Summary
Objective
The role of the anti‐Müllerian hormone (AMH) as an indicator of physical and reproductive health in men is unclear. We assessed the relationships between AMH and follicle‐stimulating hormone (FSH), luteinizing hormone (LH), testosterone, and metabolic parameters, in a cohort of expectant fathers.
Design
ORIGINS Project prospective cohort study.
Setting
Community‐dwelling men.
Participants
Partners of pregnant women attending antenatal appointments.
Main Outcome Measures
Serum AMH, FSH, LH, testosterone, and metabolic parameters.
Results
In 485 expectant fathers, median age 33 years, median AMH was 40 pmol/L (quartiles 29, 56). AMH was inversely correlated with FSH, age, and body mass index (BMI) (correlation coefficients: −.32, −.24, and −.17 respectively). The age association was nonlinear, with peak AMH between 20 and 30 years, a decline thereafter, and somewhat steady levels after 45 years. The inverse association of AMH with FSH was log‐linear and independent of age and BMI (β: −.07, SE: 0.01, p < .001). AMH was inversely correlated with waist circumference and directly associated with sex hormone‐binding globulin. Testosterone was moderately correlated with AMH (correlation coefficient: .09, β: .011, SE: 0.004, p = .014): this association was mediated by an inverse relationship with BMI (mediated proportion 0.49, p < .001).
Conclusions
In reproductively active men, lower AMH is a biomarker for advancing age, and for poorer metabolic and reproductive health. The inverse association between AMH and FSH is independent of age and BMI, whereas the association of AMH and testosterone is mediated via BMI. The utility of AMH to predict reproductive and cardiometabolic outcomes in men warrants further investigation.
Keywords: AMH, BMI, FSH, men, ORIGINS Project, testosterone, waist circumference
1. INTRODUCTION
The global increase in obesity across all age groups is expected to lead to an increase in cardiovascular disease, stroke, and diabetes and a probable decline in fertility. 1 In the 1990s, a review of 61 publications including over 14,000 men found that semen quality had declined over the prior 50 years. 2 The contribution of obesity to the decline in semen parameters continues to be explored. 3
Anti‐Müllerian hormone (AMH) is a glycoprotein of the transforming growth factor‐β family produced by Sertoli cells. 4 AMH is helpful in paediatrics for sex differentiation and in adult females for estimating female oocyte reserve. 5 In adult males, levels reflect Sertoli cell number and function. 5 , 6 AMH receptors have been found in Leydig and Sertoli cells, 5 , 6 testicular peritubular cells, 7 and hypothalamus and glial cells. 5 In mice, AMH receptors have also been found in vascular smooth muscle cells and myocardium, suggesting a possible contribution to vascular disease. 8 , 9
AMH in men is associated with specific cardiometabolic risk factors 10 with higher AMH associated with lower all‐cause mortality 11 and with lower C‐reactive protein. 12 Low AMH has been associated with increased body mass index (BMI) and obesity, 13 , 14 , 15 and there are complex relationships between AMH and the hypothalamic–pituitary–gonadal (HPG) axis with AMH also increasing gonadotrophin‐releasing hormone (GnRH) neuron activity. 5 Male reproductive capacity reflects gonadal and metabolic health, 16 and the role of AMH within these interrelationships remains unclear. Relationships between AMH, fertility hormones, and metabolic factors including BMI and waist circumference in reproductively active men are not well characterised. We studied a unique cohort of expectant fathers for metabolic risk factors and reproductive hormone profiles, and investigated the relationship of AMH to these parameters.
