Abstract
Purpose
Frailty is closely associated with biological age, concurrent medical conditions, morbidity, and decreased survival. Poor oral health is common in older individuals and is associated with frailty. Considering its potential importance, a study on the association between oral health and frailty is meaningful. Therefore, we aimed to analyze the association between major oral health factors and frailty using nationally representative samples of older adults.
Patients and Methods
This cross-sectional study included 3018 older adults (age ≥ 65 years) from the seventh Korea National Health and Nutrition Examination Survey. Oral examination results, laboratory data, handgrip strength, life style factors derived from questionnaires, and food intake survey results were analyzed. This study used the deficit accumulation model among the main operational definitions of frailty. We constructed a frailty index based on 36 items and classified participants as non-frail, pre-frail, or frail. Oral health factors included chewing difficulty, number of teeth, periodontal disease, and number of carious teeth. Logistic regression analysis was performed to determine significant factors.
Results
A total of 1222 (40.5%), 1014 (33.6%), and 782 (25.9%) individuals were classified as non-frail, pre-frail, and frail, respectively. Chewing difficulty was associated with increased risk of frailty after adjusting for age, sex, socioeconomic factors, and comorbidities (odds ratio 2.68, 95% confidence interval 2.08–3.44). Periodontal disease was positively associated with chewing difficulty (odds ratio 1.29, 95% confidence interval 1.07–1.56), and chewing difficulty decreased as the number of teeth increased (odds ratio 0.97, 95% confidence interval 0.96–0.99).
Conclusion
Chewing difficulty was significantly associated with frailty in the older population. Considering the negative effect of chewing difficulty on frailty, more attention should be focused on oral health.
Keywords: oral health, frailty, chewing difficulty, older population
Introduction
Frailty is a state of decreased physiological reserve and increased vulnerability to potential stressors as results of human aging.1,2 Reflecting the functional integrity of older individuals, studies have shown that the burden of frailty is associated with health status in various clinical circumstances and can predict adverse outcomes.3,4 Furthermore, studies suggest that the degree of frailty correlates with molecular measures of aging, supporting frailty as a marker of biological age.5
Frailty is determined by various operational definitions, and representative models include physical and biological model, deficit accumulation model, and multidimensional biopsychosocial model. In the physical and biological model,6 alterations in vitality and physical performance are assessed using operational criteria.7 In the deficit accumulation model, parameters reflecting functional, structural, or clinical alterations due to aging are aggregated to produce a frailty index.8 In both concepts of frailty, parameters reflecting nutritional status (ie, weight loss or low body mass index [BMI]) are commonly included, as nutrition is considered a cornerstone in the concept of “cycle of frailty”, a self-aggravating cycle of negative energy balance, decreased physical activity, and further decline of physical performance.7,9
Poor oral health is common in older individuals and is potentially a major harbinger of the progression of frailty by limiting proper nutritional intake.10 Dental caries and periodontal disease are the two biggest threats to oral health.11 Periodontal disease increases with age12 and is the main cause of tooth loss by loosening of teeth.13 Chewing ability is an important factor for oral health, and it is increasingly recognized as being associated with general health status because the ability to chew food may affect dietary choices and nutritional intake.14 Furthermore, oral health may affect a wide variety of health statuses beyond how individuals can properly maintain their nutritional status, by aggravating chronic inflammation.15,16 Therefore, the association between oral health and frailty is an important issue in the aged societies, and several studies have confirmed that poor oral health is associated with frailty.17,18 In recent years, oral frailty, proposed as a conceptualization of age-related gradual loss of oral function, is gaining increasing interest as a novel frailty phenotype.19
Considering its potential importance, a study on the association between oral health and frailty is meaningful. Therefore, we aimed to analyze the association between major oral health factors, including chewing difficulty, and frailty using nationally representative samples of Korean older adults. We also analyzed factors associated with chewing difficulty.
