Abstract
Background
Visual display terminal (VDT) use is a key risk factor for dry eye disease (DED). Visual display terminal (VDT) use reduces the blink rate and increases the number of incomplete blinks. However, the exact mechanisms causing DED development from VDT use have yet to be clearly described.
Purpose
The purpose of the study was to conduct a review on pathophysiological mechanisms promoting VDT‐associated DED.
Methods
A PubMed search of the literature investigating the relationship between dry eye and VDT was performed, and relevance to pathophysiology of DED was evaluated.
Findings
Fifty‐five articles met the inclusion criteria. Several pathophysiological mechanisms were examined, and multiple hypotheses were extracted from the articles. Visual display terminal (VDT) use causes DED mainly through impaired blinking patterns. Changes in parasympathetic signalling and increased exposure to blue light, which could disrupt ocular homeostasis, were proposed in some studies but lack sufficient scientific support. Together, these changes may lead to a reduced function of the tear film, lacrimal gland, goblet cells and meibomian glands, all contributing to DED development.
Conclusion
Visual display terminal (VDT) use appears to induce DED through both direct and indirect routes. Decreased blink rates and increased incomplete blinks increase the exposed ocular evaporative area and inhibit lipid distribution from meibomian glands. Although not adequately investigated, changes in parasympathetic signalling may impair lacrimal gland and goblet cell function, promoting tear film instability. More studies are needed to better target and improve the treatment and prevention of VDT‐associated DED.
Keywords: DED, dry eye disease, pathophysiology, tear film, VDT, VDT‐associated dry eye
Introduction
A healthy ocular surface is essential for good visual quality and ocular comfort (Davidson & Kuonen 2004). The tear film ensures a smooth refractive surface and protects, lubricates and nourishes the cornea (Dartt & Willcox 2013). This film consists of an inner mucoaqueous layer and an outer lipid layer (Yokoi et al. 2014). The lipid layer is primarily made up of meibum from the meibomian glands, located in the tarsal plate of the eyelids (King‐Smith et al. 2004). The lipids reduce the surface tension, diminish ocular evaporation and protect the mucoaqueous layer (Pflugfelder & Stern 2020). The mucoaqueous layer is nutrient‐rich and makes up most of the tear film volume (Fig. 1 ). The aqueous component is mainly produced by the lacrimal gland, while the conjunctival goblet cells secrete the mucins by compound exocytosis. During normal conditions, both the lacrimal gland and conjunctival goblet cells are stimulated by parasympathetic nerve fibres from the pterygopalatine ganglion (Toshida et al. 2007), while meibomian gland secretion is mainly regulated by the action of blinking (McCulley & Shine 2003).
Fig. 1.
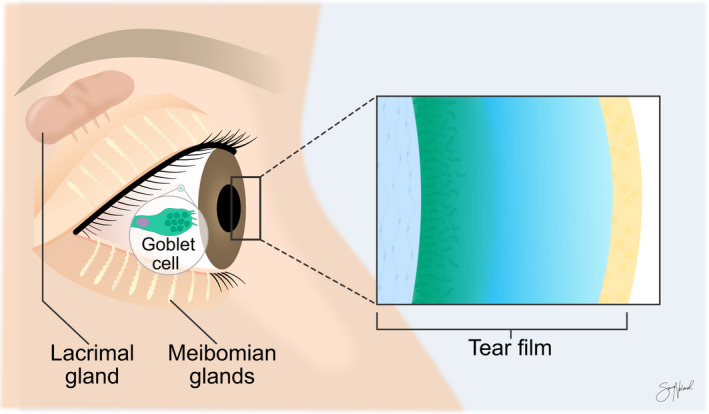
Tear film and important associated structures. Meibomian glands, the lacrimal gland and goblet cells are essential for maintaining the homeostasis of the tear film. Copyright Sara Tellefsen Nøland.
Stimulation of free nerve endings in the cornea is thought to be the origin for parasympathetic activation of conjunctival goblet cells and lacrimal gland. Primary corneal nerves also activate trigeminal brainstem nuclear complex neurons, stimulating motoneurons led by the facial nerve to the orbicularis oculi muscle and promoting contraction and the downwards motion of the upper eyelid (Wu et al. 2015). These forces compress the meibomian glands and its connected duct system pushing meibum out onto the pre‐ocular tear film (Driver & Lemp 1996). Disruption of the pre‐ocular tear film and loss of ocular surface homeostasis can lead to the development of dry eye disease (DED).
Dry eye disease (DED) is a highly prevalent condition affecting several hundred million people worldwide (Stapleton et al. 2017). The two main categories of DED are evaporative dry eye (EDE) and aqueous deficient dry eye (ADDE), although mixed types of DED are also common (Craig et al. 2017). In EDE, pathology of the eyelid structures or meibomian glands, blinking abnormalities and reduced mucin production are the main drivers of disease (Craig et al. 2017), resulting in excessive ocular evaporation despite initial normal lacrimal gland function. On the other hand, ADDE is mainly characterized by lacrimal gland hypofunction and deficient tear production despite unaltered evaporation rates (Bron et al. 2017). Mixed forms of DED include elements of both ADDE and EDE.
Dry eye disease (DED) has substantial economic and societal costs (Aggarwal & Galor 2018). In 2011, the annual indirect costs of DED in the United States alone were estimated to be 55.4 billion USD (Yu et al. 2011). Patients with DED have lower employment rates and productivity at work (Sivakumar et al. 2021). Dry eye disease (DED) also greatly reduces quality of life (Morthen et al. 2021) and can cause ocular pain, foreign body sensation and visual disturbances. Dry eye disease (DED) is tied to increased rates of depression and anxiety, further increasing the burden of the disease (van der Vaart et al. 2015).
Visual display terminal (VDT) use is an important risk factor for DED (Uchino et al. 2013). Visual display terminal (VDT) use reduces blink rates and tear film break‐up time (TBUT), which leaves the ocular surface more exposed (Bilkhu et al. 2021). The break‐up of the tear film usually triggers reflexive blinking (Ousler et al. 2008), leading to a restored tear film. However, VDT use suppresses reflexive blinking, inhibiting this response (Bilkhu et al. 2021) and causing longer intervals with an unprotected ocular surface.
The implementation of digital solutions in all parts of life has made VDT use ubiquitous. In 2005, only 17% of the global population were internet users. By 2019, this proportion had increased threefold to 51% (Bogdan‐Martin 2020; ITU 2021) with as much as 83% of Europeans (ITU 2021) using the Internet. As a result, disorders related to sedentary behaviour and increased use of VDTs are becoming increasingly prevalent (Patterson et al. 2018). This is alarming, as vocational VDT users have up to three times greater risk of DED than non‐VDT users (Doguizi et al. 2019). The COVID‐19 pandemic has accelerated this increased reliance on VDT use even more (Bahkir & Grandee 2020).
Alterations in blink patterns are likely key elements in the detrimental effects of VDT use on DED. Visual display terminal (VDT) use has been shown to decrease TBUT and increase interblink interval (Bilkhu et al. 2021) (Fig. 2). However, much is still unknown regarding the adverse effects of VDT use on the ocular surface. This review investigates possible pathophysiological mechanisms behind DED development in VDT users and highlights areas of focus for future research.
Fig. 2.
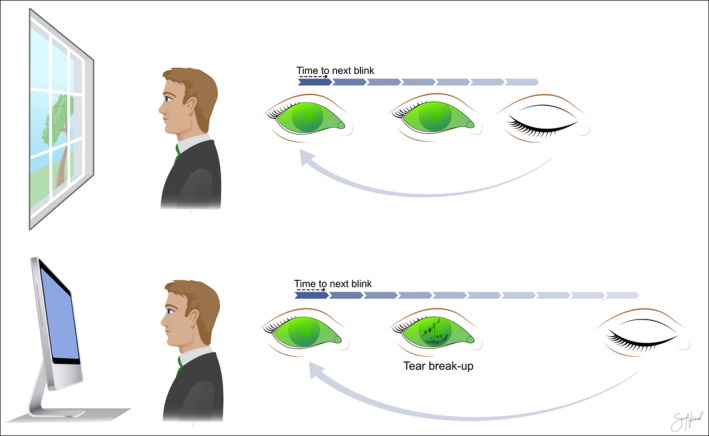
Interblink interval (IBI) and tear film break‐up time (TBUT) in two different settings. The IBI increases and the TBUT decreases with visual display terminal (VDT) use. This leaves the ocular surface epithelium exposed for longer. Copyright Sara Tellefsen Nøland.
Methods
A literature search was conducted on PubMed on the 30th of June 2021 using the following search term: “(computer OR smartphone OR display terminal OR “screen use“) AND (dry eye OR DED)”. Two researchers (KF and HF) independently reviewed articles and inconsistencies were discussed by the two researchers until a consensus was made. If necessary, further discrepancies were consulted with a third researcher (MSM) until a settlement was made. The inclusion criteria were original, peer‐reviewed articles assessing ocular signs and symptoms related to VDT use and studies investigating possible mechanisms behind these changes. Articles without available English full text, letters to the editor, review articles and case reports were excluded. Studies not including the measurement of ocular parameters, but solely the prevalence of DED based on questionnaires only were further excluded. The remaining original studies assessing dry eye signs or symptoms in VDT users, that could provide useful pathophysiological insights, were included in this study.
Findings
Overview of included articles
The search generated 1018 results. One hundred eighteen articles were not available in English. After excluding review articles, letters to the editor and case reports, 594 articles were assessed for relevance by titles and abstracts. One hundred sixty‐five full‐text articles were evaluated and checked against the inclusion criteria. Finally, 55 articles were included in this review (Fig. 3 ).
