Abstract
Chronic rhinosinusitis with nasal polyps (CRSwNP) is generally associated with eosinophilic tissue infiltration linked to type 2 inflammation and characterized by elevated levels of interleukin (IL)‐5 and other type 2 inflammatory mediators. Although distinct and overlapping contributions of eosinophils and IL‐5 to CRSwNP pathology are still being explored, they are both known to play an important role in NP inflammation. Eosinophils secrete numerous type 2 inflammatory mediators including granule proteins, enzymes, cytokines, chemokines, growth factors, lipids, and oxidative products. IL‐5 is critical for the differentiation, migration, activation, and survival of eosinophils but is also implicated in the biological functions of mast cells, basophils, innate lymphoid cells, B cells, and epithelial cells. Results from clinical trials of therapeutics that target type 2 inflammatory mediators (including but not limited to anti‐IL‐5, anti‐immunoglobulin‐E, and anti‐IL‐4/13) may provide further evidence of how eosinophils and IL‐5 contribute to CRSwNP. Finally, the association between eosinophilia/elevated IL‐5 and greater rates of NP recurrence after endoscopic sinus surgery (ESS) suggests that these mediators may have utility as biomarkers of NP recurrence in diagnosing and assessing the severity of CRSwNP. This review provides an overview of eosinophil and IL‐5 biology and explores the literature regarding the role of these mediators in CRSwNP pathogenesis and NP recurrence following ESS. Based on current published evidence, we suggest that although eosinophils play a key role in CRSwNP pathophysiology, IL‐5, a cytokine that activates these cells, also represents a pertinent and effective treatment target in patients with CRSwNP.
Keywords: antibodies, biological products, biomarkers, cytokines, immunity, inflammation, innate, monoclonal, nasal obstruction
1. INTRODUCTION
The pathology of chronic rhinosinusitis (CRS) is heterogeneous, with two main phenotypes recognized: CRS with nasal polyps (NP) (CRSwNP) and CRS without NP (CRSsNP). 1 Approximately 25%−30% of patients with CRS have CRSwNP. 1 Although crossover between phenotypes is increasingly evident, 2 CRSwNP is generally associated with an eosinophilic or type 2 inflammatory pathophysiological endotype in up to approximately 85% of patients. 3 This endotype is characterized by elevated levels of interleukin (IL)‐4, IL‐5, IL‐9, IL‐13, IL‐25, IL‐33, and increased eosinophil counts, 4 , 5 although variation in cytokine profiles is seen between different geographical regions. 6 In contrast, CRSsNP is frequently associated with a non‐type 2 inflammatory pathophysiological endotype, characterized by increased neutrophil counts and elevated interferon‐γ and/or IL‐17. 4 Symptoms overlap across both phenotypes, although CRSwNP is often associated with more severe sinonasal symptoms than CRSsNP. 1 , 7 In addition, patients with CRSwNP experience more prevalent nasal obstruction and typically have comorbid bronchial asthma. 3 , 8
Current standard of care (SoC) for CRSwNP includes intranasal corticosteroids, saline nasal douching, and short courses of systemic corticosteroids. 9 For severe CRSwNP cases, endoscopic sinus surgery (ESS) to remove the NP tissue and diseased nasal mucosa is often required. 9 However, eosinophilia and elevated IL‐5 is associated with greater rates of NP recurrence after the tissue has been removed by ESS. 10 , 11 Given the association between CRSwNP and type 2 inflammation, the pursuit of novel treatment options for CRSwNP has focused on increasing understanding of the mechanistic role of mediators in this pathway.
This review provides an overview of eosinophil and IL‐5 biology and explores the available evidence relating to the mechanistic role of eosinophils and IL‐5, in addition to other type 2 inflammatory mediators in CRSwNP pathogenesis. We also evaluate emerging evidence supporting the potential use of eosinophils and IL‐5 as biomarkers of NP recurrence following ESS.
2. EOSINOPHILS AND IL‐5
Eosinophils exist in almost all vertebrates, representing up to 6% of the bone marrow‐resident nucleated cells 12 and are involved in a plethora of cellular processes. 13 It is well established that eosinophils secrete inflammatory mediators involved in tissue damage during inflammatory responses, 12 , 14 and increased eosinophil counts are observed in the tissue and/or peripheral blood of patients with eosinophilic disease. The Local Immunity And/Or Remodeling/Repair (LIAR) hypothesis, first proposed by Lee et al., also suggested that eosinophils play a beneficial role in homeostasis. 15 The known homeostatic roles of eosinophils include: contributions to glucose homeostasis; fat deposition; tissue remodeling and development; liver and muscle repair; epithelial, neuronal, and microbiome regulation; and immunoregulation. 16 , 17 The development and maturation of eosinophils in the bone marrow is regulated by several key cytokines including IL‐5, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), and IL‐3. 13 In particular, IL‐5 is critical for the differentiation, migration, activation, and survival of eosinophils. 18 , 19
The predominant sources of IL‐5 include CD34+ progenitor cells, group 2 innate lymphoid cells (ILC‐2), Th2 lymphocytes, invariant natural killer T cells, and mast cells, 20 , 21 , 22 although IL‐5 can also be released by eosinophils in an auto/paracrine manner. 21 , 22 Following the release of eosinophils from the bone marrow, eosinophil recruitment into tissues is promoted by chemokines such as eotaxin‐1 (CCL11), ‐2 (CCL24), and ‐3 (CCL26). 23 , 24 Eosinophil activation is essential for effective responses to stimuli and can be elicited via a range of mechanisms including those induced by IL‐5, IL‐3, and GM‐CSF, resulting in the release of mediator products. 25 , 26
IL‐5 signals through IL‐5 receptors (IL‐5R), which are composed of transmembrane α and β chains; however, IL‐5 can also bind to a soluble form of the IL‐5Rα chain, which antagonizes IL‐5R signaling. 27 , 28 As such, the sensitivity of eosinophils to IL‐5 is dependent on the relative expression of transmembrane and soluble IL‐5Rα, which in turn is dependent on eosinophil activation state, maturation, and eosinophil location. 27 Tissue eosinophils have a lower expression of transmembrane IL‐5Rα (Figure 1) and demonstrate lower responsiveness to IL‐5 than eosinophils in the peripheral circulation 27 ; this could explain the larger reductions in peripheral blood versus tissue eosinophils seen with anti‐IL‐5 therapy in patients with eosinophilic disease. 29 , 30 Notably, soluble IL‐5Rα expression in blood and tissue eosinophils is increased in patients with CRSwNP, with further increases in patients with comorbid asthma. 27 , 31 Consequently, although endogenous concentrations of soluble IL‐5Rα might be insufficient to block IL‐5 activity, the higher concentrations observed in eosinophilic disease may be sufficient. It should, however, also be noted that because IL‐5 is substantially elevated in CRSwNP compared with healthy controls, 32 higher levels of IL‐5Rα may not necessarily translate to greater regulation.