Materials and Methods
Study Population
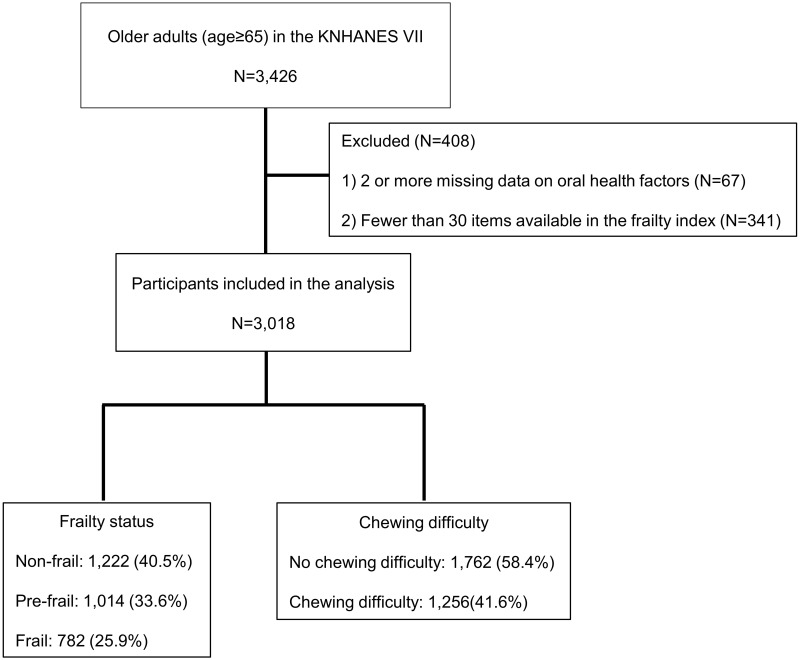
This cross-sectional study was based on data collected from the seventh Korea National Health and Nutrition Examination Survey (KNHANES VII) conducted from 2016 to 2018. This survey has been conducted periodically since 1998 to assess the health and nutritional status of Koreans, to monitor trends in health risk factors and the prevalence of major chronic diseases, and to provide data for the development and evaluation of health policies and programs in Korea.20 A total of 3426 older individuals (aged ≥ 65 years) participated in KNHANES VII. We included 3018 participants in the study after excluding 67 participants with two or more missing data on oral health factors, and 341 with fewer than 30 available items in the frailty index21 (Figure 1).
Figure 1.
Flow diagram of the study participants.
All participants provided written informed consent prior to participating in KNHANES. Personal data from the survey were de-identified before being made publicly available. This study was approved by the Institutional Review Board of Chonnam National University Bitgoeul Hospital (IRB No. CNUBH-2022-009). The study was performed in accordance with the principles of the Declaration of Helsinki.
Oral Health Evaluation
Oral examinations were performed by public health dentists who completed education and training twice a year. In the training course, simulation training using various models was conducted. The degree of agreement between standard inspectors and public health dentists was evaluated and errors were corrected. To ensure reliability of oral health examinations, another dentist conducted additional data review after the public health dentist recorded the examination results.
The number of permanent teeth was calculated by excluding wisdom and carious teeth from non-missing, natural teeth.22 Periodontal examination was performed using a dental mirror and a community periodontal index probe. A participant was considered to have periodontal disease if their periodontal tissue required treatment. The World Health Organization (WHO) community periodontal index (CPI)23 was used to assess periodontal conditions and defined periodontal disease as a CPI greater than or equal to a score of 3.24 The mouth was divided into sextants, 3 each in the maxillary and mandibular arches. Periodontal tissues of ten index teeth were evaluated and included in the examination of bleeding upon the application of 20 g of pressure using a CPI probe, the presence of dental plaque, and the presence of periodontal pockets with measurable depths. The CPI was scored on a scale of 0 to 4 as follows: 0 points for healthy periodontal tissue (no bleeding, calculus, or a pocket depth ≥4 mm); 1 point for bleeding on probing only (bleeding on probing but no calculus or pocket depth ≥4mm); 2 points for periodontal tissue with plaques (supra- or subgingival calculus, no pocket depth ≥4mm); 3 points for periodontal tissue with shallow periodontal pockets (pocket depth of 4–5mm); and 4 points for periodontal tissue with deep periodontal pockets (pocket depth of ≥6mm). After assigning scores, the highest CPI score for the 6 sextants was selected.24 A score of 3 or 4 points was defined as presence of periodontal disease, while a score of 0 to 2 points was defined as absence of periodontal disease. Chewing difficulty was assessed using questionnaires. Participants who answered the question “do you have difficulty chewing food because of problems with teeth or the mouth?” with “very difficult” or “difficult” were judged to have difficulties in chewing.
Frailty-Related Factors Evaluation
Handgrip strength was measured using a digital hand dynamometer (T.K.K 5401 Grip-D; Takei, Tokyo, Japan). Participants were instructed to stand with their elbows straight and fully extended, heads straight, wrists not flexed or extended, and feet hip-width apart and even. A total of three consecutive measurements of both hands were performed with a 60s-rest between measurements. The maximum scores of the dominant hand were obtained and recorded in kilograms.25
Blood samples were collected from the participants during the survey. Body mass index (BMI) was calculated by dividing body weight (kg) by height2 (m2). Information about household income, level of education, and life style factors was derived from a self-reported questionnaire. Smokers were defined as those who smoked five or more packs of cigarettes in their lifetime and were currently smoking. Food intake surveys were conducted based on the 24 h-dietary recall method.26 Each nutrient’s intake was calculated as the sum of that nutrient’s intake from all foods consumed during a day.