Fig. 3.
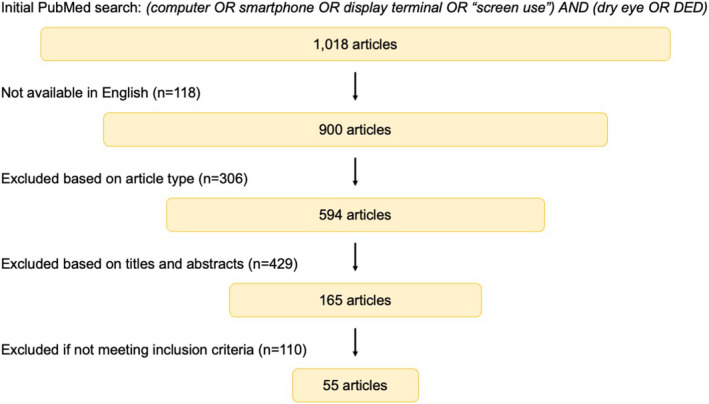
Flowchart of the stepwise search strategy and methodology.
Of the 55 included studies, 23 were single‐assessment studies (Table 1 ), while 32 included repeated assessments. Of studies that did repeated assessments, 26 studied non‐office workers (Table 2) and six studied office workers (Table 3). One study assessed the effects of blue light emitted from VDTs on human corneal cells (Núñez‐Álvarez & Osborne 2019) (not presented in tables).
Table 1.
Key outcomes found in single‐assessment studies assessing VDT use and ocular signs and symptoms.
| Study | Study design | Age, mean (SD) | Sample | Exposure times | Outcomes |
|---|---|---|---|---|---|
|
Bhargava 2014, IN (Bhargava et al. 2014) |
Cross‐sectional case–control | 26 (4) |
344 computer users 371 non‐computer users |
7 hr/day | Increased daily VDT use was associated with worse DESS, TBUT and Schirmer values. CIC and goblet cell density were also worse for computer users |
|
Cortes 2018, IT (Cortes et al. 2018) |
Cross‐sectional case–control | 47 (ND) |
120 VDT users 40 non‐VDT users |
7.8 hr/day versus 1.9 hr/day | 59% of VDT users had ocular surface disease based on OSDI, compared with none in the non‐VDT group. Significantly higher iNOS expression in tears of VDT users. NGF significantly higher for non‐VDT group, compared with symptomatic VDT group. TBUT, Schirmer and OSS showed no pathology |
|
Cremers 2021, US (Cremers et al. 2021) |
Cross‐sectional |
12 (3), 11 (2) |
17 sMGA‐children 24 healthy children |
>4 hr/day versus <4 hr/day | Increased daily VDT use associated with sMGA in children. 50% of sMGA‐children used VDTs >8 hr/day. Prolonged VDT use associated with worse meiboscores |
|
Eom 2021, KR (Eom et al. 2021) |
Cross‐sectional | 43 (14) | 158 with DED | 6.8 hr/day versus 0 hr/day | Higher DEQS for VDT users. Duration of VDT use was not correlated with signs and symptoms. Incomplete blinking associated with VDT use |
|
Fenga 2014, IT (Fenga et al. 2014) |
Cross‐sectional | 51 (8) | 64 VDT workers | ≥ 5 hr/day | 50% of subjects had a pathological OSDI score, while 58% had high tear osmolarity. TBUT was reduced in 88% of subjects, 44% had high OSS score and 38% had clinical signs of MGD. The majority had ocular surface dysfunction based on the objective measures |
|
Hu 2021, CN (Hu et al. 2021) |
Cross‐sectional | ND | 486 office workers | 0–3 hr/day, 3–6 hr/day, >6 hr/day | Most subjects had short TBUT, but normal tear secretion. Mean daily duration of VDT use associated with worse OSDI and increased Schirmer values. VDT use >6 hr/day associated with DED based on OSDI and TBUT or Schirmer |
|
Julio 2012, ES (Julio et al. 2012) |
Cross‐sectional | 41 (20) | 77 adults | <3 hr/day versus >3 hr/day | Computer use >3 hr/day was a predictor variable for high tear osmolarity |
|
Kojima 2011, JP (Kojima et al. 2011) |
Cross‐sectional case–control | 36 (7) |
69 CL wearers 102 non‐CL wearers |
<4 hr/day versus >4 hr/day | CL use and prolonged VDT associated with worse DED symptoms and TMH, but not worse OSS, Schirmer or TBUT |
|
Kumar 2013, IN (Kumar et al. 2013) |
Cross‐sectional |
24 (2), 23 (2) |
15 computer users 10 non‐computer users |
>4 hr/day versus <4 hr/day | Significantly worse CIC results with increased daily duration of computer |
|
Li 2018, CN (Li et al. 2018) |
Cross‐sectional | 20 (3) | 901 students | <8 hr/day, >8 hr/day | Symptom score, OSDI and OSS all worse for >8 hr/day group. 15% of subjects using VDTs >8 hr/day had DED based on subjective symptoms and TBUT, while only 9% of subjects had DED in group using VDT <8 hr/day. VDT use >8 hr/day associated with OSS, but not change in corneal sensation |
|
Li 2015, CN (Li et al. 2015) |
Cross‐sectional | ND | 6657 ophthalmic patients | >4 hr/day, <4 hr/day | Symptom score and OSS worse for >4 hr/day group. 56% of subjects in >4 hr/day group had DED based on Schirmer or TBUT, OSS and symptoms |
|
Moon 2014, KR (Moon et al. 2014) |
Cross‐sectional case–control | 11 (1) |
28 children with DED 260 healthy children |
2.4 hr/day versus 1.8 hr/day | Total daily duration of VDT use and daily smartphone use was associated with DED based on symptoms, TBUT and OSS. However, duration of TV or computer use, was not |
|
Nakamura 2010, JP (Nakamura et al. 2010) |
Cross‐sectional | 36 (10) | 601 office workers | >8 hr/day versus <2 hr/day | Significant decrease in Schirmer in subjects with long total and daily duration of VDT use. TBUT and DR‐1 scores showed no difference |
|
Rojas‐Carabali 2020, CO (Rojas‐Carabali et al. 2020) |
Cross‐sectional | 12 (3) | 60 children | 5.6 hr/day | All subjects had at least one pathological ocular surface test. No association between different screen devices and TBUT, Schirmer, TMH or other objective measurements |
|
Rossi 2019, IT (Rossi et al. 2019) |
Cross‐sectional | 42 (13) | 194 VDT workers | ≥ 4 hr/day versus <4 hr/day | Daily duration and total duration of VDT use were significantly longer for subjects with dry eye. Abnormal TBUT related to time spent on VDT, however OSS was not |
|
Sánchez‐Valerio 2020, MX (Sánchez‐Valerio et al. 2020) |
Cross‐sectional | 32 (8) | 108 VDT workers | 6 hr/day | Cumulative use of VDT associated with worsened TBUT, but not Schirmer. OSS, but not Schirmer, associated with mean daily duration of VDT use. Worse OSDI correlated with prolonged VDT use |
|
Tichenor 2019, US (Tichenor et al. 2019) |
Cross‐sectional | 12 (ND) | 225 adolescents | 3.1 hr/day, 3.6 hr/day, 7.0 hr/day | No correlation between usage of VDT and meibomian gland dropout score or symptoms |
|
Uchino 2018, JP (Uchino et al. 2018) |
Cross‐sectional | ND | 858 VDT workers | <8 hr/day versus >8 hr/day | 79% of subjects showed a shorter TBUT than blink interval. After adjusting for age, sex and CL use, prolonged VDT use was not significantly associated with unstable tear film. Schirmer and OSS not significant between groups |
|
Uchino 2014, JP (Uchino et al. 2014) |
Cross‐sectional | 42 (10) | 96 VDT workers | <5 hr/day, 5–7 hr/day. >7 hr/day | Significantly lower concentration of Muc5AC in the >7 hr/day group than the <5 hr/day group. No differences in Muc5AC mRNA |
|
Uchino 2013, JP (Uchino et al. 2013) |
Cross‐sectional | ND | 561 VDT workers | <4 hr/day, 4–8 hr/day, >8 hr/day | 79% showed a pathological TBUT. 17% had abnormal Schirmer values. 16% showed abnormal OSS. Meibomian glands showed no pathology in 77% |
|
Viso 2009, ES (Viso et al. 2009) |
Cross‐sectional | 64 (14) | 654 adults and elderly | ND | VDT use was associated with increased OSS, but not TBUT, Schirmer or ocular symptoms |
|
Wu 2014, CN (Wu et al. 2014) |
Cross‐sectional case–control | 31 (6) | 93 VDT workers with DED | >4 hr/day. versus ≤4 hr/day | VDT use over 4 hr/day was associated with worse TBUT, OSS, meiboscore, lid margin abnormalities and OSDI score. Schirmer showed no difference between groups |
|
Yokoi 2015, JP (Yokoi et al. 2015) |
Cross‐sectional | 43 (ND) | 561 VDT workers | ND | Subjects with DED and TBUT ≤5 and Schirmer >5 had more severe symptoms, compared with other subjects with abnormal Schirmer or OSS |
Ac = air conditioning, CIC = conjunctival impression cytology, CL: contact lens, CVS = computer vision syndrome, DED = dry eye disease, DEQS = dry eye‐related quality‐of‐life score, DESS = dry eye symptom score, iNOS = nitric oxide synthase, MGD = meibomian gland dysfunction, Muc5AC = mucin 5 AC, ND = not described, NGF = nerve growth factor, OSDI = ocular surface disease index, OSS = ocular Surface Staining, SD = standard deviation, sMGA = severe meibomian gland atrophy, TBUT = tear film break‐up time, WHS = women's health study questionnaire.
