dishes (containing invisible reward). The lid of the dish opens only in one direction. The dish is placed on a movable stand and can be moved quickly.
Figure 2.
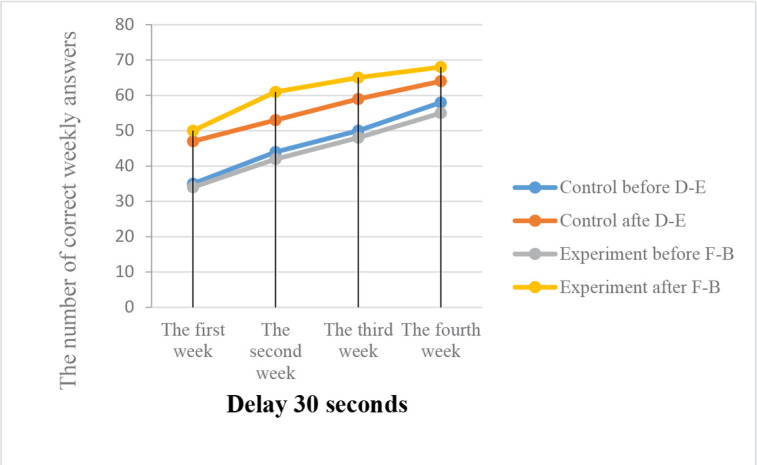
Visual memory before and after radiation under the effect of the 12-Hz wavelengths and the 0.7 μT electromagnetic field
The Figure shows the percentage of correct answers after irradiation. The visual memory response task (visible reward) of the D–E control and B–F monkeys in phase 1 (delay 30 seconds).
Figure 3.
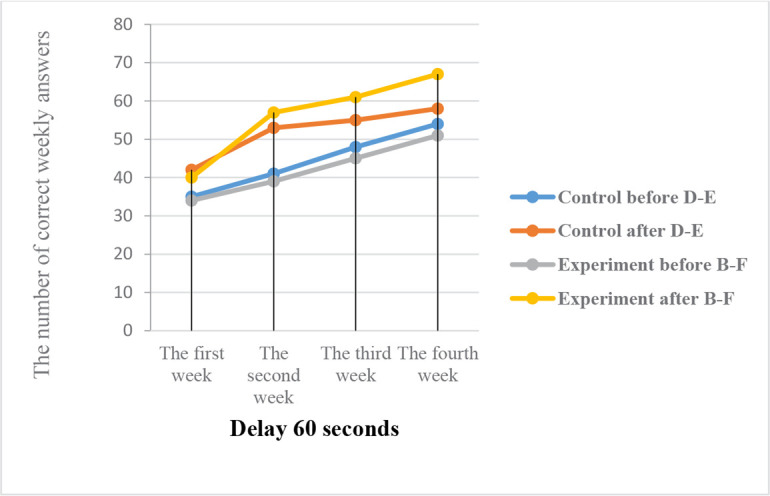
Visual memory before and after radiation under the effect of the 12-Hz wavelengths and the 0.7 μT electromagnetic field
The Figure shows the percentage of correct answers after irradiation. The visual memory response task (visible reward) of the D–E control and B–F experimental monkeys in phase 2 (delay 60 seconds).
Results of anatomical assays at the 12 HZ frequencies using the 0.7 μT field (pre and post-radiation) of the sagittal section of the left amygdala using ImageJ software (locations are indicated by the pointer) (Figures 4, 5; Table 1).
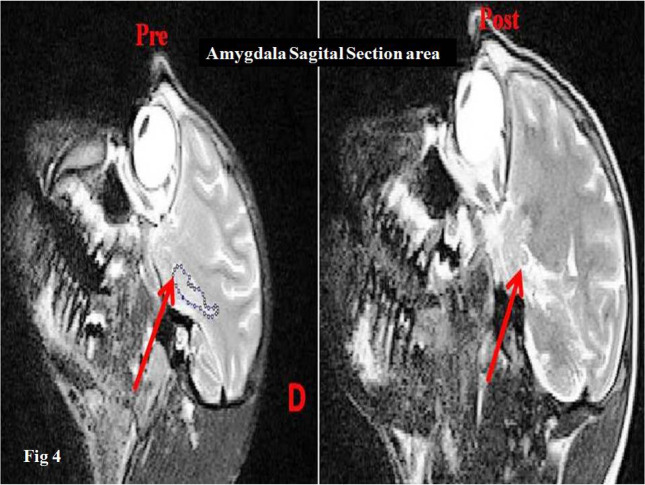
Figure 4.
Amygdala area sagittal section, before and after radiation (cod D)
Surface analysis (before and after radiation) of the left amygdala of the control monkeys, using ImageJ software.