Frailty Index
We developed the frailty index by referring to a standard procedure for creating a frailty index21 and a previous frailty index using the KNHANES data.27 The frailty index, calculated as a ratio of deficits present out of the total number of possible deficits, is given a continuous score from 0 (best) to 1 (worst).28
The frailty index in this study was composed of 36 items from KNHANES VII. The items used to calculate the index value included comorbidities, functional abilities, signs and symptoms, and laboratory test values. Comorbidities included bronchial asthma, chronic obstructive pulmonary disease, diabetes, dyslipidemia, cataract, cardiovascular disease, stroke, arthritis, anemia, cancer, and depression. Functional abilities consisted of inactivity, exercise capacity, activities of daily living limitations, social activity limitations, self-care ability, and hearing impairment. Signs and symptoms consisted of pain or discomfort, weight loss, depression or anxiety, suicidal ideation, and stress. Laboratory values consisted of systolic blood pressure, diastolic blood pressure, heart rate regularity, pulmonary function test, hemoglobin, blood urea nitrogen, creatinine, total cholesterol, triglyceride, high-density lipoprotein-cholesterol, fasting glucose, and urine protein. Additional items included current smoking state and obesity (BMI ≥ 25) (Supplementary Table 1).
Study participants were classified as non-frail (frailty index ≤ 0.15), pre-frail (0.15 < frailty index ≤ 0.25), or frail (frailty index > 0.25) according to the criteria used in previous studies.29–31
Statistical Analysis
All statistical analyses were performed using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA). In this study, a complex sample analysis method with assigned weights was used to obtain national-level statistical estimates. Continuous and discrete variables were compared using general linear model and crosstabs analysis, respectively. A multivariate logistic regression analysis was performed to identify factors associated with frailty status and chewing difficulty. When analyzing oral health factors associated with frailty status, the model was divided into a model adjusted for age and sex (Model 1) and another adjusted for age, sex, socioeconomic factors, and comorbidities (Model 2). The odds ratios (OR) and 95% confidence intervals (CI) were confirmed in both models. All statistical analyses were two-tailed, and statistical significance was set at P < 0.05.
Results
General Characteristics of the Study Participants According to Frailty Status
The mean age of the study participants was 72.7 (standard deviation 5.0) years, and 1311 (43.4%) participants were men. According to frailty status, 1222 (40.5%) participants were classified as non-frail, 1014 (33.6%) as pre-frail, and 782 (25.9%) as frail. General characteristics related to frailty status are presented in Table 1. The frailty status groups showed significant differences in age, sex ratio, income and education levels, and prevalence of various diseases. With regard to oral health factors, chewing difficulty and number of teeth showed significant differences among the frailty status groups. However, no significant differences were found in the prevalence of periodontal disease and the number of carious teeth.
Table 1.
General Characteristics of the Study Participants According to Frailty Status
| Non-Frail (N=1222) | Pre-Frail (N=1014) | Frail (N=782) | P-value | |
|---|---|---|---|---|
| Age (years), mean (SE) | 71.5 (0.3) | 73.0 (0.3) | 74.0 (0.2) | <0.001 |
| Sex (male sex), n (%) | 616 (49.1%) | 434 (42.8%) | 261 (32.9%) | <0.001 |
| Income quartile* (low/mid-low/mid-high/high), % | 39.5%/29.7%/19.0%/11.9% | 47.9%/25.2%/16.7%/10.2% | 61.8%/23.5%/9.4%/5.3% | <0.001 |
| Level of education† (1st/2nd/3rd/4th), % | 48.7%/17.3%/19.0%/15.0% | 56.7%/15.4%/17.7%/10.2% | 69.8%/13.4%/13.1%/3.7% | <0.001 |
| Smoking, n (%) | 73 (6.0%) | 108 (11.9%) | 90 (11.3%) | <0.001 |
| Hypertension, n (%) | 683 (54.3%) | 653 (63.0%) | 587 (76.1%) | <0.001 |
| Diabetes, n (%) | 168 (13.4%) | 298 (28.7%) | 318 (42.0%) | <0.001 |
| Stroke, n (%) | 25 (2.4%) | 58 (5.8%) | 101 (13.1%) | <0.001 |
| Cardiovascular disease (MI, angina), n (%) | 41 (3.2%) | 81 (7.6%) | 108 (14.3%) | <0.001 |
| BMI (kg/m2), mean (SE) | 23.5 (0.2) | 24.4 (0.2) | 24.9 (0.2) | <0.001 |
| Oral health factors | ||||
| Difficulty chewing, n (%) | 365 (29.2%) | 426 (41.3%) | 465 (57.7%) | <0.001 |
| Number of teeth, mean (SE) | 22.3 (0.4) | 21.4 (0.4) | 20.8 (0.4) | 0.001 |
| Periodontal disease, n (%) | 525 (47.5%) | 425 (46.8%) | 328 (49.2%) | 0.764 |
| Number of carious teeth, mean (SE) | 0.4 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.163 |
Notes: Continuous variables (age, BMI, number of teeth, number of carious teeth) were compared using general linear model analysis in a complex sample analysis method. Discrete variables (Sex, income quartile, level of education, smoking, hypertension, diabetes, stroke, cardiovascular disease, difficulty chewing, periodontal disease) were compared using crosstabs analysis in a complex sample analysis method. *Income quartile: household income/month, low: household income/month < 720 US dollars, mid-low: 720 US dollars ≤ household income/month < 1440 US dollars, mid-high: 1440 US dollars ≤ household income/month < 2320 US dollars, high: household income/month ≥ 2320 US dollars. †Level of education: 1st: elementary school or lower, 2nd: middle school, 3rd: high school, 4th: college or higher.