Table 2.
Changes in important clinical parameters reported in studies with repeated measurements assessing VDT use and ocular signs and symptoms.
| Study | Sample | Age, mean (SD) | Task | Duration | Symptoms | Blink parameters | TBUT | Sch | OSS | TMH | Key takeaways |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Antona 2018, ES (Antona et al. 2018) |
54 adults | 24 (3) | Smartphone versus book | 20 min | ↓5 | ND | ND | ND | ND | ND | All symptoms except headache were significantly increased after smartphone use compared with book. Dry eye symptoms increased in lower ambient light |
|
Argilés 2015, ES (Argilés et al. 2015) |
50 healthy adults | 34 (16) | Computer versus paper | 6 min × 6 | ND | ↓ | ND | ND | ND | ND | Spontaneous eye blink rate was reduced with both paper and VDT. Incomplete blinks were increased after VDT use compared with paper |
|
Cardona 2011, ES (Cardona et al. 2011) |
25 young adults | 24 (2) | Computer; Fast game, slow game | 20 min | ND | ↓ | ↓ | ND | ND | ↓ | Spontaneous eye blink rate decreased and incomplete blinks increased in both settings. More symptoms were noted after rapid compared with slow‐paced games. Tear break‐up area increased in fast‐paced game compared with baseline and slow‐paced game |
|
Choi 2018, KR (Choi et al. 2018) |
80 young adults | 26 (3) | Smartphone versus computer | 60 and 240 min | ↓1,2,5 | ND | ↓ | 0 | ND | 0 | OSDI worsened faster with smartphone use than computer. TBUT decreased in smartphone group only. VAS increased in both groups, but the smartphone group had more dryness after 1 hr. ROS significantly higher for both groups, but significantly higher for smartphone than for control at 4 hr |
|
Chu 2014, US (Chu et al. 2014) |
25 young adults | 25 (ND) | Computer versus paper | 20 min | ↓1,5 | ↓ | ND | ND | ND | ND | Incomplete blink rate and total symptom score increased with computer use, while the blink rate was not significantly changed |
|
Chu 2011, US (Chu et al. 2011) |
30 young adults | 24 (ND) | Computer versus paper | 20 min | ↓5 | ND | ND | ND | ND | ND | Mean symptom score and blurred vision worsened more with computer. No differences in ocular dryness symptom |
|
Golebiowski 2019, AU (Golebiowski et al. 2020) |
12 young adults | 19 (ND) | Smartphone | 60 min | ↓5 | ↓ | ↓ | 0 | ND | 0 | Number of Incomplete blinks increased with reading time. Complete blinks and blink rate did not change |
|
Himebaugh 2009, US (Himebaugh et al. 2009) |
16 DED and 16 non‐DED |
49 (ND), 42 (ND) |
Computer, 4 tasks | 3 min x4 | ↓4,5 | ↓ | ND | ND | ↓ | ND | VDT triggered DED symptoms and worse OSS in both groups. Blink rate decreased in both groups for user‐controlled tasks. |
|
Hirota 2013, JP (Hirota et al. 2013) |
11 young adults | 21 (3) | Computer | 60 min | ND | ↓ | ↓ | ND | ND | ND | Dynamic changes in complete blinks and incomplete blinks throughout the task |
|
Kim 2020, KR (Kim et al. 2020) |
76 adults | 40 (12) | UHD TV | 10 min | 0 | ND | ↓ | ND | ↓ | 0 | Change in TBUT with VDT use worse for non‐DED compared with DED |
|
Kim 2017, KR (Kim et al. 2017) |
59 adults | 38 (10) | Movie/ games on tablet | 60 min | ↓5 | ND | ↓ | ND | ND | ND | Total asthenopia score increased. Dry eye symptoms increased but not significantly |
|
Nakamori 1997, JP (Nakamori et al. 1997) |
161 DED and healthy adults |
49 (6), 44 (12) |
VDT | 60 min | ND | ↓ | ND | ND | ND | ND | Maximum blink interval was reduced after both 30 and 60 min of VDT use |
|
Patel 1991, GB (Patel & Port 1991) |
5 VDT users and 5 non‐VDT users | ND | Computer | >1 hr | ND | ND | 0 | ND | ND | ↑ | No association between tear film instability and tear volume |
|
Prabhasawat 2019, TH (Prabhasawat et al. 2019) |
30 adults | 32 (7) | E‐book versus book | 20 min | ↓2,5 | ND | ↓ | ND | 0 | 0 | TBUT reduced in both groups. Tearing and burning sensation were significantly worse after e‐book reading compared with paper book |
|
Rempel 2007, US (Rempel et al. 2007) |
24 young adults | 25 (4) | Computer, 3 viewing distances | 120 min x 3 | ↓5 | ND | ND | ND | ND | ND | Dry or irritated eyes and blurred vision increased with greater screen distance. Headache increased for middle distance compared with near distance |
|
Schlote 2004, DE (Schlote et al. 2004) |
30 DED patients | 45 (ND) | VDT | 30 min | ND | ↓ | 0 | 0 | ND | ND | Spontaneous eye blink rate decreased with VDT work compared with conversation |
|
Talens‐Estarelles 2020, ES (Talens‐Estarelles et al. 2020) |
31 young adults | 21 (2) | VDTs versus distant object | 15 min | ↓5 | ND | ↓ | ↓ | ND | 0 | TBUT and Schirmer worse for computer compared with control, but not other VDTs. Osmolarity and bulbar redness worse for computer than for smartphone. OSDI worse for computer than smartphone and e‐book |
|
Thorud 2012, NO (Thorud et al. 2012) |
20 students | 22 (4) | Computer | 60 and 120 min | ↓2 | ND | ND | ND | ND | ND | Significantly higher muscle activity and blood flow in m. orbicularis oculi compared with baseline. Muscle activity and blood flow tied to increased symptoms |
|
Zetterberg 2017, SE (Zetterberg et al. 2017) |
33 chronic neck pain 33 healthy adults |
39 (ND), 37 (ND) |
Computer, special lenses | 28 minutes | ↓5 | ND | ND | ND | ND | ND | Ocular discomfort and neck/shoulder pain worsened in both groups compared to baseline |
|
Bilkhu 2021, GB (Bilkhu et al. 2021) |
40 young adults | 24 (5) | Tablet use +/− treatment | 30 min | ↑3 | ↓ | 0 | ND | ND | ↑ | Tear film evaporation decreased and TBUT worsened with tablet use. Number of incomplete blinks increased and blink rate decreased with tablet use. Symptoms, DLP and LLT improved with humidity goggles |
|
Miura 2013, BR (Miura et al. 2013) |
15 DED and 15 non‐DED |
30 (5), 43 (15) |
Computer, 4 settings | 15 min | ND | ↑ | 0 | ND | 0 | ND | Blink rate increased with computer use when combined with PISC and Ac |
|
Moon 2016, KR (Moon et al. 2016) |
916 children | 10 (1) | Smartphone cessation | 1 month | ↑1 | ND | ↑ | ND | ↑ | ND | Subjects with DED spent significantly more time using VDTs than those without DED. OSDI scores and epithelial lesions improved, decreasing DED diagnoses from 100% to 0% after smartphone cessation |
|
Portello 2013, US (Portello et al. 2013) |
21 young adults | 24 (ND) | Computer | 15 min | 05 | ↑ | ND | ND | ND | ND | Negative correlation between blink rate and symptom score, and blink score and symptom score. Positive correlation between total symptom score and % of incomplete blinks. Audible tone increased mean blink rate but did not affect ocular symptoms |
|
Yee 2007, US (Yee et al. 2007) |
40 adults | ND | Computer, 4 conditions | 30 min | ↑1 | ND | ↑ | ND | 0 | ND | TBUT worsened with computer use in symptomatic but improved in asymptomatic subjects. High degree of meibomian gland dysfunction in symptomatic VDT users. MEGS improved comfort and TBUT. Artificial tears alone did not improve symptoms or objective measures |
Studies under the dotted line implemented measures to alleviate symptoms and signs related to VDT use.
↑ = Significant improvement with p < 0.005, ↓ = Significant worsening with p < 0.005, 0 = No significant alteration, ND = Not described.
1Ocular surface disease index (OSDI), 2visual analogue scale (VAS), 3symptom assessment in Dry Eye (SANDE), 4dry eye questionnaire (DEQ), 5Other.
Ac = air conditioning, DED = dry eye disease, DLP = dynamic tear film lipid layer pattern, LLT = lipid layer thickness, MEGS = microenvironment glasses, MGDS = meibomian gland dysfunction score, NEM = non‐warming eye mask, OSS = ocular surface staining, PISC = light‐emitting timer device, ROS = reactive oxygen species, SD = standard deviation, TBUT = tear film break‐up time, TMH = tear meniscus height, UHD TV = ultra‐high‐definition television.
Table 3.
