Abstract
Background and Aims
The duration of protection from hepatitis B vaccination in children and adults is not known. In 1981, we used three doses of plasma‐derived hepatitis B vaccine to immunize a cohort of 1578 Alaska Native adults and children from 15 Alaska communities who were ≥6 months old.
Approach and Results
We tested persons for antibody to hepatitis B surface antigen (anti‐HBs) levels 35 years after receiving the primary series. Those with levels <10 mIU/ml received one booster dose of recombinant hepatitis B vaccine 2–4 weeks later and were then evaluated on the basis of anti‐HBs measurements 30 days postbooster. Among the 320 recruited, 112 persons had not participated in the 22‐ or 30‐year follow‐up study (group 1), and 208 persons had participated but were not given an HBV booster dose (group 2). Among the 112 persons in group 1 who responded to the original primary series, 53 (47.3%) had an anti‐HBs level ≥10 mIU/ml. Among group 1, 73.7% (28 of 38) of persons available for a booster dose responded to it with an anti‐HBs level ≥10 mIU/ml at 30 days. Initial anti‐HBs level after the primary series was correlated with higher anti‐HBs levels at 35 years. Among 8 persons who tested positive for antibody to hepatitis B core antigen, none tested positive for HBsAg or HBV DNA.
Conclusions
Based on anti‐HBs level ≥10 mIU/ml at 35 years and a 73.7% booster dose response, we estimate that 86% of participants had evidence of protection 35 years later. Booster doses are not needed in the general population at this time.

Abbreviations
- anti‐HBc
antibody to hepatitis B core antigen
- anti‐HBs
antibody to hepatitis B surface antigen
- GMC
geometric mean concentration
INTRODUCTION
In 1981, we conducted a phase 4 HBV postlicensure vaccine trial in Alaska Native persons living along the Yukon‐Kuskokwim River System and the Bering Sea coast.[ 1 ] This population of Yupik persons are the only USA‐born population that was endemic for hepatitis B, with a prevalence of HBsAg averaging 6.4%, similar to what has been found in Southeast Asia and Sub‐Saharan Africa.[ 2 ] During that trial, 1630 Alaska Native adults and children ≥6 months living in 15 isolated communities received three doses of plasma‐derived hepatitis B vaccine. The overall response rate was high, with 97.4% of persons developing protective levels of antibody to HBV (antibody to hepatitis B surface antigen; anti‐HBs). We hypothesized at the time that an immunization series would result in lifelong protection against the adverse effects of acute HBV infection, mainly acute symptomatic hepatitis, and the acquisition of the chronic carrier state. Numerous studies have demonstrated that antibody response from the plasma‐derived vaccine is comparable to the recombinant vaccine.[ 3 , 4 ]
We visited these communities and tested for HBsAg, antibody to hepatitis B core antigen (anti‐HBc), and anti‐HBs levels in participants yearly for the first 11 years, then at years 15, 22, and 30.[ 5 , 6 , 7 , 8 , 9 , 10 ] Starting at the 22‐year follow‐up time point, we began to offer booster doses to those participants whose levels of anti‐HBs had fallen below 10 mIU/ml, the assumed protective level[ 6 , 11 ]; those participants were followed up 1 year later, then omitted from further surveillance thereafter because, although they boosted, we found that anti‐HBs levels fell rapidly in that year of follow‐up. At the time of our last report, 30 years after the initial series, 51% of persons who had never received a booster dose had anti‐HBs levels above 10 mIU/ml; and of those who did not, 88% responded to a booster dose, allowing us to estimate that >90% still had evidence of protection 30 years later.[ 12 ] We visited 12 of these 15 communities, again 35 years after the initial series. Here, we report the results of the 35‐year follow‐up period, including the proportion of participants whose antibody levels remained ≥10 mIU/ml, and among those <10 mIU/ml, the proportion who responded to a booster dose. In addition, we analyzed characteristics associated with persistent protective anti‐HBs levels.
PARTICIPANTS AND METHODS
Participant follow‐up
In 1981, a total of 1630 persons were given three doses of the Heptavax plasma‐derived HBV vaccine on a 0‐, 1‐, and 6‐month schedule. Persons ≥20 years of age received a 20‐µg dose for all three primary doses, and those <20 years received 10‐µg doses. Of these persons, 1578 remained serologically negative for HBsAg and HBcAg (anti‐HBc) throughout the primary series. We recruited persons to participate in this long‐term vaccine demonstration project from 15 study communities in a remote region of Alaska where HBV was endemic at the time.
Persons who received an inadvertent nonstudy HBV booster dose were removed from follow‐up at the time of the fourth HBV dose. Beginning at the 22‐year follow‐up, persons who had an anti‐HBs level <10 mIU/ml were offered an intramuscular 10‐µg booster HBV vaccine dose on a subsequent visit (Recombivax HB; Merck, Kenilworth, NJ, USA). We visited seven of the communities for the 22‐year follow‐up and the other eight communities (not visited at the 22‐year follow‐up) at the 30‐year follow‐up.