Abbreviations: SE, standard error; MI, myocardial infarction; BMI, body mass index.
Oral Health Factors Associated with Frailty Status
To identify the oral health factors associated with frailty status, a multivariate logistic regression analysis was performed on the factors that showed significant differences among the frailty status groups (Table 2). In models 1 and 2, participants with chewing difficulty were more likely to be frail compared with those without (Model 1: OR, 3.10; 95% CI, 2.48–3.86; Model 2: OR, 2.68; 95% CI, 2.08–3.44). However, in the adjusted models, the number of teeth was not significantly associated with frailty status. When linear regression analysis was performed to analyze factors associated with frailty index, chewing difficulty showed a significant association; however, the number of teeth did not (Supplementary Table 2). The mean frailty index of individuals with chewing difficulty was 0.22 (95% CI, 0.21–0.23), which was significantly higher than that of those without chewing difficulty (0.17; 95% CI, 0.17–0.18).
Table 2.
Oral Health Factors Associated with Frailty Status
| Prefrail | Frail | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Model 1* | ||||
| Chewing difficulty | 1.64 | 1.33–2.03 | 3.10 | 2.48–3.86 |
| Number of teeth | 0.99 | 0.98–1.01 | 0.99 | 0.97–1.00 |
| Model 2† | ||||
| Chewing difficulty | 1.49 | 1.19–1.86 | 2.68 | 2.08–3.44 |
| Number of teeth | 1.00 | 0.98–1.01 | 0.99 | 0.97–1.01 |
Notes: Logistic regression analysis was performed to identify factors associated with frailty status with non-frail as the reference. *Model 1: Adjusted for age, sex. †Model 2: Adjusted for age, sex, body mass index (BMI), smoking status, income quartile, level of education, hypertension, diabetes, stroke, and cardiovascular disease (myocardial infarction [MI], angina).
Abbreviations: CI, confidence interval; OR, odds ratio.
Factors Associated with Chewing Difficulty
Factors associated with chewing difficulty were further explored as a significant association was found between frailty and chewing difficulty. Participants with chewing difficulty were older, more likely to be currently smoking, and had higher prevalence of hypertension, diabetes, and stroke than those without chewing difficulty. Participants with chewing difficulty had fewer teeth, more carious teeth, and higher prevalence of periodontal disease than those without chewing difficulty (Table 3).
Table 3.
Clinical Characteristics of Individuals with or Without Chewing Difficulty
| No Chewing Difficulty (N=1762) | Chewing Difficulty (N=1256) | P-value | |
|---|---|---|---|
| Age (years), mean (SE) | 72.1 (0.2) | 73.3 (0.2) | <0.001 |
| Sex (male sex), n (%) | 786 (43.6%) | 525 (42.0%) | 0.437 |
| Smoking, n (%) | 130 (7.6%) | 141 (11.9%) | <0.001 |
| Hypertension, n (%) | 1090 (60.8%) | 833 (65.4%) | 0.038 |
| Diabetes, n (%) | 424 (23.5%) | 360 (28.7%) | 0.007 |
| Stroke, n (%) | 74 (4.2%) | 110 (9.2%) | <0.001 |
| Cardiovascular disease (MI, angina), n (%) | 125 (7.0%) | 105 (8.1%) | 0.331 |
| Number of teeth, mean (SE) | 22.3 (0.3) | 20.5 (0.3) | <0.001 |
| Periodontal disease, n (%) | 732 (45.2%) | 546 (51.6%) | 0.005 |
| Number of carious teeth, mean (SE) | 0.5 (0.1) | 0.7 (0.1) | 0.001 |
| Protein intake (g/day), mean (SE) | 56.9 (1.3) | 51.6 (1.0) | <0.001 |
| Grip strength (kg), mean (SE) | |||
| Male | 33.5 (0.4) | 32.0 (0.4) | <0.001 |
| Female | 20.0 (0.2) | 19.2 (0.2) | 0.001 |
| Frailty index, mean (SE) | 0.17 (0.004) | 0.22 (0.003) | <0.001 |
Notes: Continuous variables (age, number of teeth, number of carious teeth, protein intake, grip strength, frailty index) were compared using general linear model analysis in a complex sample analysis method. Discrete variables (Sex, smoking, hypertension, diabetes, stroke, cardiovascular disease, periodontal disease) were compared using crosstabs analysis in a complex sample analysis method.
