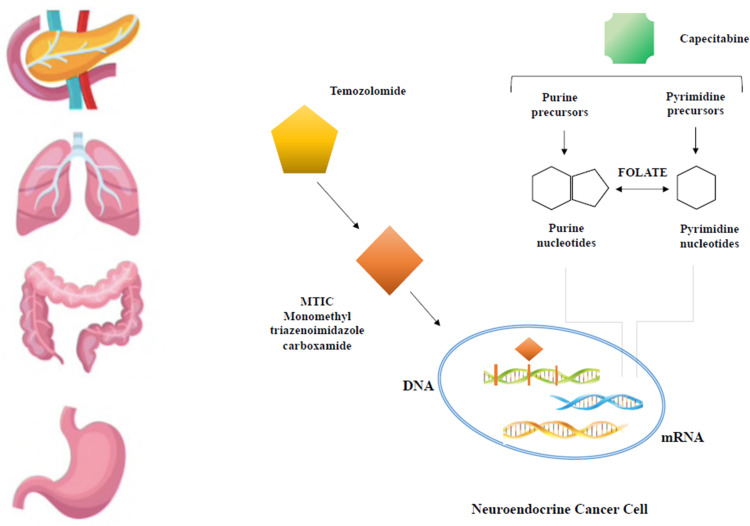
Abstract
Background
Retrospective studies and single center experiences suggest a role of capecitabine combined with temozolomide (CAPTEM) in neuroendocrine tumors (NENs).
Methods
We performed a systematic review to assess the efficacy and safety of CAPTEM in patients affected with NENs, with the aim to better clarify the role of this regimen in the therapeutic algorithm of NENs.
Results
A total of 42 articles and 1818 patients were included in our review. The overall disease control rate was 77% (range 43.5%-100%). The median progression free survival ranged from 4 to 38.5 months, while the median overall survival ranged from 8 to 103 months. Safety analysis showed an occurrence of G3-G4 toxicities in 16.4% of the entire population. The most common toxicities were hematological (27.2%), gastrointestinal (8.3%,) and cutaneous (3.2%).
Conclusion
This systematic review demonstrated that CAPTEM was an effective and relatively safe treatment for patients with advanced well-moderate differentiated NENs of gastroenteropancreatic, lung and unknown origin.
Keywords: NENs, neuroendocrine, temozolomide, capecitabine, CAPTEM, chemotherapy
Graphical Abstract
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors which have experienced a 7-fold increase in incidence in the last 40 years.1,2 Their prognosis varies in accordance with tumor morphology and Ki67 proliferation index, as well as with the primary site of origin.3–6
The most recent World Health Organization (WHO 2019) classification refers to gastroenteropancreatic NENs (GEP-NENs), that represent more than half of all NENs. GEP-NENs are classified in well differentiated neuroendocrine tumors (NETs) G1 (Ki67<3%), NETs G2 (Ki67 3–20%), NETs G3 (Ki67>20%) and poorly differentiated neuroendocrine carcinomas (NECs).3
The management of NETs should always be multidisciplinary, as treatments include curative or debulking surgery, locoregional treatments, systemic therapy with different agents like the somatostatin analogues (SSAs), tyrosine kinase inhibitors (TKIs), mammalian target of rapamycin (mTOR) inhibitors, peptide receptor radionuclide therapy (PRRT) and chemotherapy. Except chemotherapy, all of these available systemic anti-tumor treatments were approved based on randomized clinical trials, showing a significant improvement in progression free survival (PFS),7–11 although no significant advantage in terms of overall survival (OS) and tumor shrinkage has been reported.12–14
While the chemotherapy regimens with platinum/etoposide,15–17 oxaliplatin or irinotecan-based18–20 represent the standard of care for the treatment of NECs, more unclear and controversial is its role in the metastatic setting of well to moderately differentiated NETs. The use of the alkylating agent streptozocin appears to have the most antitumor activity in NENs, especially in pancreatic tumors, but at the same time a not negligible toxicity.21,22 In recent years, the oral alkylating agent temozolomide showed promising activity and tolerance profile, used either as a single agent or in combination with capecitabine, an oral prodrug for 5-fluorouracil.23,24 The mechanism of action of capecitabine plus temozolomide (CAPTEM) in NETs is still unclear, although apoptotic synergism has been demonstrated in vitro.25 The antimetabolite capecitabine incorporates 5-fluorodeoxyuridine triphosphate into the DNA, which leads to attenuation of the repair activity of MGMT through inhibition of thymidylate synthase and reduction of thymidine levels. Therefore, 5-FU treatment depletes the expression of MGMT in all cell lines, consequently the expression of low levels of MGMT correlates with more sensitivity to 5-FU.26 On the other hand, cells expressing high levels of MGMT were less sensitive to 5-FU.27,28 Literature data about the predictive role of MGMT are controverse.29–32 For these reasons, nowadays, MGMT status might not represent a useful biomarker of response.
Albeit CAPTEM is currently used in clinical practice, and has also been included in national and international guidelines such as the European Society for Medical Oncology (ESMO) and Associazione Italiana Oncologia Medica (AIOM),33,34 no high-quality evidences or prospective randomized Phase III controlled trials have been published so far. Most of the data come from retrospective studies35–71 and single center experiences, whereas only four Phase I–II studies were published.72–75 Furthermore, the efficacy of CAPTEM was mostly investigated in GEP-NETs but some evidences suggested a role also in NETs of lung and thymic origin and unknown primary site, irrespective of the treatment line.61,65,76
We performed a systematic review to assess the efficacy and safety of CAPTEM, including long-term outcomes in patients affected with NENs, with the aim to better clarify the role of this regimen in the therapeutic algorithm of NENs.
Materials and Methods
Search Strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) guidelines.77 The literature search in PubMed, Cochrane Central Register of Controlled Trials, SCOPUS, Web of Science and Google Scholar, was performed in April 2021 to identify available articles, both published and in abstract form, that evaluated the efficacy of capecitabine and temozolomide (CAPTEM) for the treatment of advanced neuroendocrine neoplasms (NENs). For PubMed the searching strategy was as follows: (“temozolomide” OR “Temodal” OR “TMZ”) AND (“Capecitabine” OR “Xeloda”) AND (“Neuroendocrine Tumors” OR “Carcinoma, Neuroendocrine” OR “Carcinoid Tumor” OR “Neuroendocrine Tumor” OR “Tumor, Neuroendocrine” OR “Tumors, Neuroendocrine” OR “Neuroendocrine Carcinoma” OR “Neuroendocrine Carcinomas” OR “Carcinoid Tumors” OR “Tumor, Carcinoid” OR “Tumors, Carcinoid” OR “Carcinoid), while key words “temozolomide, capecitabine, neuroendocrine” were used for searching in Cochrane Central Register of Controlled Trials, SCOPUS, Web of Science and Google Scholar.
Meeting abstracts, for a 5-year period (2016 to January 2021) including those of the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), European Neuroendocrine Tumor Society (ENETS), Associazione Italiana Oncologia Medica (AIOM) and North American Neuroendocrine Tumor Society (NANETS) were also checked.
Study Selection Criteria and Data Extraction
The following criteria were used to identify eligible studies for our review: studies describing CAPTEM for the treatment for advanced NENs; studies reporting tumor response outcome measures and/or toxicities, and studies clearly reporting World Health Organization (WHO) grading of patients. The search, restricted to the English language, was then limited to prospective clinical trials and retrospective or prospective cohort series including more than 10 patients. Case reports and case series, editorials, commentaries, meta-analyses, review articles, and animal studies were excluded. After this selection process, the selected studies and abstracts were independently screened by two authors (G.A. and M.V.). Finally, full-text articles were reviewed for all studies that met the inclusion criteria.
The primary endpoint was to evaluate the disease control rate (DCR), defined as the percentage of patients who experienced partial response (PR), complete response (CR), or stable disease (SD), according to the RECIST 1.1 criteria. Secondary endpoints were: a) median PFS, defined as the time from study enrollment to the first evidence of disease progression, or death from any cause, b) overall survival (OS), defined as the time from study enrollment to death due to any cause, c) grade 3/4 toxicities, according to Common Terminology Criteria for Adverse Events (CTCAE 4.0). Graphical histograms were generated to better visualize pooled tumor response and toxicities.
Data were extracted independently by 2 authors (G.B. and M.R.) and entered into a standardized, predesigned Microsoft Excel form. The following data were recorded: author, publication year and study design; number of total patients; median age of patients; dose and schedule of CAPTEM; lines of treatment; site of primary tumor and histotypes according to WHO; median OS; median PFS; DCR among all evaluable patients. Each author also assessed the quality of reporting.