2. MATERIALS AND METHODS
2.1. Participants
Partners of pregnant women involved in the ORIGINS Project at Joondalup Health Campus (JHC), Perth, WA, Australia were invited to participate. The ORIGINS Project is a community intervention birth cohort‐based at the JHC, aiming to recruit mothers and follow up their offspring for 5 years. 17 Men whose partners are part of ORIGINS were invited to participate in a study of expectant fathers: the Cardiovascular Risk Evaluation in Expectant Fathers (CARE‐Dads) Study. Men requiring male factor fertility assistance were not included in the study. Thus, the study cohort was drawn from healthy, fertile couples recruited after pregnancy had occurred. There were 503 expectant fathers participating in this study, from which 18 were excluded, to provide a total of 485 reproductively active men in the analysis cohort. Exclusions included four females, 11 participants with missing data, and three with outlying hormone data (two men with luteinizing hormone [LH] < 1 IU/L, and one with a follicle‐stimulating hormone [FSH] elevated well above the reference interval of 24.8 IU/L). This study was approved by the Ramsay Health Care Human Research Ethics Committee (Approval Number 1536). All participants provided written informed consent.
2.2. Measurements
Participants completed a questionnaire assessing medical history and lifestyle behaviours. Participants were recorded as having hypertension, high cholesterol, or diabetes if they self‐reported a medical diagnosis of the condition, or were on specific medications for the treatment of the condition. A physical assessment was conducted measuring height, weight, and waist circumference (using a standard tape measure at the midpoint between the lowest lateral margin of the ribs and the highest lateral margin of the iliac crests, in the horizontal plane). BMI was calculated as mass (kg) divided by height squared (m2). Heart rate and blood pressure (BP) were measured on the right arm in the seated position with the elbow loosely flexed, after 5 min of rest, using an automated digital BP monitor (Omron HEM7130; Omron Corporation).
2.3. Laboratory analysis
Blood samples were collected between 6:30 AM and 3:40 PM (lower quartile [LQ] 9:30 AM, median 10:45 AM and upper quartile [UQ] 11:50 AM) into 5 ml serum separator tubes (BD Vacutainer®; Becton Dickinson), clotted at room temperature and then centrifuged within 30 min at 1200 g for 10 min. Serum was decanted and frozen at −20°C until analysis. AMH was analysed according to manufacturer's guidelines using the electrochemiluminescence assay on Roche Modular E170 initially and then on cobas e 601 from December 2019 (Roche Diagnostics GmbH). Coefficients of variation (CV) from our internal quality controls at concentrations of 6.7, 32, and 97 pmol/L were 4.2%, 5.0%, and 4.9%, respectively. To convert AMH values in pmol/L to ng/ml or µg/L divide results by 7.14. Serum LH, FSH, sex hormone‐binding globulin (SHBG), and estradiol (E2) were analysed on the Roche Modular E170 and cobas e 601 (Roche Diagnostics, GmbH) according to the manufacturer's guidelines. The same reagent kits were compatible with and used on both the E170 and the e 601. Testosterone, dihydrotestosterone (DHT), androstenedione, 17‐hydroxyprogesterone (17‐OHP), and progesterone were analysed using liquid chromatography on Waters Xevo TQ‐S tandem quadrupole mass spectrometer (liquid chromatography with tandem mass spectrometry) (Waters Corporation). The CVs for testosterone were both 2.6% at testosterone concentrations of 5.3 and 28 nmol/L. LH values less than 8 U/L were defined as normal, consistent with the laboratory reference interval. Testosterone ranges vary with age. Baseline testosterone was within age‐specific reference intervals in all men according to published data on men aged 21–35 years (range 10.4–30.1 nmol/L) 18 and additional age‐specific reference intervals recently reported. 19 The AMH reference intervals for men in our laboratory are 20–120 pmol/L for 19–40‐year‐olds and for >40 years 15–72 pmol/L. These intervals were derived from local data, Roche method sheet data on male reference intervals, and literature. 20
2.4. Statistical analysis
Descriptive statistics are given as mean (SD) or, for skewed variables, median and LQ and UQ. Spearman correlation coefficients (ρ) were calculated to evaluate relationships between AMH and reproductive hormones, BMI, waist circumference, and age. Linear regression was used to refine the relationship between AMH and these variables, with adjustment for age and BMI. AMH and other hormone variables were log‐transformed where necessary before analysis to ensure statistical validity of regression models. BMI measurements were grouped into the categories <25, 25–29, 30–34, and ≥35 kg/m2 or treated as a continuous variable, as required. Similarly, age was categorised as ≤30, 31–35, 36–40, or ≥41 years or treated as a continuous variable. In multiple regression analysis, predictors were checked for collinearity by evaluating variance inflation factors (VIF). For the predictors BMI, testosterone, and SHBG, we evaluated the possibility of mediatory effects on AMH using mediation analysis. In this analysis, a predictor is partitioned into its direct effect on an outcome and its effect due to mediation by a third variable, the significance of each part is evaluated and the mediated proportion calculated.