Abbreviations: SE, standard error; MI, myocardial infarction.
To identify the factors associated with chewing difficulty, a multivariate logistic regression analysis was performed on the factors that showed significant differences depending on the presence or absence of chewing difficulty (Table 4). Frailty index was significantly associated with chewing difficulty (OR, 1.57; 95% CI, 1.42–1.75). In addition, participants with periodontal disease tended to have difficulty in chewing (OR, 1.29; 95% CI, 1.07–1.56). Participants with higher number of teeth had significantly lesser difficulty in chewing (OR, 0.97; 95% CI, 0.96–0.99).
Table 4.
Factors Associated with Chewing Difficulty
| Chewing Difficulty (Univariate Analysis) | Chewing Difficulty (Multivariate Analysis)* | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age (1 year higher) | 1.05 | (1.03–1.07) | <0.001 | 1.01 | (0.99–1.03) | 0.168 |
| Smoking | 1.64 | (1.25–2.14) | <0.001 | 1.36 | (0.96–1.91) | 0.080 |
| Hypertension | 1.22 | (1.01–1.47) | 0.039 | 0.99 | (0.80–1.23) | 0.945 |
| Diabetes | 1.31 | (1.08–1.60) | 0.007 | 1.01 | (0.81–1.26) | 0.946 |
| Stroke | 2.31 | (1.64–3.25) | <0.001 | 1.48 | (0.99–2.20) | 0.056 |
| Number of teeth | 0.96 | (0.95–0.98) | <0.001 | 0.97 | (0.96–0.99) | 0.001 |
| Periodontal disease | 1.30 | (1.08–1.56) | 0.005 | 1.29 | (1.07–1.56) | 0.009 |
| Number of carious teeth | 1.09 | (1.03–1.16) | 0.006 | 1.07 | (0.99–1.15) | 0.076 |
| Frailty index (0.1 higher) | 1.68 | (1.53–1.84) | <0.001 | 1.57 | (1.42–1.75) | <0.001 |
Notes: Logistic regression analysis was performed to identify factors associated with chewing difficulty. Values in bold indicate statistical significance. *All variables in the univariate analysis were entered in this multivariate analysis.
Abbreviations: CI, confidence interval; OR, odds ratio.
Discussion
In this study, we showed that chewing difficulty was significantly associated with frailty status. Older adults with chewing difficulty were more than twice as likely to be frail than those without chewing difficulty. To our knowledge, this study is the first to show the association between chewing difficulty with frailty and other clinical factors in a nationwide population of Korean older adults.
Chewing ability affects food choices in older adults, as shown by studies suggesting a significant relationship between chewing ability and nutrient intake.32 In our study, individuals with chewing difficulty had significantly lower protein intake than those without chewing difficulty, which is consistent with the result of a previous study.33 Considering that appropriate protein intake is essential for the prevention and treatment of sarcopenia,25,34 this may at least partially explain why the participants with chewing difficulty had lower grip strength than those without. Furthermore, masticatory force, a biomechanical component of the chewing process, has been associated with grip strength in prior studies.35,36 Therefore, a decrease in masticatory force may potentially mediate lower grip strength and chewing difficulty. However, masticatory force was not measured in KNHANES. Considering these associations, chewing difficulty can be regarded as both a consequence and a driver of the progression of the “cycle of frailty.”
The number of teeth had a significant association with chewing difficulty in our study. Insufficient number of teeth is known to give rise to functional disabilities,37 including chewing disability.38 A previous study showing that the number of teeth is significantly associated with handgrip strength supports the fact that factors related to chewing ability affect muscle strength.39 Although no direct association was found between the number of teeth and frailty in our study, several studies have confirmed this association.17,37,40 Since sarcopenia is a key mechanism of frailty and the core of physical frailty,7,41 factors related to chewing ability may be significantly associated with frailty. A recent systematic review confirmed that chewing difficulty and the number of teeth were strongly associated with frailty.19 However, the results of this study emphasize that chewing difficulty caused by a decrease in the number of teeth has a more important association with frailty than the number of teeth itself.