At the 35‐year follow‐up, we visited 15 remote communities plus three urban areas during the fall of 2016. Persons who had received an HBV booster dose at the 22‐ or 30‐year follow‐up were not actively recruited at the 35‐year time point. The study team made three visits to each of the 15 study communities: the first to recruit participants and draw HBV serology; the second visit to offer a booster dose to persons whose anti‐HBs level fell below 10 mIU/ml; and the third, 1 month after the second, to check the anti‐HBs level in those participants who had received a booster dose.
Laboratory testing
Serological specimens from participants were tested at the Centers for Disease Control and Prevention Arctic Investigations Program laboratory for anti‐HBs (ETI‐AB‐AUK PLUS; DiaSorin, Saluggia, Italy) and anti‐HBc (ORTHO HBc ELISA; ORTHO Diagnostics, Raritan, NJ, USA), using methods described.[ 12 ] An IgG anti‐HBs linear standard curve ranging from 5 to 160 mIU/ml was generated with each test run (ABAU‐STD‐SET; DiaSorin), with the lower limit of detection ≥5 mIU/ml. Specimens were initially screened for antibody to anti‐HBc antibody by qualitative ELISA; then, if specimens tested positive for anti‐HBc, the participant was referred to the Alaska Native Medical Center for follow‐up diagnostic testing.
Statistical analysis
Characteristics of persons recruited at the 35‐year follow‐up were compared to persons living in study communities, but who were not recruited.
An anti‐HBs level ≥10 mIU/ml was considered protective, based on randomized‐control studies conducted before U.S. Food and Drug Administration approval.[ 13 , 14 ] Additionally, a successful response was defined as a participant with an anti‐HBs level ≥10 mIU/ml 30 days following the administration of the booster dose. Statistical analyses were conducted on the proportion of persons with anti‐HBs ≥10 mIU/ml as well as on the geometric mean concentration (GMC) of anti‐HBs. The proportion of persons recruited and with anti‐HBs ≥10 mIU/ml was compared by use of the likelihood‐ratio chi‐square test for categorical variable and the Cochran‐Armitage trend test for continuous variables. p values are exact when the expected counts were <5.
We used two methods to assess the proportion of the cohort with protective antibody levels at the 35‐year follow‐up; We included persons who had participated in the 22‐ and 30‐year follow‐up who were not boosted and had anti‐HBs levels ≥10 mIU/ml at the time of that previous follow‐up. For this group, we recruited them at 35 years to determine their anti‐HBs level. If it had fallen to <10 mIU/ml, we offered them a booster dose. Because of this study‐induced selection bias (not recruiting persons who, at the two previous time points, had received a booster dose), in direct association with anti‐HBs levels (and omitted persons whose levels had in earlier studies fallen below 10 IU/ml), we first evaluated the proportion of persons with protective anti‐HBs at 35 years only among persons who had not participated in either the 22‐ or 30‐year follow‐up. Second, we used data from all time points on the entire cohort in a survival model examining the time until anti‐HBs dropped to <10 mIU/ml following the primary three‐dose HBV vaccine series. In this group, persons who received a study booster dose at the 22‐ or 30‐year follow‐up were included in the analysis until their mIU/ml dropped to <10 mIU/ml following the primary series, before the booster dose administration. The start of follow‐up was the date of the third HBV vaccine in the primary series in the survival analysis. In persons who dropped below 10 mIU/ml, the end of follow‐up was the interval between their last anti‐HBs ≥10 mIU/ml and their first anti‐HBs <10 mIU/ml. In persons who did not drop below 10 mIU/ml, the end of follow‐up was the date of their last anti‐HBs measurement. We used the Gamma distribution for the survival model, and the model fit was evaluated using the Akaike information criterion statistic.
We assessed the overall long‐term protection of the HBV vaccine series by examining the proportion of persons who either still had evidence of a protective antibody level or humoral immune memory defined by a response to the booster dose. We present this overall long‐term protection of the HBV vaccine series at the 35‐year follow‐up and, additionally, at the 22‐ and 30‐year follow‐up for comparative evaluation. We assessed exposure to HBV infection in all participants by testing them for anti‐HBc. Those who were found to be positive were tested for HBsAg and HBV DNA. For persons found to be anti‐HBc positive, we reviewed the community health charts, regional hospital charts, the tertiary care hospital, Alaska Native Medical Center in Anchorage, as well as the electronic health records used by all these facilities (Cerner) for any evidence of acute hepatitis. All statistical analyses were conducted using SAS software (version 9.4; Statistical Analysis System, Raleigh, NC). All p values are two‐sided, and a value <0.05 was used to define statistical significance.
Approvals and informed consent
This study was approved by the Alaska Area and the Centers for Disease Control and Prevention institutional review boards, as well as the Yukon‐Kuskokwim Health Corporation and the Norton Sound regional health boards. Additionally, the study was approved by the Southcentral Foundation and the Alaska Native Tribal Health Consortium. At the 35‐year follow‐up, the continued study objectives were described, and written informed consent was obtained from each participant. In addition, this study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review boards.