FIGURE 1.
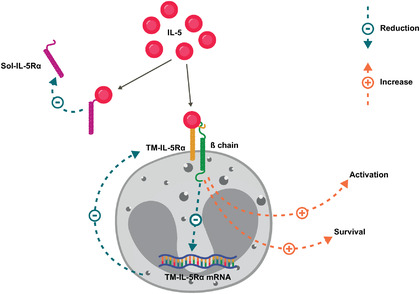
IL‐5 regulation of IL‐5Rα isoforms. 27 , 28
IL‐5 promotes eosinophil activation and survival and reduces the responsiveness of eosinophils to IL‐5 by regulating expression of the soluble and transmembrane IL‐5Rα isoforms.
mRNA, messenger ribonucleic acid; sol‐IL‐5Rα, soluble interleukin‐5 alpha receptor; TM‐IL‐5Rα; transmembrane interleukin‐5 alpha receptor
Beyond its role in eosinophil biology, IL‐5 can act on several other cell types with important biological functions. Functionally active IL‐5R has been identified on tissue‐resident human‐activated B cells (plasma cells) from patients with non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease (N‐ERD). 33 Moreover, IL‐5Rα chain expression is upregulated in activated human B cells. 34 These findings suggest a critical role for IL‐5 in the regulation of lymphocyte responses, which will be important for future studies to investigate. Single‐cell sequencing of nasal sinus tissue has demonstrated that IL‐5R are expressed on plasma cells, mast cells, and ciliated epithelial cells. 35 In addition, stimulation of cultured human airway epithelial cells with IL‐5 leads to downregulation of a range of cell adhesion molecules; this suggests a direct effect of IL‐5 on epithelial cells that may weaken epithelial barrier integrity through the modification of cell‐to‐cell and cell–matrix adhesions. 36 As IL‐5 stimulation also decreases epithelial epidermal growth factor receptor (EGFR) gene expression 36 and EGFR is upregulated during epithelial repair, 37 local IL‐5 release may cause NP epithelial cells to have a persistent epithelial damage/repair phenotype. Despite advances in our understanding of the involvement of eosinophil and IL‐5 biology in type 2 inflammatory pathways, their role in CRSwNP pathophysiology is only starting to be uncovered.
3. MECHANISTIC ROLE OF EOSINOPHILS, IL‐5, AND OTHER TYPE 2 INFLAMMATORY MEDIATORS IN CRSwNP
An accumulation of eosinophils infiltrating the nasal mucosa is characteristically observed in the majority of patients with CRSwNP. 38 , 39 , 40 This accumulation may be facilitated by prolonged survival, likely due to protection from cell death by locally produced cytokines, including IL‐5, in the nasal tissue. 41 Eosinophils are activated upon recruitment to NP tissue, and eosinophil activation status is important for disease pathology, as evidenced by the positive correlation between expression of the eosinophil activation marker CD69 and NP scores, in addition to the negative correlation between CD69 expression and lung function. 42 Following activation, eosinophils generate and secrete a plethora of factors including granule proteins, enzymes, cytokines, chemokines, growth factors, lipids, and oxidative products that contribute to type 2 inflammatory responses (Figure 2; Figure 3; Table 1). 12 , 14 , 43 In particular, IL‐5 is upregulated in NP tissue compared with control samples, 31 , 32 , 44 and higher levels of IL‐5 have been associated with more severe NP. 45 Interestingly, significant correlations have been reported between the number of epithelial eosinophils and the extent of epithelial damage or sense of smell impairment in patients with NP. 46 , 47 Moreover, the ability of IL‐5 to downregulate the expression of cell adhesion molecules 36 may increase epithelial susceptibility to eosinophil‐directed damage. Epithelial damage in response to eosinophil mediators triggers a repair response, leading to growth factor generation, fibroblast activation, and collagen deposition. 14 , 48 Consistent with this, there is a positive correlation between eosinophilic NP inflammation and the extent of fibrosis within polyps. 47
FIGURE 2.
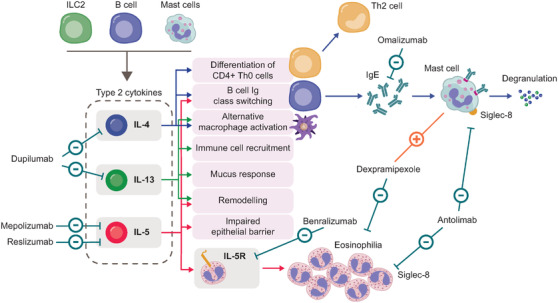
Type 2 inflammatory mediators and biologic therapies targeting type 2 inflammation in CRSwNP. 47 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59
CRSwNP, chronic rhinosinusitis with nasal polyps; Ig, immunoglobin; IL, interleukin; ILC2, group 2 innate lymphoid cell; Th, T helper
FIGURE 3.
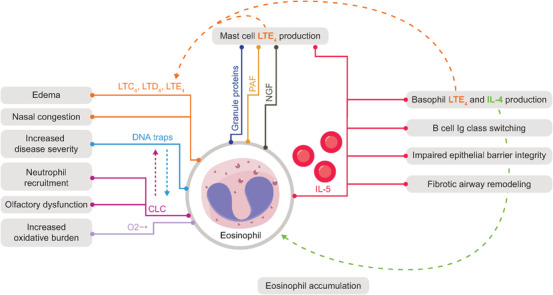
The role of IL‐5 and eosinophils in the pathophysiology of CRSwNP. 14 , 44 , 47 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70
CLC, Charcot–Leyden crystals; CRSwNP, chronic rhinosinusitis with nasal polyps; DNA, deoxyribonucleic acid; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; Ig, immunoglobulin; IL, interleukin; ILC2, group 2 innate lymphoid cell; LTC4, leukotriene C4; LTD4, leukotriene D4; LTE4, leukotriene E4; NGF, nerve growth factor; O2−•, superoxide radical anion; PAF, platelet‐activating factor
TABLE 1.