To improve chewing ability, factors associated with chewing difficulty can be potentially targeted for interventions. In this study, we found that chewing difficulty decreased as the number of teeth increased, supporting the potential role of proper dental preventive measures for preserving natural teeth from tooth loss caused by periodontal disease and dental caries.13,42 Untreated carious teeth are known to have a negative effect on chewing ability.43 However, in our study, no significant association was found between the number of carious teeth and chewing difficulty. This null association might be due to the evidently higher effect of teeth loss and the number of natural teeth itself on chewing ability when compared with the effect of dental caries on the remaining teeth.12,13 In this study, no significant differences were observed in the presence or absence of periodontal disease according to frailty status. In previous studies, the association between periodontal disease and frailty has been reported to be inconsistent,17 with several studies showing a significant association between the two,44,45 while others did not.40,46 Differences in defining periodontal disease between studies may have influenced the study results. A representative study to confirm the association analyzed the association between severe periodontitis defined as at least three teeth with a pocket depth of 5.5 mm or more and frailty.44
In addition to oral health factors, diseases, such as diabetes and stroke, and current smoking status were significantly associated with chewing difficulty in the univariate analyses. Stroke is a representative disease that causes functional disability,47 and diabetes is also closely related to functional decline.48,49 In particular, since diabetes is associated with an increase in loss of teeth,50 patients with diabetes require more attention to maintain their chewing ability. Smoking cessation is important to reduce chewing difficulty as it can lower the risk of tooth loss,51,52 and as smoking is a major risk factor for periodontal disease.53
From our observation, in establishing public health and welfare to prevent the progression of frailty, chewing difficulties in older adults should be considered. With nutrition and exercise comprising the core element of multidimensional frailty prevention measures, older individuals need a customized diet plan to meet their nutritional needs based on chewing ability. Future longitudinal or interventional studies considering chewing and swallowing ability in Korean older population are warranted.
This study has several strengths. First, the analysis was performed using data from nationally representative samples of older adults in Korea, supporting the generalizability of the study. Twenty-three households across 192 survey districts were selected annually, and approximately 10,000 individuals aged 1 year and over were targeted by KNHANES VII. To reduce the limitation of seasonal variations, KNHANES was conducted continuously throughout the year. Additionally, a complex sample analysis method with assigned weights was used to obtain national-level statistical estimates. Second, the analysis was performed including the functional factor such as chewing difficulty among oral health factors, and significant results were obtained even after adjusting multiple covariates. Third, by analyzing specific factors associated with chewing difficulty, we presented potential clinical factors which would be targeted in the interventional study to reduce chewing difficulty in older adults. This study also has some limitations. First, we could not determine a causal relationship between oral health factors and frailty because of the limitations inherent in the cross-sectional design. Second, since several data of the frailty index were obtained from the self-reported questionnaire included in KNHANES VII, limitations, such as the participant’s recall bias, might exist. We used secondary data analysis and even if there was a recall bias, it was difficult to address the bias. Third, we developed the frailty index by referring to a standard procedure for creating a frailty index and items used in previously published studies that used KNHANES data to create a frailty index. However, there was no additional validation of the frailty index used in this study. Fourth, we were not able to evaluate the potential mechanistic links between oral health factors and frailty in our study, as the study lacked for biomarkers. Fifth, generalizability in other populations is limited as our study was from observations in community-dwelling Korean population.
Conclusion
Among oral health factors, chewing difficulty was significantly associated with frailty in older population. Since chewing difficulty can restrict appropriate nutritional intake essential in preventing the progression of the “cycle of frailty”, proper management of factors associated with chewing difficulty is imperative. Reducing risk factors for tooth loss by regular oral examinations with attention focused on chronic conditions, such as stroke and diabetes, is recommended.
Funding Statement
This study was funded by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through high value-added food technology development program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant number: 321031031HD030) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C2383).
Data Sharing Statement
The datasets used and/or analyzed during the current study are publicly available from https://knhanes.kdca.go.kr/.
Ethics Approval
All participants provided written informed consent prior to participating in KNHANES. Personal data from the survey were de-identified before being made publicly available. This study was approved by the Institutional Review Board of Chonnam National University Bitgoeul Hospital (IRB No. CNUBH-2022-009). The study was performed in accordance with the principles of the Declaration of Helsinki.
Disclosure
Hee-Won Jung cofounded Dyphi Inc., a startup company based on sensor technology. The other author claims no conflicts of interest in this work.