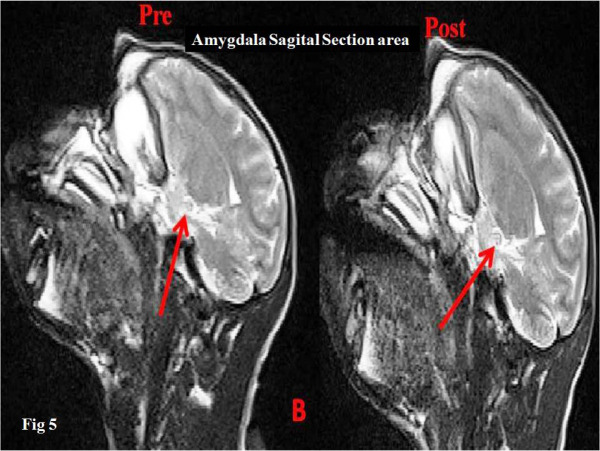
Figure 5.
Amygdala area sagittal section, before and after radiation (cod B)
Surface analysis (pre- and post-radiation) of the left amygdala of the experimental monkeys, using Image J software
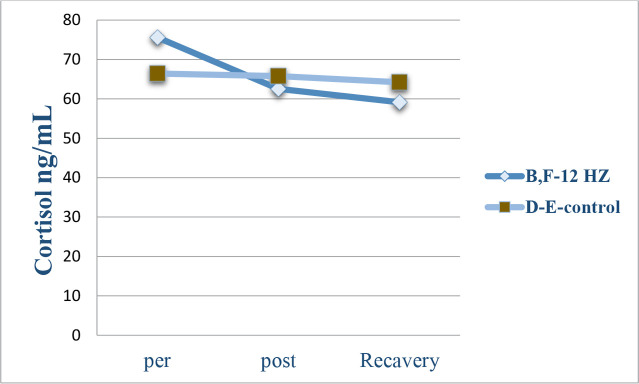
Variations of cortisol under the impact of the 12 Hz and the 0.7 μT field in the B–F experimental monkeys decreased considerably following the radiation. Besides, cortisol changes in the D –E control monkeys showed an inconsiderable following. The recovery of both primates involved restoring their states to the pre-radiation phase (Figure 6)
Figure 6.
The level of plasma cortisol in the control and experimental groups
It shows variations of cortisol levels in B–F monkeys before and after radiation with the 12-Hz wavelengths and the 0.7 μT field magnitude
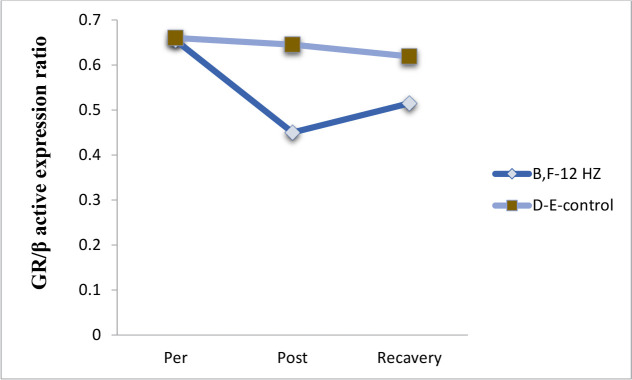
Effects of radiation (12 Hz with the 0.7 μT field) on the expression of GR in B–F experimental monkeys showed a decrease in the expression of GR. In addition, variations of the GR gene in the D–E monkeys showed an inconsiderable. The recovery of both primates involved restoring their states to the pre-radiation phase (Figure 7).
Figure 7.
The expression of the GR receptor gene in the control and experimental group
It shows changes in expression of the GR receptor in the D–E control and B–F experimental monkeys before and after radiation with the 12-Hz wavelength and the 0.7 μT field.
4. Discussion
With the increased presence of electromagnetic fields in modern industrial societies, the study of the biological effect of extremely low-frequency electromagnetic fields (as essential factors influencing environments) with different magnitudes has gained the attention of researchers in the past decades. We studied the effects of low-frequency (12 Hz) electromagnetic waves with the 0.7 μT magnetic field magnitude on the cognitive abilities of the primates.
Results of studying the effects of ELF-MF waves on animal and human models are contradictory. According to the literature, the effects of electromagnetic fields depend on wave properties, radiation period, and the biological and physiological properties of humans and animals ( Baharara and Zahedifar, 2012a; Wingenfeld and Wolf, 2015). Also, magnetic fields with a frequency of 50 Hz impair memory and learning. Results of research by other researchers have also revealed that ELF waves with 10 and 30 Hz frequencies and a field magnitude of 2 μT improved memory and learning in the experimental group of rats significantly compared to the control group ( An et al., 2015; Casile, 2013; D’Angelo, Costantini, E., Kamal, & Reale, 2015; Rimbach, Link, Montes-Rojas, Di Fiore, Heistermann, & Heymann, 2014; Sakhnini, L., Al-Ghareeb, Khalil, Ahmed, Ameer, & Kamal, 2014). In this research, the effects of ELF on the most important memory cognitive factor were studied at two different frequencies, which matched the delta (1 Hz) and alpha (12 Hz) brain wave frequencies. Visual information reaches the occipital cortex. This information, directly and indirectly, flows to the hippocampus, which plays a significant role in learning processes and memory consolidation (especially spatial memory). Mammals use environmental signals and visual signals to learn and memorize a position in the space or their physical position in the space or the environment ( Sakhnini et al., 2014; Kiorpes and Movshon, 2004).