RESULTS
Cohort follow‐up
Since the start of long‐term follow‐up in 1982, 342 persons have died (none from hepatitis B), and 225 persons have received a nonstudy HBV booster dose (Figure 1). At the 22‐ and 30‐year follow‐up, study personnel administered an HBV vaccine booster dose to 298 participants in total whose levels of anti‐HBs had fallen below 10 mIU/ml. There remained 712 potential study participants at the 35‐year follow‐up; 320 were recruited during 2016. Study personnel are only in the remote communities for 2 days, so the primary reason that persons are not recruited is availability during the short recruiting window. Mean age of persons recruited at 35 years was 48.8 years, and 158 (49%) were female. The 320 persons who were recruited did not differ from those who were eligible but not recruited in terms of age, sex, and their anti‐HBs level following the primary series nor at the 10‐year follow‐up time point (Table 1). Mean age at the time of receiving the initial series was 13.1 years (range, 0–54) among the 320 persons who were recruited and 13.7 years (range, 0–62) among the 392 persons who were not recruited (p = 0.41). Among the 320 recruited, 112 persons had not participated in the 22‐ or 30‐year follow‐up study (group 1; Figure 1), and 208 persons had participated but were not given an HBV booster dose (group 2).
FIGURE 1.
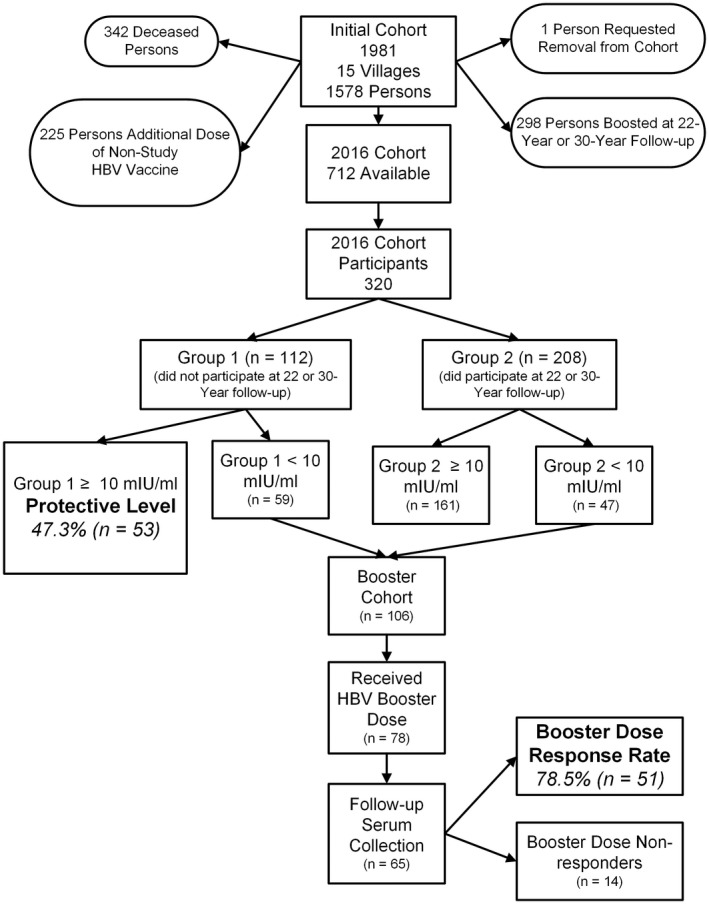
Participant flow chart in a 35‐year follow‐up study of 1578 persons receiving three doses of plasma‐derived HBV vaccine in Alaska in 1981.
TABLE 1.
Characteristics of persons recruited at the 35‐year follow‐up (n = 320) compared to persons living in study communities that were eligible but who we did not recruit (n = 392), Alaska
| Characteristic | Vax demo 35 cohort (n = 320) % (N) | Persons who were eligible but not recruited (n = 392) % (N) | p value a |
|---|---|---|---|
| Female | 49.4 (158) | 49.2 (193) | 0.97 |
| Mean age at 35‐year follow‐up, years | 48.8 | 49.4 | 0.42 |
| Age group at booster vaccination (age at primary vaccination), years | |||
| <40 (<5) | 13.4 (43) | 14.8 (58) | 0.049 |
| 40 to <45 (5 to <10) | 22.8 (73) | 25.0 (98) | |
| 45 to <55 (10 to <20) | 46.6 (149) | 40.8 (160) | |
| ≥55 (≥20) | 17.2 (55) | 19.4 (76) | |
| Anti‐HBs after primary series group, mIU | |||
| 10–199 | 9.1 (29) | 13.1 (48) | 0.16 |
| 200–499 | 9.7 (31) | 8.2 (30) | |
| 500–999 | 12.5 (40) | 14.5 (53) | |
| ≥1000 | 68.7 (220) | 64.2 (235) | |
| 10‐year anti‐HBs level, mIU | n = 248 | n = 285 | |
| <10 | 10.1 (25) | 18.6 (53) | 0.28 |
| 10–199 | 47.2 (117) | 42.5 (121) | |
| 200–499 | 21.0 (52) | 17.5 (50) | |
| 500–999 | 9.2 (23) | 7.7 (22) | |
| ≥1000 | 12.5 (31) | 13.7 (39) |
Female and age group were tested using the likelihood‐ratio chi‐square test. The Cochran‐Armitage trend test was used to analyze anti‐HBs levels after the HBV primary series and 10‐year anti‐HBs level.