Changes in important ocular signs and symptoms reported in studies focusing on office workers.
| Study | Sample | Age, mean (SD) | Task | Duration | Symptoms | Blink parameters | TBUT | Schirmer | OSS | TMH | Key takeaways |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Akkaya 2018, TR (Akkaya et al. 2018) |
60 |
30 (4), 28 (5) |
Computer | 1 workday | 01 | ND | ↓ | 0 | ND | ND | TBUT significantly worsened for VDT users compared with non‐VDT users |
|
Chlasta‐Twardzik 2021, PL (Chlasta‐Twardzik et al. 2021) |
150 | 47 (8) | VDT use | 1 workday | 01 | ND | 0 | ↓ | ND | ↓ | After 1 year, TMH and conjunctival hyperemia worsened but not OSDI or Schirmer. TBUT lower for subjects working >4 hr/day before work |
|
Doguizi 2019, TR (Doguizi et al. 2019) |
102 |
39 (6), 38 (6) |
VDT use | 1 workday | ND | ND | ND | ND | ND | ↓ | At baseline, TBUT, OSS, Schirmer and symptoms were significantly worse in VDT user than the control group. TMH and tear meniscus area were significantly reduced post‐vocationally for the VDT group |
|
Yazici 2015, TR (Yazici et al. 2015) |
77 |
31 (6), 34 (6) |
Computer | 1 workday | ↓1 | ND | ↓ | ↓ | ND | ND | One day of VDT work significantly worsened signs and symptoms |
|
Fujita 2019, JP (Fujita et al. 2019) |
10 | 41 (9) | Increased blind work time | 60 min | ↑4 | ↑ | ND | ND | ND | ND | Interblink interval decreased after 20 min of blind work, compared with baseline, and normal VDT use. Ocular symptoms were significantly improved during blind work |
|
Vaz 2019, PT (Vaz et al. 2019) |
77 | 34 (ND) | Behavioural intervention | 1 month |
01 ↑2 |
ND | ↑ | ↑ | ↑ | ND | Significant differences between subjects using computers >2 hr compared with <2 hr. Improvement of objective measures after behavioural intervention |
Studies under the dotted line implemented measures to alleviate symptoms and signs related to VDT use.
↑ Significant improvement with p < 0.005, ↓ Significant worsening with p < 0.005, 0 No significant alteration.
1Ocular surface disease index (OSDI), 2Portuguese group of ergophthalmology (PGE), 3Symptom score, 4Visual analogue scale (VAS).
DED = dry eye disease, ND = not described, OSS = ocular surface staining TBUT = tear break‐up time, SD = standard deviation, TMA = tear meniscus area, TMH = tear meniscus height.
Table 1 shows the key clinical elements of 23 single‐assessment studies. Across studies, prolonged VDT use was associated with decreased TBUT in five out of 11 studies (Bhargava et al. 2014; Moon et al. 2014; Wu et al. 2014; Rossi et al. 2019; Sánchez‐Valerio et al. 2020). In studies assessing prevalence, TBUT ≤5 seconds in VDT workers, was a frequent finding (Uchino et al. 2013; Fenga et al. 2014; Uchino et al. 2014; Hu et al. 2021). On the other hand, associations between prolonged VDT use and decreased Schirmer scores were only present in two out of 13 studies (Nakamura et al. 2010; Bhargava et al. 2014). Ocular surface staining (OSS) was linked with prolonged VDT use in six out of 11 studies (Viso et al. 2009; Moon et al. 2014; Wu et al. 2014; Li et al. 2015; Li et al. 2018; Sánchez‐Valerio et al. 2020), and prevalence of extensive OSS in VDT workers was 8% (Uchino et al. 2014), 16% (Uchino et al. 2013) and 44% (Fenga et al. 2014). Prolonged VDT use was associated with meibomian gland dysfunction (MGD) in two out of three studies (Wu et al. 2014; Cremers et al. 2021). In other studies, prevalence of clinical signs of MGD in VDT workers ranged from 23% (Uchino et al. 2013) to 38% (Fenga et al. 2014) (see Table 1 for more details). These objective clinical parameters are important in order to assess the pathological changes in VDT users.
Table 2 presents the outcomes of the 26 studies with repeated measures performed in non‐office workers. Blink parameters were investigated in 12 studies (Tsubota et al. 1996; Nakamori et al. 1997; Schlote et al. 2004; Himebaugh et al. 2009; Cardona et al. 2011; Hirota et al. 2013; Miura et al. 2013; Portello et al. 2013; Chu et al. 2014; Argilés et al. 2015; Bilkhu et al. 2021; Golebiowski et al. 2020). Two studies (Chu et al. 2014; Argilés et al. 2015) compared the number of incomplete blinks and blink rates seen with the use of hardcopies and VDTs, finding more incomplete blinks with VDTs. However, blink rates were similar with the use of hardcopies. Another study compared blink rates in VDT work with the average number of blinks seen in conversation. The authors found that blink rates were lower in the setting of VDT work, but TBUT and Schirmer were not reduced (Schlote et al. 2004). Tear film break‐up time (TBUT) was negatively influenced by VDT usage in 11 out of the 13 studies investigating this parameter (Cardona et al. 2011; Hirota et al. 2013; Moon et al. 2016; Kim et al. 2017; Choi et al. 2018; Prabhasawat et al. 2019; Bilkhu et al. 2021; Golebiowski et al. 2020; Kim et al. 2020; Sun et al. 2020; Talens‐Estarelles et al. 2020). Ocular surface staining (OSS) was linked to VDT use in three out of four studies (Himebaugh et al. 2009; Moon et al. 2016; Kim et al. 2020). One study found high degrees of MGD in symptomatic VDT users (Yee et al. 2007), while another found lipid layer thickness to be unchanged with tablet use (Bilkhu et al. 2021). Interventions such as the use of a light‐emitting timer device (Miura et al. 2013), smartphone cessation (Moon et al. 2016), eyelid steamers (Sun et al. 2020) and periocular isolation with microenvironment glasses (Yee et al. 2007) improved blink rate and signs and symptoms related to VDT use. Overall, VDT use appear to adversely affect blink parameters and meibomian glands, TBUT, ocular discomfort and signs of ocular surface damage.
Table 3 highlights the results of the six articles assessing changes in ocular signs and symptoms in office workers. When ocular signs were assessed, a day of VDT work appeared to reduce TBUT (Yazici et al. 2015; Akkaya et al. 2018; Doguizi et al. 2019) and tear meniscus height (Doguizi et al. 2019; Chlasta‐Twardzik et al. 2021). One study found lower TBUT baseline values for subjects working with VDT for more than 4 hr per day, compared with subjects working less (Chlasta‐Twardzik et al. 2021). Visual display terminal (VDT)‐related ocular symptoms, however, showed inconclusive results. Five out of six studies measured symptoms related to VDT use (Yazici et al. 2015; Akkaya et al. 2018; Fujita et al. 2019; Vaz et al. 2019; Chlasta‐Twardzik et al. 2021). One study showed that a day of VDT work worsened dry eye symptoms compared with non‐VDT work (Yazici et al. 2015). However, two studies found no worsening in OSDI with 1 day of VDT work (Akkaya et al. 2018; Chlasta‐Twardzik et al. 2021). In four studies, Schirmer scores were negatively affected by VDT use or improved by measures aiming to improve VDT‐associated symptoms and signs (Yazici et al. 2015; Doguizi et al. 2019; Vaz et al. 2019; Chlasta‐Twardzik et al. 2021). One study found no decrease in Schirmer when conducting a 1‐year follow‐up (Chlasta‐Twardzik et al. 2021). In addition, one study did not find any changes in Schirmer values with a day of VDT work (Akkaya et al. 2018). Meibomian gland dysfunction (MGD) was assessed in two studies, with one of the studies finding that subjects working on VDT for 4 hr per day were more likely to develop MGD (Chlasta‐Twardzik et al. 2021). Two studies found behavioural interventions to alleviate signs and symptoms of VDT use (Fujita et al. 2019; Vaz et al. 2019). These studies show that subjects with occupations relying on VDT use for work are susceptible to DED‐related signs and symptoms and should implement preventive measures.
A brief overview of the proposed mechanisms behind the adverse effects of VDT use on ocular dryness
Across articles, four separate mechanisms for how VDT use promotes dry eye were proposed. Reduced blink rates and more incomplete blinks during VDT use was the most frequently suggested pathway, and only six out of 55 articles did not mention these mechanisms (Viso et al. 2009; Zetterberg et al. 2017; Tichenor et al. 2019; Kim et al. 2020; Talens‐Estarelles et al. 2020). Of the 49 studies that did, five proposed incomplete blinking as the main contributor to harmful consequences (Hirota et al. 2013; Portello et al. 2013; Chu et al. 2014; Golebiowski et al. 2020; Eom et al. 2021). Two studies hypothesized that VDT use caused DED‐related signs and symptoms through suppression of parasympathetic stimulation and reduced lacrimal gland secretion (Nakamura et al. 2010; Doguizi et al. 2019), and a neurogenic inflammation theory was suggested in another article (Yazici et al. 2015). The ocular toxicity of blue light exposure from VDTs was mentioned in three articles (Wu et al. 2014; Kim et al. 2017; Núñez‐Álvarez & Osborne 2019). In four articles, no mechanism was suggested (Viso et al. 2009; Zetterberg et al. 2017; Tichenor et al. 2019; Kim et al. 2020). The results of the proposed mechanisms are increased tear film instability, exposure and evaporation (Cardona et al. 2011; Uchino et al. 2013), caused by reduced lacrimal gland function (Nakamura et al. 2010), decreased blink rate, increased incomplete blinking (Wu et al. 2014) and reduced mucin secretion (Uchino et al. 2014). These changes may contribute to the vicious cycle of DED. Figure 4 illustrates an overview of the proposed mechanisms, and more details about each are provided in sections 3.3 to 3.6 below.
Fig. 4.