Cognitive functions (such as attention and visual memory) increase processing speed and relationships between different brain parts. Another outcome is the improvement of communications between different brain members, especially the hippocampus (the origin of memory), the prefrontal cortex (the origin of concentration and thinking), the cerebellum (controls conscious body movements), and optic tracts ( Murphy, Arnsten, Jentsch, & Roth, 1996; Kalin, Shelton, & Davidson, 2004). Every learning process starts with attention and develops with the activation of the working memory. Without adequate information, the person experiences learning impairments. Attention is one of the important sublime mental activities and main dimensions of the cognitive structure. It plays a significant role in the structure of intelligence, memory, and perception ( Basile and Hampton, 2013; Hampton, 2001; Lavenex and Lavenex, 2006; Pipa et al., 2009).
Results of cognitive tests show that after radiation, visual memory (visible) increased significantly after 30 and 60 seconds at the 12 Hz frequency as compared to the pre-radiation state. Besides, the behavior displayed by the B–F monkeys after exposure to the 12 Hz frequency was interesting. The B–F monkeys were highly restless, reckless, and noisy, and everybody was complaining about their noisiness. However, they were very calm and careful after being radiated at the 12 Hz frequency. The visual memory of these monkeys did not change significantly in the control groups, and no significant difference was also observed in their behavior. In cognitive tests, attention and concentration play substantial roles in vision, and the activity of mirror neurons is the result of careful observation. Mirror neurons are located in the F5 prefrontal region ( Caggiano, Pomper, Fleischer, Fogassi, Giese, & Thier, 2013; Fabbri-Destro and Rizzolatti, 2008; Tallon-Baudry, Mandon, Freiwald, & Kreiter, 2004).
Research results indicate that in different phases of memorization, numerous structures are involved in the brain. Although there is no special memory center in the brain, the role of the frontal lobe, hippocampus, and amygdala in memory is more significant. Studies on vision or the encoding phase are primarily centered on the left hemisphere, which is more involved in retrieving information ( McLeod, 2007; Keller and Roberts, 2009; Newman, J. D., Kenkel, Aronoff, Bock, Zametkin, & Silva, 2009; Tae et al., 2008). Amygdala substantially contributes to spatial memory and emotional learning, while GR receptors of the amygdala and hippocampus are extensively involved in consolidating spatial and emotional information ( Chou et al., 2002; Sandi, 1998). Afterward, the amygdala was studied using MRI and the T2 technique based on sagittal sections of the D–E and B–F monkeys. Results of volumetric assessments of the left amygdala in the B–F monkeys (12-Hz frequency) and the D–E (control group) did not show a drastic change in the post-radiation phase compared to the pre-radiation phase.
In humans/primates and rats, cortisol and corticosterone significantly regulate cognitive processes ( Kazemi et al., 2018; Aliyari et al., 2018b; Tekieh et al., 2017). Other reports suggest that rats in stressful environments show increased plasma corticosteroid concentration and eventually learning and memory impairments. Also, exposure of rats to radio waves increases plasma corticosterone levels and results in spatial memory impairment ( Akirav, Kozenicky, Tal, Sandi, Venero, & Richter-Levin, 2004; Baker and Kim, 2002). Research results suggest that under the effect of ELF waves (at the 12 Hz frequency), the level of corticosterone hormone decreased in rats ( Mahdavi, Rezaei-Tavirani, Nikzamir, & Ardeshirylajimi, 2014). Concerning cortisol levels in the B–F monkeys and the D–E control monkeys revealed that the cortisol level decreased in the B–F monkeys (12 Hz frequency) following radiation. This finding is also in line with findings reported by other researchers. However, changes in cortisol levels in control monkeys were not significant. Restoration of hormonal changes to the pre-radiation state in the recovery phase reflects unstable changes, which can be both positive and negative. If the purpose of radiation is to enhance memory function, this result is a negative outcome because it is unstable. However, if radiation is meant to destroy, it is a positive outcome as it is not stable and is transient. It is worth stating that cortisol is an essential hormone secreted by the adrenal cortex in humans in response to stress factors. When the brain considers a factor to be a threat factor (stress factor), the amygdala is activated and stimulates the hypothalamic par ventricular nucleus, and the neurons of this nucleus discharge the corticotropin-releasing hormone into the blood ( Balbo, Leproult, & Van Cauter, 2010; Sandi, 1998).
