Abstract
The first part of this paper presented the current knowledge on two very significant respiratory diseases with high pandemic potential, COVID-19 and influenza. The second part reviews other pathogens that cause acute respiratory viral infections, ARVI, including parainfluenza viruses, adenoviruses, pneumoviruses and specifically respiratory syncytial virus, enteroviruses, rhinoviruses, bocaviruses, and seasonal coronaviruses. The review presents modern data on the structure and replication of viruses, epidemiology and immunopathogenesis of diseases, diagnostics, preventive vaccination, and antiviral drugs. Topical issues regarding ARVI vaccination and the search for new broad-spectrum antiviral drugs are discussed.
Keywords: ARVI, respiratory viruses, SARS-CoV-2, viral replication, immunopathogenesis, ARVI prophylaxis
BIBLIOMETRICS IN THE STUDY OF RESPIRATORY VIRUSES
Influenza viruses are widespread in human populations and circulate in wild-animal species. Influenza A viruses have a high potential to cause pandemics, and, hence, they are the most studied respiratory viruses, as is shown in a number of bibliometric analysis [1–3]. The rest of viruses that cause acute respiratory diseases have been studied significantly less, which is probably because they do not have so many negative health effects on the human population. Bursts of research are commonly caused by the increasing threat to the population in cases of epidemics and pandemics. A significant rise in the number of publications on influenza viruses was observed in 2009–2010, including up to 650 publications per year, which was associated with the pandemic caused by the A/H1N1pdm09 influenza virus. However, the emergence of the SARS-CoV-2 virus at the end of 2019 and outbreak of the COVID-19 pandemic led to an unseen avalanche-like growth in the number of studies and, hence, publications devoted to this novel pathogen and the search for and creation of therapeutic and preventive medicines against the new disease (with there being more than 23 000 publications in 2020) [4, 5].
The main properties of the most common respiratory viruses are shown in Table 1.
Table 1. .
Characterization of the most widespread human viruses that cause ARVI
| Causative agent of ARVI |
Family, genus | Type/subtype/serotype | Nucleic acid | Virion size/ genome size |
Lipid envelope | Illness | Etiotropic drugs/vaccines |
|---|---|---|---|---|---|---|---|
| Influenza viruses |
Family Orthomyxoviridae Genus Alphainfluenzavirus, Betainfluenzavirus, Gammainfluenzavirus |
Types (species) A, B, C. Type A: 18 subtypes of HA, 11 subtypes of NA. Type B: B/Victoria, B/Yamagata |
Segmented single-stranded (–) RNA |
90–120 nm/ 13.6 kb* |
Yes | Common cold, laryngitis, pharyngitis, bronchitis, bronchiolitis, pneumonia | Neuraminidase inhibitors/live, inactivated, subunit influenza vaccines |
| Coronaviruses |
Family Coronaviridae Genus Alphacoronavirus, Betacoronavirus |
α-coronaviruses 229E, NL63; β-coronaviruses OC43, HKU1, SARS-CoV, MERS-CoV, SARS-CoV-2 |
Nonsegmented single-stranded (+) RNA |
120–125 nm/ 27–33.5 kb |
Yes | Common cold, pharyngitis, laryngitis, bronchitis, bronchiolitis, pneumonia, SARS, MERS, COVID-19 | None/vaccines against SARS-CoV-2 |
| Rhinoviruses |
Family Picornaviridae Genus Enterovirus |
Types (species): A, B, C. About 160 serotypes |
Nonsegmented single-stranded (+) RNA |
25 nm/7.2 kb | No | Common cold, pharyngitis, bronchiolitis, bronchitis, pneumonia | None/none |
| Enteroviruses |
Family Picornaviridae Genus Enterovirus |
Types A, B, D. More than 100 serotypes |
Nonsegmented single-stranded (+) RNA |
30 nm/7.2–8.5 kb | No | Common cold, conjunctivitis, acute pharyngitis, serous (aseptic) meningitis, encephalitis | None/vaccine based on inactivated EV-A71 (China) |
| Parainfluenza viruses |
Family Paramyxoviridae Genus Respirovirus |
Types 1, 2, 3, 4 |
Nonsegmented single-stranded (–) RNA |
150–200 nm/15 kb | Yes | Common cold, laryngitis, otitis media, pharyngitis, bronchiolitis, bronchitis, laryngotracheobronchitis (croup), pneumonia | None/none |
| Respiratory syncytial viruses |
Family Pneumoviridae Genus Orthopneumovirus |
Serotypes: A and B. 11 genotypes of serotype A, 23 genotypes of serotype B |
Nonsegmented single-stranded (–) RNA |
210 nm/13.3 kb | Yes | Common cold, otitis media, bronchiolitis, bronchitis, pneumonia | None/none (ribavirin only in critical situation) |
| Metapneumoviruses |
Family Pneumoviridae Genus Metapneumovirus |
Genotypes A and B. 4 subtypes |
Nonsegmented single-stranded (–) RNA |
150–250 nm/ 36 kb |
Yes | Common cold, laryngitis, bronchiolitis, bronchitis, pneumonia | None/none |
| Adenoviruses |
Family .Adenoviridae Genus Mastadenovirus |
Species A, B, C, D, E, F, G. More than 50 serotypes |
Nonsegmented double-stranded DNA |
80–110 nm/ 36 kb |
No | Pharyngitis, laryngitis, bronchitis, bronchiolitis, pneumonia | None/none for extensive use |
| Bocaviruses |
Family Parvoviridae Genus Bocaparvovirus |
Subtypes (serotypes) 1, 2, 3, 4 |
Nonsegmented single-stranded DNA |
18–26 nm/ 5.5 kb |
No | Common cold, asthma exacerbation, bronchiolitis, pneumonia | None/none |
*Kilobase, kb, a unit of measurement in molecular biology equal to 1000 nucleotides in RNA or single-stranded DNA or 1000 bp of double-stranded DNA.