Anti‐HBs levels 35 years after primary series vaccination
At 35 years following the primary HBV vaccine series, among group 1, 47.3% of persons (n = 53) maintained an anti‐HBs level ≥10, and the GMC was 10.6 mIU/ml (Table 2). This compares to 51.4% of persons with anti‐HBs ≥10 mIU/ml at the 30‐year follow‐up and 60.4% at the 22‐year follow‐up (Figure 2). Fifty‐three percent of males had an anti‐HBs level ≥10 mIU/ml and 41.2% of females, which was not statistically significant. There was no difference in anti‐HBs level at 35 years according to overall age class; however, a higher proportion of persons who were 10–19 years old at the time when they received the primary vaccine series had protective antibody levels (anti‐HBs, ≥10 mIU/ml) 35 years later compared to older and younger age groups (p = 0.009). However, the anti‐HBs level at 35 years remains significantly associated with the anti‐HBs level after the primary HBV vaccination series. Among persons who initially responded to an anti‐HBs level ≥1000 mIU/ml, 69.6% (48 of 69) had an anti‐HBs level ≥10 at 35 years compared to ≤20% in all other groups (p < 0.0001; Table 2). We used survival analysis with the entire cohort to predict the probability of having an anti‐HBs ≥10 mIU/ml at 35 years. The outcome was the time until anti‐HBs dropped below 10 mIU/ml. The estimated probability of having an anti‐HBs ≥10 mIU/ml at 35 years among all participants was 43.8% (95% CI, 40.3, 48.2) compared with 47.3% (95% CI, 38.1, 56.6) estimated among the 112 participants in group 1.
TABLE 2.
Level of anti‐HBs 35 years after a primary series of hepatitis B vaccine, Alaska 2016–2017 (among persons recruited into 35‐year cohort who did not participate at 22‐ or 30‐year follow‐up)
| Characteristic | N (%) of Cohort | Anti‐HBs Level (mIU/ml) | p value a | |
|---|---|---|---|---|
| GMC | % With ≥10 (N) | |||
| Overall | 112 | 10.6 | 47.3 (53) | |
| Sex | ||||
| Female | 51 (45.5) | 8.3 | 41.2 (21) | 0.23 b |
| Male | 61 (54.5) | 12.9 | 52.5 (32) | |
| Age at the time of anti‐HBs follow‐up (age at primary vaccination), years | ||||
| <40 (<5) | 20 (17.9) | 3.8 | 30.0 (6) | 0.05 |
| 40 to <45 (5 to <10) | 25 (22.3) | 7.9 | 44.0 (11) | |
| 45 to <55 (10 to <20) | 49 (43.8) | 21.6 | 61.2 (30) | |
| ≥55 (≥20) | 18 (16.1) | 7.1 | 33.3 (6) | |
| Anti‐HBs level after primary series, mIU/ml | ||||
| 10–199 | 17 (15.2) | 1.5 | 5.6 (1) | <0.0001 |
| 200–499 | 16 (14.3) | 2.1 | 12.5 (2) | |
| 500–999 | 10 (8.9) | 3.5 | 20.0 (2) | |
| ≥1000 | 69 (61.6) | 29.0 | 69.6 (48) | |
Tested using the likelihood‐ratio chi‐square test. The Cochran‐Armitage trend test was used to analyze anti‐HBs levels after the HBV primary series.
After adjustment for anti‐HBs level after primary series, p values for sex and age remained >0.05.
FIGURE 2.
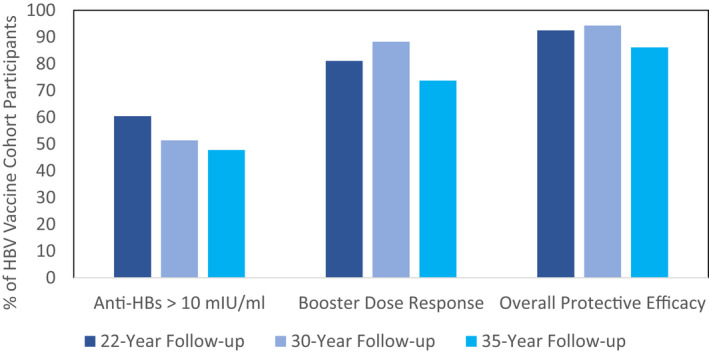
Protective level, booster dose response, and overall protective efficacy proportions for 22‐, 30‐, and 35‐year follow‐ups after a primary three‐dose series of HBV vaccine, Alaska.