Results
Literature Search and Included Studies
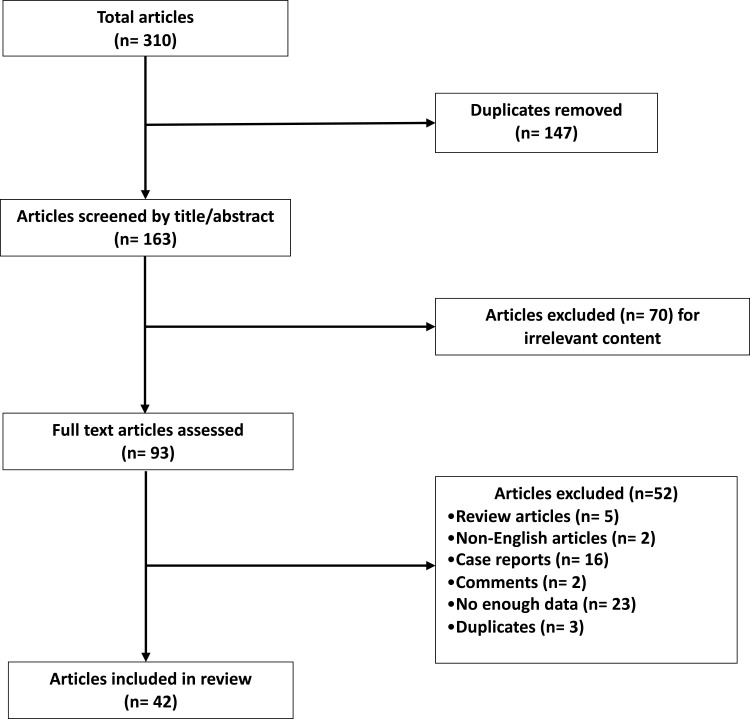
Overall, a total of 310 records has been identified using the aforementioned search strategy, and we excluded 147 duplicate articles. After screening the titles and abstracts, 70 records were excluded for irrelevant content. Of the remaining 93 potentially relevant studies, we excluded 5 review articles, 16 case reports and 2 comments. Furthermore, 2 records were excluded because of non-English language, 23 for not having enough data available and 3 duplicates. Finally, we included 42 articles involving 1912 patients with advanced NENs in our review. The selection process was showed in Figure 1. The 42 selected articles were published between 2003 and 2021 and comprised 38 retrospective studies, 2 Phase II studies and 2 phase I–II studies. A total of 1818 patients were studied in this review. The main characteristics of each study are listed in Table 1, and their detailed eligibility criteria and results have been previously reported.
Figure 1.
Flow diagram representing the systemic review process performed according to PRISMA statement.
Notes: Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535.77
Table 1.
Characteristics of Trials Included in the Review
| First Author, Year of Publication | Type of the Study | Treatment Regimen | Patients (N) | Median Age, y | Site of Primary Tumor | Histotypes (WHO) | Line of Treatment (I or > I Line) | Duration of Treatment |
|---|---|---|---|---|---|---|---|---|
| Fine, 200535 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 1500 mg/m2 1–14 | 10 ˜6 | -- | GI NET, panNET, lung, others | WD NET | >I line | -- |
| Isacoff, 200636 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 1000 mg/m2 BID 1–14 | 17 §16 | 54 | panNET | NET | >I line | -- |
| Strosberg 201137 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 30 | 58 | panNET | NET G1-G2 | >/= I line | 11 pts until PD, 15 until treatment break (non-specified) |
| Welin, 201138 | Retrospective | TEM 150 mg/m2 10–14 + CAP 750–1000 mg/m2 BID 1–14 | 25 | 55 | GI NET, panNET, lung, unknown | NEC, atypical carcinoid (ki-67>20%) | >I line | -- |
| Claringbold, 201274 | Phase I–II | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 35 *34 | 63 | GI NET, panNET, lung | WD NET | >/= I line | 4 cycles |
| Ganetsky, 201239 | Retrospective | -- | 20 | 64 | GI NET, panNET, lung, others | NET G1-G2-G3 | >I line | -- |
| Fine, 201340 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 600 mg/m2 BID 1–14 | 18 | 54 | GI NET, panNET | WD NET | >I line | Until PD |
| Oberstein, 201341 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 1000 mg/m2 BID 1–14 | 18 | 55 | GI NET, panNET, others | NET G1-G2 (Ki67<10%) | >I line | -- |
| Abbasi, 201442 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 600 mg/m2 BID 1–14 | 21 | 47 | panNET, GI NET | WD NET (Ki67<10) | >I line | Until PD or death |
| Fine, 201443 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 1500 mg/m2 1–14 | 28 | -- | GI NET, panNET, lung, others | WD NET (ki-67 ≤20%) | >I line | -- |
| Ramirez, 201544 | Retrospective | -- | 30 | 58.5 | GI NET, panNET, lung, others | NET | -- | > 1 cycle |
| Spada, 201523 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 1500 mg/m2 BID 1–14 | 58 | 58 | GI NET, panNET, lung, unknown | NET G1-G2-G3, typical, atypical carcinoid | >I line | -- |
| Chaves, 201645 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 10 | 59 | GI NET, panNET | NET G1-G2-G3 | >/= I line | -- |
| Cives, 201646 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 143 | 59 | panNET | NET G1-G2-G3 | >/= I line | 9 cycles (median number) |
| Claringbold, 201675 | Phase I–II | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 30 | 60 | panNET | NET G1-G2 | >I line | 4 cycles (8 weeks) |
| Crespo, 201647 | retrospective | -- | 25 †21 | 56 | GI NET, panNET, lung, others | NEN (60% Ki67≤50%) | >/= I line | -- |
| Ramirez, 201648 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 29 | 58 | GI NET, panNET, lung | NET G1-G2-G3 | -- | 1 cycle at least |
| Crespo, 201749 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 65 | 62 | GI NET, panNET, lung, others | NET G1-G2 | >/= I line | 1 cycle at least |
| Lamarca, 201750 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 60 | 63.6 | GI NET, panNET, lung, others | NET G1-G2-G3 | >/= I line | 6 cycles |
| Owen, 201751 | Retrospective | TEM 200 mg/m2 10–14 + CAP 1500 mg/m2 1–14 | 38 #29 | 53 | panNET | NET G1-G2-G3 | >/= I line | At least 1 cycle (median number 4 cycles) |
| Chauhan, 201852 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 12 | 62 | unknown | NET G1-G2-G3, NEC | >/= I line | 6 cycles (median number) |
| Campana, 201853 | Retrospective | TEM 10–14 + CAP 1–14 | 95 | 62 | panNET, lung, others | NET G1-G2, NEC | >/= I line | 6 (1–45) cycles (median number) |
| Smiroldo, 201854 | Retrospective | TEM 75 mg/m2 BID 10–14 + CAP 600 mg/m2 BID 1–14 | 27 | 61 | GI NET, panNET, lung, others | NET | >/= I line | 6 (2–25) cycles (median number) |
| Soulen 201855 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 600 mg/m2 BID 1–14 | 21 °19 | 58 | GI NET, panNET, lung, unknown | NET G2 | >/= I line | -- |
| De Mestier, 201956 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 153 | 57.7 | GI NET, panNET | NET G1-G2-G3 | >/= I line | 6 cycles (median number) |
| Rogowski, 201957 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 600 mg/m2 BID 1–14 | 32 | 55 | GI NET | NET G3, NEC | >/= I line | -- |
| Sahu, 201958 | Retrospective | TEM 100 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 32 | 58 | GI NET, panNET, lung, unknown | NET G2-G3 | >/= I line | 6 (2–16) cycles (median number) |
| Yordanova, 201959 | Retrospective | TEM 150–250 mg/m2 10–14 + CAP 500–1000 mg/m2 BID 1–14 | 12 ‡ 11 | 54 | GI NET, panNET, others | NET G2-G3 | >/= I line | Until PD |
| Al Toubaah, 202060 | Retrospective | -- | 32 | -- | GI NET | -- | -- | 8 (0–73) months (median number) |
| Al Toubaah, 202061 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 20 | -- | lung | Typical, atypical NET, LCNEC | >/= I line | Until PD, max response, physician choice |
| Chatzellis, 202062 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 79 | 60 | GI NET, panNET, lung, tymic | NEN G1-G2-G3 | >/= I line | 12.1 (0.6–55.6) months (median number) |
| De Mestier, 202063 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 100 | 57 | panNET | NET G1-G2-G3 | >/= I line | 7 ± 4.5 cycles |
| Ostwal, 202064 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 29▯24 | 48 | GI NET, panNET | NET G2-G3 | >/= I line | 4 (1–15) cycles (median number) |
| Papaxoinis, 202065 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 33 | -- | lung | Typical, atypical carcinoid | >I line | 6 cycles (median number) |
| Squires, 202066 | Retrospective | TEM 200 mg/m2 10–14 + CAP 1500 mg/m2 1–14 | 30 | panNET | NET G1-G2-G3 | I line (neoadj) | ||
| Thomas, 202067 | Retrospective | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 116 | 56 | GI NET, panNET, lung, others, unknown | WD NET, NEC | >/= I line | 9.5 cycles (median number) |
| Wang W, 202068 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 750–850 mg/m2 BID 1–14 | 151 | 49 | GI NET, panNET, others, unknown | NET G1-G2-G3, NEC | >/= I line | 5 (2–44) cycles (median number) |
| Dogan, 202169 | Retrospective | -- | 43 | 59 | GI NET, panNET | WD NET, NEC | >I line | -- |
| Jeong, 202172 | Phase II | TEM 200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 30 | 55 | GI NET, panNET | NET G3, NEC | >/= I line | Until PD, max toxicity |
| Liu, 202170 | Retrospective | -- | 20 | 59.5 | GI NET, panNET, unknown | NET G3 | I line | -- |
| Spada, 202171 | Retrospective | TEM 150–200 mg/m2 10–14 + CAP 750 mg/m2 BID 1–14 | 114 | 59 | GI NET, panNET, lung, unknown | NET G1-G2-G3, NEC | >/= I line | Until PD, max toxicity |
| Zrajkowska, 202173 | Phase II | -- | 21“0 | 58.6 | panNET midgut |
NET G1-G2-G3 | >I line | -- |
Notes: *34 pts evaluated for response rate (RR); †21 pts evaluated for RR; ˜6 pts evaluated for RR; §16 pts evaluated for RR; ▯24 pts evaluated for RR; #29 pts evaluated for RR; °19 pts evaluated for RR; ‡ 11 pts evaluated for RR; “pts evaluated for RR.