Evidence for the mechanistic role of eosinophil‐associated type 2 inflammatory mediators in CRSwNP
| Inflammatory mediator | Role in CRSwNP |
|---|---|
| IL‐3 | |
| IL‐5 |
|
| IL‐13 |
|
| GM‐CSF | |
| CCL11, CCL13, CCL24, CCL26 |
|
| ECP | |
| Eosinophil‐derived neurotoxin |
|
| LTs | |
| PGE2 | |
| PAF | |
| O2−• |
|
| NADPH oxidase 1 and 4 |
|
| DNA traps and CLC |
|
Abbreviations: CCL11, eotaxin‐1; CCL24, eotaxin‐2; CCL26, eotaxin‐3; CD69, cluster of differentiation 69 (an eosinophil activation marker); CLC, Charcot–Leyden crystals; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; ECP, eosinophil cationic protein; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IL, interleukin; LT, leukotrienes; NADPH, nicotinamide adenine dinucleotide phosphate; NP, nasal polyps; O2−•, a superoxide radical anion; PAF, platelet activating factor; PGE2, the anti‐inflammatory prostaglandin.
Eosinophils release lipid mediators (Table 1), which are found in higher levels in the NP of patients with CRSwNP compared with patients with CRSsNP. 75 Among these, cysteinyl leukotrienes (LT) promote edema and nasal congestion via the induction of vascular leakage and vasodilation, as well as airway remodeling and inflammatory cell recruitment. 67 Oxidative products released by eosinophils (Table 1) are significantly upregulated in eosinophilic NP tissue compared with healthy controls, suggesting increased oxidative burden in patients with CRSwNP. 65 Eosinophils also generate extracellular DNA traps and release Charcot–Leyden crystals (CLC), both of which are strongly associated with CRSwNP disease severity. 62 , 64 , 66 , 69 In patients with CRSwNP, a correlation between the number of neutrophils and the extent of eosinophil extracellular DNA traps and CLC has been observed. 62 Moreover, elevated levels of CLC gene expression in patients with CRSwNP inversely correlate with olfactory threshold. 44 This may suggest that CLC contribute to olfactory dysfunction and loss of smell, which is more common in patients with CRSwNP than patients with CRSsNP. 46 , 79
Beyond eosinophils, several other IL‐5R‐expressing immune cells including mast cells, basophils, ILC‐2, and IgG+ B cells are elevated in patients with CRSwNP, 68 , 80 , 81 suggesting a potential role of these cells and IL‐5 in disease pathology (Figure 3). In several eosinophilic disorders, eosinophils have the capacity to modulate mast cell functions via mediators including IL‐5, platelet activating factor, granule proteins, and nerve growth factor. 82 Conversely, mast cells can release de novo‐synthesized mediators including IL‐5 and GM‐CSF, which can modulate eosinophils and other cells expressing the IL‐5R. 82 In the context of allergic skin disorders, activated basophils modulate eosinophil tissue infiltration via the delivery of IL‐4 to the endothelium, inducing endothelial vascular cell adhesion molecule‐1, which is required for eosinophil accumulation. 61 Furthermore, both basophils and mast cells upregulate leukotriene E4 (LTE4) production in response to IL‐5. 60 , 63 In eosinophilic asthma, ILC2s are a source of IL‐5 and may drive the initiation of eosinophil processes, via IL‐5 signaling. 68 In patients with unexplained eosinophilia, eosinophil and B‐cell counts are correlated, suggesting that factors secreted by activated eosinophils may lead to increased B‐cell proliferation. 83 Additionally, in plasma cells from the NP tissue of patients with CRSwNP and N‐ERD, IL‐5 stimulation leads to the upregulation of transcripts involved in cell proliferation, suggesting that IL‐5 may increase immunoglobulin (Ig) generation in NP tissue. 33
4. IMPLICATIONS FOR TREATMENT
Several therapeutic agents that directly or indirectly target eosinophils, including intranasal and systemic corticosteroids, biologics, and dexpramipexole (mechanism unknown) have been studied in patients with CRSwNP (Figure 2). Corticosteroids have a broad mechanism of action, improving the clinical symptoms of CRSwNP and reducing tissue eosinophil counts, eosinophil cationic protein (ECP) and IL‐5 levels. 84 However, long‐term use of systemic corticosteroids is associated with an increased risk of health complications. 85 Biologic therapies targeting type 2 inflammation may therefore represent an important treatment approach for patients with CRSwNP who have had surgery and fail to respond to short courses of systemic corticosteroids. 86
Several biologics targeting IL‐5 or the IL‐5‐R are associated with clinical benefits in patients with CRSwNP. For example, the anti‐IL‐5 monoclonal antibodies (mAbs) mepolizumab and reslizumab reduce eosinophil counts, nasal and peripheral IL‐5, soluble IL‐5Rα, and ECP levels in patients with CRSwNP. 50 , 51 Mepolizumab also reduces several other local and systemic markers of type 2 inflammation, such as IgE and periostin, in addition to nasal matrix metallopeptidase 9 and myeloperoxidase. 50 Moreover, mepolizumab decreases numbers of circulating basophils and nasal levels of the proinflammatory mediators prostaglandin D2 (PGD2), prostaglandin F2 alpha (PGF2α), leukotriene B4 (LTB4), and thromboxane in patients with CRSwNP and N‐ERD. 35 Although there are currently no data available on the impact of mepolizumab on B cells in CRSwNP, results from the MATERIAL trial in patients with mild asthma and rhinovirus infection suggested that mepolizumab can reduce blood and alveolar lavage fluid B‐cell counts. 87 An initial investigation of the anti‐IL‐5 receptor mAb benralizumab suggested that treatment improves clinical symptoms and decreases eosinophil counts in patients with CRSwNP. 58 The subsequent phase III, randomized OSTRO study demonstrated that compared with placebo, benralizumab in addition to SoC reduced nasal polyp score, nasal blockage, and difficulties with sense of smell in patients with CRSwNP. 38 However, statistically significant improvements in sinonasal outcome test (SNOT)‐22 score at week 40, time to first sinonasal surgery, and systemic corticosteroid use for NP were not observed between treatment groups. These results therefore warrant further investigation into the effects of benralizumab on type 2 inflammation in CRSwNP.