Response to an HBV booster dose and overall protective levels
There were 106 persons from both groups 1 and 2 with anti‐HBs <10 mIU/ml who were eligible for a booster dose. Of these persons, 78 (73.6%) received an HBV vaccine booster dose and 65 had a follow‐up serum draw a mean of 32 days after their boost. Of the persons with complete follow‐up, 34 (52.3%) were female and their mean age at the time of boost was 48.5 years. At 35 years following the primary series, 78.5% (51 of 65; 95% CI, 68.5, 88.5) of participants (groups 1 and 2 with anti‐HBs <10 mIU/ml) responded to the booster dose to a level of anti‐HBs ≥10 mIU/ml. The GMC 1 month after the boost was 91.3 mIU/ml. The proportion who responded to the booster dose did not differ by sex, age class at the time of the boost, or whether they had participated in the 22‐ or 30‐year draw (group 1 vs. group 2; Table 3). Among group 1, 73.7% (28 of 38) of persons responded to the booster dose. This compared to 88.2% who responded to the booster dose at the 30‐year follow‐up and 81.1% at the 22‐year follow‐up (Figure 2). The higher the anti‐HBs level following the primary three‐dose series, the more likely persons were to respond to a booster dose 35 years later. Among persons who initially responded to the three‐dose series to an anti‐HBs level >1000 mIU/ml, 100% (26 of 26) responded to the booster dose, compared to 27.3% (3 of 11) among persons with an initial response between 10 and 199 mIU/ml (p value for trend, =<0.001; Table 3). Level of anti‐HBs before the booster dose was also associated with the likelihood of response. Among persons with a preboost level between 5 and 10 mIU/ml, 100% responded compared to 64.9% (24 of 37) among persons with an anti‐HBs <2 mIU/ml (p value for trend = 0.0021; Table 3).
TABLE 3.
Level of anti‐HBs after hepatitis B vaccine booster dose in 65 persons with anti‐HBs level <10 mIU/ml 35 years after a primary hepatitis B vaccination series—Alaska 2016–2017
| Characteristic | N (%) of cohort | Anti‐HBs level (mIU/ml) | p value a | |
|---|---|---|---|---|
| GMC | % With ≥10 (N) | |||
| Overall | 65 | 91.3 | 78.5 (51) | |
| Sex | ||||
| Female | 34 (52.3) | 79.3 | 82.4 (28) | 0.42 |
| Male | 31 (47.7) | 106.7 | 74.2 (23) | |
| Age at booster vaccination (age at primary vaccination), years | ||||
| <40 (<5) | 12 (18.5) | 74.1 | 83.3 (10) | 0.50 |
| 40 to <45 (5 to <10) | 11 (16.9) | 235.0 | 81.8 (9) | |
| 45 to <55 (10 to <20) | 27 (41.5) | 79.0 | 77.8 (21) | |
| ≥55 (≥ 20) | 15 (23.1) | 70.1 | 73.3 (11) | |
| Anti‐HBs level after primary series, mIU/ml | ||||
| 10–199 | 11 (16.9) | 9.8 | 27.3 (3) | <0.001 b |
| 200–499 | 15 (23.1) | 46.5 | 80.0 (12) | |
| 500–999 | 13 (20.0) | 45.8 | 76.9 (10) | |
| ≥1000 | 26 (40.0) | 488.7 | 100.0 (26) | |
| Preboost Anti‐HBs, mIU/ml | ||||
| 0 to <2 | 37 (56.9) | 29.1 | 64.9 (24) | 0.0021 |
| 2 to <5 | 10 (15.4) | 225.6 | 90.0 (9) | |
| 5 to <10 | 18 (27.7) | 581.2 | 100.0 (18) | |
| Follow‐up group | ||||
| Did not participate in 22‐ or 30‐year follow‐up (group 1) | 38 (58.5) | 58.7 | 73.7 (28) | 0.26 |
| Did participate in 22‐ or 30‐year follow‐up (group 2) | 27 (41.5) | 170.2 | 85.2 (23) | |
Tested using the likelihood‐ratio chi‐square test with sufficient numbers. Fisher’s exact test was performed when expected counts were <5. The Cochran‐Armitage trend test was used to analyze anti‐HBs levels after the HBV primary series and preboost anti‐HBs.
After adjustment for anti‐HBs level after primary series, no other variables were statistically significant.
For group 1, 47.3% (53 of 112) had evidence of long‐term immune efficacy against HBV because their anti‐HBs still remained above the assumed protective level of 10 mIU/ml. Among the 59 with anti‐HBs <10 mIU/ml, of persons who received a booster dose, 73.7% responded. If we apply this response rate to the entire group of 59, we estimate that 86.1% of participants still have protection against HBV, as evidenced by a level of protective antibodies or humoral immune memory (Figure 2). This overall proportion with evidence of protection at 35 years compares to an estimated 94.3% at the 30‐year follow‐up and 92.5% at the 22‐year follow‐up.