Abbreviations: TEM, temozolomide; CAP, capecitabine; BID, bis in die; GI NET, gastrointestinal (non-pancreatic) neuroendocrine tumor; panNET, pancreatic neuroendocrine tumor; NEC, neuroendocrine carcinoma; G, histological grading; PD, progression disease; max, maximum.
Patients’ Characteristics and Treatment Regimens
The whole number of NEN patients in this review was 1912, ranged in each study from 10 to 151. The age of patients ranged from 47 to 63 years. In about 90% of records (N = 39/42) GEP-NENs patients were enrolled, while two studies involved patients with lung NENs and one involved unknown primary origin NENs.
Regarding WHO classification, most studies involved NET G1-G2-G3, both typical and atypical lung carcinoid; 26% of studies (N =11/42) included also NEC. In one trial histopathological differentiation was not reported.
A CAPTEM regimen was administered in all studies until either disease progression or unacceptable toxicity levels. The most used schedule of treatment was temozolomide (TEM) 200 mg/m2 on days 10–14 + capecitabine (CAP) 750 mg/m2 BID on days 1–14. All CAPTEM schedules are summarized in Table 1. CAPTEM was administered in the second or subsequent lines of therapy, except for one study where patients, naïve for treatment, were enrolled.70
In about 10% of the studies (N =4/42) CAPTEM was administered concomitantly or sequentially to Peptide Receptor Radionuclide Therapy (PRRT) (Table 1). 59,64,73,74
Efficacy (DCR, PFS, OS)
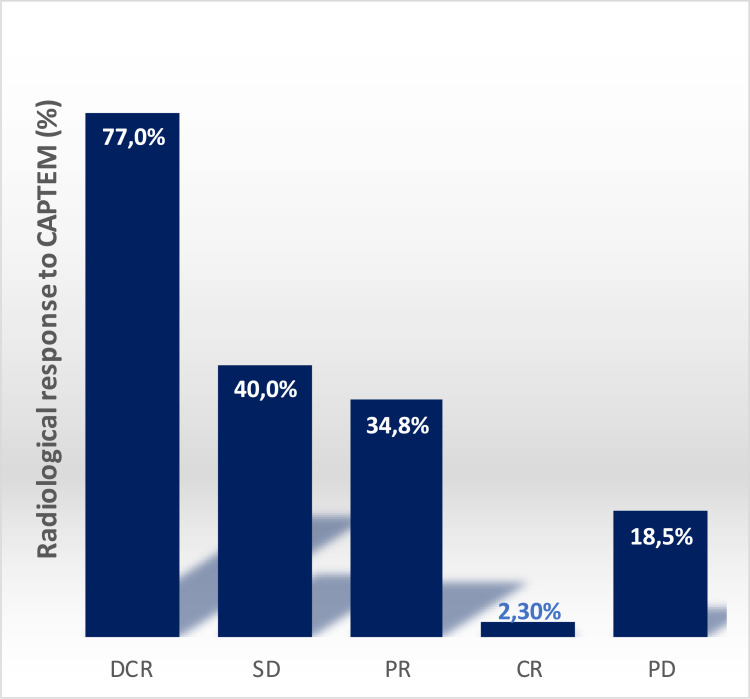
According to RECIST 1.1 criteria, tumor response was assessed in 1864 patients from forty-one studies (Table 2). Overall, DCR was 77% (range 43.5%-100%), SD was 40%; PR was 34.8% and CR was 2.3%, PD was observed in 18.5% of patients (see Figure 2).
Table 2.
Summary of Tumor Responses with CAPTEM Treatment from the Analyzed Studies
| Study, Year | Patients (N) | mOS [95% CI] (Months) | mPFS [95% CI] (Months) | DCR | CR | PR | SD | PD |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | ||||
| Fine, 200535 | 6 | -- | -- | 5 (83) | 1 (17) | 2 (33) | 2 (33) | 1 (17) |
| Isacoff, 200636 | 16 | 8 (4–17) | -- | 13 (77) | 1 (6) | 9 (53) | 3 (18) | 3 (18) |
| Strosberg 201137 | 30 | 38 (32–46) | 13 (11–23) | 85 (74) | 1 (1) | 23 (20) | 61(53) | 31 (27) |
| Welin, 201138 | 25 | 25.3 (13.7–36.9) | 7.1 (3.6–10.6) | 6 (54.5) | 0 (0) | 1 (9) | 5 (45.5) | 5 (45.5) |
| Claringbold, 201274 | 34 | NR | 31 (21–33) | 31 (91) | 5 (15) | 13 (38) | 13 (38) | 3 (9) |
| Ganetsky, 201239 | 20 | -- | 16 | 3 (65) | 0 (0) | 6 (30) | 7 (35) | 7 (35) |
| Fine, 201340 | 18 | 83 (19–140) | 14 (4–18) | 15 (83) | 1 (6) | 10 (55) | 4 (22) | 3 (17) |
| Oberstein, 201341 | 18 | -- | 14 (4–18) | 15 (84) | 1 (6) | 10 (56) | 4 (22) | 3 (16) |
| Abbasi, 201442 | 21 | -- | 16.5 | 17 (80) | 0 (0) | 12 (57) | 5 (23) | 4 (19) |
| Fine, 201443 | 28 | 25 | 20 | 27 (97) | 3 (11) | 9 (32) | 15 (54) | 1 (3) |
| Ramirez, 201544 | 30 | -- | 11 | 22 (73) | 0 (0) | 10 (33) | 12 (40) | 8 (27) |
| Spada, 201523 | 58 | 37 (30–56) | 15 (10–21) | 92 (81) | 1 (1) | 37 (33) | 54 (47) | 22 (19) |
| Chaves, 201645 | 10 | 48 | -- | 5 (50) | 1 (10) | -- | 3 (30) | 1 (10) |
| Cives, 201646 | 143 | 73 (52–81) | 17 (15–25) | 127 (89) | 0 (0) | 77 (54) | 50 (35) | 16 (11) |
| Claringbold, 201675 | 30 | NR | 48 | 30 (100) | 4 (13) | 20 (67) | 6 (20) | 0 (0) |
| Crespo, 201647 | 21 | 8 (5–11) | 4 (4–5) | 12 (44) | 0 (0) | 1 (4) | 11 (42.3) | 10 (38.5) |
| Ramirez, 201648 | 29 | NR | 12 | 19 (65) | 0 (0) | 5 (17) | 14 (48) | 10 (35) |
| Crespo, 201749 | 65 | 38 (25–52) | 16 (11–22) | 58 (90) | 2 (3) | 29 (45) | 27 (42) | 10 (10) |
| Lamarca, 201750 | 60 | 27 (16-NR) | 10 (7–14) | 43 (71) | 0 (0) | 14 (23) | 29 (48) | -- |
| Owen, 201751 | 29 | 29.3 (17.7–45.3) | 13 (5.6–17) | 26 (90) | 0 (0) | 11 (38) | 15 (52) | 3 (10) |
| Chauhan, 201852 | 12 | -- | -- | 9 (75) | 0 (0) | 6 (50) | 3 (25) | 3 (25) |
| Campana, 201853 | 95 | 33 (20–46) | 10 (5.6–14.4) | 68 (71.6) | 0 (0) | 26 (27.4) | 42 (44.2) | 27 (28.4) |
| Smiroldo, 201854 | 27 | NR | 4 | 16 (59) | 0 (0) | -- | -- | -- |
| Soulen 201855 | 19 | NR | 38.5 (29.8–47) | 19 (100) | 3 (16) | 11 (58) | 5 (26) | 0 (0) |
| De Mestier, 201956 | 153 | 60.5 (54.3–66.8) | 18.3 (13.8–21.7) | 128 (84) | 4 (3) | 60 (39) | 64 (42) | 25 (16) |
| Rogowski, 201957 | 32 | 15.6 (8–22) | 7 (3–15) | 18 (56) | 0 (0) | 11 (34) | 7 (22) | 14 (44) |
| Sahu, 201958 | 32 | 24 (17–30.8) | 10 (3.7–16.2) | 20 (63) | 4 (13) | 11 (34) | 5 (16) | 12 (37) |
| Yordanova, 201959 | 11 | NR | 4 | 16 (59) | 0 (0) | -- | -- | -- |
| Al Toubaah, 202060 | 32 | -- | -- | 21 (67) | 0 (0) | 6 (18.7) | 15 (46.8) | 5 (15.6) |
| Al Toubaah, 202061 | 20 | 68 (35–101) | 13 (4–22) | 17 (85) | 0 (0) | 6 (30) | 11 (55) | 3 (15) |
| Chatzellis, 202062 | 79 | 103 (43–163) | 10 (6–14) | 47 (59) | 0 (0) | 23 (29) | 24 (30) | 28 (35) |
| De Mestier, 202063 | 100 | 75 (58.5–92) | 21.4 (12.5–27.4) | 87 (87) | 2(2) | 49(49) | 36 (36) | 13 (13) |
| Ostwal, 202064 | 24 | NR | 34 (22–46) | 18 (61) | 3 (10) | 14 (48) | 1 (3) | 6 (25) |
| Papaxoinis, 202065 | 33 | 30.4 (25.6–35) | 9 (4.6–13.4) | 25 (76) | 0 (0) | 6 (18) | 19 (58) | 8 (24) |
| Squires, 202066 | 30 | -- | 18 (9–31) | 29 (97) | 0 (0) | 21 (70) | 8 (27) | 1 (3) |
| Thomas, 202067 | 116 | 74 | 12 | 115 (77) | 3 (2) | 37 (25) | 75 (50) | 36 (23) |
| Wang W, 202068 | 151 | 22 (8–27) | 6 (4–14) | 17 (71) | 1 (4) | 7 (29) | 9 (38) | 7 (29) |
| Dogan, 202169 | 43 | -- | 18 | 32 (76) | 1 (3) | 16 (38) | 16 (38) | -- |
| Jeong, 202172 | 30 | NR (10.5-NR) | 5.9 (4–11) | 23 (77) | 1 (3) | 8 (27) | 14 (47) | 7 (23) |
| Liu, 202170 | 20 | 41 (17-NR) | 9 (3–16) | 13 (65) | 0 (0) | 7 (35) | 6 (30) | 7 (35) |
| Spada, 202171 | 114 | 40 (23–122) | -- | 43 (74) | 0 (0) | 13 (22) | 30 (52) | -- |
Abbreviations: NR, not reached; --, not available; mOS, median overall survival; mPFS, median progression free survival; DCR, disease control rate; CR, complete response; PR, partial response; SD, stable disease; PD, progression disease.