It is worth stating that the glucocorticoid and glutamate systems of the brain are among the most important parts of the nervous and hormonal systems of the body, which are influenced by environmental conditions. Since the expression of gene and receptor proteins of these two systems in the peripheral blood lymphocytes are similar to the nervous system and change under the effect of ELF waves, these two properly manifest the performance of the two systems mentioned above. The expression of the GR gene was tested using the PCR technique. White blood cells in monkeys are mostly formed of lymphocytes, and GRs in lymphocyte cells are similar to the GRs in neurons of the central nervous system. Therefore, the frequency (%) of lymphocytes in the blood of monkeys allows for the examination of the expression of the GR gene and the examination of the effects of ELF waves on the two receptor genes. Expression of the GR gene is associated with cognitive behavior, including memory and learning ( Conrad, 2005; Heijtz et al., 2010; Magee, Blum, Lates, & Jusko, 2002; Vugmeyster, Howell, Bakshi, Flores, Hwang, & McKeever, 2004). Beta-actin is widely used as a local standard in biological research regardless of animal and tissue types. GR gene beta-actin was used as an internal control ( Al-Bader and Al-Sarraf, 2005; Gantasala et al., 2013). One of the most important functions of stress hormones is the excessive activation of the brain glutamate system, followed by extensive destruction of different brain regions. Therefore, researchers believe that a decrease in memory following chronic stress is caused by the effects of stress hormones on the increased activity of the glutamate system. Previous research also suggests that glucocorticoids affect many cognitive processes. For example, acute administration of compounds such as corticosterone increases long-term memory consolidation dose-dependent ( Phillips et al., 2006; Sandi, 1998; Pipa et al., 2009). The effect of ELF waves on the expression of the GR gene in the B–F monkeys (12 Hz) was studied with the 0.7 μT field. The investigations showed that GR gene expression in the B–F primate (12-Hz frequency) decreased slightly following the radiation, and GR gene expression in the control group did not change. Besides, the recovery involved restoration to the pre-radiation state. One of the mechanisms of action of glucocorticoids is the alteration of gene expression following activation of intracellular receptors. GRs are widely scattered in the brains of reptiles, and their concentration is higher in the hippocampus, par ventricular nucleus, and hypothalamus. Recent research indicates that the effects of glucocorticoids on memory processes are efficacious. These immediate effects are seemingly caused by the interaction of glucocorticoids with several neurotransmitters in the brain. Hence, the effects of glucocorticoids on memory and memory consolidation may be exerted via excitatory amino acids and their receptors ( Finsterwald and Alberini, 2014; Conrad, 2005; Patel et al., 2008). On the other hand, the role of calcium ions and calcium channels in memory has also been confirmed because glucocorticoids increase the concentration of calcium ions and activation of calcium channels. Therefore, one of the mechanisms involved in the influence of glucocorticoids on memory may be the increase in the activity of calcium channels. ELF-MF fields, like environmental factors, probably have inhibitory effects on the expression of the GR gene. ELF-MF waves impair glucocorticoid receptors. Stress, fear, and anxiety exert negative impacts on cognition and function of the central nervous system. Moreover, cortisol (the stress system) and the adrenal sympathetic system (the emotion system) can also have drastic effects ( Lewczuk, Redlarski, G., Żak, Ziółkowska, Przybylska-Gornowicz, & Krawczuk, 2014; Sandi, 2011; Touitou and Selmaoui, 2012; Mahdavi et al., 2014). The prefrontal cortex receives sensory inputs from the limbic system. After processing information and experience, the resulting data are stored in the form of memory in certain regions. The processed data are stored within several seconds in the form of information in the short-term memory and then in the long-term memory in a more stable form and eventually in the hippocampus. Similar to the present research, visual memory was tested for 30 seconds and 60 seconds after exposure to ELF-MF, respectively ( Kandel, Dudai, & Mayford, 2014; Mayford, Siegelbaum, & Kandel, 2012; Pipa et al., 2009). Based on cortisol assessment research, visual memory is directly related to the cognitive behavior of the monkey. Abnormal increase and decrease in cortisol levels result in cognitive changes in the monkey relative to the effective dose. In 2018, Hamed Aliyari et al. reported that the electric field of the high-voltage fields’ tower caused an excessive reduction in cortisol levels compared to basal cortisol, which represent of cognitive impairment in monkey (weakening of visual working memory) and depression. Nevertheless, in this study, the cortisol concentration was lower than the basal level, improved cognitive index (visual memory). Electromagnetic magnitude reduced the concentration of cortisol (effective dose). An effective dose and any stimulus that changes the concentration of cortisol results in cognitive changes in humans and animals ( Tekieh et al., 2017; Aliyari et al., 2018a). Reduced expression of the GR genes enhances the cognitive memory of the visual memory in the monkey. Results of radiation at the 12-Hz frequency with the 0.7 μT field decreased the expression of the GR gene. The decrease in expression of the GR gene and cortisol concentration improved visual memory in the B–F monkeys following exposure to an electromagnetic field at the 12-Hz frequency. The result of the cognitive behavior of B–F monkeys showed that they were more relaxed, vivacious, and careful. So, it can be said that all the extremely low-frequency electromagnetic fields are not harmful because their harm depends on frequency, field magnitude, radiation lengths, and other factors. Perhaps further research may identify other useful frequencies, such as the frequencies used in neuro-feedback. However, the attainment of this goal calls for more fundamental studies by interested researchers.