Other HBV viral endpoints
All participants were tested for anti‐HBc at the 35‐year time point, of whom 8 persons were positive for anti‐HBc (Table 4). Mean age of these 8 persons was 47.8 years. Four of these persons had never been positive for anti‐HBc on any of their follow‐up visits, whereas the 4 others had been positive for anti‐HBc on ≥1 follow‐up visit. None of the 8 persons were positive for HBsAg or HBV DNA. One person had abnormal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) at the time of the positive result (presumed because of alcohol use; after a period of sobriety, ALT and AST levels returned to normal). None revealed any clinical signs or symptoms of hepatitis on chart review. Over the course of all follow‐up time points, 28 persons have had a positive result for anti‐HBc on one or more visit. The overall rate of anti‐HBc core positivity within the cohort is 1.48 (95% CI, 0.98, 2.14) per 1000 person‐years of follow‐up. This rate did not differ by age or sex, but was higher among persons who did not initially respond to the three‐dose primary series (5.96 per 1000 person‐years) compared to persons who did respond (p < 0.001; 1.18 per 1000 person‐years). During the course of the 35 years of follow‐up, none of the 28 persons with evidence of a breakthrough infection had evidence, by history or in their medical records, for acute clinical hepatitis or developed chronic HBV infection.
TABLE 4.
Characteristics of persons who were positive for antibodies to hepatitis B core 35 years following a primary three‐dose series of HBV vaccine
| Sex | Age | Anti‐HBs at 35 years | Highest anti‐HBs after primary series (Year) | Core positive | ALT (SGPT) U/L | AST (SGOT) U/L | HBsAg | HBV PCR |
|---|---|---|---|---|---|---|---|---|
| Male | 37 | 12.202 | 608.3 (1982) | 1986–1996, 2003, 2016 | 24 | 35 | Nonreactive | Not detected |
| Female | 57 | <0.000 | 32.9 (1986) | 1982, 1984, 1986, 2011, 2016 | 14 | 20 | Nonreactive | Not detected |
| Female | 50 | 28.027 | 431.4 (1984) | 2016 (last vax demo visit 2011) | 15 | 18 | Nonreactive | Not detected |
| Female | 37 | 13.056 | 1011.1 (1982) | 1988–1997, 2003, 2016 | 10 | 14 | Nonreactive | Not detected |
| Male | 50 | 88.654 | 2637.1 (1985) | 2016 (last vax demo visit 1997) | 137 | 86 | Nonreactive | Not detected |
| Female | 47 | 40.182 | 2658.0 (1982) | 2016 (last vax demo visit 2004) | 11 | 14 | Nonreactive | Not detected |
| Male | 52 | 26.036 | 991.1 (1987) | 2016 (last vax demo visit 1997) | 15 | 25 | Nonreactive | Not detected |
| Male | 52 | 12.581 | 198.4 (1991) | 1989–1991, 2003, 2011, 2016 | 22 | 28 | Nonreactive | Not detected |
DISCUSSION
This study is the longest cohort study on long‐term protection from hepatitis B vaccination in the world. It demonstrates that protection continues through 35 years; 86% of persons had protective antibody levels of >10 mIU/ml of anti‐HBs or response to a booster dose of vaccine. Age at primary vaccination and initial anti‐HBs levels after primary series was correlated with higher anti‐HBs levels at 35 years. No chronic HBV infections were documented. During the first 22 years of follow‐up for this cohort, we documented 22 breakthrough infections. Since year 22, we have not identified any new instances of transient anti‐HBc, HBsAg, or HBV DNA. This could be related to the finding that HBV‐DNA viral levels have fallen dramatically in the infected persons living in these communities.[ 15 ]
Findings from other long‐term HBV cohorts with 20–30 years of follow‐up show similar long‐term protection from use of HBV vaccine.[ 16 , 17 , 18 , 19 , 20 ] Some of these studies included infants, adolescents, or adults; our cohort included children ≥6 months of age. Although there was no difference found in anti‐HBs level at 35 years according to overall age class, we found that a higher proportion of persons who were 10–19 years old at the time that they received the primary vaccine series had protective antibody levels (anti‐HBs, ≥10 mIU/ml) 35 years later compared to older and younger age groups. These findings are particularly relevant for young adults (often vaccinated for occupational safety reasons) and children vaccinated in catch‐up programs.[ 21 , 22 ]
We found that among participants with anti‐HBs levels <10 mIU/ml, those with a higher initial antibody level after the primary series and those with a higher preboost anti‐HBs level were more likely to demonstrate a booster response (anti‐HBs, ≥10 mIU/ml) when compared to persons with a lower initial antibody level after the primary series and those with lower preboost anti‐HBs levels, respectively.
What constitutes an appropriate booster response is still not clear; however, in our study, 78.5% of participants in groups 1 and 2 who received a booster dose responded with levels of anti‐HBs ≥10 mIU/ml. In addition, we found that even if anti‐HBs levels had dropped to <10 mIU/ml 35 years after the primary vaccination, the closer the antibody level was to 10 mIU/ml, the higher the probability that the boost would succeed at raising anti‐HBs levels to ≥10 mIU/ml.