Figure 2.
Graphical histogram of responses to CAPTEM in the analyzed studies: disease control rate (DCR), stable disease (SD), partial response (PR), complete response (CR).
The mPFS was reported in 35 of the included studies, ranging from 4 to 38.5 months, while mOS was reported in 32 studies, ranging from 8 to 103 months. In 8 studies mOS was not reached (Table 2).
Safety
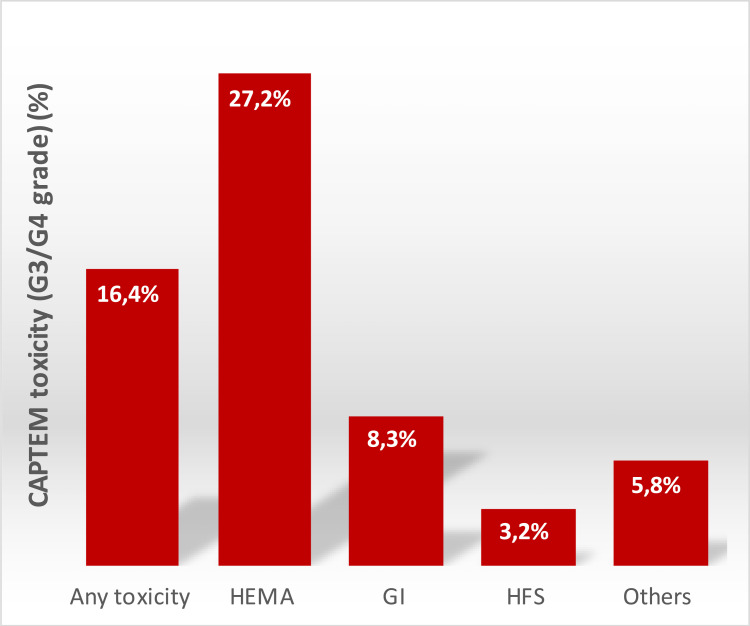
The safety analysis included 1133 patients from 29 studies (Table 3). Thirteen records were excluded because no safety data have been reported. 16.4% of the entire population reported G3-G4 toxicities. Among them, the most common were hematological (27.2% of patients), gastrointestinal (8.3%), and cutaneous (Hand-Foot Syndrome, 3.2%). Other toxicities such us insomnia, anorexia, asthenia, and mucositis were observed in 5.5% of patients. A complete list of safety profile was reported in the Figure 3.
Table 3.
Summary of Toxicities (Grade 3 to 4) with CAPTEM in the Analyzed Studies
| Study, Year | Patients (N) | Toxicities (all) | Gastrointestinal N (%) | Hematological N (%) | Cutaneous N (%) | Others N (%) |
|---|---|---|---|---|---|---|
| Fine, 200535 | 10 | 1(10) | -- | -- | 1(10) | -- |
| Isacoff, 200636 | 17 | -- | -- | 2(12) | -- | 1(6) |
| Strosberg 201137 | 30 | 4 (12) | 1 (3) | 2 (6) | -- | 1 (3) |
| Welin, 201138 | 25 | 3 (12) | 1 (4) | 1(4) | -- | 1(4) |
| Ganetsky, 201239 | 20 | -- | 4(20) | 1 (5) | -- | 3(15) |
| Fine, 201340 | 18 | -- | -- | 2 (11) | -- | -- |
| Oberstein, 201341 | 18 | -- | -- | 2(11) | -- | -- |
| Fine, 201443 | 28 | -- | 1(3) | 10(35) | -- | 4(15) |
| Claringbold, 201675 | 30 | -- | -- | 3(10) | -- | -- |
| Crespo, 201647 | 25 | 4 (15.2) | -- | 4 (15.2) | -- | -- |
| Ramirez, 201648 | 29 | -- | 3(10) | 9(31) | -- | -- |
| Crespo, 201749 | 65 | 9(13.8) | 1 (1.5) | 14 (21.6) | -- | -- |
| Owen, 201751 | 38 | -- | 2(5) | 9(24) | -- | -- |
| Chauhan, 201852 | 12 | -- | -- | -- | -- | 1(8) |
| Smiroldo, 201854 | 27 | 2(7) | -- | 2 (7) | -- | --9) |
| De Mestier, 201956 | 146 | 36 (24.7) | 6(4.2) | 28 (19.2) | 3 (2.1) | 7(4.8) |
| Rogowski, 201957 | 32 | -- | 2(6) | 8(25) | 2 (6) | 1 (3) |
| Sahu, 201958 | 32 | 10 (31.3) | 8(25) | 10(31.3) | -- | 4(12.5) |
| Yordanova, 201959 | 12 | -- | 4 (26.6) | -- | -- | -- |
| Al Toubaah, 202060 | 32 | -- | 9 (28) | 13 (40.6) | -- | 2 (6.2) |
| Al Toubaah, 202061 | 20 | -- | 1(5) | 2(10) | -- | -- |
| Chatzellis, 202062 | 79 | -- | -- | 4(5) | -- | -- |
| De Mestier, 202063 | 94 | 21 (22.3) | 4(4.2) | 19 (20.3) | 3(2.3) | -- |
| Ostwal, 202064 | 29 | -- | 4 (14) | 10 (35) | -- | 1 (3) |
| Papaxoinis, 202065 | 33 | -- | 1 (3) | 6 (18.2) | -- | -- |
| Squires, 202066 | 30 | 4(12) | 2(6) | 2(6) | -- | -- |
| Wang W, 202068 | 151 | 7(4.6) | -- | -- | -- | -- |
| Jeong, 202172 | 30 | 8(26.7) | 5(16.6) | 6(20) | -- | 1(3.3) |
| Zrajkowska, 202173 | 21 | -- | - (18) | -(9) | -- | -- |
Abbreviation: --, not available.
Figure 3.
Graphical histogram of gastrointestinal (GI), hematological (HEMA), hand-foot syndrome (HFS), and any other G3-G4 toxicities that occurred within the analyzed studies.
Discussion
Our systematic review showed that CAPTEM could represent an effective and relatively safe treatment for patients with advanced NENs regardless of the site of origin and should be prioritized for well to moderate differentiated NENs considering its best outcomes in these subgroups of patients.
Efficacy and safety analysis of the CAPTEM chemotherapy regimen demonstrated a DCR of 77% (range: 54.5–100%) and a severe toxicity rate of 16.4% in NENs of gastrointestinal, lung and unknown origin.