Ethical Considerations
Compliance with ethical guidelines
All ethical standards were met based on the international ir.bmsu.rec.1394.112 (Code: 12345).
Acknowledgments
The author would like to express their gratitude to the Neuroscience Research Center of Baqiyatallah University of Medical Sciences.
Footnotes
Funding
This research was conducted with the financial support of the Neuroscience Research Center, Baqiyatallah Medical Science University.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Akirav I., Kozenicky M., Tal D., Sandi C., Venero C., Richter-Levin G. (2004). A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learning & Memory, 11(2), 188–95. [DOI: 10.1101/lm.61704] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bader M. D., Al-Sarraf H. A. (2005). Housekeeping gene expression during fetal brain development in the rat-validation by semi-quantitative RT-PCR. Developmental Brain Research, 156(1), 38–45. [DOI: 10.1016/j.devbrainres.2005.01.010] [PMID ] [DOI] [PubMed] [Google Scholar]
- Al-Akhras M. A., Darmani H., Elbetieha A. (2006). Influence of 50 Hz magnetic field on sex hormones and other fertility parameters of adult male rats. Bioelectromagnetics, 27(2), 127–31. [DOI: 10.1002/bem.20186] [PMID ] [DOI] [PubMed] [Google Scholar]
- Aliyari H., Hosseinian S., Sahraei H., Menhaj M. (2018a). Effect of proximity to high-voltage fields: results of the neural network model and experimental model with macaques. International Journal of Environmental Science and Technology, 16(8), 4315–26. [DOI: 10.1007/s13762-018-1830-8] [DOI] [Google Scholar]
- Aliyari H., Sahraei H., Daliri M. R., Minaei-Bidgoli B., Kazemi M., Agaei H., et al. (2018b). The beneficial or harmful effects of computer game stress on cognitive functions of players. Basic and Clinical Neuroscience, 9(3), 177–86. [DOI: 10.29252/nirp.bcn.9.3.177] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G. Z., Xu H., Zhou Y., Du L., Miao X., Jiang D. P., et al. (2015). Effects of long-term 50Hz power-line frequency electromagnetic field on cell behavior in Balb/c 3T3 cells. PloS One, 10(2), e0117672. [DOI: 10.1371/journal.pone.0117672] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharara J., Zahedifar Z. (2012a). [The effect of low-frequency electromagnetic fields on some biological activities of animals (Persian)]. Journal of Arak University of Medical Sciences, 15(66), 80–93. http://jams.arakmu.ac.ir/article-1-1144-en.html [Google Scholar]
- Baharara J., Zahedifar Z. (2012b). [The effect of low-frequency electromagnetic fields on some biological activities of animals (Persian)]. Journal of Arak University of Medical Sciences, 15(7), 80–93. http://jams.arakmu.ac.ir/article-1-1144-en.html [Google Scholar]
- Baker K. B., Kim J. J. (2002). Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning & Memory, 9(2), 58–65. [DOI: 10.1101/lm.46102] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbo M., Leproult R., Van Cauter E. (2010). Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. International Journal of Endocrinology, 2010, 759234. [DOI: 10.1155/2010/759234] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile B. M., Hampton R. R. (2013). Dissociation of active working memory and passive recognition in rhesus monkeys. Cognition, 126(3), 391–6. [DOI: 10.1016/j.cognition.2012.10.012] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano V., Pomper J. K., Fleischer F., Fogassi L., Giese M., Thier P. (2013). Mirror neurons in monkey area F5 do not adapt to the observation of repeated actions. Nature Communications, 4, 1433. [DOI: 10.1038/ncomms2419] [PMID ] [DOI] [PubMed] [Google Scholar]
- Casile A. (2013). Mirror neurons (and beyond) in the macaque brain: An overview of 20 years of research. Neuroscience Letters, 540, 3–14. [DOI: 10.1016/j.neulet.2012.11.003] [PMID ] [DOI] [PubMed] [Google Scholar]
- Chou C. F., Tegenfeldt J. O., Bakajin O., Chan S. S., Cox E. C., Darnton N., et al. (2002). Electrodeless dielectrophoresis of single-and double-stranded DNA. Biophysical Journal, 83(4), 2170–9. [DOI: 10.1016/S0006-3495(02)73977-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C. D. (2005). The relationship between acute glucocorticoid levels and hippocampal function depends upon task aversiveness and memory processing stage. Nonlinearity in Biology, Toxicology, Medicine, 3(1), 57–78. [DOI: 10.2201/nonlin.003.01.004] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C., Procyk E. (2004). The primate working memory networks. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 444–65. [DOI: 10.3758/CABN.4.4.444] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C., Thomas A., Prato F. (2002). Human electrophysiological and cognitive effects of exposure to ELF magnetic and ELF modulated RF and microwave fields: A review of recent studies. Bioelectromagnetics, 23(2), 144–57. [DOI: 10.1002/bem.107] [PMID ] [DOI] [PubMed] [Google Scholar]