CIRCULATION OF RESPIRATORY VIRUSES DURING THE COVID-19 PANDEMIC
At the end of 2019, the novel coronavirus (SARS-CoV-2) originated in China and, then, spread around the world. This led to the COVID-19 pandemic, which continues to this day. Adoption of strict public health and social measures (for example, remote work, cancellation of mass gatherings, improved hand hygiene, wearing masks, etc.) rapidly mitigated the circulation of seasonal respiratory viruses.
To illustrate, in Parma (northern Italy), in the epidemic season of 2019 until February 2020, flulike illness was caused mainly by the respiratory syncytial virus (HRSV). The percentage of tests positive for this virus was then reduced dramatically, and, starting at the end of February 2020, only SARS-CoV-2 was detected in patients. Mixed infections were very rare during 2020–2021 compared to the prepandemic period and were found only in children less than 1 year of age. Low circulation of non-SARS-CoV2 respiratory viruses was also observed in Lombardy (northern Italy) [6]. Influenza viruses and HRSV circulated until the end of February (week 9 of 2020), when they suddenly ceased to circulate 7 weeks earlier than during the previous five influenza seasons. SARS-CoV-2 remained the only respiratory virus identified in patients from week 10 of 2020 to the end of the year [7]. A similar mode of circulation was observed in Southeast Asia [8].
Intriguing data were collected by a group of Canadian scientists. They analyzed data from the Canadian Respiratory Virus Detection Surveillance System from August 30, 2014, to February 13, 2021. It was shown that, during the pandemic, the circulation of influenza A and B virus, respiratory syncytial virus, paramyxovirus, seasonal coronavirus, metapneumovirus, and adenovirus was reduced significantly. Meanwhile, enterovirus/rhinovirus continued to circulate, with regional differences in the epidemic intensity [9].
In the period from week 40 of 2020 to week 8 of 2021, the WHO European Office received data from 37 countries and territories where more than 25 thousand samples from sentinel surveillance sources were tested, which included only 33 samples positive for the influenza virus. More than 400 thousand samples were tested from nonsentinel surveillance sources, of which 679 tested were positive for the influenza virus. Most positive samples, i.e., 488 (72%), were collected in the United Kingdom [10]. Experts of the WHO European Office believe that the strict anti-epidemic measures implemented in European countries following outbreak of the COVID-19 pandemic led to this sharp reduction in the circulation of influenza virus. Herein, it is unclear why anti-epidemic measures had an insignificant effect on the circulation of enterovirus/rhinovirus. This is apparently because of the biology of the pathogens.
ENTEROVIRUSES
The Enterovirus genus is one of 35 genera in the family Picornaviridae and includes 13 species, seven of which are human pathogens: Enterovirus A–D (more than 100 types) and Rhinovirus A–C (more than 160 types) [11].
Enteroviruses (Fig. 1b) are an abundant group of pathogens with a high mutation rate and recombination frequency, which leads to the emergence of new viral strains.
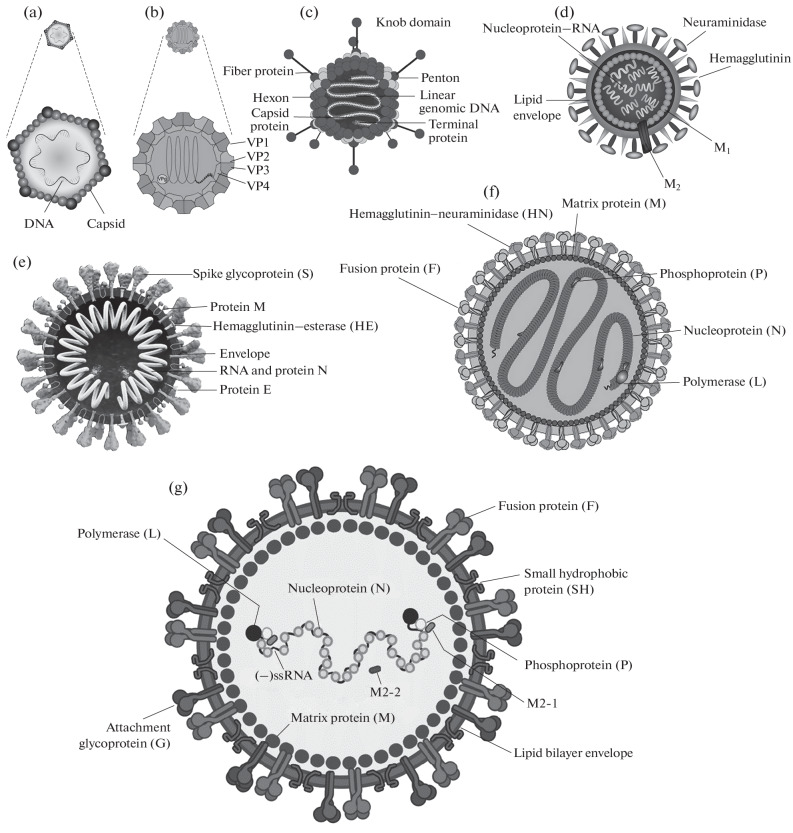
Fig. 1.