In the management of NENs, surgical resection represents the gold standard treatment, but not all patients are candidates due to late diagnosis or high tumor burden.1,78,79 Systemic anti-tumor treatments available for advanced GEP-NENs, including SSAs, TKIs, mTOR inhibitors and PRRT - commonly used in well to moderate differentiated NENs management - were approved on the basis of randomized clinical trials,7–11 while chemotherapy is widely used in poorly differentiated NENs, based on mostly retrospective experiences.23,37,71,80,81 Indeed, the vast majority of randomized trials about chemotherapy were conducted in the 1980s and 1990s and no placebo-controlled randomized trials have been published.21,22,82–86
The role of chemotherapy in NENs has progressively increased with the development of recent systemic therapies, and it is now used not only in NECs but also in NETs. Although several chemotherapy regimens have been associated with antitumor activity, there is not a consensus regarding the optimal use and indications of cytotoxic agents in NENs. Platinum/etoposide, 5-FU based protocols and capecitabine/temozolomide are the most commonly used regimens in current practice. Treatment choice and schedule may be different depending on patient and tumor characteristics, tumor grading and primary site, but the therapeutic algorithm is still a matter of ongoing debate.
The 2012 NORDIC NEC retrospective analysis highlighted that G3 NENs represented a heterogeneous group of neoplasms: G3 NENs with a Ki-67 index between 20% and 55% seemed to have a less aggressive behavior and sensitivity to platinum-based chemotherapy than those with a Ki-67 index > 55%.87,88 Chemotherapy protocol with cisplatin and etoposide showed a better response (ORR = 67%) in NEC than NETs (ORR = 7%)89 and streptozotocin-based therapies prolonged survival in NETs.83
Although the definition of NETs G3 and NEC has recently been revised and a different response to chemotherapy was reported in the literature, treatment strategies are substantially the same between these clinical subgroups. Nowadays the distinction between NETs G3 and NEC, appears extremely important in order to better tailor treatment options for patients with NETs, adding chemotherapy to TKIs, SSAa and PRRT in therapeutic management.
As mentioned before, there is a lack of prospective, randomized trials, but this has led to the widespread use of several alkylating agents and their combinations - including temozolomide alone or in association with 5FU-based chemotherapy - in the treatment of advanced or metastatic NENs.32,39,90 According to its mechanism of action, and considering its favorable toxicity profile, the CAPTEM regimen, is now routinely used in clinical practice especially in G2-G3 NETs.58,67
A recent single-arm phase II trial, including only unresectable or metastatic GEP-NENs G3 with a Ki-67 labeling index >20% and <55% treated with CAPTEM, showed a significantly PFS and OS improvement in NETs compared to NEC (9.3 months versus 3.5 months, P = 0.005, not reached versus 6.2 months, P = 0.004, respectively). Furthermore, patients with NEC had both a lower ORR (14.3% versus 34.8%, P = 0.393) and DCR (42.9% versus 87.0%, P = 0.033) than NETs G3, supporting the role of CAPTEM as preferred treatment for patients with well-differentiated G3 NETs.72
When compared to 5-FU and platinum-based chemotherapy regimens, CAPTEM showed a better DCR in G3 NETs (DCR: 65%, 57.1% and 50% in CAPTEM, FOLFOX and cisplatin plus etoposide arm, respectively) although this did not translate into a survival benefit. A possible explanation for the lack of survival benefit may lie in the different sample size for each treatment group, which were underpowered to assess OS benefits. When stratified by the treatment line and Ki67 index, naïve patients and NETs with Ki‐67 <55% showed an OS and PFS benefit, confirming a more favorable profile of CAPTEM in the first line setting of G3 NETs with Ki‐67 20–54%.70
A retrospective Italian multicentric experience,71 showed a global lower ORR of 28%, median PFS of 14.7 months and median OS of 35.6 months in TEM-based chemotherapy treated patients, without significant difference between TEM alone and CAPTEM groups. Although the heterogeneity of population with NENs in terms of primary sites, tumor grade and different schedules, does not allow to drawn definitive conclusions, this study confirmed a worse prognosis for patients who had received more than two lines of chemotherapy before CAPTEM. Interestingly, the favorable outcomes reported in lung NETs suggested a role of TEM-based chemotherapy in this setting.
An Italian “Real-World” data analysis91 reported no significant difference in survival in all NENs, in relation to chemotherapy protocol and to the primary site of disease when treated with TEM or CAPTEM. The ORR of 44.1% and the DCR of 70.9%, suggested that TEM-based regimen could be used to reduce the tumor burden and palliate symptoms.
One of the most recent randomized phase II trial (E2211) comparing CAPTEM to TEM monotherapy in 144 patients with advanced low or intermediate grade pNETs, established CAPTEM as standard chemotherapy in advanced pNETs. Although no significant difference in ORR (33.3% for CAPTEM vs 27.8% for TEM, p = 0.47) was reported between the two treatment arms, the combination was associated with a significantly longer median PFS than TEM monotherapy (22.7 vs 14.4 months).92 The imbalance between the two arms, as the patients included in the CAPTEM arm had pNETs of a significantly lower grade, could justify the similar data of ORR. A propensity score-based retrospective analysis including 138 patients with advanced, progressive pNETs demonstrated a similar PFS between TEM and CAPTEM arms, but ORR (34.2 vs 51%, p = 0.088) and DCR (73.7 vs 87%, p = 0.075) tended to be higher in the CAPTEM group.63
It is noteworthy that, although not statistically significant in the aforementioned studies, ORR was higher in NENs treated with CAPTEM compared to most approved therapy (≈ 30%). To our knowledge, there have been no prospective, randomized clinical trial comparing CAPTEM to single-agent tyrosine kinase inhibitors, so the optimal sequence of treatment is still a matter of debate.9,93–95
In the current literature, both the pancreatic origin of NETs, and the absence of prior chemotherapy were associated with higher efficacy of CAPTEM.96 The majority of published studies included in our review confirmed the role of site origin of neoplasm in treatment response.29,30,32,37,40,42,48,49,93,97,98 The increased pNETs chemosensitivity, that justify the most common clinical use in this site origin of tumors, is thought to be partially attributed to absent or low levels of MGMT, more commonly reported in pNETs than in non-pNETs.28,29,93 About this hypothesis, a recent systematic review and meta-analysis suggested that in NETs MGMT status may be predictive of TEM efficacy, however remarking that the current evidence is not enough to justify a routine detection of MGMT before starting treatment in clinical practice. Patients with pNET showed more favorable overall response rate to the CAPTEM regimen (mean ORR 46.4%) than small intestinal tumors (siNETs) (ORR 0%).99 In survival analysis was reported a median PFS of 20.6 months and 6.9 months in pNETs and siNETs respectively, while not significantly OS difference between the two groups was observed.56 An ORR of 70% was observed in patients with low- and intermediate-grade pNETs naive to chemotherapy treatment, with a median duration of response of 20 months and a median PFS of 18 months.37 More common favorable responses - defined as PR + SD -, although not statistically significant between different primary tumor locations, was reported in patients with pNENs and lung/thymic NENs reaching 70 and 64.7%, respectively. Moreover, a pancreas or lung/thymus primary site demonstrated a significant prognostic factor for both PFS (p < 0.0001) and OS (p < 0.0001).62 Although low grade tumors, pancreatic origin, and tumors with low levels of MGMT expression seemed to have higher response rates to CAPTEM regimen; responses were still observed also in patients with non-pancreatic origin NENs, high grade tumors and higher levels of MGMT expression.29 Most of the efficacy data of CAPTEM regimen were reported in pNETs, but other tumor sites also seemed to show encouraging response rates to this chemotherapy regimen, as suggested above. Two recent retrospective studies have investigated the activity of CAPTEM in a selected population of lung NETs. Pretreated typical/atypical lung carcinoids and large‐cell NECs showed a high response rate (ORR 30%, DCR 85%), with mPFS of 13 months and mOS of 68 months, when treated with the CAPTEM regimen.61 Instead, more modest response rate (DCR 75.8%) was reported in a selected typical/atypical lung carcinoid population, with longer survival outcomes than that described with TEM alone (mPFS 9 months, mOS 30.4 months). Median PFS and OS did not differ significantly between patients with typical and atypical carcinoids.65
Although chemotherapy protocols appeared to be less effective in advanced lung carcinoids100–103 the survival outcomes observed with CAPTEM in the aforementioned studies, appear similar when compared to the survival outcomes with everolimus.94,104 Despite the common perception that the CAPTEM regimen is particularly active in pNETs, and given the scarcity of the therapeutic landscape in lung NETs, the present review highlighted CAPTEM as a valid therapeutic alternative to TKIs and SSAa, mostly when the aim is tumor stabilization rather than an objective response.65
With the aim to define a valid therapeutic algorithm, the identification of biomarkers able to identify patients who are likely to benefit from specific therapies, is an unmet need still. Data reported in literature about the predictive and prognostic role of Ki67 index are heterogeneous, despite treatment is often based on the Ki67 level.105 Better ORR to CAPTEM was reported in pNETs with Ki-67 >5%,106 and in NENs with Ki67 2–20%23 suggesting a stronger role of chemotherapy in G2-G3 NETs. On the contrary, other studies reported no influence by tumor proliferation on the response to CAPTEM32 and survival.93.