Other biologics targeting type 2 inflammation include the anti‐IgE mAb, omalizumab, and the anti‐IL‐4Rα (the common signaling receptor subunit of IL‐4 and IL‐13) mAb, dupilumab. 52 , 55 Studies have shown that omalizumab improves clinical symptoms of CRSwNP and reduces serum periostin, ECP, and soluble IL‐5Rα levels, but with limited effect on local IL‐5 and blood eosinophil counts. 50 , 52 Dupilumab improves clinical symptoms along with decreasing NP levels of CCL24, CCL26, ECP, IL‐5, IgE, and pulmonary and activation‐regulated chemokine. 55 , 88 The SINUS‐24/52 studies showed improvements in symptoms with dupilumab, despite a transient increase in blood eosinophil counts, which returned to pretreatment baseline by 52 weeks post treatment initiation. 88 This may indicate dupilumab is effective in inhibiting the recruitment of eosinophils from the blood into tissue, which is the proposed mechanism of dupilumab treatment benefits in severe asthma. 89 However, an analysis of patients in SINUS‐52 revealed no significant association between higher blood eosinophil counts and treatment response to dupilumab, suggesting that eosinophils may not be a key mediator of dupilumab treatment benefits in CRSwNP. 90
Studies of biologics that target type 2 inflammation may also provide more specific evidence of the roles of eosinophils and type 2 inflammatory mediators in CRSwNP pathophysiology. For example, the consistently demonstrated efficacy of IL‐5‐targeting biologics in reducing NP size and relieving symptoms in patients with CRSwNP 53 , 54 , 58 , 79 suggests that IL‐5 may be a particularly important treatment target for CRSwNP. Accordingly, patients with CRSwNP or eosinophilic CRS who have higher levels of IL‐5 demonstrate greater responses to anti‐IL‐5 therapy. 51 , 91 Further insight into the role of eosinophils in CRSwNP may be gained from two recently investigated molecules: antolimab and dexpramipexole. Phase I results suggested that antolimab, which targets siglec‐8 (a cell surface receptor expressed by human eosinophils and mast cells) can decrease blood eosinophil counts in a dose‐dependent manner, 57 whilst Phase II results suggested that it reduces NP score and has a similar safety profile to placebo. 92 However, CRSwNP has not been further pursued as an indication of antolimab. Dexpramipexole is a synthetic aminobenzothiazole developed as an oral treatment for amyotrophic lateral sclerosis (ALS), which, despite promising early results, was discontinued for this indication following failure to meet the primary endpoint in a large phase III trial. 93 However, it was observed that dexpramipexole reduced blood eosinophil counts in patients with ALS by an unknown mechanism of action 94 ; therefore, it was further investigated in patients with eosinophil‐associated disease. 56 , 94 In a proof‐of‐concept study of 16 patients with CRSwNP, dexpramipexole reduced blood and NP tissue eosinophils by up to 94% and 97%, respectively, while significantly increasing mast cell numbers. 56 Despite these observations, dexpramipexole was not associated with decreased NP size in a subgroup analysis of 10 patients. 56 As markers of eosinophil activation status such as ECP were not reported, it remains uncertain if this lack of clinical benefit was due to a limited impact on eosinophil activation status. 95 Moreover, given the open‐label design and small sample size of the proof‐of‐concept study, further, more rigorous investigation into the effects of dexpramipexole in CRSwNP is warranted. 56
The results for dexpramipexole in patients with CRSwNP suggest that decreasing eosinophil counts alone is not sufficient for treatment benefits. Importantly, they also raise the question of whether eosinophils themselves represent the main target for effective NP treatments or whether in type 2 disease, the action of IL‐5 on other cell populations, including B cells 96 and epithelial cells, 35 is key to the influence of anti‐IL‐5 biologic therapy. For example, an observed effect of mepolizumab treatment is the upregulation of nasal epithelial tight‐junction genes, consistent with this treatment improving epithelial barrier integrity. 35 Another potential factor might be the stage of the disease; it is plausible that although eosinophils drive initial NP formation, polyps become more resistant to therapeutic intervention once fibrosis has occurred.
5. EOSINOPHIL COUNTS AS A BIOMARKER OF NP RECURRENCE RISK
The importance of eosinophils in CRSwNP pathophysiology is supported by data indicating that eosinophils play a prominent role in NP recurrence after surgery. 97 , 98 , 99 , 100 , 101 , 102 In a study conducted by Brescia et al., elevated levels of serum eosinophils and basophils were correlated with increased risk of NP recurrence, with patients with serum eosinophil counts ≥ 3.8% more than twice as likely to experience NP recurrence than those with counts < 3.8%. 97 Other studies have also demonstrated a significant association between the presence of tissue or blood eosinophils and NP recurrence. 98 , 99 , 100 , 101 , 102 Two studies have demonstrated an association between tissue IL‐5 levels and NP recurrence. 11 , 98 Together, these findings suggest eosinophils and IL‐5 levels may have utility as biomarkers of NP recurrence in patients with CRSwNP.