Schematic structure of main respiratory viruses. (a) Bocavirus, (b) enterovirus, (с) adenovirus, (d) influenza virus, (e) coronavirus, (f) parainfluenza virus, and (g) pneumovirus.
Enteroviruses are transmitted by the fecal–oral or respiratory routes. They are able to advance from the primary site of infection (gastrointestinal or respiratory tract) to infect other organs, including the central nervous system. Most people with respiratory enterovirus do not get sick or have mild symptoms, like those of the common cold. The symptoms may include fever, runny nose, sneezing, cough, skin rash, mouth blisters, and/or muscle aches. Occasionally, these viruses can cause more serious illness, including neurological injury (encephalitis, viral meningitis, and peripheral paralysis) [12]. The D68 (EV-D68) and A71 (EV-A71) are the two most common types of respiratory enteroviruses.
With the exception of two inactivated EV-A71 enterovirus vaccines recently licensed in China, there are no effective measures to prevent or treat respiratory enterovirus infection [13].
RHINOVIRUSES
Human rhinoviruses (RVs) were first discovered in the 1950s in an effort to identify the etiology of the common cold. Human rhinoviruses (Fig. 1b), similarly to all members of the family Picornaviridae, are viruses with a single strand of positive-sense RNA having approximately 7200 bp in its genome. The viral genome consists of a single gene translated into a single polypeptide that is cleaved by viral proteases to produce 11 individual proteins [14]. The viral capsid contains four structural proteins, i.e., VP1, VP2, VP3, and VP, and encloses the genome. The remaining proteins are nonstructural and participate in genome replication and viral-particle assembly. Proteins VP1, VP2, and VP3 account for the virus antigenic diversity, and VP4 creates viral RNA-capsid interface. Based on genome similarity, more than 160 RV strains were classified into three genetic clades, which are now recognized as three species (A, B, and C): RV-A (80 serotypes), RV-B (32 serotypes), and RV-C (55 serotypes) [15].
After binding to cell-surface receptors, viruses are internalized via endocytosis or macropinocytosis. Exposure to acid pH in the endosome leads to uncoating of the virus to release its genome into the cytosol. RNA also enables both the production of virus-specific proteins and synthesis of the antigenomes, which serve to generate RNA genome of new virus particles. New virions are assembled and packaged in intracellular plasma–membrane compartments and are exported via cell lysis [16].
Rhinovirus infection is transmitted mainly via direct contact or infected objects (fomites). Rhinoviruses cause lytic infection of airway epithelial cells. Rhinoviruses are mainly the etiological agents of upper respiratory tract infections because they grow better at 34°C than at 37°C. Rhinoviruses are the causative agents of from half to two-thirds of common colds [17]. Children may have from 8 to 12 rhinovirus infections and adults two or three infections per year; peaks of illness occur throughout the year [18]. Although most rhinovirus infections are mild, rhinovirus is a pathogen associated with bronchiolitis in infants, pneumonia with immunosuppression, and viral-induced exacerbations of respiratory diseases, such as asthma and chronic obstructive lung disease [19].
There are current\ly no approved antiviral therapies for rhinovirus infection, and treatment remains primarily supportive, which includes amelioration of the symptoms of the illness.
PARAINFLUENZA VIRUSES
Human parainfluenza viruses (HPIVs) (Fig. 1f) were first discovered in the 1950s [20]. They belong to the family Paramyxoviridae, Respirovirus genus. HPIVs are classified antigenically and genetically into four types (HPIV-1, -2, -3, and -4) and two subtypes (HPIV-4a and HPIV-4b). HPIV types 1 and 3 are classified as belonging to the Respirovirus genus, while HPIV types 2 and 4 are in the Rubulavirus genus [21].
The genome of parainfluenza viruses encodes eight proteins [22]. The three surface proteins are the fusion (F) protein, the receptor-binding protein hemagglutinin–neuraminidase (HN), and the small hydrophobic (SH) protein. Mature virions consist of the nucleocapsid and the matrix (M) protein surrounded by the lipid envelope derived from the host plasma membrane. The nucleocapsid is made of RNA enwrapped with the N protein to form the ribonucleoprotein (RNP); the P phosphoprotein; the V protein, which is a suppressor of the host innate immune system; and the RNA-dependent RNA polymerase. The N protein encapsidates the RNA genome to protect and prevent it from degradation by nucleases. The RNP serves as the template for the viral polymerase. The life cycle of the virus is similar to the infectious cycle of the respiratory syncytial virus described below [23].