According to the safety profile of similar chemotherapy agents, the CAPTEM regimen is known to be associated with toxicities such as myelosuppression and gastrointestinal toxicities. Notably, our meta-analysis showed a G3-G4 rate of any toxicities in 16.4% of patients. The most frequent chemotherapy-related side effects were hematological, gastrointestinal and cutaneous toxicity in 27.2%, 8.3% and 3.2% respectively. The mostly hematological toxicity profile was confirmed in previous reviews.107,108
Our review has some limitations: firstly, the nature of the included studies, which are mostly retrospective hence prone to error through issues with selection bias and reporting. The absence of placebo-controlled phase III randomized studies can only allow indirect comparisons with other treatment regimens, but definitive conclusion cannot be drawn.
Furthermore, the included studies had a variety of endpoints and diverse cohort size. Albeit some degree of heterogeneity is always to be expected, it diminishes the validity of the combined data set and subsequent results. A previous review explored the role of temozolomide-based combination therapy and confirmed its effective profile in NENs with similar results in term of DCR and survival, but the lack of homogeneity among different studies with regard to accompanying drugs made across-trial comparisons difficult.108 A more recent review107 about the safety and efficacy of CAPTEM, included only fifteen studies and the new data about lung NENs were not reported.61,65
Conclusion
Although more robust efficacy data are found in pNETs, which have led to the consolidation of the use of CAPTEM regimen in cancers with pancreatic origin, this systematic review confirms CAPTEM as effective and relatively safe treatment for patients with advanced well-moderately differentiated NENs of gastrointestinal, lung and unknown origin.
Future efforts should focus on the research of best candidates for the CAPTEM regimen in terms of disease characteristics and previous treatments. Moreover, the identification of potential prognostic and predictive biomarkers would be very useful to personalize treatments. Finally, randomized phase III trials are especially needed to define a better therapeutic algorithm.
Disclosure
Prof. Dr. Antongiulio Faggiano reports grants, personal fees from Triple AAA, non-financial support from Ipsen, outside the submitted work. Prof. Dr. Paolo Marchetti reports grants, personal fees from ROCHE, grants, personal fees from MSD, grants, personal fees from BMS, personal fees from ASTRA ZENECA, personal fees from BOEHRINGER INGELHEIM, personal fees from LILLY, grants from NOVARTIS, grants from PFIZER, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Yao JC, Hassan M, Phan A., et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagtegaal ID, Odze RD, Klimstra D, et al.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol. 2015;10(9):1240–1242. doi: 10.1097/JTO.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 5.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: prostate and Bladder Tumours. Eur Urol. 2016;70(1):106–119. doi: 10.1016/j.eururo.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 6.Elder DE, Massi D, Scolyer RA, Willemze R. WHO Classification of Skin Tumours. 4th. Vol. 11. WHO Classification of Tumours; 2015. [Google Scholar]
- 7.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510 [DOI] [PubMed] [Google Scholar]
- 8.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158 [DOI] [PubMed] [Google Scholar]
- 9.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors [published correction appears. N Engl J Med. 2011;364(6):501–513. doi: 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 11.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125–135. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinke A, Wittenberg M, Schade-Brittinger C, et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): results of Long-Term Survival. Neuroendocrinology. 2017;104(1):26–32. doi: 10.1159/000443612 [DOI] [PubMed] [Google Scholar]
- 13.Caplin ME, Pavel M, Phan AT, et al. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: final results of the CLARINET open-label extension study. Endocrine. 2021;71(2):502–513. doi: 10.1007/s12020-020-02475-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mestier L, Dromain C, d’Assignies G, et al. Evaluating digestive neuroendocrine tumor progression and therapeutic responses in the era of targeted therapies: state of the art. Endocr Relat Cancer. 2014;21(3):R105–R120. doi: 10.1530/ERC-13-0365 [DOI] [PubMed] [Google Scholar]
- 15.Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81(8):1351–1355. doi: 10.1038/sj.bjc.6690325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fjällskog ML, Granberg DP, Welin SL, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer. 2001;92(5):1101–1107. doi: [DOI] [PubMed] [Google Scholar]
- 17.Iwasa S, Morizane C, Okusaka T, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 2010;40(4):313–318. doi: 10.1093/jjco/hyp173 [DOI] [PubMed] [Google Scholar]
- 18.Hentic O, Hammel P, Couvelard A, et al. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 2012;19(6):751–757. doi: 10.1530/ERC-12-0002 [DOI] [PubMed] [Google Scholar]
- 19.Hadoux J, Malka D, Planchard D, et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22(3):289–298. doi: 10.1530/ERC-15-0075 [DOI] [PubMed] [Google Scholar]
- 20.Bongiovanni A, Liverani C, Pusceddu S, et al. Randomised phase II trial of CAPTEM or FOLFIRI as SEcond-line therapy in NEuroendocrine CArcinomas and exploratory analysis of predictive role of PET/CT imaging and biological markers (SENECA trial): a study protocol. BMJ Open. 2020;10(7):e034393. doi: 10.1136/bmjopen-2019-034393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303(21):1189–1194. doi: 10.1056/NEJM198011203032101 [DOI] [PubMed] [Google Scholar]
- 22.Engstrom PF, Lavin PT, Moertel CG, Folsch E, Douglass HO. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol. 1984;2(11):1255–1259. doi: 10.1200/JCO.1984.2.11.1255 [DOI] [PubMed] [Google Scholar]
- 23.Spada F, Antonuzzo L, Marconcini R, et al. Chemotherapy with capecitabine plus temozolomide (CAP-TEM) in patients with advanced neuroendocrine neoplasms (NENs): an Italian multicenter retrospective analysis. Neuroendocrinology. 2015;43(3):121. [Google Scholar]
- 24.Lopez Lopez C, Jimenez Fonseca P, Crespo G, et al. Temozolomide plus capecitabine as salvage treatment for patients with advanced neuroendocrine tumors (NETs) in the community setting. J Clin Oncol. 2013;31:e15169. doi: 10.1200/jco.2013.31.15_suppl.e15169 [DOI] [Google Scholar]
- 25.Fine RL, Gulati AP, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol. 2014;32:179. doi: 10.1200/jco.2014.32.3_suppl.179 [DOI] [Google Scholar]
- 26.Murakami J, Lee YJ, Kokeguchi S, et al. Depletion of O6-methylguanine-DNA methyltransferase by O6-benzylguanine enhances 5-FU cytotoxicity in colon and oral cancer cell lines. Oncol Rep. 2007;17(6):1461–1467. [PubMed] [Google Scholar]
- 27.Fine RL, Fogelman DR, Schreibman SM. Effective treatment of neuroendocrine tumors with temozolomide and capecitabine. J Clin Oncol. 2005;23(Suppl 16):S4216. doi: 10.1200/jco.2005.23.16_suppl.4216 [DOI] [Google Scholar]
- 28.Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15(1):338–345. doi: 10.1158/1078-0432.CCR-08-1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt AM, Pavel M, Rudolph T, et al. Prognostic and predictive roles of MGMT protein expression and promoter methylation in sporadic pancreatic neuroendocrine neoplasms. Neuroendocrinology. 2014;100(1):35–44. doi: 10.1159/000365514 [DOI] [PubMed] [Google Scholar]
- 30.Cros J, Hentic O, Rebours V, et al. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23(8):625–633. doi: 10.1530/ERC-16-0117 [DOI] [PubMed] [Google Scholar]
- 31.Campana D, Walter T, Pusceddu S, et al. Correlation between MGMT promoter methylation and response to temozolomide-based therapy in neuroendocrine neoplasms: an observational retrospective multicenter study. Endocrine. 2018;60(3):490–498. doi: 10.1007/s12020-017-1474-3 [DOI] [PubMed] [Google Scholar]
- 32.Cives M, Ghayouri M, Morse B, et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23(9):759–767. [DOI] [PubMed] [Google Scholar]
- 33.Linee guida Neoplasie Neuroendocrini Aiom, Available from: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Neuroendocrini.pdf. Accessed 11, February 2022.