Data from this 35‐year cohort are most applicable to young children, young adults, adult travelers to HBV‐endemic countries, and health care workers vaccinated for HBV. Data from other studies[ 20 , 23 , 24 ] show continued protection from disease among vaccinated persons and therefore do not support the need for periodic population screening or boosting. Data from this study demonstrate strong evidence that protection from disease lasts at least 35 years and support current Advisory Committee on Immunization Practices recommendations that booster doses are not needed.[ 25 ]
Some of the limitations of this study are loss to follow‐up over 35 years. This occurred mainly because of death, receiving a booster dose in the 22‐ or 30‐year cohort, or receiving an additional dose of nonstudy HBV vaccine; this reduced the original cohort by 55%. Another limitation was that we used plasma‐derived vaccine, but numerous studies have demonstrated that antibody response from the plasma‐derived vaccine is comparable to the recombinant vaccine.[ 3 , 4 ] In addition, we used recombinant vaccine as the booster dose, and excellent immunological responses were demonstrated among persons whose initial series was with plasma‐derived vaccine.
The long‐term immunity we found at 35 years could, in part, be attributable to boosting because persons in this study have continued to reside in communities that have residents infected with chronic HBV. During the first 10 years of following this cohort on a yearly basis, we found that 8% of participants had a 4‐fold rise in anti‐HBs levels not accompanied by detectable anti‐HBc, suggesting that continuing exposure to HBV in these communities might help to maintain anti‐HBs levels.[ 26 ] Examination of a subset of these participants 32 years after the original vaccine series found that all participants exhibited T‐cell recognition of HBsAg and a proportion had T‐cell responses to HBcAg.[ 27 ] These findings suggest that long‐term immunity lasts longer in persons living in endemic areas or working in environments where exposure to HBV is more likely. It would be of interest to test for both humoral and cellular immunity in persons living and working in settings where risk of HBV exposure is very low.
CONFLICT OF INTEREST
Nothing to report.
AUTHOR CONTRIBUTIONS
Michael G. Bruce: Substantial contribution to conception, design, data acquisition, analysis and interpretation, drafting the article, revision of the article and final approval. Dana Bruden: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Debby Hurlburt: Substantial contribution to data acquisition, analysis, drafting the article. Julie Morris: Substantial contribution to laboratory data acquisition, production and analysis. Sara Bressler: Substantial contribution to data analysis and interpretation, drafting the article, revision of the article. Gail Thompson: Substantial contribution to data acquisition, analysis and interpretation. Danielle Lecy: Substantial contribution to data acquisition, analysis and interpretation. Karen Rudolph: Substantial contribution to laboratory data acquisition, analysis and interpretation. Lisa Bulkow: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Thomas Hennessy: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Brenna C. Simons: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Mark K. Weng: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Noele Nelson: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Brian J. McMahon: Substantial contribution to conception, design, data acquisition, analysis and interpretation, drafting the article, revision of the article.
ACKNOWLEDGMENT
We thank the Yukon Kuskokwim Health Corporation and the Norton Sound Health Corporation for their participation. We also thank the many community health aides in the study communities and the nurses at the Arctic Investigations Program and the Alaska Native Medical Center who over the years gave their valuable time to the study. This study is supported by in‐kind personnel support and from a cooperative agreement (U50/CCU022279) between the Centers for Disease Control and Prevention and the Alaska Native Tribal Health Consortium. The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
Bruce MG, Bruden D, Hurlburt D, Morris J, Bressler S, Thompson G, et al. Protection and antibody levels 35 years after primary series with hepatitis B vaccine and response to a booster dose. Hepatology. 2022;76:1180–1189. 10.1002/hep.32474
Funding information
Supported by in‐kind personnel support and from a cooperative agreement (U50/CCU022279) between the Centers for Disease Control and Prevention and the Alaska Native Tribal Health Consortium.