A 5% significance level was used throughout. To allow for a maximum of four comparisons when assessing differences among BMI or age categories, we adjusted the significance level to α' = .05/4 = .0125. All analysis was completed in the R statistical computing environment (R version 4.0.0), 21 including the mediation 22 package.
3. RESULTS
3.1. Baseline characteristics of the study population
Descriptive statistics for the 485 reproductively active men were stratified according to BMI and are shown in Table 1. The median age was 33 years, 32% of men had one or more previous children, and rates of hypertension, hypercholesterolemia, and diabetes were low. Median AMH was 40 pmol/L (LQ: 29, UQ: 56), and this decreased across categories of increasing BMI (Figure 1). Median values for testosterone and SHBG decreased across increasing categories of BMI. Median LH was lowest in men with BMI 25–29 kg/m2.
Table 1.
Demographic information for the whole CARE‐Dads cohort, then for BMI subgroups
| All | BMI < 25 | BMI 25–29 | BMI 30–34 | BMI ≥ 35 | p | |
|---|---|---|---|---|---|---|
| N | 485 | 113 | 226 | 108 | 38 | |
| Age (years) | 33 (30, 37) | 32 (30, 35) | 34 (30,37) | 35 (31, 39) | 32 (29, 38) | .005 |
| Existing children | 155 (32%) | 40 (35%) | 74 (33%) | 37 (34%) | 4 (11%) | .008 |
| Smoker (yes) | 59 (12%) | 11 (10%) | 31 (14%) | 13 (12%) | 4 (11%) | .79 |
| Alcohol: Drinks/session | 3.5 (2, 3.5) | 3.5 (2, 3.5) | 3.5 (2, 5.5) | 3.5 (2.0, 3.5) | 3.5 (2, 5.5) | .676 |
| Alcohol: Drinks/day | 0.3 (0.1, 1) | 0.3 (0.1, 0.6) | 0.4 (0.1, 1.3) | 0.6 (0.1, 1.3) | 0.3 (0.1, 0.6) | .077 |
| High cholesterol | 26 (5%) | 3 (3%) | 12 (5%) | 8 (7%) | 3 (8%) | .292 |
| High BP | 20 (4%) | 1 (1%) | 7 (3%) | 8 (7%) | 4 (11%) | .011 |
| Diabetes | 4 (1%) | 0 | 1 (0.4%) | 1 (1%) | 2 (5%) | .027 |
| BMI (kg/m2) | 28 (25, 31) | |||||
| Waist (cm) | 97 (89, 106) | 85 (81, 89) | 96 (92, 101) | 108 (103, 111) | 123 (117, 132) | <.001 |
| Systolic BP (mmHg) | 128 (120, 135) | 124 (117, 131) | 127 (119, 133) | 132 (124, 139) | 135 (129, 144) | <.001 |
| Diastolic BP (mmHg) | 80 (73, 86) | 75 (68,81) | 80 (74, 85) | 83.5 (76, 89) | 86 (78, 93) | <.001 |
| HbA1c (%) | 5.1 (4.9, 5.3) | 5.1 (4.9, 5.2) | 5.1 (4.9, 5.3) | 5.1 (4.9, 5.3) | 5.2 (5, 5.6) | .023 |
| AMH (pmol/L) | 40 (29, 56) | 45 (33, 62) | 40 (30, 56) | 37 (24, 50) | 39 (23, 50) | .002 |