Parainfluenza viruses can infect people of any age group and commonly cause mild respiratory illnesses in adults, but frequently result in pediatric hospitalizations for lower respiratory infections in children up to 5 years of age. HPIV-1 is the most common cause (in more than 50% of cases) of severe croup (laryngotracheobronchitis). HPIV-3 is second only to respiratory syncytial virus as a cause of pneumonia and bronchiolitis in young children [24].
There are no vaccines and licensed treatments against parainfluenza viruses. Paracetamol or ibuprofen may be used for relief of symptoms.
PNEUMOVIRUSES
Paramyxoviruses and pneumoviruses used to be classified in the same family Paramyxoviridae. However, in 2016, the International Committee on Taxonomy of Viruses (ICTV) separated these viruses into distinct families (Paramyxoviridae and Pneumoviridae) [25]. The family Pneumoviridae includes two genera: Metapneumovirus and Orthopneumovirus.
Respiratory Syncytial Viruses (HRSVs)
In 2016, the ICTV gave a new Latin name to the viruses, i.e., Human Orthopneumovirus, and classified them on the Orthopneumovirus genus within the family Pneumoviridae [26]. HRSVs are a globally prevalent cause of lower respiratory tract infection in infants [27]. The virus was first isolated in 1956. The virus’ name is derived from its ability to produce syncytia, that is, is the large cells that are formed when infected cells fuse.
The HRSV genome is a single-stranded, nonsegmented, linear RNA genome of negative polarity 15.2 kb in size. The RNA contains ten genes and encodes for 11 proteins. The proteins are as follows: eight proteins are internal and include a matrix protein (M); two are nonstructural proteins (NS1 and NS2); a nucleocapsid-associated transcription factor (M2-1) and the associated polypeptide involved in genome replication (M2-2), which are both transcribed from the same M2 gene; nucleoprotein (N), and phosphoprotein (P) (Fig. 1g). N and P proteins interact with the RNA-dependent RNA polymerase to promote nucleocapsid formation. The three remaining proteins accumulate in the viral envelope: the small hydrophobic (SH) protein forms a ion channel pore that facilitates ion transport across host cell membranes, the HRSV attachment (G) glycoprotein mediates viral attachment, and the fusion (F) protein mediates viral and host membrane fusion. Compared to influenza viruses, HRSVs are antigenically relatively stable, and only the G protein is important antigenically and exhibits antigenic drift similar to the influenza virus hemagglutinin and neuraminidase. This antigenic variability led to emergence of two major antigenic groups of circulating HRSVs: A and B. Group A consists of 11 genotypes, and 23 genotypes were described for HRSV-B [28]. It is believed that this genetic and serological diversity is the main cause of a large number of repeated infections throughout life. The antigenic and serologic differences that occur among these viruses may contribute to the ability of HRSVs to establish reinfections throughout life and lack of long-term immunity [21].
The infectious cycle of HRSVs begins upon attachment of the virion to the cell surface receptors. Surfactant protein A, annexin II, and CX3CR1 have been shown to bind HRSV G protein, and these proteins have been proposed to be the receptors for HRSVs [29]. In contrast to the influenza virus, acidic pH is not a rigger of HRSV F proteins for activity. Fusion can occur at the cell surface or within an endosomal compartment. Once the nucleocapsid has entered the cytoplasm, a viral positive-sense RNA is generated for protein synthesis and genome replication. Viral proteins are translated by both free ribosomes (internal proteins) and by ribosomes bound to the endoplasmic reticulum (surface proteins). Viral glycoproteins are transported to the plasma membrane. At the plasma membrane, viral structural proteins and genomic RNA coordinate with viral glycoproteins to form new virus particles, which are then released.
To date, ribavirin is the only approved antiviral treatment of HRSVs [30]. At the same time, clinical trials have not found sufficient evidence of ribavirin efficacy in the treatment of HRSV infection in children less than 1 year of age. In addition, ribavirin is a toxic medication and, hence, indications for its inhalation include severe laboratory-confirmed HRSV infection in young children and patients with congenital heart disease [31].
Currently, palivizumab is the only product available to prevent HRSVs. Palivizumab, an antiviral monoclonal antibody, reduces HRSV hospitalizations in high-risk infants by up to 80% [32]. However, due to its high cost, it is recommended for high-risk infants only. To date, several candidate vaccines are engineered, but none of them have yet been licensed [33].
Metapneumoviruses
Human metapneumovirus (HMPV) (Fig. 1g) was first reported in 2001 in samples taken from children with acute illnesses of the lower respiratory tract [34]. Symptoms commonly associated with HMPVs include cough, fever, nasal congestion, and shortness of breath. Clinical symptoms of HMPV infection may progress to bronchitis or pneumonia and are similar to other upper and lower respiratory tract infections. The estimated incubation period is 3–6 days, and the median duration of illness can vary depending upon severity, but it is similar to other respiratory infections. HMPVs can cause upper and lower respiratory disease in people of all ages, especially among young children, older adults, and people with weakened immune systems [35].
The genome organization of HMPV and HRSV is similar, but HMPV possesses several genes different from that of HRSV, in addition to lacking nonstructural proteins NS1 and NS2 [36]. The effects of metapneumovirus proteins on the host immune system have not been fully characterized. Nevertheless, G-protein, one of the two proteins responsible for virus entry into the target cell, has been well studied, since it plays a key role in immune evasion by inhibiting interferon synthesis in an infected cell [37]. This distinguishes it from HRSV, in which nonstructural proteins NS1 and NS2 hinder the host’s innate immune response.