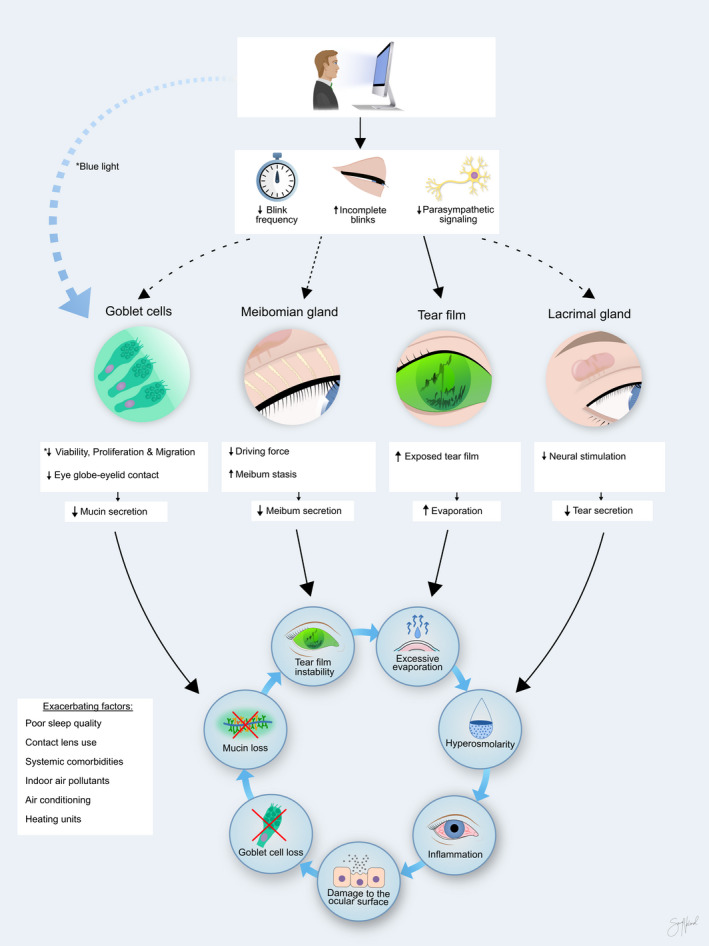
Possible mechanisms involved in visual display terminal (VDT)‐associated dry eye development. Visual display terminal (VDT) use causes decreased blink frequency, increased incomplete blinks and exposure to blue light. This may induce excessive evaporation and several noxious reactions in the goblet cells, meibomian glands and lacrimal glands, feeding into the vicious cycle of dry eye disease. These reactions can be exacerbated by several factors, including contact lens wear and air conditioning. Copyright Sara Tellefsen Nøland.
Effects on the ocular protection index
The ocular protection index (OPI) is a useful tool when assessing tear film function (Bron et al. 2017). The OPI is TBUT divided by the interblink interval (IBI) (Ousler et al. 2008). An OPI <1 indicates that the tear film breaks up before the next blink occurs and that the ocular surface is unprotected during a blink cycle (Fig. 5). The use of VDT devices reduces TBUT and increases IBI, substantially lowering the OPI (Bilkhu et al. 2021). Furthermore, VDT use increases the tear break‐up areas (Cardona et al. 2011) and is tied to worse osmolarity scores (Julio et al. 2012; Fenga et al. 2014; Yazici et al. 2015).
Fig. 5.
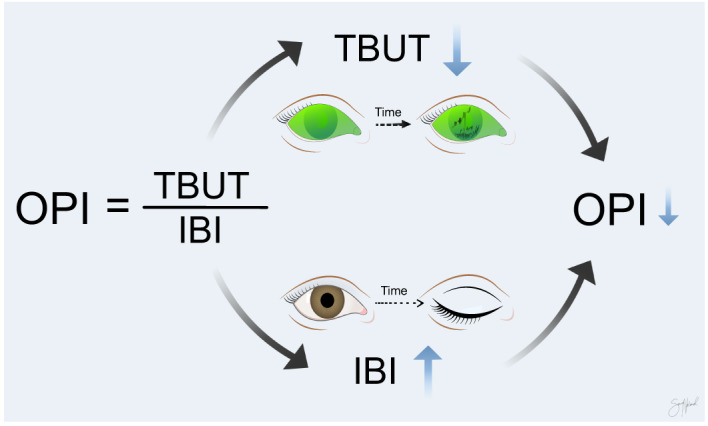
Ocular protection index (OPI) is the ratio between the tear film break‐up time (TBUT) and the interblink interval (IBI). Both tear film break‐up time and interblink interval worsen during visual display terminal (VDT) use. Copyright Sara Tellefsen Nøland.
The increased cognitive demand and the gathering of visual information during VDT use reduce blink rates and promote incomplete blinking (Rosenfield et al. 2015). Incomplete blinking is known to reduce ocular lubrication, increase friction and contribute to lid wiper epitheliopathy (McMonnies 2007). Additionally, greater ocular surface exposure increases tear film evaporation and hyperosmolar stress independent of VDT use (Bron et al. 2017). Voluntary squinting which decreases blink rates (Sheedy et al. 2005) and higher angles of gaze further exacerbate this in VDT workers (Kojima et al. 2011). In sum, altered blink patterns and increased ocular surface exposure directly affect the tear film, promoting DED development.
Lacrimal gland and aqueous layer
Despite many studies finding no association between VDT use and Schirmer scores, seven out of 23 studies found decreased Schirmer scores associated with VDT use (Nakamura et al. 2010; Bhargava et al. 2014; Yazici et al. 2015; Doguizi et al. 2019; Vaz et al. 2019; Talens‐Estarelles et al. 2020; Chlasta‐Twardzik et al. 2021). An additional four studies found reduced tear meniscus height after VDT exposure (Cardona et al. 2011; Kojima et al. 2011; Doguizi et al. 2019; Chlasta‐Twardzik et al. 2021). Thus, a change in the aqueous layer and lacrimal gland function could contribute to DED in VDT use, but this needs to be further investigated.
The lacrimal gland is stimulated by postganglionic parasympathetic nerve fibres derived from the pterygopalatine ganglion, which in turn receives preganglionic axons from the greater superficial petrosal nerve (Piagkou et al. 2012). Hyperosmolar stress and cooling of the cornea can stimulate corneal sensory afferent nerves which in turn can activate this nerve loop (Meng & Kurose 2013). In addition, sensory input from intraocular neurovasculature with bright light can also activate parasympathetic nerve fibres and reflex lacrimation (Okamoto et al. 2012). Two studies hypothesized that the suppression of parasympathetic stimulation of lacrimal gland secrete during VDT use caused the observed changes (Nakamura et al. 2010; Doguizi et al. 2019). In a study of 601 office workers, Nakamura et al. (2010) proposed that this suppression stemmed from a disuse‐dysfunction, caused by reduced blinking and reduced lacrimal gland stimulation during VDT use. In the same study, the researchers used a rat model to show that the lacrimal gland morphology deteriorated with exposure to the VDT model. They also showed increased ocular surface staining of the eyes of the rats. In a later study of human lacrimal gland samples, the research group found morphological differences and a greater accumulation of secretory vesicles in VDT users compared with controls, implying impaired protein secretion from lacrimal glands of VDT users (Kamoi et al. 2012). Both the disruption of lacrimal gland morphology and the accumulation of secretory vesicles may be signs of disturbance of parasympathetic innervation of the gland (Toshida et al. 2007). Other studies have confirmed the importance of parasympathetic signalling to the lacrimal gland in mice and humans (Dartt 2009; Jin et al. 2020). Thus, reduced parasympathetic stimulation during high‐focus tasks (Bruya & Tang 2018) could potentially play a role in VDT‐associated DED and should be further investigated. In a different article (Yazici et al. 2015), it was proposed that the lacrimal hypofunction seen in the study by Nakamura et al. (2010) could be due to neurogenic inflammation. They proposed that ocular surface dryness continuously stimulates the lacrimal functional unit neural arch, leading to inflammation and subsequent fibrosis and atrophy of the gland. Electrophysiological experiments in corneal nerve endings and lacrimal gland nerves and measurements of autonomic activity by ECG, blood pressure, pupil dilation and sweat responses, during VDT use, could provide useful insights.
Conjunctival goblet cells and mucin production
The water‐binding capacity of mucins is vital for maintaining the tear film volume (Hori 2018). Visual display terminal (VDT) use may negatively affect conjunctival goblet cells and the concentration of Mucin 5 AC (Muc5AC), a key mucin protein and marker of overall mucin status (Bhargava et al. 2014; Uchino et al. 2014). Despite similar amounts of messenger RNA for Muc5Ac, the Muc5Ac protein concentration in tears was lower in subjects using VDTs for more than 7 hr per day than those using VDTs less than 5 hr per day (Uchino et al. 2014). The authors attributed this to reduced blinking during VDT use and lower humidity in office environments. However, the effect of blink rates and VDT use on the Muc5AC concentration in tears need to be directly studied before casual relations can be concluded.
Daily VDT work was further associated with reduced goblet cell density (Bhargava et al. 2014). Decreased density of goblet cells in the conjunctival epithelium could be a result of either increased goblet cell secretion, increased goblet cell death and/or decreased goblet cell development (Dartt 2002). A possible mechanism responsible for reduced goblet cell density is exposure to blue light. Blue light, like that emitted by VDTs, can be harmful to the epithelial cells on the ocular surface and reduce viability, proliferation and migration of human corneal epithelial cells in vitro (Núñez‐Álvarez & Osborne 2019) (Fig. 6 ). Exposure to higher intensity blue light causes oxidative stress and cell death (Núñez‐Álvarez & Osborne 2019). Interestingly, patients with DED could be particularly sensitive to blue light, as preexisting hyperosmolar stress has been shown to increase the ocular toxicity of blue light (Marek et al. 2018). In vivo studies assessing the direct effects of blue light on the ocular surface during normal VDT use should be conducted.
Fig. 6.