- Cvetkovic D., Cosic I. (2009). Alterations of human electroencephalographic activity caused by multiple extremely low frequency magnetic field exposures. Medical & Biological Engineering & Computing, 47(10), 1063–73. [DOI: 10.1007/s11517-009-0525-1] [PMID ] [DOI] [PubMed] [Google Scholar]
- Cvetkovic D., Fang Q., Cosic I. (2008). Multiple human electrophysiological responses to extremely low frequency pulsed electromagnetic field exposures: A pilot study. Estonian Journal of Engineering, 14(2), 138–53. [DOI: 10.3176/eng.2008.2.04] [DOI] [Google Scholar]
- D’Angelo C., Costantini E., Kamal M., Reale M. (2015). Experimental model for ELF-EMF exposure: Concern for human health. Saudi Journal of Biological Sciences, 22(1), 75–84. [DOI: 10.1016/j.sjbs.2014.07.006] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorge J., Grissett J. (1977). Behavioral effects in monkeys exposed to extremely low frequency electromagnetic fields. International Journal of Biometeorology, 21(4), 357–65. [DOI: 10.1007/BF01555197] [PMID ] [DOI] [PubMed] [Google Scholar]
- Fabbri-Destro M., Rizzolatti G. (2008). Mirror neurons and mirror systems in monkeys and humans. Physiology, 23(3), 171–9. [DOI: 10.1152/physiol.00004.2008] [PMID ] [DOI] [PubMed] [Google Scholar]
- Fang X., Zhang Y., Zhang R., Yang L., Li M., Ye K., et al. (2011). Genome sequence and global sequence variation map with 5.5 million SNPs in Chinese rhesus macaque. Genome Biology, 12(7), R63. [DOI: 10.1186/gb-2011-12-7-r63] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwald C., Alberini C. M. (2014). Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiology of Learning and Memory, 112, 17–29. [DOI: 10.1016/j.nlm.2013.09.017] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantasala N. P., Papolu P. K., Thakur P. K., Kamaraju D., Sreevathsa R., Rao U. (2013). Selection and validation of reference genes for quantitative gene expression studies by real-time PCR in eggplant (Solanum melongena L). BMC Research Notes, 6, 312. [DOI: 10.1186/1756-0500-6-312] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton R. R. (2001). Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences, 98(9), 5359–62. [DOI: 10.1073/pnas.071600998] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton R. R., Hampstead B. M., Murray E. A. (2005). Rhesus monkeys (Macaca mulatta) demonstrate robust memory for what and where, but not when, in an open-field test of memory. Learning and Motivation, 36(2), 245–59. [DOI: 10.1016/j.lmot.2005.02.004] [DOI] [Google Scholar]
- Heijtz R. D., Fuchs E., Feldon J., Pryce C. R., Forssberg H. (2010). Effects of antenatal dexamethasone treatment on glucocorticoid receptor and calcyon gene expression in the prefrontal cortex of neonatal and adult common marmoset monkeys. Behavioral and Brain Functions, 6, 18. [DOI: 10.1186/1744-9081-6-18] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann A. G., Ramdas J., Multanen K., Norman M. R., Harmon J. M. (2000). Glucocorticoid receptor gene mutations in leukemic cells acquired in vitro and in vivo. Cancer Research, 60(7), 2056–62. [PMID ] [PubMed] [Google Scholar]
- Hosseini H. M., Soleimanirad J., Aghdam E. M., Amin M., Fooladi A. A. I. (2015). Texosome-anchored superantigen triggers apoptosis in original ovarian cancer cells. Medical Oncology, 32(1), 409. [DOI: 10.1007/s12032-014-0409-6] [PMID ] [DOI] [PubMed] [Google Scholar]
- Kalin N. H., Shelton S. E., Davidson R. J. (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of Neuroscience, 24(24), 5506–15. [DOI: 10.1523/JNEUROSCI.0292-04.2004] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Dudai Y., Mayford M. R. (2014). The molecular and systems biology of memory. Cell, 157(1), 163–86. [DOI: 10.1016/j.cell.2014.03.001] [PMID ] [DOI] [PubMed] [Google Scholar]