- 34.Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–860. doi: 10.1016/j.annonc.2020.03.304 [DOI] [PubMed] [Google Scholar]
- 35.Fine RL, Fogelman DR, Schreibman SM. Effective treatment of neuroendocrine tumors with temozolomide and capecitabine. J Clin Oncol. 2005;23:4216. [Google Scholar]
- 36.Isacoff WH, Moss RA, Pecora AL, Fine RL. Temozolomide/capecitabine therapy for metastatic neuroendocrine tumors of the pancreas. A retrospective review. J Clin Oncol. 2006;24:14023. doi: 10.1200/jco.2006.24.18_suppl.14023 [DOI] [Google Scholar]
- 37.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268–275. doi: 10.1002/cncr.25425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117(20):4617–4622. doi: 10.1002/cncr.26124 [DOI] [PubMed] [Google Scholar]
- 39.Ganetsky A, Adel NG, Do KG, Reidy DL. The efficacy of capecitabine and temozolomide for the treatment of metastatic neuroendocrine tumors: memorial Sloan-Kettering Cancer Center experience. JCO. 2012;30(4_suppl):363. doi: 10.1200/jco.2012.30.4_suppl.363 [DOI] [Google Scholar]
- 40.Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71(3):663–670. doi: 10.1007/s00280-012-2055-z [DOI] [PubMed] [Google Scholar]
- 41.Oberstein PE, Gulati PA, Krantz BA, et al. The efficacy and safety of the capecitabine/temozolomide (CAPTEM) regimen in the treatment of well-differentiated neuroendocrine tumors with liver metastasis after failure of previous therapy: Columbia University Medical Center experience. J Clin Oncol. 2013;31:308. doi: 10.1200/jco.2013.31.4_suppl.30823233718 [DOI] [Google Scholar]
- 42.Abbasi S, Kashashna A, Albaba H. Efficacy of capecitabine and temozolomide combination in well-differentiated neuroendocrine tumors: Jordan experience. Pancreas. 2014;43(8):1303–1305. doi: 10.1097/MPA.0000000000000174 [DOI] [PubMed] [Google Scholar]
- 43.Fine RL, Gulati PA, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol. 2014;32:179. [Google Scholar]
- 44.Ramirez RA, Boudreaux JP, Wang Y, et al. Combination capecitabine/temozolomide (CAPTEM) in patients with neuroendocrine tumors (NETs): a single institution review. J Clin Oncol. 2015;33(15_suppl):e15184. doi: 10.1200/jco.2015.33.15_suppl.e15184 [DOI] [Google Scholar]
- 45.Chaves A, Garcia A, Ribeiro J, et al. P-178: capecitabine and temozolomide (CAPTEM) in patients with advanced neuroendocrine tumors: the experience of a Portuguese cancer center. Ann Oncol. 2016;27(Suppl 2):ii53. doi: 10.1093/annonc/mdw199.171 [DOI] [Google Scholar]
- 46.Cives M, Ghayouri M, Morse B, et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23(9):759–767. doi: 10.1530/ERC-16-0147 [DOI] [PubMed] [Google Scholar]
- 47.Crespo GH, Lopez C, Jimenez-Fonseca P, et al. Capecitabine-Temozolomide in G3 Neuroendocrine Neoplasms. 13th Annual ENETS conference 2016; 2016. [Google Scholar]
- 48.Ramirez RA, Beyer DT, Chauhan A, Boudreaux JP, Wang YZ, Woltering EA. The Role of Capecitabine/Temozolomide in Metastatic Neuroendocrine Tumors. Oncologist. 2016;21(6):671–675. doi: 10.1634/theoncologist.2015-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crespo G, Jiménez-Fonseca P, Custodio A, et al. Capecitabine and temozolomide in grade 1/2 neuroendocrine tumors: a Spanish multicenter experience. Future Oncol. 2017;13(7):615–624. doi: 10.2217/fon-2016-0434 [DOI] [PubMed] [Google Scholar]
- 50.Lamarca A, Barriuso J, McCallum J, et al. Temozolomide-capecitabine (TemCap) chemotherapy for neuroendocrine neoplasms (NENs): time to maximum response and optimal treatment duration. Ann Oncol. 2017;28:v151. doi: 10.1093/annonc/mdx368.024 [DOI] [Google Scholar]
- 51.Owen DH, Alexander AJ, Konda B, et al. Combination therapy with capecitabine and temozolomide in patients with low and high grade neuroendocrine tumors, with an exploratory analysis of O6-methylguanine DNA methyltransferase as a biomarker for response. Oncotarget. 2017;8(61):104046. doi: 10.18632/oncotarget.22001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chauhan A, Farooqui Z, Murray LA, et al. Capecitabine and Temozolomide in Neuroendocrine Tumor of Unknown Primary. J Oncol. 2018;2018:3519247. doi: 10.1155/2018/3519247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campana D, Walter T, Pusceddu S, et al. Correlation between MGMT promoter methylation and response to temozolomide-based therapy in neuroendocrine neoplasms: an observational retrospective multicenter study. Endocrine. 2018;60(3):490–498. [DOI] [PubMed] [Google Scholar]
- 54.Smiroldo V, Carnaghi C, Rimassa L, et al. Efficacy of oral chemotherapy with capecitabine and temozolomide (CapTem) in metastatic neuroendocrine tumors (NETs): a single-institution experience. J Clin Oncol. 2018;36(4_suppl):487. doi: 10.1200/JCO.2018.36.4_suppl.487 [DOI] [Google Scholar]
- 55.Soulen MC, van Houten D, Teitelbaum UR, Damjanov N, Cengel KA, Metz DC. Safety and Feasibility of Integrating Yttrium-90 Radioembolization With Capecitabine-Temozolomide for Grade 2 Liver-Dominant Metastatic Neuroendocrine Tumors. Pancreas. 2018;47(8):980–984. doi: 10.1097/MPA.0000000000001115 [DOI] [PubMed] [Google Scholar]
- 56.de Mestier L, Walter T, Brixi H, et al. Comparison of Temozolomide-Capecitabine to 5-Fluorouracile-Dacarbazine in 247 Patients with Advanced Digestive Neuroendocrine Tumors Using Propensity Score Analyses. Neuroendocrinology. 2019;108(4):343–353. doi: 10.1159/000498887 [DOI] [PubMed] [Google Scholar]
- 57.Rogowski W, Wachuła E, Gorzelak A, et al. Capecitabine and temozolomide combination for treatment of high-grade, well-differentiated neuroendocrine tumour and poorly-differentiated neuroendocrine carcinoma - retrospective analysis. Endokrynol Pol. 2019;70(4):313–317. doi: 10.5603/EP.a2019.0010 [DOI] [PubMed] [Google Scholar]
- 58.Sahu A, Jefford M, Lai-Kwon J, Thai A, Hicks RJ, Michael M. CAPTEM in Metastatic Well-Differentiated Intermediate to High Grade Neuroendocrine Tumors: a Single Centre Experience. J Oncol. 2019;2019:9032753. doi: 10.1155/2019/9032753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yordanova A, Ahrens H, Feldmann G, et al. Peptide Receptor Radionuclide Therapy Combined With Chemotherapy in Patients With Neuroendocrine Tumors. Clin Nucl Med. 2019;44(5):e329–e335. doi: 10.1097/RLU.0000000000002532 [DOI] [PubMed] [Google Scholar]
- 60.Al-Toubah T, Morse B, Strosberg J. Efficacy of Capecitabine and Temozolomide in Small Bowel (Midgut) Neuroendocrine Tumors. Curr Oncol. 2022;29(2):510–515. doi: 10.3390/curroncol29020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Toubah T, Morse B, Strosberg J. Capecitabine and Temozolomide in Advanced Lung Neuroendocrine Neoplasms. Oncologist. 2020;25(1):e48–e52. doi: 10.1634/theoncologist.2019-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatzellis E, Angelousi A, Daskalakis K, et al. Activity and Safety of Standard and Prolonged Capecitabine/Temozolomide Administration in Patients with Advanced Neuroendocrine Neoplasms. Neuroendocrinology. 2019;109(4):333–345. doi: 10.1159/000500135 [DOI] [PubMed] [Google Scholar]
- 63.de Mestier L, Walter T, Evrard C, et al. Temozolomide Alone or Combined with Capecitabine for the Treatment of Advanced Pancreatic Neuroendocrine Tumor. Neuroendocrinology. 2020;110(1–2):83–91. doi: 10.1159/000500862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ostwal V, Basu S, Bhargava P, et al. Capecitabine-Temozolomide in Advanced Grade 2 and Grade 3 Neuroendocrine Neoplasms: benefits of Chemotherapy in Neuroendocrine Neoplasms with Significant 18FDG Uptake. Neuroendocrinology. 2021;111(10):998–1004. doi: 10.1159/000511987 [DOI] [PubMed] [Google Scholar]
- 65.Papaxoinis G, Kordatou Z, McCallum L, et al. Capecitabine and Temozolomide in Patients with Advanced Pulmonary Carcinoid Tumours. Neuroendocrinology. 2020;110(5):413–421. doi: 10.1159/000502864 [DOI] [PubMed] [Google Scholar]
- 66.Squires MH, Worth PJ, Konda B, et al. Neoadjuvant Capecitabine/Temozolomide for Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49(3):355–360. doi: 10.1097/MPA.0000000000001500 [DOI] [PubMed] [Google Scholar]
- 67.Thomas K, Voros BA, Meadows-Taylor M, et al. Outcomes of Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs). Cancers. 2020;12(1):206. doi: 10.3390/cancers12010206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, Zhang Y, Peng Y, et al. A Ki-67 Index to Predict Treatment Response to the Capecitabine/Temozolomide Regimen in Neuroendocrine Neoplasms: a Retrospective Multicenter Study. Neuroendocrinology. 2021;111(8):752–763. doi: 10.1159/000510159 [DOI] [PubMed] [Google Scholar]