In general, the replication of human metapneumovirus is similar to HRSV replication. Several laboratories in different countries are developing vaccines and medicines for the prevention and treatment of HMPV infection, but so far no drug has been approved for regular clinical use [38].
Human Adenoviruses
Adenoviruses (AdVs) are a large group of viruses that have a double-stranded DNA genome. These viruses are members of the family Adenoviridae in the Mastadenovirus genus. The first human adenovirus was isolated in 1953, when an infectious agent was detected from adenoidal tissue. To date, more than 100 human adenovirus (HAdV) serotypes have been classified, divided into seven types, A–G [39].
Adenovirus virions (Fig. 1c) are nonenveloped particles 80–100 nm in diameter. The icosahedral capsid is made up of 252 subunits called “capsomeres,” including 240 hexon proteins that are trimers, each interacting with six other trimers to form the 20 surfaces and 12 penton proteins that interact with five hexon capsomers and form 12 vertices of the capsid. Each penton bears a penton base and a knobbed fiber. An icosahedral protein shell surrounds a protein core that contains the linear, double-stranded DNA linked to four (internal) proteins: Mu, VII, V, and terminal protein (TR). The adenovirus terminal protein (TP) is covalently linked to the 5'-ends of the adenovirus genome [40].
The genome of adenoviruses is about 36 kbp in length. The genome encodes 10–12 proteins. However, 40 proteins are synthesized in infected cells, since both strands of the double-stranded DNA code for specific viral functions, with adenovirus also making extensive use of alternative RNA splicing. A mature virion has 13 proteins; the rest belong to nonstructural proteins and are found only in an infected cell. The assembly of this virion structure takes place in the nucleus, where the viral DNA is “dressed up” in the new capsid. Adenoviruses induce lysis of the host cell to release viral progeny. HAdVs can produce persistent or latent infections; it was the latent virus that was discovered in lymphoid tissues [40].
HAdV causes a wide range of diseases depending on the species, but respiratory infections are among the most common, especially in children under 5 years of age. Approximately 5–7% of respiratory-tract infections worldwide in children are caused by HAdVs. The adenoviral infections usually cause only mild common cold symptoms and get better on their own without medical treatment. However, in high-risk groups, HAdVs can also cause more severe diseases, such as pneumonia, bronchitis, and croup [41].
Respiratory HAdVs usually spread from infected people to others with respiratory droplets released in sneezes and coughs, as well as through contaminated surfaces. Adenoviruses have a high degree of environmental resistance; they are stable in the pH range of 5–9 and are resistant to isopropyl alcohol, ether, and chloroform. HAdVs can persist on surfaces for weeks at room temperature and longer at lower temperatures. A person infected with HAdVs is contagious during the incubation period, which is usually from 4 to 8 days, but can last up to 24 days depending on HAdV serotype [42].
Currently, there are no licensed etiotropic drugs for the treatment of adenovirus infection. A live attenuated oral vaccine has been developed in the United States, but the vaccine is approved for military personnel 17 through 50 years of age [43].
Human Bocaviruses
The first human bocavirus (HBoV) (Fig. 1a) was described in the laboratory of Karolinska University (Stockholm, Sweden) in 2005 during molecular assays of nasopharyngeal wash collected to detect new respiratory tract viruses [44]. The analysis made it possible to identify a previously unknown member of the family Parvoviridae referred to as “human bocavirus.” In subsequent assays, three more subtypes of HBoV (HBoV-2, -3, and -4) were detectable in the stool samples.
HBoVs belong to the family Parvoviridae, which is represented by viruses with single-stranded DNA and are classified to the Bocaparvovirus genus [45]. Parvoviruses are a group of very small viruses, since they package a genome of about 5–5.5 kbp, in addition to having a small viral particle size, i.e., 18 to 26 nm. Parvoviruses are nonenveloped. The capsid is composed of 60 copies of three capsid proteins. The HBoV genome has three reading frames. The first two encode nonstructural proteins NS1–4 and NP1, and the third reading frame encodes three capsid proteins VP1–3.
The life cycle of HBoV has not yet been fully characterized. Replication occurs in the nucleus and, similarly to other parvoviruses, replication of HBoV requires many components that are used by the cell to replicate cellular DNA. Progeny virions leave the cell by lysis, but progeny particles may also be exported by exocytosis; the factors that contribute to these life cycles remain unclear [46].
A large meta-analysis of epidemiological studies conducted from 2005 to 2016 showed that HBoV is detected in about 6% of cases of respiratory infections and more than 50% of cases are a combined infection.
HBoV respiratory infection is clinically indistinguishable from other respiratory infections and can only be diagnosed using molecular assays. Symptoms associated with HBoVs range from mild respiratory illness to severe conditions. The most common symptoms reported in clinical studies are cough, fever, runny nose, asthma exacerbation, bronchiolitis, acute wheezing, and pneumonia.
Human bocaviruses are stable in the environment; they are abundant in urban sewage [47]. There is no clinically approved antiviral agent against bocavirus infections.