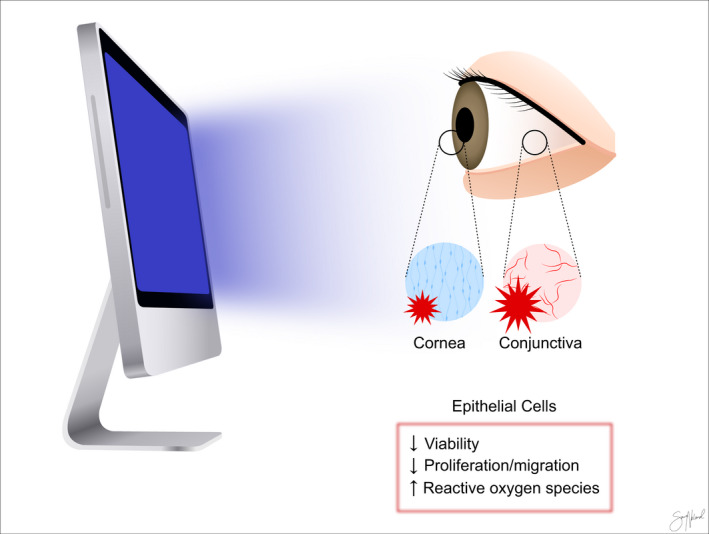
Possible effects of blue light on the ocular surface. Blue light emitted by visual display terminal (VDT) devices is shown to have direct cytotoxic effects on the corneal and conjunctival cells in vitro. Copyright Sara Tellefsen Nøland.
Similar to the lacrimal gland, parasympathetic nerves are thought to provide the main neural stimulation of the conjunctival goblet cells (Dartt 2004). Additionally, increased incomplete blinking may inhibit the spread of mucin secreted by the tarsal goblet cells (McMonnies 2007). Thus, altered parasympathetic stimuli and blink patterns might also play a role in the reduced mucin concentrations observed with VDT use (Bhargava et al. 2014; Uchino et al. 2014) and should be explored further in future studies.
Meibomian gland and the lipid layer
The lipids secreted from the meibomian glands promote tear film stability and minimize ocular evaporation (Willcox et al. 2017). Three out of five studies found links between prolonged VDT use and meibomian gland dysfunction (Wu et al. 2014; Chlasta‐Twardzik et al. 2021; Cremers et al. 2021). Two studies found limited associations between meibomian gland pathology and VDT use despite finding decreased TBUT values (Doguizi et al. 2019; Bilkhu et al. 2021). However, Wu et al. (2014) reported impaired meibum expression, increased lid abnormality and decreased meiboscores, in addition to reduced TBUT values, in subjects using VDTs for more than 4 hr per day. Reduced blink rate during VDT use was suggested to cause stagnant meibum to build up and block the meibomian glands (Wu et al. 2014). Furthermore, as incomplete blinks are slower than complete blinks, they create less driving force (Nakamura et al. 2008; Hirota et al. 2013), which reduces the secretion of the meibum (Hirota et al. 2013). Moreover, increasing age is tied to a decreased spontaneous eye blink rate (Sun et al. 1997; Argilés et al. 2015) and lower peak velocity of spontaneous blinks (Sun et al. 1997), which could reduce meibum secretion and exacerbate the effects of VDT use in older VDT users.
In addition to computer use, reading from smartphones, tablets, e‐books and even a printed book decrease TBUT values (Kim et al. 2017; Prabhasawat et al. 2019). This may indicate that the attention level required to perform the task is responsible for the decreased TBUT. One study found that subjects playing a fast‐paced game had a greater reduction in TBUT than those playing a less attention‐demanding one (Cardona et al. 2011). The researchers ascribed these changes to the amount of visual information being processed and the resulting altered blinking patterns (Cardona et al. 2011).
Combined, these findings show that tear film stability and meibomian gland function may be adversely affected by VDT use, likely due to changes in blink patterns because of the elevated attention required with VDT use. Blink exercises recently showed positive effects on DED in other settings (Kim et al. 2021), and their impact on ocular discomfort during VDT use should be studied further.
Exacerbating factors
The use of VDTs in dark environments worsened dry eye symptoms (Tsubota et al. 1996; Antona et al. 2018). Poor illumination of the screen (Tsubota et al. 1996) and squinting was thought to reduce the blink rate (Antona et al. 2018). Antona et al. (2018) also discovered that reading on a smartphone induced more discomfort than reading from a book, possibly due to the loss of sharpness and increased visual searching during scrolling. Blink rates have been shown to decrease with increased voluntary squinting (Sheedy et al. 2005), which is a challenge with older VDT devices with poor screen quality, where squinting might improve visual resolution (Rosenfield et al. 2015). Difficulties with convergence and accommodation in response to glare and small font on screen can worsen ocular discomfort (Thorud et al. 2012). Additionally, glare on the screen is associated with increased symptoms of computer vision syndrome (Altalhi et al. 2020). In sum, poor lighting, low screen quality and greater glare on screen with VDT use could induce or worsen dry eye symptoms.
Contact lens wear (Bazeer et al. 2019) and exposure to air conditioning and heating units (Titiyal et al. 2018; Huang et al. 2020) are exacerbating factors of DED that are common for office workers. As a result, VDT workers wearing contact lenses were especially prone to ocular discomfort in environments with air conditioning or heating units (Kojima et al. 2011). Furthermore, sedentary behaviour and systemic comorbidities in VDT users have also been tied to increased risk of DED and could reflect a negative additive effect to VDT use (Kawashima 2018; Bazeer et al. 2019). The background for these likely exacerbating factors is shown in Table S1. Based on this, addressing comorbidities and exacerbating factors might be essential when grappling with VDT‐associated DED.
Conclusion
Visual display terminal (VDT) use causes more incomplete blinks and decreased blink rates, triggering several pathophysiological mechanisms that promote DED development. Increased exposure of the tear film and decreased meibum distribution could cause an increase in tear film instability and ocular surface stress. Although not sufficiently studied, inadequate blinking may lead to altered parasympathetic signalling to the lacrimal gland and goblet cells and reduced secretion. Combined with possible harmful effects of blue light, these changes may disrupt tear film homeostasis and promote DED development. More studies are needed to further illuminate the pathways involved and find appropriate preventive measures for VDT‐associated DED.
Supporting information
Table S1 Background for the exacerbating factors presented in the review.
Supplementary Materials
The authors want to thank Marie Wangen Beining, Emily Moschowits and Alejandra Cuero for their valuable input and help.
Irrespective of potential conflict of interest, for the sake of transparency: Tor Paaske Utheim is co‐founder and co‐owner of The Norwegian dry eye clinic and the Clinic of eye health, Oslo, Norway, which delivers talks for and/or receives financial support from the following: ABIGO, Alcon, Allergan, AMWO, Bausch&Lomb, Bayer, European school for advanced studies in ophthalmology, InnZ Medical, Medilens Nordic, Medistim, Novartis, Santen, Specsavers, Shire Pharmaceuticals and Thea Laboratories. He has served on the global scientific advisory board for Novartis and Alcon as well as the European advisory board for Shire Pharmaceuticals. Utheim is the Norwegian Global Ambassador for Tear Film and Ocular Surface Society (TFOS), a Board Member of the International Ocular Surface Society, a Consultant at the Norwegian Association for the Blind and Partially Sighted, and the Editor‐in‐Chief of Oftalmolog, an eye journal distributed to all eye doctors in the Nordic region since 1980.
This research was partly funded by NFR 271555 grant by the Norwegian Research Council, through the Medical Student Research Program. D.A. Dartt was supported by grants from the US NIH: R01 EY019470, R01 EY029789 and R01 EY021292.
References
- Aggarwal S & Galor A (2018): What's new in dry eye disease diagnosis? Current advances and challenges. F1000Res 7: F1000 Faculty Rev‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya S, Atakan T, Acikalin B, Aksoy S & Ozkurt Y (2018): Effects of long‐term computer use on eye dryness. North Clin Istanb 5: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altalhi A, Khayyat W, Khojah O, Alsalmi M & Almarzouki H (2020): Computer vision syndrome among health sciences students in saudi arabia: prevalence and risk factors. Cureus 12: e7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antona B, Barrio AR, Gascó A, Pinar A, González‐Pérez M & Puell MC (2018): Symptoms associated with reading from a smartphone in conditions of light and dark. Appl Ergon 68: 12–17. [DOI] [PubMed] [Google Scholar]
- Argilés M, Cardona G, Pérez‐Cabré E & Rodríguez M (2015): Blink rate and incomplete blinks in six different controlled hard‐copy and electronic reading conditions. Invest Ophthalmol Vis Sci 56: 6679–6685. [DOI] [PubMed] [Google Scholar]
- Bahkir FA & Grandee SS (2020): Impact of the COVID‐19 lockdown on digital device‐related ocular health. Indian J Ophthalmol 68: 2378–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazeer S, Jansonius N, Snieder H, Hammond C & Vehof J (2019): The relationship between occupation and dry eye. Ocul Surf 17: 484–490. [DOI] [PubMed] [Google Scholar]
- Bhargava R, Kumar P, Kaur A, Kumar M & Mishra A (2014): The diagnostic value and accuracy of conjunctival impression cytology, dry eye symptomatology, and routine tear function tests in computer users. J Lab Physicians 6: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkhu P, Wolffsohn J & Purslow C (2021): Provocation of the ocular surface to investigate the evaporative pathophysiology of dry eye disease. Cont Lens Anterior Eye 44: 24–29. [DOI] [PubMed] [Google Scholar]
- Bogdan‐Martin D (2020): Measuring digital development: International Telecommunication Union; 2019.