| Testosterone (nmol/L) | 16 (12, 20) | 18 (15, 22) | 16 (12, 20) | 14 (12, 17) | 11 (8.9, 14) | <.001 |
| DHT (nmol/L) | 1.0 (0.8, 1.4) | 1.2 (1.0, 1.6) | 1.1 (0.8, 1.4) | 0.9 (0.7, 1.1) | 0.7 (0.4, 0.9) | <.001 |
| E2 (pmol/L) | 110 (88, 129) | 108 (84, 125) | 108 (87, 127) | 113 (92, 132) | 119 (103, 138) | .018 |
| SHBG (nmol/L) | 34 (25, 44) | 42 (32, 52) | 34 (25, 43) | 31 (24, 38) | 26 (17, 35) | <.001 |
| LH (IU/L) | 4.8 (3.6, 6.3) | 5.0 (3.9, 6.9) | 4.5 (3.6, 5.8) | 5.0 (3.6, 6.4) | 5.2 (3.8, 6.6) | .027 |
| FSH (IU/L) | 3.9 (2.9, 5.2) | 4.0 (2.9, 5.4) | 3.8 (3.0, 5.0) | 3.9 (2.7, 5.0) | 4.12 (2.9, 6.2) | .621 |
| Androstenedione (nmol/L) | 2.4 (1.9, 3.0) | 2.5 (2, 3) | 2.5 (1.9, 3.1) | 2.4 (1.9, 2.9) | 2.1 (1.7, 2.8) | .085 |
| 17‐Hydroxyprogesterone (nmol/L) | 2.0 (1.4, 2.8) | 2.4 (1.6, 3.4) | 2 (1.4, 2.7) | 2 (1.5, 2.6) | 1.5 (1.1, 2.1) | <.001 |
| Progesterone (nmol/L) | 0.13 (0.08, 0.20) | 0.15 (0.08, 0.22) | 0.13 (0.09, 0.20) | 0.12 (0.08, 0.17) | 0.09 (0.05, 0.15) | .006 |
Note: Continuous variables: Median (LQ, UQ). Categorical variables: Count (%).
p‐value is from a Kruskal–Wallis test for continuous variables or Fisher's exact test for categorical variables.
Abbreviations: AMH, anti‐Müllerian hormone; BMI, body mass index; BP, blood pressure; CARE‐Dads, Cardiovascular Risk Evaluation in Expectant Fathers; DHT, dihydrotestosterone; E2, estradiol; FSH, follicle‐stimulating hormone; HbA1c, hemoglobin A1c; LH, luteinizing hormone; LQ, lower quartile; SHBG, sex hormone‐binding globulin; UQ, upper quartile.
Figure 1.
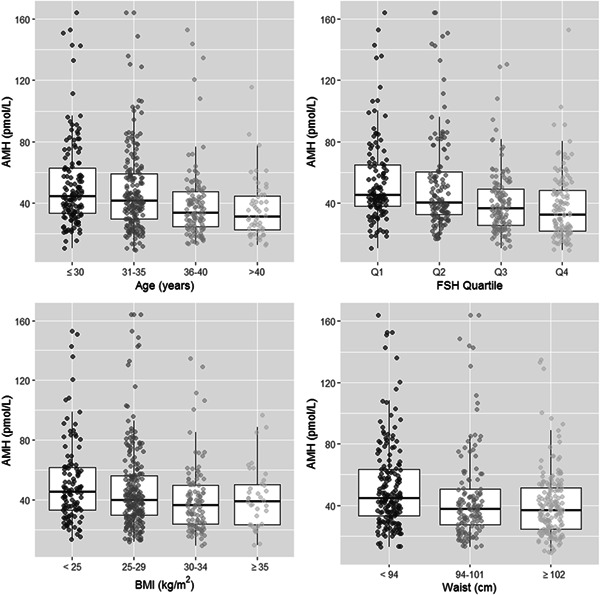
Distribution of anti‐Mullerian hormone (AMH) concentrations in expectant fathers, stratified according to age, follicle‐stimulating hormone (FSH), body mass index (BMI), and waist circumference categories. Data are shown as boxplots with AMH on the y‐axis, with median and interquartile ranges for each category of age, FSH, BMI, and waist circumference. Quartile limits for FSH are: Q1: <2.9, Q2: 2.9–3.8, Q3: 3.9–5.1, Q4: >5.1 pmol/L
3.2. Inverse associations of AMH with FSH, age, waist circumference, and BMI
Correlations between AMH and other variables are shown in Table 2. AMH was inversely correlated with FSH, age, waist circumference, and BMI (Spearman correlation coefficients [ρ]: −.32, −.24, −.20, and −.17, respectively). These associations are illustrated in Figure 1 and Figure S1.