NP tissue can be assessed during removal to directly quantify local eosinophil counts; however, peripheral blood eosinophil counts may also be a useful biomarker for NP recurrence. In patients with severe asthma, blood eosinophil counts are more predictive of anti‐IL‐5 treatment responses than tissue eosinophil counts; in chronic obstructive pulmonary disease, the two measures are correlated. 103 , 104 , 105 However, the relationship between peripheral blood and tissue eosinophils in patients with CRSwNP remains unclear, with most studies reporting a weak to moderate correlation between the two measures and one study finding no correlation. 39 , 40 , 106 , 107 , 108 , 109 The European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA) and Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC) have suggested that peripheral blood eosinophil count be incorporated into algorithms for the diagnosis and assessment of CRSwNP severity. 3 , 110 Although more challenging to measure, tissue eosinophil counts could be used as an alternative biomarker, as peripheral blood eosinophil counts can be impacted by factors such as systemic corticosteroid use. 111 Other alternatives for measuring tissue eosinophil counts include measuring ECP in nasal secretions 11 , 50 and analyzing mucus secretions for eosinophils. 101 , 112 However, at present, these techniques are mainly used for research purposes rather than in routine clinical practice.
Further insight into the use of blood eosinophil counts as a biomarker, including the determination of a relevant cutoff to assess the risk of NP recurrence in patients with CRSwNP, may come from clinical trials such as the Phase III SYNAPSE study. 54 In SYNAPSE, patients with CRSwNP in need of revision ESS demonstrated significant improvements from baseline in total endoscopic NP score and nasal obstruction visual analog score with mepolizumab versus placebo (both in addition to SoC). Whether there is an association between treatment benefit and specific blood eosinophil counts remains to be determined. Blood eosinophil counts ≥300 cells/μl and/or the presence of comorbid asthma have been proposed as part of an algorithm to determine whether patients have type 2 inflammation, which may be useful to guide treatment decisions. 3
6. CONCLUSIONS
Despite advances in our understanding of eosinophil biology and the involvement of eosinophils in type 2 inflammatory pathways, their exact role in CRSwNP pathophysiology requires further investigation. Currently, evidence suggests that eosinophil activation status or type 2 inflammatory cytokines, such as IL‐5, play a key role in CRSwNP pathophysiology. Moreover, IL‐5 is likely to act on immune cells other than eosinophils that are involved in type 2 inflammatory responses. Therefore, we suggest that in addition to eosinophils, IL‐5 also represents a pertinent and effective treatment target in patients with CRSwNP. Evidence suggests blood eosinophil counts in addition to comorbid asthma may be a useful marker for assessing the risk of NP recurrence following ESS. Eosinophilia in CRSwNP may be best measured by less invasive techniques including blood eosinophil counts and nasal secretions. However, further research is needed to confirm these findings and to drive the development of effective treatment and nasal and systemic monitoring options in patients with CRSwNP.
CONFLICT OF INTEREST
Philippe Gevaert has participated in advisory boards and received speaker fees from ALK‐Abelló, Argenx, AstraZeneca, Genentech, GSK, Novartis, Regeneron, Roche, Sanofi Genzyme, and Stallergenes‐Greer; Joseph K. Han has received consultancy fees from Sanofi Genzyme, Regeneron, Genentech, AstraZeneca, GSK, and Gossamer Bio; Steven G. Smith, Ana R. Sousa, Peter H. Howarth, Steven W. Yancey, and Robert Chan are employees of GSK and own stocks/shares; Claus Bachert has participated in advisory boards and received speaker fees from Sanofi, Novartis, AstraZeneca, GlaxoSmithKline, ALK‐Abelló, and Meda Pharmaceuticals.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of this review article, in addition to writing, editing, and providing final approval of the submitted version of the article.
ACKNOWLEDGMENTS
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Bianca Paris, PhD, at Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GlaxoSmithKline (GSK).
Gevaert P, Han JK, Smith SG, et al. The roles of eosinophils and interleukin‐5 in the pathophysiology of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2022;12:1413–1423. 10.1002/alr.22994
REFERENCES
- 1. Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016; 4(4): 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delemarre T, Holtappels G, De Ruyck N, et al. Type 2 inflammation in chronic rhinosinusitis without nasal polyps: another relevant endotype. J Allergy Clin Immunol. 2020; 146(2): 337–343. e336. [DOI] [PubMed] [Google Scholar]
- 3. Bachert C, Han JK, Wagenmann M, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: definitions and management. J Allergy Clin Immunol. 2021; 147(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 4. Bachert C, Akdis CA. Phenotypes and emerging endotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2016; 4(4): 621–628. [DOI] [PubMed] [Google Scholar]
- 5. Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype‐driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018; 141(5): 1543–1551. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Zhang N, Bo M, et al. Diversity of T(H) cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; 138(5): 1344–1353. [DOI] [PubMed] [Google Scholar]
- 7. Deal RT, Kountakis SE. Significance of nasal polyps in chronic rhinosinusitis: symptoms and surgical outcomes. Laryngoscope. 2004; 114(11): 1932–1935. [DOI] [PubMed] [Google Scholar]
- 8. Alobid I, Cardelus S, Benítez P, et al. Persistent asthma has an accumulative impact on the loss of smell in patients with nasal polyposis. Rhinology. 2011; 49(5): 519–524. [DOI] [PubMed] [Google Scholar]
- 9. Hellings PW, Scadding G, Bachert C, et al. EUFOREA treatment algorithm for allergic rhinitis. Rhinology. 2020; 58(6): 618–622. [DOI] [PubMed] [Google Scholar]