References
- 1.Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nature Aging. 2021;1(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitnitski A, Collerton J, Martin-Ruiz C, et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161. doi: 10.1186/s12916-015-0400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41(2):141–149. doi: 10.1016/j.archger.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 5.Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dibello V, Lozupone M, Sardone R, et al. Clinical indicators of oral frailty: a domain-specific frailty phenotype. Curr Top Med Chem. 2022;22(29):2391–2394. doi: 10.2174/1568026622666220615145647 [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol a Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 9.Goisser S, Guyonnet S, Volkert D. The role of nutrition in frailty: an overview. J Frailty Aging. 2016;5(2):74–77. [DOI] [PubMed] [Google Scholar]
- 10.Lim J, Park H, Lee H, et al. Longitudinal impact of oral health on geriatric syndromes and clinical outcomes in community-dwelling older adults. BMC Geriatr. 2021;21(1):482. doi: 10.1186/s12877-021-02416-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin RM. Oral health: the silent epidemic. Public Health Reports. 2010;125(2):158–159. doi: 10.1177/003335491012500202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eke PI, Wei L, Borgnakke WS, et al. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontology. 2016;72(1):76–95. doi: 10.1111/prd.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratthall D, Petersen PE, Stjernswärd JR, et al. Oral and Craniofacial Diseases and Disorders. In: Jamison DT, Breman JG, Measham AR, editors. Disease Control Priorities in Developing Countries. Washington (DC) New York: The International Bank for Reconstruction and Development / The World Bank Oxford University Press Copyright © 2006, The International Bank for Reconstruction and Development/The World Bank Group; 2006. [Google Scholar]
- 14.Inukai M, John MT, Igarashi Y, Baba K. Association between perceived chewing ability and oral health-related quality of life in partially dentate patients. Health Qual Life Outcomes. 2010;8:118. doi: 10.1186/1477-7525-8-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018;130(1):98–104. doi: 10.1080/00325481.2018.1396876 [DOI] [PubMed] [Google Scholar]
- 16.Cecoro G, Annunziata M, Iuorio MT, Nastri L, Guida L. Periodontitis, low-grade inflammation and systemic health: a scoping review. Medicina. 2020;56:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakeem FF, Bernabé E, Sabbah W. Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology. 2019;36(3):205–215. doi: 10.1111/ger.12406 [DOI] [PubMed] [Google Scholar]
- 18.Tôrres LH, Tellez M, Hilgert JB, Hugo FN, de Sousa MD, Ismail AI. Frailty, frailty components, and oral health: a systematic review. J Am Geriatr Soc. 2015;63(12):2555–2562. doi: 10.1111/jgs.13826 [DOI] [PubMed] [Google Scholar]
- 19.Dibello V, Zupo R, Sardone R, et al. Oral frailty and its determinants in older age: a systematic review. Lancet Healthy Longevity. 2021;2(8):e507–e520. doi: 10.1016/S2666-7568(21)00143-4 [DOI] [PubMed] [Google Scholar]
- 20.Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014;43(1):69–77. doi: 10.1093/ije/dyt228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim ES, Kim BI, Jung HI. Age, period and cohort trends in oral health status in South Korean adults. Community Dent Oral Epidemiol. 2021;49(2):136–143. doi: 10.1111/cdoe.12585 [DOI] [PubMed] [Google Scholar]
- 23.World Health O. Oral Health Surveys: Basic Methods. 4th ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 24.Shin YU, Lim HW, Hong EH, et al. The association between periodontal disease and age-related macular degeneration in the Korea National health and nutrition examination survey: a cross-sectional observational study. Medicine. 2017;96(14):e6418. doi: 10.1097/MD.0000000000006418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e302. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 26.Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. doi: 10.4178/epih/e2014009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang MG, Kim SW, Yoon SJ, Choi JY, Kim KI, Kim CH. Association between frailty and hypertension prevalence, treatment, and control in the elderly Korean population. Sci Rep. 2017;7(1):7542. doi: 10.1038/s41598-017-07449-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60(3):464–470. doi: 10.1016/j.archger.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 29.Won CW, Lee Y, Lee S, Kim M. Development of Korean Frailty Index for Primary Care (KFI-PC) and Its Criterion Validity. Ann Geriatric Med Res. 2020;24(2):125–138. doi: 10.4235/agmr.20.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol a Biol Sci Med Sci. 2019;74(8):1271–1276. doi: 10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbard RE, Goodwin VA, Llewellyn DJ, Warmoth K, Lang IA. Frailty, financial resources and subjective well-being in later life. Arch Gerontol Geriatr. 2014;58(3):364–369. doi: 10.1016/j.archger.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 32.Walls AW, Steele JG. The relationship between oral health and nutrition in older people. Mech Ageing Dev. 2004;125(12):853–857. doi: 10.1016/j.mad.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 33.Nagai H, Shibata H, Haga H, et al. 地域老人におけるそしゃく能力と栄養摂取ならびに食品摂取との関連 [The relationship of chewing ability to nutrient and food intakes in the community elderly]. Japanese J Public Health. 1991;38(11):853–858. [PubMed] [Google Scholar]