Table 2.
Relationships between AMH and other variables
| Correlation | Linear regression adjustment | ||||
|---|---|---|---|---|---|
| Relationship of AMH with | ρ | Univariate β (p) | Age β (p) | Age and BMI β (p) | R 2 |
| FSH | −.32 | −.076 (<.001) | −.069 (<.001) | −.071 (<.001) | 11.3% |
| Age | −.24 | −.021 (<.001) | a | −.019 (<.001) | 4.9% |
| Waist | −.20 | −.008 (<.001) | −.007 (<.001) | b | 4.1% |
| BMI | −.17 | −.019 (<.001) | −.016 (.002) | c | 3.2% |
| LH | −.09 | −.16 (.010) | −.12 (.039) | −.13 (.030) | 1.4% |
| SHBG | .08 | .12 (.040) | .18 (.001) | .14 (.022) | 0.9% |
| DHT | .10 | .12 (.010) | .11 (.014) | NS | 1.4% |
| Testosterone | .09 | .011 (.014) | .008 (.054) | NS | 1.2% |
| Estradiol | −.07 | −.14 (.108) | −.19 (.025) | NS | 0.5% |
| Androstenedione | .04 | NS | NS | NS | 0.7% |
| Progesterone | .09 | NS | NS | NS | 0.6% |
| 17‐OHP | .05 | NS | NS | NS | 0.5% |
Note: ρ is the Spearman correlation coefficient. Linear regression coefficient (β) and p‐value are from linear regression of log‐transformed AMH against a given variable. LH, SHBG, and oestrogen were also log‐transformed for regression analysis. R 2 is from univariate regression analysis.
“NS” indicates the variable was not significant in the AMH regression model.
Abbreviations: 17‐OHP, 17‐hydroxyprogesterone; AMH, anti‐Müllerian hormone; BMI, body mass index; DHT, dihydrotestosterone; FSH, follicle‐stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone‐binding globulin.
Linear model cannot be adjusted for age.
Linear model not adjusted for BMI, as waist and BMI are collinear.
Linear model cannot be adjusted for BMI
There was a significant downward trend in log‐transformed AMH with increased FSH concentration (β = −.069, p < .001, age adjusted; Figure 1). There was no evidence that the AMH‐FSH relationship differed in men with BMI < 30 kg/m2 and those with BMI ≥ 30 kg/m2 (p interaction = .875).
Although age was correlated with both BMI and FSH, there was no evidence of collinearity when all were included in a multiple linear regression model (all VIF < 1.02), indicating that each is an independent predictor of AMH. Of the variables examined, FSH accounted for the largest proportion of variance in AMH with an R 2 of 11.3%, compared with 4.9% for age and 3.2% for BMI.
3.3. Associations of AMH with metabolic parameters
Peak AMH occurred between 20 and 30 years of age and declined thereafter (β = −.019, p < .001, for trend). There were significant differences in AMH between the 20–30 year group and both the 36–40 and > 41‐year groups (both p < .001), but no significant difference between the youngest group and the 31–35‐year‐olds (p = .070). After adjustment for age, AMH showed a significant decrease over the BMI range (β = −.016, p = .002); however, there was no evidence that the downward trend continued beyond the 30–34 kg/m2 category (p = .966 for comparison between 30 and 34 and ≥35 kg/m2 BMI categories; Figure 1).