- 10. Philpott C, Hopkins C, Erskine S, et al. The burden of revision sinonasal surgery in the UK—data from the Chronic Rhinosinusitis Epidemiology Study (CRES): a cross‐sectional study. BMJ open. 2015; 5(4): e006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calus L, Van Bruaene N, Bosteels C, et al. Twelve‐year follow‐up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin and Trans Allergy. 2019; 9(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramirez GA, Yacoub M‐R, Ripa M, et al. Eosinophils from physiology to disease: a comprehensive review. Biomed Res Int. 2018; 2018:9095275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nature Rev Immunol. 2013; 13(1): 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McBrien CN, Menzies‐Gow A. The biology of eosinophils and their role in asthma. Front Med. 2017; 4: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JJ, Jacobsen EA, McGarry MP, et al. Eosinophils in health and disease: the LIAR hypothesis. Clin Experiment Allergy. 2010; 40(4): 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophils to human health and disease. Annu Rev Pathol. 2020; 15: 179–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weller PF, Spencer LA. Functions of tissue‐resident eosinophils. Nat Rev Immunol. 2017; 17(12): 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kung T, Stelts D, Zurcher J, et al. Involvement of IL‐5 in a murine model of allergic pulmonary inflammation: prophylactic and therapeutic effect of an anti‐IL‐5 antibody. Am J Resp Cell Molecular Bio. 1995; 13(3): 360–365. [DOI] [PubMed] [Google Scholar]
- 19. Pelaia C, Paoletti G, Puggioni F, et al. Interleukin‐5 in the pathophysiology of severe asthma. Frontiers Physiol. 2019; 10: 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Symowski C, Voehringer D. Interactions between innate lymphoid cells and cells of the innate and adaptive immune system. Frontiers Immunol. 2017; 8: 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nussbaum JC, Van Dyken SJ, Von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013; 502(7470): 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bagnasco D, Ferrando M, Varricchi G, et al. Anti‐interleukin 5 (IL‐5) and IL‐5Ra biological drugs: efficacy, safety, and future perspectives in severe eosinophilic asthma. Front Med. 2017; 4: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menzies‐Gow A, Ying S, Sabroe I, et al. Eotaxin (CCL11) and eotaxin‐2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early‐and late‐phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002; 169(5): 2712–2718. [DOI] [PubMed] [Google Scholar]
- 24. Yamada T, Miyabe Y, Ueki S, et al. Eotaxin‐3 as a plasma biomarker for mucosal eosinophil infiltration in chronic rhinosinusitis. Frontiers Immunol. 2019; 10: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fulkerson PC, Rothenberg ME. Eosinophil development, disease involvement, and therapeutic suppression. Adv Immunol. 2018; 138: 1–34. [DOI] [PubMed] [Google Scholar]
- 26. van der Bruggen T, Kok PT, Raaijmakers JA, et al. Cytokine priming of the respiratory burst in human eosinophils is Ca2+ independent and accompanied by induction of tyrosine kinase activity. J Leukocyte Biol. 1993; 53(4): 347–353. [DOI] [PubMed] [Google Scholar]
- 27. Gevaert P, Hellman C, Lundblad L, et al. Differential expression of the interleukin 5 receptor alpha isoforms in blood and tissue eosinophils of nasal polyp patients. Allergy. 2009; 64(5): 725–732. [DOI] [PubMed] [Google Scholar]
- 28. Gregory B, Kirchem A, Phipps S, et al. Differential regulation of human eosinophil IL‐3, IL‐5, and GM‐CSF receptor alpha‐chain expression by cytokines: iL‐3, IL‐5, and GM‐CSF down‐regulate IL‐5 receptor alpha expression with loss of IL‐5 responsiveness, but up‐regulate IL‐3 receptor alpha expression. J Immunol. 2003; 170(11): 5359–5366. [DOI] [PubMed] [Google Scholar]
- 29. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012; 380(9842): 651–659. [DOI] [PubMed] [Google Scholar]
- 30. Roufosse F. Targeting the interleukin‐5 pathway for treatment of eosinophilic conditions other than asthma. Front Med. 2018; 5: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gevaert P, Bachert C, Holtappels G, et al. Enhanced soluble interleukin‐5 receptor alpha expression in nasal polyposis. Allergy. 2003; 58(5): 371–379. [DOI] [PubMed] [Google Scholar]
- 32. Bachert C, Wagenmann M, Hauser U, Rudack C. IL‐5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997; 99(6): 837–842. [DOI] [PubMed] [Google Scholar]
- 33. Buchheit KM, Dwyer DF, Ordovas‐Montanes J, et al. IL‐5Rα marks nasal polyp IgG4‐ and IgE‐expressing cells in aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol. 2020; 145(6): 1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emslie D, D'Costa K, Hasbold J, et al. Oct2 enhances antibody‐secreting cell differentiation through regulation of IL‐5 receptor alpha chain expression on activated B cells. J Exp med. 2008;205(2):409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buchheit KM, Lewis E, Gakpo D, et al. Mepolizumab targets multiple immune cells in aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol. 2021;148(2):574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barretto KT, Brockman‐Schneider RA, Kuipers I, et al. Human airway epithelial cells express a functional IL‐5 receptor. Allergy. 2020; 75(8): 2127–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004; 59(11): 992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: a randomized, placebo‐controlled trial. J Allergy Clin Immunol. 2021;S0091‐6749(21)01459‐7.S0091‐6749(21):01459–7. [DOI] [PubMed] [Google Scholar]
- 39. Gitomer SA, Fountain CR, Kingdom TT, et al. Clinical examination of tissue eosinophilia in patients with chronic rhinosinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2016; 155(1): 173–178. [DOI] [PubMed] [Google Scholar]
- 40. Wang K, Deng J, Yang M, et al. Concordant systemic and local eosinophilia relates to poorer disease control in patients with nasal polyps. World Allergy Organ J. 2019; 12(8): 100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simon H‐U, Yousefi S, Schranz C, et al. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997; 158(8): 3902–3908. [PubMed] [Google Scholar]
- 42. Yun Y, Kanda A, Kobayashi Y, et al. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol Int. 2020; 69(2): 232–238. [DOI] [PubMed] [Google Scholar]
- 43. Bochner BS, Stevens WW. Biology and function of eosinophils in chronic rhinosinusitis with or without nasal polyps. Allergy Asthma Immunol Res. 2021; 13(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lavin J, Min JY, Lidder AK, et al. Superior turbinate eosinophilia correlates with olfactory deficit in chronic rhinosinusitis patients. Laryngoscope. 2017; 127(10): 2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; 137(5): 1449–1456. [DOI] [PubMed] [Google Scholar]