Coronaviruses
As was mentioned in the first part of the article, coronaviruses (CoVs) are members of the subfamily Orthocoronavirinae (family Coronaviridae, order Nidovirales). According to ICTV, the subfamily consists of four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. α- and β-coronaviruses infect only mammals. Gamma- and delta-coronaviruses are largely associated with avian hosts, but some of them can also be detected in mammalian species. The first human coronaviruses (HCoV-229E and HCoV-OC43) were isolated almost 50 years ago, whereas HCoV-NL63 and HCoV-HKU were identified only following the outbreak of SARS-CoV in 2002–2003 [48]. Usually, seasonal coronaviruses cause mild common colds. They are more dangerous for patients from high-risk groups. HCoV-NL63 can cause acute laryngotracheitis (croup) [49].
The structure of seasonal coronaviruses is similar to the structure of SARS-CoV-2 described in the first part of the article. Analysis of the molecular clocks of human coronaviruses showed that HCoV-NL63 and HCoV-229E emerged about 500–800 and 200 years ago, respectively [50, 51]. As for SARS-CoV and MERS-CoV, they must have separated from bat CoV in the past 3–4 decades [52]. However, these dates are approximate, and confidence intervals are often wide.
Prospects for Prevention and Treatment of ARVI
The urgent need for preventive and antiviral medications to treat COVID-19 has accelerated vaccine development worldwide substantially. It is possible that novel approaches to vaccine development (RNA vaccines, viral vectors, and protein vaccines with potent adjuvants) combined with the advances of immunotherapy will in the near future become a solution to some concerns of modern society, such as emerging viral infections, including those caused by respiratory viruses. Nevertheless, many obstacles remain on this path. Thus, RNA vaccines against COVID-19 went through preclinical trials in a record 66 days and there was progress from phase I to II of clinical trials in less than 5 months that obtained promising data on immunogenicity and efficacy in human beings after 10 months [53].
In the case of viral vectors, a synthetic gene encoding the target protein is inserted into the genome of one of the viruses that cannot replicate in the human host. The virus is then grown in cell culture and used to deliver a synthetic gene during vaccination. Adenoviruses, measles virus, modified Ankara smallpox vaccine virus, vesicular stomatitis virus, and cytomegalovirus are usually used as vectors [54]. On average, a vector vaccine can be created in 3–4 week, and 3–4 months are needed before the start of clinical trials. It is noteworthy that the immune response to the vector is a major challenge with vector vaccines.
When creating protein vaccines, mammalian or plant cells are used as expression systems for the production of recombinant proteins, which are then purified, combined with adjuvants, and used as a vaccine. It took two months to obtain producer cells and another six months for preclinical trials before the first vaccine based on the SARS-COV-2 S-protein was approved for human trials [55]. Therefore, effective anti-COVID-19 vaccines were developed in a record short time, and mass vaccination began less than a year after the outbreak of the pandemic. However, in the case of an epidemic caused by a highly pathogenic influenza A virus, 8–10 months without a vaccine is an overly long period, and the consequences can be catastrophic.
In our opinion, in the near future, we can expect the appearance of effective drugs both against a particular pathogen and with broad antiviral activity. One approach is to use computer technology to select substances from a huge variety of chemical compounds that are likely to bind to viral proteins. Computers simulate the interaction of the tested substances with the binding sites in three-dimensional models of target proteins. The most promising compounds are then tested experimentally in cell culture and on animals. Successful preclinical and clinical trials allow us to recommend some of them for the treatment of viral diseases [56].
The search for drugs based on their structure has played an important role in antiviral drug design. For example, viracept is an antiviral medicine discovered in the 1990s for the treatment of HIV infection was designed using computer simulation. Since the 1990s, the computational power of supercomputers has increased by millions of times. More than a billion compounds can now be analyzed in a few days [57].
Therefore, in the near future, we can expect that a range of highly effective vaccines and new antiviral drugs will be engineered, and we look forward to the appearance of potent medicines against a multitude of respiratory viruses among these new antiviral medications.
FUNDING
This study was funded by Russian Foundation for Basic Research in the framework of research project no. 20-115-50029 (review part) and state order no. FSUS-2020-0035 (development of new vaccines and antiviral drugs).