After adjustment for age, AMH showed a significant decrease over the waist measurement range (β = −.007, p < .001; Figure 1). Compared with males with a waist below 94 cm, those with a waist measurement of 94–101 cm had AMH levels 12% lower (β = −.13, p = .036), while males with a waist of 102 cm or more had AMH levels 17.5% lower on average (β = −.19, p < .001). Waist measurement and BMI were highly collinear predictors with respect to AMH (both VIF > 2.4), and AMH showed a slightly stronger association with waist than BMI (R 2 Waist = 4.1% vs. R 2 BMI = 3.2%).
3.4. Associations of AMH with reproductive hormones
As expected, serum testosterone was inversely correlated with age, waist circumference, and BMI (ρ = −.12, −.38, and −.37, respectively; Table 3), and positively correlated with SHBG, DHT, and E2 (ρ = .59, .75, and .36, respectively; Table 3). There was only a modest positive association between serum testosterone and AMH (ρ = .09; Table 2). While in univariate analysis the AMH‐testosterone association was significant (β = .011, p = .014), this result did not persist after adjustment for age and BMI (p = .376). We assessed whether this observation might reflect a case of mediation and found no evidence that testosterone mediated the effect of BMI on AMH (p = .248); conversely, there was strong statistical evidence that BMI mediated 49% of the effect of testosterone on AMH (p < .001; estimate of the testosterone effect on AMH mediated by BMI was 0.005, 95% confidence interval: 0.002–0.010).
Table 3.
Correlation matrix for study variables
| Age | BMI | Waist | T | DHT | 17‐OHP | E2 | SHBG | LH | FSH | |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI | 0.13** | |||||||||
| Waist | 0.22** | 0.89** | ||||||||
| T | −0.12** | −0.37** | −0.38** | |||||||
| DHT | −0.04 | −0.38** | −0.39** | 0.75** | ||||||
| 17‐OHP | 0.01 | −0.20** | −0.19** | 0.60** | 0.37** | |||||
| E2 | −0.11* | 0.13** | 0.12** | 0.36** | 0.16** | 0.23** | ||||
| SHBG | 0.16** | −0.34** | −0.31** | 0.59** | 0.67** | 0.26** | 0.01 | |||
| LH | 0.11* | 0.00 | 0.04 | 0.07 | 0.00 | 0.14** | 0.10* | 0.05 | ||
| FSH | 0.18** | −0.02 | 0.02 | 0.00 | 0.00 | 0.01 | −0.08 | 0.09 | 0.46** | |
| AMH | −0.24** | −0.17** | −0.20** | 0.09* | 0.10* | 0.05 | −0.07 | 0.08 | −0.09* | −0.32** |
Note: Age (years), BMI (kg/m2), waist circumference (cm), testosterone (T, nmol/L), dihydrotestosterone (DHT, nmol/L), estradiol (E2, pmol/L), sex hormone‐binding globulin (SHBG, nmol/L), luteinising hormone (LH, IU/L), follicle‐stimulating hormone (FSH, IU/L), and anti‐Müllerian hormone (AMH, pmol/L) in 485 expectant fathers. Spearman correlation coefficients (ρ) are shown.
Abbreviations: 17‐OHP, 17‐hydroxyprogesterone; BMI, body mass index.
p < .05.
p < .01.
Testosterone and SHBG were collinear and directly associated with AMH. There was a modest positive association between serum SHBG and AMH (ρ = .08). Serum SHBG was inversely correlated with BMI (ρ = −.34) and positively correlated with age (ρ = .16). When adjusted for age, the AMH–SHBG association was significant (β = .18, p = .001); however, this result was slightly attenuated when further adjustment was made for BMI (β = .14, p = .022). This attenuation was attributed to a degree of collinearity between SHBG and BMI (both VIF > 1.18).