- 34.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kugimiya Y, Iwasaki M, Ohara Y, et al. Relationship between Oral Hypofunction and Sarcopenia in Community-Dwelling Older Adults: the Otassha Study. Int J Environ Res Public Health. 2021;18:12. doi: 10.3390/ijerph18126666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita K, Tsuka H, Kato K, et al. Factors related to masticatory performance in healthy elderly individuals. J Prosthodont Res. 2018;62(4):432–435. doi: 10.1016/j.jpor.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 37.Albani V, Nishio K, Ito T, et al. Associations of poor oral health with frailty and physical functioning in the oldest old: results from two studies in England and Japan. BMC Geriatr. 2021;21(1):187. doi: 10.1186/s12877-021-02081-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bortoluzzi MC, Traebert J, Lasta R, Da Rosa TN, Capella DL, Presta AA. Tooth loss, chewing ability and quality of life. Contemp Clin Dent. 2012;3(4):393–397. doi: 10.4103/0976-237X.107424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin HS. Handgrip strength and the number of teeth among Korean population. J Periodontol. 2019;90(1):90–97. doi: 10.1002/JPER.18-0242 [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Lee E, Lee SW. Association between oral health and frailty: results from the Korea National Health and Nutrition Examination Survey. BMC Geriatr. 2022;22(1):369. doi: 10.1186/s12877-022-02968-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. doi: 10.3389/fnagi.2014.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broadbent JM, Thomson WM, Poulton R. Progression of dental caries and tooth loss between the third and fourth decades of life: a birth cohort study. Caries Res. 2006;40(6):459–465. doi: 10.1159/000095643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decerle N, Nicolas E, Hennequin M. Chewing deficiencies in adults with multiple untreated carious lesions. Caries Res. 2013;47(4):330–337. doi: 10.1159/000348397 [DOI] [PubMed] [Google Scholar]
- 44.Castrejón-Pérez RC, Jiménez-Corona A, Bernabé E, et al. Oral Disease and 3-Year Incidence of Frailty in Mexican Older Adults. J Gerontol a Biol Sci Med Sci. 2017;72(7):951–957. doi: 10.1093/gerona/glw201 [DOI] [PubMed] [Google Scholar]
- 45.Clark D, Kotronia E, Ramsay SE. Frailty, aging, and periodontal disease: basic biologic considerations. Periodontology. 2021;87(1):143–156. doi: 10.1111/prd.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsay SE, Papachristou E, Watt RG, et al. Influence of Poor Oral Health on Physical Frailty: a Population-Based Cohort Study of Older British Men. J Am Geriatr Soc. 2018;66(3):473–479. doi: 10.1111/jgs.15175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schimmel M, Ono T, Lam OL, Müller F. Oro-facial impairment in stroke patients. J Oral Rehabil. 2017;44(4):313–326. doi: 10.1111/joor.12486 [DOI] [PubMed] [Google Scholar]
- 48.Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25(1):61–67. doi: 10.2337/diacare.25.1.61 [DOI] [PubMed] [Google Scholar]
- 49.Kim IS, Han TR. Influence of mastication and salivation on swallowing in stroke patients. Arch Phys Med Rehabil. 2005;86(10):1986–1990. doi: 10.1016/j.apmr.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 50.López-Gómez SA, González-López BS, Scougall-Vilchis RJ, et al. Tooth loss in patients with and without diabetes: a large-scale, cross-sectional study of Mexican adults. J Am Dental Assoc. 2020;151(4):276–286. doi: 10.1016/j.adaj.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 51.Souto MLS, Rovai ES, Villar CC, Braga MM, Pannuti CM. Effect of smoking cessation on tooth loss: a systematic review with meta-analysis. BMC Oral Health. 2019;19(1):245. doi: 10.1186/s12903-019-0930-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Ansari A. Smoking cessation is effective in reducing the risk of tooth loss. Evid Based Dent. 2020;21(4):120–121. doi: 10.1038/s41432-020-0130-6 [DOI] [PubMed] [Google Scholar]
- 53.Qandil R, Sandhu HS, Matthews DC. Tobacco smoking and periodontal diseases. J Canadian Dental Assoc. 1997;63(3):187–192, 194–185. [PubMed] [Google Scholar]