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human beings performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Ilyicheva, T.N., Netesov, S.V., Gureyev, V.N., COVID-19, influenza, and other acute respiratory viral infections: etiology, immunopathogenesis, diagnosis, and treatment. Part I. COVID-19 and influenza, Molecular Genetics, Microbiology and Virology, 2022, vol. 37, no. 1, pp. 1–9. 10.3103/S0891416822010025
REFERENCES
- 1.Chen N., Liu Y., Cheng Y., Liu L., Yan Z., Tao L. Technology resource, distribution, and development characteristics of global influenza virus vaccine: A patent bibliometric analysis. PLoS One. 2015;10:e0136953. doi: 10.1371/journal.pone.0136953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fricke R., Uibel S., Klingelhoefer D., Groneberg D.A. Influenza: A scientometric and density-equalizing analysis. BMC Infect. Dis. 2013;13:454. doi: 10.1186/1471-2334-13-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gureyev V.N., Mazov N.A., Ilyicheva T.N., Bazhan S.I. An informetric analysis of studies on influenza vaccines and vaccination. OnLine J. Biol. Sci. 2017;17:372–381. doi: 10.3844/ojbsci.2017.372.381. [DOI] [Google Scholar]
- 4.Haghani M., Bliemer M.C.J., Goerlandt F., Li J. The scientific literature on Coronaviruses, COVID-19 and its associated safety-related research dimensions: A scientometric analysis and scoping review. Saf. Sci. 2020;129:104806. doi: 10.1016/j.ssci.2020.104806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou J., Tian S.J., Niu S.M., Kang X.Q., Lian H.X., Zhang L.X., Zhang J.J. Coronavirus disease 2019: A bibliometric analysis and review. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3411–3421. doi: 10.26355/eurrev_202003_20712. [DOI] [PubMed] [Google Scholar]
- 6.Calderaro A., De Conto F., Buttrini M., Piccolo G., Montecchini S., Maccari C. Human respiratory viruses, including SARS-CoV-2, circulating in the winter season 2019–2020 in Parma, Northern Italy. Int. J. Infect. Dis. 2021;102:79–84. doi: 10.1016/j.ijid.2020.09.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli C., Pellegrinelli L., Bubba L., Primache V., Anselmi G., Delbue S. When the covid-19 pandemic surges during influenza season: Lessons learnt from the sentinel laboratory-based surveillance of influenza-like illness in Lombardy during the 2019–2020 season. Viruses. 2021;13:695. doi: 10.3390/v13040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilyicheva T.N., Gureyev V.N. The impact of anti-epidemic measures against coronavirus disease 2019 (COVID-19) on the seasonal influenza epidemic. Chin. Med. J. 2021;134:879–880. doi: 10.1097/CM9.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groves, H.E., Piché-Renaud, P., Peci, A., Farrar, D.S., Buckrell, S., Bancej, C., et al., The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada, medRxiv, 2021. 10.1101/2021.04.15.21255591 [DOI] [PMC free article] [PubMed]
- 10.Adlhoch C., Mook P., Lamb F., Ferland L., Melidou A., Amato-Gauci A.J. Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021. Eurosurveillance. 2021;26:1–8. doi: 10.2807/1560-7917.ES.2021.26.11.2100221. [DOI] [Google Scholar]
- 11.Baggen J., Thibaut H.J., Strating J.R.P.M., Van Kuppeveld F.J.M. The life cycleof non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018;16:368–381. doi: 10.1038/s41579-018-0005-4. [DOI] [PubMed] [Google Scholar]
- 12.Xue Y.C., Feuer R., Cashman N., Luo H. Enteroviral infection: The forgotten link to amyotrophic lateral sclerosis? Front. Mol. Neurosci. 2018;11:63. doi: 10.3389/fnmol.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim H.X., Poh C.L. Insights into innate and adaptive immune responses in vaccine development against EV-A71. Ther. Adv. Vaccines Immunother. 2019;7:251513551988899. doi: 10.1177/2515135519888998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis-Rogers N., Seger J., Adler F.R. Human rhinovirus diversity and evolution: How strange the change from major to minor. J. Virol. 2017;91:e01659–16. doi: 10.1128/JVI.01659-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ledford R.M., Patel N.R., Demenczuk T.M., Watanyar A., Herbertz T., Collett M.S., Pevear D.C. VP1 sequencing of all human rhinovirus serotypes: Insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 2004;78:3663–3674. doi: 10.1128/JVI.78.7.3663-3674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs S.E., Lamson D.M., Kirsten S., Walsh T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mäkelä M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner R.B. Epidemiology, pathogenesis, and treatment of the common cold. Ann. Allergy, Asthma, Immunol. 1997;78:531–540. doi: 10.1016/S1081-1206(10)63213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jartti T., Gern J.E. Rhinovirus-associated wheeze during infancy and asthma development. Curr. Respir. Med. Rev. 2011;7:160–166. doi: 10.2174/157339811795589423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henrickson K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weston S., Frieman M.B. Encyclopedia of Microbiology. 2019. [Google Scholar]
- 22.Lamb R.A., Parks G.D. Fields Virology. Philadelphia, PA: Wolters Kluwer/Lippincott, Williams, and Wilkins; 2013. [Google Scholar]
- 23.Alayyoubi M., Leser G.P., Kors C.A., Lamb R.A. Structure of the paramyxovirus parainfluenza virus 5 nucleoprotein-RNA complex. Proc. Natl. Acad. Sci. U. S. A. 2015;112:1792–1799. doi: 10.1073/pnas.1503941112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell C.J., Simões E.A.F., Hurwitz J.L. Vaccines for the paramyxoviruses and pneumoviruses: Successes, candidates, and hurdles. Viral Immunol. 2018;31:133–141. doi: 10.1089/vim.2017.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amarasinghe G.K., Bào Y., Basler C.F., Bavari S., Beer M., Bejerman N. Taxonomy of the order Mononegavirales: update 2017. Arch. Virol. 2017;162:2493–2504. doi: 10.1007/s00705-017-3311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mammas I.N., Spandidos D.A. Updating taxonomy changes: RSV is now known as Orthopneumovirus. Int. J. Mol. Med. 2019;44:S312019. [Google Scholar]
- 27.Li Y., Reeves R.M., Wang X., Bassat Q., Brooks W.A., Cohen C. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Global Health. 2019;7:1031–1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 28.Ascough S., Paterson S., Chiu C. Induction and subversion of human protective immunity: Contrasting influenza and respiratory syncytial virus. Front. Immunol. 2018;9:323. doi: 10.3389/fimmu.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson S.M., McNally B.A., Ioannidis I., Flano E., Teng M.N., Oomens A.G. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog. 2015;11:e1005318. doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Association for Respiratory Care Aerosol consensus statement-1991. Respir. Care. 1991;36:916–921. [PubMed] [Google Scholar]
- 31.Krivitskaya V.Z. Respiratory syncytial virus infection. Pathogenesis peculiarities, prevention and treatment strategies. Vopr. Sovrem. Pediatr. 2013;12:35–43. doi: 10.15690/vsp.v12i2.618. [DOI] [Google Scholar]
- 32.Wang, D., Cummins, C., Bayliss, S., Sandercock, J., and Burls, A., Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: A systematic review and economic evaluation, Health Technol. Assess., 2008, vol. 12, no. 36. 10.3310/hta12360 [DOI] [PubMed]
- 33.RSV Clinical Trial Tracker, 2020. https://www.path.org/ resources/rsv-and-mab-trial-tracker. Accessed April 20, 2021.