- 46. Hauser LJ, Chandra RK, Li P, Turner JH. Role of tissue eosinophils in chronic rhinosinusitis–associated olfactory loss. Int For Allergy Rhinol. 2017; 7(10): 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saitoh T, Kusunoli T, Yao T, et al. Relationship between epithelial damage or basement membrane thickness and eosinophilic infiltration in nasal polyps with chronic rhinosinusitis. Rhinology. 2009; 47(3): 275–279. [DOI] [PubMed] [Google Scholar]
- 48. Leoni G, Neumann P, Sumagin R, et al. Wound repair: role of immune–epithelial interactions. Mucosal immunology. 2015; 8(5): 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bachert C, Lund VJ, Scadding GK, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017; 140(4): 1024–1031. e1014. [DOI] [PubMed] [Google Scholar]
- 50. De Schryver E, Derycke L, Calus L, et al. The effect of systemic treatments on periostin expression reflects their interference with the eosinophilic inflammation in chronic rhinosinusitis with nasal polyps. Rhinology. 2017; 55(2): 152–160. [DOI] [PubMed] [Google Scholar]
- 51. Gevaert P, Lang‐Loidolt D, Lackner A, et al. Nasal IL‐5 levels determine the response to anti–IL‐5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006; 118(5): 1133–1141. [DOI] [PubMed] [Google Scholar]
- 52. Gevaert P, Corren J, Mullol J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020; 146(3): 595–605. [DOI] [PubMed] [Google Scholar]
- 53. Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti‐IL‐5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011; 128(5): 989–995. e981‐988. [DOI] [PubMed] [Google Scholar]
- 54. Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Res Med. 2021. 8(10):1141–1153. 10.1016/s2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 55. Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro‐inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019; 74(4): 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Laidlaw TM, Panettieri RA, Lee S, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019; 129(2): e61–66. [DOI] [PubMed] [Google Scholar]
- 57. Rasmussen HS, Tomasevic N, Bebbington C. A randomized, double‐blind, placebo‐controlled, ascending dose phase 1 study of AK002, a novel Siglec‐8 selective monoclonal antibody, in healthy subjects. J Allergy Clin Immunol. 2018; 141(2): AB403. [Google Scholar]
- 58. Tversky J, Lane AP, Azar A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): a randomized double‐blind placebo‐controlled trial. Clin Exp Allergy. 2021;51(6):836‐844. [DOI] [PubMed] [Google Scholar]
- 59. Hulse KE, Stevens WW, Tan BK. et al. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015; 45(2):328–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bischoff SC, Brunner T, De Weck AL, Dahinden CA. Interleukin 5 modifies histamine release and leukotriene generation by human basophils in response to diverse agonists. J Exp Med. 1990; 172(6): 1577–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheng LE, Sullivan BM, Retana LE, et al. IgE‐activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Experiment Med. 2015; 212(4): 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Delemarre T, Holtappels G, De Ruyck N, et al. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2021; 147(1): 179–188. [DOI] [PubMed] [Google Scholar]
- 63. Hsieh FH, Lam BK, Penrose JF, et al. T helper cell type 2 cytokines coordinately regulate immunoglobulin E‐dependent cysteinyl leukotriene production by human cord blood‐derived mast cells: profound induction of leukotriene C(4) synthase expression by interleukin 4. J Exp Med. 2001; 193(1): 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hwang CS, Park SC, Cho HJ, et al. Eosinophil extracellular trap formation is closely associated with disease severity in chronic rhinosinusitis regardless of nasal polyp status. Sci Rep. 2019; 9(1): 8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moon JH, Kim TH, Lee HM, et al. Overexpression of the superoxide anion and NADPH oxidase isoforms 1 and 4 (NOX1 and NOX4) in allergic nasal mucosa. Am J Rhinol Allergy. 2009; 23(4): 370–376. [DOI] [PubMed] [Google Scholar]
- 66. Persson EK, Verstraete K, Heyndrickx I, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science. 2019; 364(6442):eaaw4295. [DOI] [PubMed] [Google Scholar]
- 67. Pezato R, Świerczyńska‐Krępa M, Niżankowska‐Mogilnicka E, et al. Role of imbalance of eicosanoid pathways and staphylococcal superantigens in chronic rhinosinusitis. Allergy. 2012; 67(11): 1347–1356. [DOI] [PubMed] [Google Scholar]
- 68. Salter BM, Aw M, Sehmi R. The role of type 2 innate lymphoid cells in eosinophilic asthma. J Leukocyte Biol. 2019; 106(4): 889–901. [DOI] [PubMed] [Google Scholar]
- 69. Ueki S, Tokunaga T, Melo RCN, et al. Charcot‐Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018; 132(20): 2183–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dougan M, Dranoff G, Dougan SK. GM‐CSF, IL‐3, and IL‐5 family of cytokines: regulators of inflammation. Immunity. 2019; 50(4): 796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Soman KV, Stafford SJ, Pazdrak K, et al. Activation of human peripheral blood eosinophils by cytokines in a comparative time‐course proteomic/phosphoproteomic study. J Proteome Res. 2017; 16(8): 2663–2679. [DOI] [PubMed] [Google Scholar]
- 72. Allen JS, Eisma R, Leonard G. et al. Interleukin‐3 interleukin‐5, and granulocyte‐macrophage colony‐stimulating factor expression in nasal polyps. Am J Otolaryngol. 1997; 18(4): 239–246. [DOI] [PubMed] [Google Scholar]
- 73. Rudack C, Bachert C, Stoll W. Effect of prednisolone on cytokine synthesis in nasal polyps. J Interferon Cytokine Res. 1999; 19(9): 1031–1035. [DOI] [PubMed] [Google Scholar]
- 74. Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin‐exacerbated respiratory disease. Am J Respir Crit Care Med. 2015; 192(6): 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pérez‐Novo CA, Watelet JB, Claeys C, et al. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005; 115(6):1189–1196. [DOI] [PubMed] [Google Scholar]
- 76. Pérez‐Novo CA, Claeys C, Van Cauwenberge P, Bachert C. Expression of eicosanoid receptors subtypes and eosinophilic inflammation: implication on chronic rhinosinusitis. Respir Res. 2006; 7(1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Roca‐Ferrer J, Garcia‐Garcia FJ, Pereda J, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin‐intolerant asthma. J Allergy Clin Immunol. 2011; 128(1): 66–72.e61. [DOI] [PubMed] [Google Scholar]