REFERENCES
- 1. Heyward WL, Bender TR, McMahon BJ, Hall DB, Francis DP, Lanier AP, et al. The control of hepatitis‐B virus‐infection with vaccine in Yupik Eskimos. Demonstration of safety, immunogenicity, and efficacy under field conditions. Am J Epidemiol. 1985;121:914–23. [DOI] [PubMed] [Google Scholar]
- 2. Schreeder MT, Bender TR, McMahon BJ, Moser MR, Murphy BL, Sheller MJ, et al. Prevalence of hepatitis B in selected Alaskan Eskimo villages. Am J Epidemiol. 1983;118:543–9. [DOI] [PubMed] [Google Scholar]
- 3. Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]
- 4. Keck J, Bulkow L, Raczniak G, Negus SE, Zanis CL, Bruce MG, et al. Hepatitis B virus antibody levels 7 to 9 years after booster vaccination in Alaska native persons. Clin Vaccine Immunol. 2014;21:1339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15‐year follow‐up. Ann Intern Med. 2005;142:333–41. [DOI] [PubMed] [Google Scholar]
- 6. McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine: results of a 22‐year follow‐up study and response to a booster dose. J Infect Dis. 2009;200:1390–6. [DOI] [PubMed] [Google Scholar]
- 7. McMahon BJ, Heyward WL, Ritter D, Wainwright RB, Rhoades ER, Tower E, et al. A comprehensive programme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan natives. Lancet. 1987;2:1134–6. [DOI] [PubMed] [Google Scholar]
- 8. Wainwright RB, Bulkow LR, Parkinson AJ, Zanis C, McMahon BJ. Protection provided by hepatitis B vaccine in a Yupik Eskimo population–results of a 10‐year study. J Infect Dis. 1997;175:674–7. [DOI] [PubMed] [Google Scholar]
- 9. Wainwright RB, McMahon BJ, Bulkow LR, Hall DB, Fitzgerald MA, Harpster AP, et al. Duration of immunogenicity and efficacy of hepatitis B vaccine in a Yupik Eskimo population. JAMA. 1989;261:2362–6. [PubMed] [Google Scholar]
- 10. Wainwright RB, McMahon BJ, Bulkow LR, Parkinson AJ, Harpster AP. Protection provided by hepatitis B vaccine in a Yupik Eskimo population. Seven‐year results. Arch Intern Med. 1991;151:1634–6. [PubMed] [Google Scholar]
- 11. Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–92. [DOI] [PubMed] [Google Scholar]
- 12. Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30‐year follow‐up study and response to a booster dose. J Infect Dis. 2016;214:16–22. [DOI] [PubMed] [Google Scholar]
- 13. Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high‐risk population in the United States. N Engl J Med. 1980;303:833–41. [DOI] [PubMed] [Google Scholar]
- 14. Francis DP, Hadler SC, Thompson SE, Maynard JE, Ostrow DG, Altman N, et al. The prevention of hepatitis B with vaccine. Report of the Centers for Disease Control multi‐center efficacy trial among homosexual men. Ann Intern Med. 1982;97:362–6. [DOI] [PubMed] [Google Scholar]
- 15. Livingston S, Simonetti J, Bulkow L, Homan CE, Snowball MM, Cagle HH, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology. 2007;133:1452–7. [DOI] [PubMed] [Google Scholar]
- 16. Borràs E, Urbiztondo L, Carmona G, Jané M, Barrabeig I, Rosa Sala M, et al. Effectiveness and impact of the hepatitis B vaccination program in preadolescents in Catalonia 21 years after its introduction. Vaccine. 2019;37:1137–41. [DOI] [PubMed] [Google Scholar]
- 17. Van Damme P, Leroux‐Roels G, Suryakiran P, Folschweiller N, Van Der Meeren O. Persistence of antibodies 20 y after vaccination with a combined hepatitis A and B vaccine. Hum Vaccin Immunother. 2017;13:972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moghadami M, Dadashpour N, Mokhtari AM, Ebrahimi M, Mirahmadizadeh A. The effectiveness of the national hepatitis B vaccination program 25 years after its introduction in Iran: a historical cohort study. Braz J Infect Dis. 2019;23:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma JC, Wu ZW, Zhou HS, Gao Z, Hao ZY, Jin F, et al. Long‐term protection at 20–31 years after primary vaccination with plasma‐derived hepatitis B vaccine in a Chinese rural community. Hum Vaccin Immunother. 2020;16:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cocchio S, Baldo V, Volpin A, Fonzo M, Floreani A, Furlan P, et al. Persistence of Anti‐Hbs after up to 30 years in health care workers vaccinated against hepatitis B virus. Vaccines (Basel). 2021;9:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Funderburke PL, Spencer L. Hepatitis B immunity in high risk health care workers. Seven years post vaccination. AAOHN J. 2000;48:325–30. [PubMed] [Google Scholar]
- 22. Tohme RA, Ribner B, Huey MJ, Spradling PR. Hepatitis B vaccination coverage and documented seroprotection among matriculating healthcare students at an academic institution in the United States. Infect Control Hosp Epidemiol. 2011;32:818–21. [DOI] [PubMed] [Google Scholar]
- 23. Gilca V, De Serres G, Boulianne N, Murphy D, De Wals P, Ouakki M, et al. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine. 2013;31:448–51. [DOI] [PubMed] [Google Scholar]
- 24. Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, et al.; Centers for Disease Control and Prevention (CDC) . CDC guidance for evaluating health‐care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep. 2013;62:1–19. [PubMed] [Google Scholar]
- 25. Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bulkow LR, Wainwright RB, McMahon BJ, Parkinson AJ. Increases in levels of antibody to hepatitis B surface antigen in an immunized population. Clin Infect Dis. 1998;26:933–7. [DOI] [PubMed] [Google Scholar]
- 27. Simons BC, Spradling PR, Bruden DJ, Zanis C, Case S, Choromanski TL, et al. A longitudinal hepatitis B vaccine cohort demonstrates long‐lasting hepatitis B virus (HBV) cellular immunity despite loss of antibody against HBV surface antigen. J Infect Dis. 2016;214:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