- 34.Van Den Hoogen B.G., De Jong J.C., Groen J., Kuiken T., De Groot R., Fouchier R.A.M., Osterhaus A.D.M.E. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Human Metapneumovirus (HMPV) Clinical Features, 2021. https://www.cdc.gov/surveillance/nrevss/hmpv/ clinical.html. Accessed April 20, 2021.
- 36.Hamelin M.È., Abed Y., Boivin G. Human metapneumovirus: A new player among respiratory viruses. Clin. Infect. Dis. 2004;38:983–990. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao X., Liu T., Shan Y., Li K., Garofalo R.P., Casola A. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog. 2008;4:e1000077. doi: 10.1371/journal.ppat.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar P., Srivastava M. Prophylactic and therapeutic approaches for human Metapneumovirus. Virus Dis. 2018;29:434–444. doi: 10.1007/s13337-018-0498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto S., Gonzalez G., Harada S., Oosako H., Hanaoka N., Hinokuma R., Fujimoto T. Recombinant type human mastadenovirus D85 associated with epidemic keratoconjunctivitis since 2015 in Japan. J. Med. Virol. 2018;90:881–889. doi: 10.1002/jmv.25041. [DOI] [PubMed] [Google Scholar]
- 40.Ghebremedhin B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014;4:26–33. doi: 10.1556/eujmi.4.2014.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014;27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crenshaw B.J., Jones L.B., Bell C.R., Kumar S., Matthews Q.L. Perspective on adenoviruses: Epidemiology, pathogenicity, and gene therapy. Biomedicines. 2019;7:1–18. doi: 10.3390/biomedicines7030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudhry A., Mathena J., Albano J.D., Yacovone M., Collins L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine. 2016;34:4558–4564. doi: 10.1016/j.vaccine.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ICTV Taxonomy, 2021. https://talk.ictvonline.org/taxonomy. Accessed April 20, 2021.
- 46.Guido M., Tumolo M.R., Verri T., Romano A., Serio F., De Giorgi M. Human bocavirus: Current knowledge and future challenges. World J. Gastroenterol. 2016;22:8684–8697. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamza H., Leifels M., Wilhelm M., Hamza I.A. Relative abundance of human bocaviruses in urban sewage in Greater Cairo, Egypt. Food Environ. Virol. 2017;9:304–313. doi: 10.1007/s12560-017-9287-3. [DOI] [PubMed] [Google Scholar]
- 48.Fehr, A.R. and Perlman, S., Coronaviruses: An overview of their replication and pathogenesis, in Methods in Molecular Biology, New York, NY: Humana Press, 2015, vol. 1282. 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed]
- 49.Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huynh J., Li S., Yount B., Smith A., Sturges L., Olsen J.C. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J. Virol. 2012;86:12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfefferle S., Oppong S., Drexler J.F., Gloza-Rausch F., Ipsen A., Seebens A. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerging Infect. Dis. 2009;15:1377–1384. doi: 10.3201/eid1509.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z., Shen L., Gu X. Evolutionary dynamics of MERS-CoV: potential recombination, positive selection and transmission. Sci. Rep. 2016;6:25049. doi: 10.1038/srep25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 54.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheryl, K., Gregory, M.G., Gary, A., Iksung, C., and Andreana, R., Patricia, R., et al., First-in-human trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine, medRxiv, 2020. 10.1101/2020.08.05.20168435
- 56.Parks J.M., Smith J.C. How to discover antiviral drugs quickly. N. Engl. J. Med. 2020;382:2261–2264. doi: 10.1056/NEJMcibr2007042. [DOI] [PubMed] [Google Scholar]
- 57.Amaro R.E., Baudry J., Chodera J., Demir Ö., McCammon J.A., Miao Y., Smith J.C. Ensemble docking in drug discovery. Biophys. J. 2018;114:2271–2278. doi: 10.1016/j.bpj.2018.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]