- 78. Furukawa M, Ogura M, Tsutsumi T, et al. Presence of platelet‐activating factor in nasal polyps and eosinophils. Acta Otolaryngol. 2002; 122(8): 872–876. [PubMed] [Google Scholar]
- 79. Stevens WW, Peters AT, Tan BK, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2019; 7(8): 2812–2820. e2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ho J, Bailey M, Zaunders J, et al. Cellular comparison of sinus mucosa vs polyp tissue from a single sinus cavity in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015; 5(1): 14–27. [DOI] [PubMed] [Google Scholar]
- 81. Takabayashi T, Kato A, Peters AT, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012; 130(2): 410–420. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Galdiero MR, Varricchi G, Seaf M, et al. Bidirectional mast cell–eosinophil interactions in inflammatory disorders and cancer. Front Med. 2017; 4: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kuang FL, Makiya M, Rampertaap S, et al. Exploring a relationship between B cells and eosinophils. J Allergy Clin Immunol. 2019; 143(2): AB289. [Google Scholar]
- 84. Zhang Y, Lou H, Wang Y, et al. Comparison of corticosteroids by 3 approaches to the treatment of chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res. 2019; 11(4): 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long‐term oral corticosteroid therapy and its side‐effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Resp J. 2018; 52(4) :1800703. [DOI] [PubMed] [Google Scholar]
- 86. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; 58(S29): 1–464. [DOI] [PubMed] [Google Scholar]
- 87. Sabogal Piñeros YS, Bal SM, van de Pol MA, et al. Anti‐IL‐5 in mild asthma alters rhinovirus‐induced macrophage, b‐cell, and neutrophil responses (MATERIAL). A placebo‐controlled, double‐blind study. Am J Respir Crit Care Med. 2019; 199(4): 508–517. [DOI] [PubMed] [Google Scholar]
- 88. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019; 394(10209): 1638–1650. [DOI] [PubMed] [Google Scholar]
- 89. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018; 378(26): 2486–2496. [DOI] [PubMed] [Google Scholar]
- 90. Fujieda S, Matsune S, Takeno S, et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS‐52 is unaffected by eosinophilic status. Allergy. 2021;77(1):186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Numata T, Nakayama K, Utsumi H, et al. Efficacy of mepolizumab for patients with severe asthma and eosinophilic chronic rhinosinusitis. BMC Pulm Med. 2019; 19(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Clinicaltrials.gov Study to evaluate multiple doses in patients with nasal polyposis. Accessed 15 March 2021. Available at: https://clinicaltrials.gov/ct2/show/results/NCT02734849
- 93. Cudkowicz ME, van den Berg LH, Shefner JM, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double‐blind, phase 3 trial. Lancet Neurol. 2013;12(11):1059–1067 [DOI] [PubMed] [Google Scholar]
- 94. Dworetzky SI, Hebrank GT, Archibald DG, et al. The targeted eosinophil‐lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis. 2017; 63: 62–65. [DOI] [PubMed] [Google Scholar]
- 95. Lou H, Zhang N, Bachert C, Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol. 2018; 8(11): 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kariyawasam HH, James LK. Do B cells rather than eosinophils drive chronic rhinosinusitis with nasal polyps? Lancet Respir med. 2021. ;9(10):e97. [DOI] [PubMed] [Google Scholar]
- 97. Brescia G, Barion U, Zanotti C, et al. The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int For Allergy Rhinol. 2017; 7(3): 261–267. [DOI] [PubMed] [Google Scholar]
- 98. Rosati D, Rosato C, Pagliuca G, et al. Predictive markers of long‐term recurrence in chronic rhinosinusitis with nasal polyps. Am J Otolaryngol. 2020; 41(1): 102286. [DOI] [PubMed] [Google Scholar]
- 99. Nakayama T, Yoshikawa M, Asaka D, et al. Mucosal eosinophilia and recurrence of nasal polyps ‐ new classification of chronic rhinosinusitis. Rhinology. 2011; 49(4): 392–396. [DOI] [PubMed] [Google Scholar]
- 100. Lou H, Meng Y, Piao Y, et al. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015; 29(5): 350–356. [DOI] [PubMed] [Google Scholar]
- 101. Vlaminck S, Vauterin T, Hellings PW, et al. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a 3‐year prospective observational study. Am J Rhinol Allergy. 2014; 28(3): 260–264. [DOI] [PubMed] [Google Scholar]
- 102. Tosun F, Arslan HH, Karslioglu Y et al. Relationship between postoperative recurrence rate and eosinophil density of nasal polyps. Ann Ontol Rhinol Laryngol. 2010;119(7):455–459. [DOI] [PubMed] [Google Scholar]
- 103. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Res med. 2016; 4(7): 549–556. [DOI] [PubMed] [Google Scholar]
- 104. Kolsum U, Damera G, Pham TH, et al. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol. 2017; 140(4): 1181–1184. [DOI] [PubMed] [Google Scholar]
- 105. Katz LE, Gleich GJ, Hartley BF, et al. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014; 11(4): 531–536. [DOI] [PubMed] [Google Scholar]
- 106. Han DH, Kim SW, Cho SH, et al. Predictors of bronchial hyperresponsiveness in chronic rhinosinusitis with nasal polyp. Allergy. 2009; 64(1): 118–122. [DOI] [PubMed] [Google Scholar]
- 107. Sreeparvathi A, Kalyanikuttyamma LK, Kumar M, et al. Significance of blood eosinophil count in patients with chronic rhinosinusitis with nasal polyposis. J Clin Diagn Res. 2017; 11(2): Mc08–mc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tanaka S, Hirota T, Kamijo A, et al. Lung functions of Japanese patients with chronic rhinosinusitis who underwent endoscopic sinus surgery. Allergol Int. 2014; 63(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 109. Zhao QL, Yu L, Zhi LL, et al. The relationship between lung function and the clinical and histopathological features in Chinese patients with nasal polyps. J Laryngol Otol. 2017; 131(10): 880–888. [DOI] [PubMed] [Google Scholar]
- 110. Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015; 70(8): 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ortega H, Llanos J‐P, Lafeuille M‐H, et al. Effects of systemic corticosteroids on blood eosinophil counts in asthma: real‐world data. J Asthma. 2019; 56(8): 808–815. [DOI] [PubMed] [Google Scholar]
- 112. Vlaminck S, Acke F, Prokopakis E, et al. Surgery in nasal polyp patients: outcome after a minimum observation of 10 years. Am J Rhinol Allergy. 2021; 35(4): 449–457. [DOI] [PubMed] [Google Scholar]