- Kanthaswamy S., Ng J., Ross C. T., Trask J. S., Smith D. G., Buffalo V. S., et al. (2013). Identifying human-rhesus macaque gene orthologs using heterospecific SNP probes. Genomics, 101(1), 30–7. [DOI: 10.1016/j.ygeno.2012.09.001] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M., Sahraei H., Aliyari H., Tekieh E., Saberi M., Tavacoli H., et al. (2018). Effects of the extremely low frequency electromagnetic fields on NMDA-receptor gene expression and visual working memory in male rhesus macaques. Basic and Clinical Neuroscience, 9(3), 167–76. [DOI: 10.29252/NIRP.BCN.9.3.167] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. S., Roberts N. (2009). Measurement of brain volume using MRI: Software, techniques, choices and prerequisites. Journal of Anthropological Sciences, 87, 127–51. [PMID ] [PubMed] [Google Scholar]
- Kiorpes L., Movshon J. A. (2004). Development of sensitivity to visual motion in macaque monkeys. Visual Neuroscience, 21(06), 851–9. [DOI: 10.1017/S0952523804216054] [PMID ] [DOI] [PubMed] [Google Scholar]
- Kula B., Sobczak A., Kuska R. (2002). Effects of electromagnetic field on free-radical processes in steelworkers. Part I: Magnetic field influence on the antioxidant activity in red blood cells and plasma. Journal of Occupational Health, 44(4), 226–9. [DOI: 10.1539/joh.44.226] [DOI] [Google Scholar]
- Lavenex P., Lavenex P. B. (2006). Spatial relational memory in 9-month-old macaque monkeys. Learning & Memory, 13(1), 84–96. [DOI: 10.1101/lm.97606] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk B., Redlarski G., Żak A., Ziółkowska N., Przybylska-Gornowicz B., Krawczuk M. (2014). Influence of electric, magnetic, and electromagnetic fields on the circadian system: current stage of knowledge. BioMed Research International, 2014, 169459. [DOI: 10.1155/2014/169459] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. (2004). Long-term potentiation and memory. Physiological Reviews, 84(1), 87–136. [DOI: 10.1152/physrev.00014.2003] [PMID ] [DOI] [PubMed] [Google Scholar]
- Magee M. H., Blum R. A., Lates C. D., Jusko W. J. (2002). Pharmacokinetic/pharmacodynamic model for prednisolone inhibition of whole blood lymphocyte proliferation. British Journal of Clinical Pharmacology, 53(5), 474–84. [DOI: 10.1046/j.1365-2125.2002.01567.x] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi S. M., Rezaei-Tavirani M., Nikzamir A., Ardeshirylajimi A. (2014). 12 Hz electromagnetic field changes stress-related hormones of rat. Journal of Paramedical Sciences, 5(4), 83–8. [DOI: 10.22037/jps.v5i4.78] [DOI] [Google Scholar]
- Marino A. A., Becker R. O. (1977). Biological effects of extremely low frequency electric and magnetic fields: A review. Physiological Chemistry and Physics, 9(2), 131–47. [PMID ] [PubMed] [Google Scholar]
- Mayford M., Siegelbaum S. A., Kandel E. R. (2012). Synapses and memory storage. Cold Spring Harbor Perspectives in Biology, 4(6), a005751. [DOI: 10.1101/cshperspect.a005751] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod S. (2007). Stages of memory-encoding storage and retrieval. Retrieved from http://blogs.spsk12.net/8576/files/2015/11/Day-1-Memory-homework-reading.docx [Google Scholar]
- Mehrdad J., Yousef S., Ahmad H., Naser N., Abbas P. (2008). Working memory learning method and astrocytes number in different subfields of rat’s Hippocampus. American Journal of Animal and Veterinary Sciences, 3(1), 28–31. [DOI: 10.3844/ajavsp.2008.28.31] [DOI] [Google Scholar]
- Mitchell J. F., Leopold D. A. (2015). The marmoset monkey as a model for visual neuroscience. Neuroscience Research, 93, 20–46. [DOI: 10.1016/j.neures.2015.01.008] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. L., Arnsten A. F., Jentsch J. D., Roth R. H. (1996). Dopamine and spatial working memory in rats and monkeys: Pharmacological reversal of stress-induced impairment. The Journal of Neuroscience, 16(23), 7768–75. [DOI: 10.1523/JNEUROSCI.16-23-07768.1996] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. D., Kenkel W. M., Aronoff E. C., Bock N. A., Zametkin M. R., Silva A. C. (2009). A combined histological and MRI brain atlas of the common marmoset monkey, Callithrix jacchus. Brain Research Reviews, 62(1), 1–18. [DOI: 10.1016/j.brainresrev.2009.09.001] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova N., Duzel E., Deutch A. Y., Lisman J. (2013). The hippocampal-VTA loop: The role of novelty and motivation in controlling the entry of information into long-term memory. In Baldassarre G., Mirolli M.(Eds.), Intrinsically Motivated Learning in Natural and Artificial Systems. Berlin: Springer; (pp. 235–54). [DOI: 10.1007/978-3-642-32375-1_10] [DOI] [Google Scholar]