- Bron AJ, de Paiva CS, Chauhan SK et al. (2017): TFOS DEWS II pathophysiology report. Ocul Surf 15: 438–510. [DOI] [PubMed] [Google Scholar]
- Bruya B & Tang Y‐Y (2018): Is attention really effort? Revisiting Daniel Kahneman's Influential 1973 book attention and effort. Front Psychol 9: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona G, García C, Serés C, Vilaseca M & Gispets J (2011): Blink rate, blink amplitude, and tear film integrity during dynamic visual display terminal tasks. Curr Eye Res 36: 190–197. [DOI] [PubMed] [Google Scholar]
- Chlasta‐Twardzik E, Górecka‐Nitoń A, Nowińska A & Wylęgała E (2021): The influence of work environment factors on the ocularsurface in a one‐year follow‐up prospective clinical study. Diagnostics (Basel) 11: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Li Y, Kim SH, Jin R, Kim YH, Choi W, You IC & Yoon KC (2018): The influences of smartphone use on the status of the tear film and ocular surface. PLoS One 13: e0206541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Rosenfield M, Portello JK, Benzoni JA & Collier JD (2011): A comparison of symptoms after viewing text on a computer screen and hardcopy. Ophthalmic Physiol Opt 31: 29–32. [DOI] [PubMed] [Google Scholar]
- Chu CA, Rosenfield M & Portello JK (2014): Blink patterns: reading from a computer screen versus hard copy. Optom Vis Sci 91: 297–302. [DOI] [PubMed] [Google Scholar]
- Cortes M, Esposito G, Sacco R, Gillet VB, Ianni A & Micera A (2018): NGF and iNOS changes in tears from video display terminal workers. Curr Eye Res 43: 1119–1125. [DOI] [PubMed] [Google Scholar]
- Craig JP, Nichols KK, Akpek EK et al. (2017): TFOS DEWS II definition and classification report. Ocul Surf 15: 276–283. [DOI] [PubMed] [Google Scholar]
- Cremers SL, Khan ARG, Ahn J, Cremers L, Weber J, Kossler AL, Pigotti C & Martinez A (2021): New indicator of children's excessive electronic screen use and factors in meibomian gland atrophy. Am J Ophthalmol 229: 63–70. [DOI] [PubMed] [Google Scholar]
- Dartt DA (2002): Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res 21: 555–576. [DOI] [PubMed] [Google Scholar]
- Dartt DA (2004): Control of mucin production by ocular surface epithelial cells. Exp Eye Res 78: 173–185. [DOI] [PubMed] [Google Scholar]
- Dartt DA (2009): Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res 28: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA & Willcox MDP (2013): Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res 117: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson HJ & Kuonen VJ (2004): The tear film and ocular mucins. Vet Ophthalmol 7: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doguizi S, Sekeroglu MA, Inanc M & Yılmazbas P (2019): Evaluation of tear meniscus dimensions using anterior segment optical coherence tomography in video terminal display workers. Clin Exp Optom 102: 478–484. [DOI] [PubMed] [Google Scholar]
- Driver PJ & Lemp MA (1996): Meibomian gland dysfunction. Surv Ophthalmol 40: 343–367. [DOI] [PubMed] [Google Scholar]
- Eom Y, Hyon JY, Lee HK, Song JS & Kim HM (2021): A multicenter cross‐sectional survey of dry eye clinical characteristics and practice patterns in Korea: the DECS‐K study. Jpn J Ophthalmol 65: 261–270. [DOI] [PubMed] [Google Scholar]
- Fenga C, Aragona P, Di Nola C & Spinella R (2014): Comparison of ocular surface disease index and tear osmolarity as markers of ocular surface dysfunction in video terminal display workers. Am J Ophthalmol 158: 41–48.e42. [DOI] [PubMed] [Google Scholar]
- Fujita H, Sano K, Baba T, Tanaka T & Ohno‐Matsui K (2019): Blind working time in visual display terminal users. J Occup Health 61: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiowski B, Long J, Harrison K, Lee A, Chidi‐Egboka N & Asper L (2020): Smartphone use and effects on tear film, blinking and binocular vision. Curr Eye Res 45: 428–434. [DOI] [PubMed] [Google Scholar]
- Himebaugh NL, Begley CG, Bradley A & Wilkinson JA (2009): Blinking and tear break‐up during four visual tasks. Optom Vis Sci 86: E106–E114. [DOI] [PubMed] [Google Scholar]
- Hirota M, Uozato H, Kawamorita T, Shibata Y & Yamamoto S (2013): Effect of incomplete blinking on tear film stability. Optom Vis Sci 90: 650–657. [DOI] [PubMed] [Google Scholar]
- Hori Y (2018): Secreted mucins on the ocular surface. Invest Ophthalmol Vis Sci 59: Des151–Des156. [DOI] [PubMed] [Google Scholar]
- Hu JW, Zhu XP, Pan SY, Yang H & Xiao XH (2021): Prevalence and risk factors of dry eye disease in young and middle‐aged office employee: a Xi'an Study. Int J Ophthalmol 14: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Janecki J, Galor A, Rock S, Menendez D, Hackam AS, Jeng BH & Kumar N (2020): Association of the indoor environment with dry eye metrics. JAMA Ophthalmol 138: 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITU (2021): Digital trends in Europe 2021.
- Jin K, Imada T, Hisamura R, Ito M, Toriumi H, Tanaka KF, Nakamura S & Tsubota K (2020): Identification of lacrimal gland postganglionic innervation and its regulation of tear secretion. Am J Pathol 190: 1068–1079. [DOI] [PubMed] [Google Scholar]
- Julio G, Lluch S, Cardona G, Fornieles A & Merindano D (2012): Item by item analysis strategy of the relationship between symptoms and signs in early dry eye. Curr Eye Res 37: 357–364. [DOI] [PubMed] [Google Scholar]
- Kamoi M, Ogawa Y, Nakamura S et al. (2012): Accumulation of secretory vesicles in the lacrimal gland epithelia is related to non‐Sjögren's type dry eye in visual display terminal users. PloS Onse 7: e43688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M (2018): Systemic health and dry eye. Investig Ophthalmol Vis Sci 59: Des138–Des142. [DOI] [PubMed] [Google Scholar]
- Kim AD, Muntz A, Lee J, Wang MTM & Craig JP (2021): Therapeutic benefits of blinking exercises in dry eye disease. Cont Lens Anterior Eye 44: 101329. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Lim CY, Gu N & Park CY (2017): Visual fatigue induced by viewing a tablet computer with a high‐resolution display. Korean J Ophthalmol 31: 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Yang HK, Seo JM, Lee S & Hwang JM (2020): Effect of ultra‐high‐definition television on ocular surface and fatigue. Korean J Ophthalmol 34: 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King‐Smith E, Fink B, Hill R, Koelling K & Tiffany J (2004): The thickness of the tear film. Curr Eye Res 29: 357–368. [DOI] [PubMed] [Google Scholar]
- Kojima T, Ibrahim OM, Wakamatsu T, Tsuyama A, Ogawa J, Matsumoto Y, Dogru M & Tsubota K (2011): The impact of contact lens wear and visual display terminal work on ocular surface and tear functions in office workers. Am J Ophthalmol 152: 933–940.e932. [DOI] [PubMed] [Google Scholar]
- Kumar S, Bansal R, Khare A, Malik KP, Malik VK, Jain K & Jain C (2013): Conjunctival impression cytology in computer users. Nepal J Ophthalmol 5: 33–37. [DOI] [PubMed] [Google Scholar]
- Li J, Zheng K, Deng Z, Zheng J, Ma H, Sun L & Chen W (2015): Prevalence and risk factors of dry eye disease among a hospital‐based population in southeast china. Eye Contact Lens 41: 44–50. [DOI] [PubMed] [Google Scholar]
- Li S, He J, Chen Q, Zhu J, Zou H & Xu X (2018): Ocular surface health in Shanghai University students: a cross‐sectional study. BMC Ophthalmol 18: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek V, Mélik‐Parsadaniantz S, Villette T, Montoya F, Baudouin C, Brignole‐Baudouin F & Denoyer A (2018): Blue light phototoxicity toward human corneal and conjunctival epithelial cells in basal and hyperosmolar conditions. Free Radic Biol Med 126: 27–40. [DOI] [PubMed] [Google Scholar]
- McCulley JP & Shine WE (2003): Meibomian gland function and the tear lipid layer. Ocul Surf 1: 97–106. [DOI] [PubMed] [Google Scholar]
- McMonnies CW (2007): Incomplete blinking: exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lenses. Cont Lens Anterior Eye . 30: 37–51. [DOI] [PubMed] [Google Scholar]
- Meng ID & Kurose M (2013): The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res 117: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura DL, Hazarbassanov RM, Yamasato CK, Bandeira e Silva F, Godinho CJ & Gomes J (2013): Effect of a light‐emitting timer device on the blink rate of non‐dry eye individuals and dry eye patients. Br J Ophthalmol 97: 965–967. [DOI] [PubMed] [Google Scholar]
- Moon JH, Kim KW & Moon NJ (2016): Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol 16: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JH, Lee MY & Moon NJ (2014): Association between video display terminal use and dry eye disease in school children. J Pediatr Ophthalmol Strabismus 51: 87–92. [DOI] [PubMed] [Google Scholar]
- Morthen MK, Magno MS, Utheim TP, Snieder H, Hammond CJ & Vehof J (2021): The physical and mental burden of dry eye disease: A large population‐based study investigating the relationship with health‐related quality of life and its determinants. Ocul Surf 21: 107–117. [DOI] [PubMed] [Google Scholar]