- 69.Dogan I, Tastekin D, Karabulut S, et al. Temozolomide and capecitabine (CAPTEM) is effective in metastatic well-differentiated gastrointestinal neuroendocrine tumors. J Clin Oncol. 2021;39:366. doi: 10.1200/JCO.2021.39.3_suppl.366 [DOI] [PubMed] [Google Scholar]
- 70.Liu AJ, Ueberroth BE, McGarrah PW, et al. Treatment Outcomes of Well-Differentiated High-Grade Neuroendocrine Tumors. Oncologist. 2021;26(5):383–388. doi: 10.1002/onco.13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spada F, Maisonneuve P, Fumagalli C, et al. Temozolomide alone or in combination with capecitabine in patients with advanced neuroendocrine neoplasms: an Italian multicenter real-world analysis. Endocrine. 2021;72(1):268–278. doi: 10.1007/s12020-020-02421-2 [DOI] [PubMed] [Google Scholar]
- 72.Jeong H, Shin J, Jeong JH, et al. Capecitabine plus temozolomide in patients with grade 3 unresectable or metastatic gastroenteropancreatic neuroendocrine neoplasms with Ki-67 index <55%: single-arm phase II study. ESMO Open. 2021;6(3):100119. doi: 10.1016/j.esmoop.2021.100119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zrajkowska A, Kolasinska-ćwikła A, Nowicki M, et al. Low rate of toxicity of combined PRRT and CAPTEM therapy in patients with advanced, non resectable, progressive pancreatic and midgut neuroendocrine tumors (NET). ENETS Annual Congress Barcelona, 2021. [Google Scholar]
- 74.Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27(9):561–569. doi: 10.1089/cbr.2012.1276 [DOI] [PubMed] [Google Scholar]
- 75.Claringbold PG, Turner JH. Pancreatic Neuroendocrine Tumor Control: durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology. 2016;103(5):432–439. doi: 10.1159/000434723 [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Li Y, Duan J, et al. Capecitabine and Temozolomide as a Promising Therapy for Advanced Thymic Atypical Carcinoid. Oncologist. 2019;24(6):798–802. doi: 10.1634/theoncologist.2018-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yalcin S. Introduction to Neuroendocrine Tumours. In: Yalcin S, Öberg K, editors. Neuroendocrine Tumours. Berlin, Heidelberg: Springer; 2015. [Google Scholar]
- 79.Boudreaux JP. Surgery for gastroenteropancreatic neuroendocrine tumors (GEPNETS). Endocrinol Metab Clin North Am. 2011;40(1):163–71, ix. doi: 10.1016/j.ecl.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 80.Frizziero M, Spada F, Lamarca A, et al. Carboplatin in Combination with Oral or Intravenous Etoposide for Extra-Pulmonary, Poorly-Differentiated Neuroendocrine Carcinomas. Neuroendocrinology. 2019;109(2):100–112. doi: 10.1159/000497336 [DOI] [PubMed] [Google Scholar]
- 81.Elvebakken H, Perren A, Scoazec JY, et al. A Consensus-Developed Morphological Re-Evaluation of 196 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and Its Clinical Correlations. Neuroendocrinology. 2021;111(9):883–894. doi: 10.1159/000511905 [DOI] [PubMed] [Google Scholar]
- 82.Moertel CG, Hanley JA. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials. 1979;2(4):327–334. [PubMed] [Google Scholar]
- 83.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326(8):519–523. doi: 10.1056/NEJM199202203260804 [DOI] [PubMed] [Google Scholar]
- 84.Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG; Eastern Cooperative Oncology Group. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23(22):4897–4904. doi: 10.1200/JCO.2005.03.616 [DOI] [PubMed] [Google Scholar]
- 85.Meyer T, Qian W, Caplin ME, et al. Capecitabine and streptozocin ± cisplatin in advanced gastroenteropancreatic neuroendocrine tumours. Eur J Cancer. 2014;50(5):902–911. doi: 10.1016/j.ejca.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 86.Dahan L, Bonnetain F, Rougier P, et al. Phase III trial of chemotherapy using 5-fluorouracil and streptozotocin compared with interferon alpha for advanced carcinoid tumors: FNCLCC-FFCD 9710. Endocr Relat Cancer. 2009;16(4):1351–1361. doi: 10.1677/ERC-09-0104 [DOI] [PubMed] [Google Scholar]
- 87.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–160. doi: 10.1093/annonc/mds276 [DOI] [PubMed] [Google Scholar]
- 88.Kos-Kudła B, Blicharz-Dorniak J, Handkiewicz-Junak D, et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2013;64(6):418–443. doi: 10.5603/EP.2013.0028 [DOI] [PubMed] [Google Scholar]
- 89.Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227–232. doi: [DOI] [PubMed] [Google Scholar]
- 90.Okusaka T, Ueno H, Morizane C, et al. Cytotoxic chemotherapy for pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci. 2015;22(8):628–633. doi: 10.1002/jhbp.257 [DOI] [PubMed] [Google Scholar]
- 91.Bongiovanni A, Liverani C, Foca F, et al. Temozolomide Alone or Combined with Capecitabine for the Treatment of Metastatic Neuroendocrine Neoplasia: a “Real-World” Data Analysis. Neuroendocrinology. 2021;111(9):895–906. doi: 10.1159/000513218 [DOI] [PubMed] [Google Scholar]
- 92.Kunz P, Catalano P, Nimeiri H, et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: a trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol. 2018;36(15(suppl)):4004. doi: 10.1200/JCO.2018.36.15_suppl.4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peixoto RD, Noonan KL, Pavlovich P, Kennecke HF, Lim HJ. Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non-PNETs. J Gastrointest Oncol. 2014;5(4):247–252. doi: 10.3978/j.issn.2078-6891.2014.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi: 10.1016/S0140-6736(15)00817-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiedmann MW, Mössner J. Safety and efficacy of sunitinib in patients with unresectable pancreatic neuroendocrine tumors. Clin Med Insights Oncol. 2012;6:381–393. doi: 10.4137/CMO.S7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kotteas EA, Syrigos KN, Saif MW. Profile of capecitabine/temozolomide combination in the treatment of well-differentiated neuroendocrine tumors. Onco Targets Ther. 2016;9:699–704. doi: 10.2147/OTT.S72155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saif MW, Kaley K, Brennan M, Garcon MC, Rodriguez G, Rodriguez T. A retrospective study of capecitabine/temozolomide (CAPTEM) regimen in the treatment of metastatic pancreatic neuroendocrine tumors (pNETs) after failing previous therapy. JOP. 2013;14(5):498–501. doi: 10.6092/1590-8577/1589 [DOI] [PubMed] [Google Scholar]
- 98.Walter T, van Brakel B, Vercherat C, et al. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: prognostic relevance and association with response to alkylating agents. Br J Cancer. 2015;112(3):523–531. doi: 10.1038/bjc.2014.660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trillo Aliaga P, Spada F, Peveri G, et al. Should temozolomide be used on the basis of O6-methylguanine DNA methyltransferase status in patients with advanced neuroendocrine tumors? A systematic review and meta-analysis. Cancer Treat Rev. 2021;99:102261. doi: 10.1016/j.ctrv.2021.102261 [DOI] [PubMed] [Google Scholar]
- 100.Chong CR, Wirth LJ, Nishino M, et al. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer. 2014;86(2):241–246. doi: 10.1016/j.lungcan.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crona J, Fanola I, Lindholm DP, et al. Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology. 2013;98(2):151–155. doi: 10.1159/000354760 [DOI] [PubMed] [Google Scholar]
- 102.Bello Roufai D, Planchard D, Walter T, et al. Antitumour Efficacy of Temozolomide in Patients with Metastatic Pulmonary Carcinoids. Neuroendocrinology. 2016;103(suppl 1):65. [Google Scholar]
- 103.Tabaksblat E, Temozolomide-Based Second-Line LM. Chemotherapy in Patients with Advanced Bronchopulmonary Neuroendocrine Tumours. Neuroendocrinology. 2016;103(suppl 1):72. [Google Scholar]
- 104.Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, Phase 2 trial. Lancet Oncol. 2017;18(12):1652–1664. doi: 10.1016/S1470-2045(17)30681-2 [DOI] [PubMed] [Google Scholar]
- 105.Vilar E, Salazar R, Pérez-García J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer. 2007;14(2):221–232. doi: 10.1677/ERC-06-0074 [DOI] [PubMed] [Google Scholar]
- 106.Strosberg JR, Cives M, Brelsford M, et al. Identification of response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. J Clin Oncol. 2015;33(15_suppl):4099. doi: 10.1200/jco.2015.33.15_suppl.409926324360 [DOI] [Google Scholar]
- 107.Lu Y, Zhao Z, Wang J, et al. Safety and efficacy of combining capecitabine and temozolomide (CAPTEM) to treat advanced neuroendocrine neoplasms: a meta-analysis. Medicine. 2018;97(41):e12784. doi: 10.1097/MD.0000000000012784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdel-Rahman O, Fouad M. Temozolomide-based combination for advanced neuroendocrine neoplasms: a systematic review of the literature. Future Oncol. 2015;11(8):1275–1290. doi: 10.2217/fon.14.302 [DOI] [PubMed] [Google Scholar]