- Patel P. D., Katz M., Karssen A. M., Lyons D. M. (2008). Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology, 33(3), 360–7. [DOI: 10.1016/j.psyneuen.2007.12.003] [PMID ] [PMCID] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. J., McGorry P. D., Garner B., Thompson K. N., Pantelis C., Wood SJ, et al. (2006). Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: Implications for the development of psychotic disorders. Australian and New Zealand Journal of Psychiatry, 40(9), 725–41. [DOI: 10.1080/j.1440-1614.2006.01877.x] [PMID ] [DOI] [PubMed] [Google Scholar]
- Pipa G., Städtler E. S., Rodriguez E. F., Waltz J. A., Muckli L., Singer W., et al. (2009). Performance-and stimulus-dependent oscillations in monkey prefrontal cortex during short-term memory. Frontiers in Integrative Neuroscience 3, 25. [DOI: 10.3389/neuro.07.025.2009] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G., Akirav I. (2000). Amygdala-hippocampus dynamic interaction in relation to memory. Molecular Neurobiology, 22(1–3), 11–20. [DOI: 10.1385/MN:22:1-3:011] [PMID ] [DOI] [PubMed] [Google Scholar]
- Rimbach R., Link A., Montes-Rojas A., Di Fiore A., Heistermann M., Heymann E. W. (2014). Behavioral and physiological responses to fruit availability of spider monkeys ranging in a small forest fragment. American Journal of Primatology, 76(11), 1049–61. [DOI: 10.1002/ajp.22292] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhnini L., Al-Ghareeb S., Khalil S., Ahmed R., Ameer A. A., Kamal A. (2014). Effects of exposure to 50Hz electromagnetic fields on Morris water-maze performance of prenatal and neonatal mice. Journal of the Association of Arab Universities for Basic and Applied Sciences, 15(1), 1–5. [DOI: 10.1016/j.jaubas.2013.05.004] [DOI] [Google Scholar]
- Sandi C. (1998). The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plasticity, 6(3), 41–52. [DOI: 10.1155/NP.1998.41] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C. (2011). Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends in Neurosciences, 34(4), 165–76. [DOI: 10.1016/j.tins.2011.01.006] [PMID ] [DOI] [PubMed] [Google Scholar]
- Shanthi V., Singh D. Estimation of hippocampus volume from MRI using ImageJ for Alzheimer’s diagnosis. Atlas Journal of Medical & Biological Sciences, 1(1), 15–20. https://www.researchgate.net/profile/L-Jaba-Sheela/publication/289824108_Estimation_of_Hippocampus_Volume_from_MRI_Using_ImageJ_for_Alzheimer’s_Diagnosis/links/5dccc2564585156b351033dc/Estimation-of-Hippocampus-Volume-from-MRI-Using-ImageJ-for-Alzheimers-Diagnosis.pdf [Google Scholar]
- Sobczak A., Kula B., Danch A. (2002). Effects of electromagnetic field on free-radical processes in steelworkers. Part II: Magnetic field influence on vitamin A, E and selenium concentrations in plasma. Journal of Occupational Health, 44(4), 230–3. [DOI: 10.1539/joh.44.230] [DOI] [Google Scholar]
- Tae W. S., Kim S. S., Lee K. U., Nam E. C., Kim K. W. (2008). Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology, 50(7), 569–81. [DOI: 10.1007/s00234-008-0383-9] [PMID ] [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Mandon S., Freiwald W. A., Kreiter A. K. (2004). Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cerebral Cortex, 14(7), 713–20. [DOI: 10.1093/cercor/bhh031] [PMID ] [DOI] [PubMed] [Google Scholar]
- Tekieh E., Riahi E., Kazemi M., Sahraei H., Tavakoli H., Aliyary H., et al. (2017). Role of basal stress hormones and amygdala dimensions in stress coping strategies of male rhesus monkeys in response to a hazard-reward conflict. Iranian Journal of Basic Medical Sciences, 20(8), 951–7. [DOI: 10.22038/IJBMS.2017.9120] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touitou Y., Selmaoui B. (2012). The effects of extremely low-frequency magnetic fields on melatonin and cortisol, two marker rhythms of the circadian system. Dialogues in Clinical Neuroscience, 14(4), 381–99. [DOI: 10.31887/DCNS.2012.14.4/ytouitou] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugmeyster Y., Howell K., Bakshi A., Flores C., Hwang O., McKeever K. (2004). B-cell subsets in blood and lymphoid organs in Macaca fascicularis. Cytometry Part A, 61(1), 69–75. [DOI: 10.1002/cyto.a.20039] [PMID ] [DOI] [PubMed] [Google Scholar]
- Wingenfeld K., Wolf O. T. (2015). Effects of cortisol on cognition in major depressive disorder, posttraumatic stress disorder and borderline personality disorder-2014 Curt Richter Award Winner. Psychoneuroendocrinology, 51, 282–95. [DOI: 10.1016/j.psyneuen.2014.10.009] [PMID ] [DOI] [PubMed] [Google Scholar]
- Zare S., Hayatgeibi H., Alivandi S., Ebadi A. (2005). Effects of whole-body magnetic field on changes of glucose and cortisol hormone in guinea pigs. American Journal of Biochemistry Biotechnology, 1(4), 217–9. [DOI: 10.3844/ajbbsp.2005.209.211] [DOI] [Google Scholar]