- Nakamori K, Odawara M, Nakajima T, Mizutani T & Tsubota K (1997): Blinking is controlled primarily by ocular surface conditions. Am J Ophthalmol 124: 24–30. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kinoshita S, Yokoi N et al. (2010): Lacrimal hypofunction as a new mechanism of dry eye in visual display terminal users. PLoS One 5: e11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Matsuda J, Suzuki K, Toyoda H, Hakamata N, Shimamoto T & Kinoshita S (2008): Measurement of spontaneous blinks with a high‐speed blink analyzing system. Nippon Ganka Gakkai Zasshi 112: 1059–1067. [PubMed] [Google Scholar]
- Núñez‐Álvarez C & Osborne NN (2019): Enhancement of corneal epithelium cell survival, proliferation and migration by red light: Relevance to corneal wound healing. Exp Eye Res 180: 231–241. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Tashiro A, Thompson R, Nishida Y & Bereiter DA (2012): Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci 36: 3492–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousler GWI, Hagberg KW, Schindelar M, Welch D & Abelson MB (2008): The ocular protection index. Cornea 27: 509–513. [DOI] [PubMed] [Google Scholar]
- Patel S & Port MJ (1991): Tear characteristics of the VDU operator. Optom Vis Sci 68: 798–800. [DOI] [PubMed] [Google Scholar]
- Patterson R, McNamara E, Tainio M et al. (2018): Sedentary behaviour and risk of all‐cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta‐analysis. Eur J Epidemiol 33: 811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC & Stern ME (2020): Biological functions of tear film. Exp Eye Res 197: 108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piagkou M, Demesticha T, Troupis T et al. (2012): The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract 12: 399–412. [DOI] [PubMed] [Google Scholar]
- Portello JK, Rosenfield M & Chu CA (2013): Blink rate, incomplete blinks and computer vision syndrome. Optom Vis Sci 90: 482–487. [DOI] [PubMed] [Google Scholar]
- Prabhasawat P, Pinitpuwadol W, Angsriprasert D, Chonpimai P & Saiman M (2019): Tear film change and ocular symptoms after reading printed book and electronic book: a crossover study. Jpn J Ophthalmol 63: 137–144. [DOI] [PubMed] [Google Scholar]
- Rempel D, Willms K, Anshel J, Jaschinski W & Sheedy J (2007): The effects of visual display distance on eye accommodation, head posture, and vision and neck symptoms. Hum Factors 49: 830–838. [DOI] [PubMed] [Google Scholar]
- Rojas‐Carabali W, Uribe‐Reina P, Muñoz‐Ortiz J, Terreros‐Dorado JP, Ruiz‐Botero ME, Torres‐Arias N, Reyes‐Guanes J, Rodriguez Zarante A, Arteaga‐Rivera JY, Mosos C, Gutiérrez ÁM, N Molano‐González, G Marroquín & A de‐la‐Torre (2020): High prevalence of abnormal ocular surface tests in a healthy pediatric population. Clin Ophthalmol 14: 3427–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield M, Jahan S, Nunez K & Chan K (2015): Cognitive demand, digital screens and blink rate. Comput Hum Behav 51: 403–406. [Google Scholar]
- Rossi GCM, Scudeller L, Bettio F, Pasinetti GM & Bianchi PE (2019): Prevalence of dry eye in video display terminal users: a cross‐sectional Caucasian study in Italy. Int Ophthalmol 39: 1315–1322. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Valerio MDR, Mohamed‐Noriega K, Zamora‐Ginez I, Baez Duarte BG & Vallejo‐Ruiz V (2020): Dry eye disease association with computer exposure time among subjects with computer vision syndrome. Clin Ophthalmol 14: 4311–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlote T, Kadner G & Freudenthaler N (2004): Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol 242: 306–312. [DOI] [PubMed] [Google Scholar]
- Sheedy JE, Gowrisankaran S & Hayes JR (2005): Blink rate decreases with eyelid squint. Optom Vis Sci 82: 905–911. [DOI] [PubMed] [Google Scholar]
- Sivakumar GK, Patel J, Malvankar‐Mehta MS & Mather R (2021): Work productivity among Sjögren's Syndrome and non‐Sjögren's dry eye patients: a systematic review and meta‐analysis. Eye 35: 3243–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Alves M, Bunya VY et al. (2017): TFOS DEWS II epidemiology report. Ocul Surf 15: 334–365. [DOI] [PubMed] [Google Scholar]
- Sun CC, Lee CY, Hwang YS, Michihito I, Tagami K & Hsiao CH (2020): Effect of warming eyelids on tear film stability and quality of life in visual display terminal users: a randomized controlled trial. Sci Rep 10: 16919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WS, Baker RS, Chuke JC, Rouholiman BR, Hasan SA, Gaza W, Stava MW & Porter JD (1997): Age‐related changes in human blinks. Passive and active changes in eyelid kinematics. Invest Ophthalmol Vis Sci 38: 92–99. [PubMed] [Google Scholar]
- Talens‐Estarelles C, Sanchis‐Jurado V, Esteve‐Taboada JJ, Pons ÁM & García‐Lázaro S (2020): How do different digital displays affect the ocular surface? Optom Vis Sci 97: 1070–1079. [DOI] [PubMed] [Google Scholar]
- Thorud HM, Helland M, Aarås A, Kvikstad TM, Lindberg LG & Horgen G (2012): Eye‐related pain induced by visually demanding computer work. Optom Vis Sci 89: E452–E464. [DOI] [PubMed] [Google Scholar]
- Tichenor AA, Ziemanski JF, Ngo W, Nichols JJ & Nichols KK (2019): Tear film and meibomian gland characteristics in adolescents. Cornea 38: 1475–1482. [DOI] [PubMed] [Google Scholar]
- Titiyal JS, Falera RC, Kaur M, Sharma V & Sharma N (2018): Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index‐based cross‐sectional hospital study. Indian J Ophthalmol 66: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshida H, Nguyen DH, Beuerman RW & Murakami A (2007): Evaluation of novel dry eye model: preganglionic parasympathetic denervation in rabbit. Invest Ophthalmol Vis Sci 48: 4468–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota K, Toda I & Nakamori K (1996): Poor illumination, VDTs, and desiccated eyes. Lancet 347: 768–769. [DOI] [PubMed] [Google Scholar]
- Uchino M, Kawashima M, Uchino Y, Tsubota K & Yokoi N (2018): Association between tear film break up time and blink interval in visual display terminal users. Int J Ophthalmol 11: 1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino M, Yokoi N, Uchino Y, Dogru M, Kawashima M, Komuro A, Sonomura Y, Kato H, Kinoshita S, Schaumberg DA & Tsubota K (2013): Prevalence of dry eye disease and its risk factors in visual display terminal users: the osaka study. Am J Ophthalmol 156: 759–766.e751. [DOI] [PubMed] [Google Scholar]
- Uchino Y, Uchino M, Yokoi N et al. (2014): Alteration of tear mucin 5AC in office workers using visual display terminals: the Osaka study. JAMA Ophthalmol 132: 985–992. [DOI] [PubMed] [Google Scholar]
- van der Vaart R, Weaver MA, Lefebvre C & Davis RM (2015): The association between dry eye disease and depression and anxiety in a large population‐based study. Am J Ophthalmol 159: 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz FT, Henriques SP, Silva DS, Roque J, Lopes AS & Mota M (2019): Digital asthenopia: Portuguese group of ergophthalmology survey. Acta Med Port 32: 260–265. [DOI] [PubMed] [Google Scholar]
- Viso E, Rodriguez‐Ares MT & Gude F (2009): Prevalence of and associated factors for dry eye in a spanish adult population (The Salnes Eye Study). Ophthalmic Epidemiol 16: 15–21. [DOI] [PubMed] [Google Scholar]
- Willcox MDP, Argüeso P, Georgiev GA et al. (2017): TFOS DEWS II tear film report. Ocul Surf 15: 366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang Y, Dong N, Yang F, Lin Z, Shang X & Li C (2014): Meibomian gland dysfunction determines the severity of the dry eye conditions in visual display terminal workers. PLoS One 9: e105575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Begley CG, Port N, Bradley A, Braun R & King‐Smith E (2015): The effects of increasing ocular surface stimulation on blinking and tear secretion. Invest Ophthalmol Vis Sci 56: 4211–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici A, Sari ES, Sahin G, Kilic A, Cakmak H, Ayar O & Ermis SS (2015): Change in tear film characteristics in visual display terminal users. Eur J Ophthalmol 25: 85–89. [DOI] [PubMed] [Google Scholar]
- Yee RW, Sperling HG, Kattek A, Paukert MT, Dawson K, Garcia M & Hilsenbeck S (2007): Isolation of the ocular surface to treat dysfunctional tear syndrome associated with computer use. Ocul Surf 5: 308–315. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Bron AJ & Georgiev GA (2014): The precorneal tear film as a fluid shell: the effect of blinking and saccades on tear film distribution and dynamics. Ocul Surf 12: 252–266. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Uchino M, Uchino Y et al. (2015): Importance of tear film instability in dry eye disease in office workers using visual display terminals: the Osaka study. Am J Ophthalmol 159: 748–754. [DOI] [PubMed] [Google Scholar]
- Yu J, Asche CV & Fairchild CJ (2011): The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 30: 379–387. [DOI] [PubMed] [Google Scholar]
- Zetterberg C, Forsman M & Richter HO (2017): Neck/shoulder discomfort due to visually demanding experimental near work is influenced by previous neck pain, task duration, astigmatism, internal eye discomfort and accommodation. PLoS One 12: e0182439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Background for the exacerbating factors presented in the review.
Supplementary Materials