The relationship between AMH and LH concentrations followed that of AMH and FSH, but with a much weaker degree of association (ρ = −.09): there was a significant downward trend in age‐adjusted AMH with increasing LH concentration (β = −.12, p = .039, LH log‐transformed), which persisted after further adjustment for BMI (β = −.13, p = .030).
There was a modest positive association between serum DHT and AMH (ρ = .10; Table 2). In univariate analysis, the AMH‐DHT association was significant (β = .12, p = .010); however, this result did not persist after adjustment for age and BMI (p = .138). There was strong statistical evidence that BMI mediated 44% of the effect of DHT on AMH (p = .008). Serum DHT was inversely correlated with BMI and positively correlated with SHBG (ρ = −.38, .67, respectively; Table 3).
There was no significant association between serum 17‐OHP and AMH, either before or after adjustment for age and BMI (p = .118, .307, respectively). Serum 17‐OHP was inversely correlated with BMI (ρ = −.20; Table 3).
3.5. Supplementary analyses
Of the 485 blood samples, 363 were analysed on the E170 and 122 on the e 601. Median (LQ and UQ) AMH and FSH were comparable between the two analysers (AMH 41.3 [29.7, 55.4] vs. 36.6 [24.4, 57.8] pmol/L, FSH 3.8 [2.8, 5.2] vs. 4.2 [3.1, 5.2] IU/L, both p > .05). There were differences between analysers for SHBG, LH, and E2 (35.5 [26.4, 44.9] vs. 30.2 [23.1, 39.9] nmol/L; 4.6 [3.5, 6.2] vs. 5.2 [4.0, 6.7] IU/L; 108 [85, 125] vs. 119 [98, 139] pmol/L; all p < .05). However, these differences did not significantly modify the AMH–analyte relationships (AMH vs. SHBG, p = .273; AMH vs. LH, p = .239; AMH vs. E2, p = .299).
When time of blood sampling was tested as an explanatory variable for each hormone, there was no association with AMH, FSH, LH, DHT, or SHBG (all p > .05). Only testosterone (p < .001) and E2 (p = .020) were associated with time. When time was tested as an adjustment variable in the relationship between AMH and each of these hormones, there was no evidence of confounding from time of blood sampling (AMH vs. testosterone p = .908; vs. FSH p = .512; vs. LH p = .759; vs. E2 p = .633; vs. DHT p = .947; vs. SHBG p = .779). Furthermore, there was no evidence of hormone–time interaction in the relationships between AMH and each of these hormones (all p interaction > .05).
4. DISCUSSION
In a large group of expectant fathers, we found AMH was inversely associated with FSH, age, waist circumference, and BMI. The inverse association of AMH with FSH was the strongest, was independent of age and BMI, and was consistent across the range of FSH values. Testosterone and SHBG were both associated with AMH, with evidence that for testosterone the association with AMH was mediated via the inverse association of testosterone with BMI.
There are strong genetic influences on AMH levels in men after puberty 13 and a high degree of interpersonal variation. 23 Our study confirmed a cross‐sectional decline in AMH with increasing age, which is consistent with previous studies. Both testosterone production and spermatogenesis demonstrate impairment during the course of male ageing. 24 , 25 Our results are consistent with previous observations that reduced AMH in older men may reflect age‐related reductions in Sertoli cell function. 20 , 25 , 26 Of note a study of 970 young men (median age 19 years) found no association between serum AMH and semen quality, except for a trend for a lower percentage of normal morphology with higher AMH. 27
We found an inverse relationship between AMH and BMI and AMH and waist circumference. There was a direct relationship between AMH and SHBG. The relationships between BMI, waist circumference, and AMH are of interest given impaired reproductive health with increasing obesity. 14 , 28 , 29 BMI increases with age, particularly in middle‐aged adults. 30 , 31 BMI is inversely associated with testosterone, SHBG, and inhibin B 14 , 15 , 28 and semen quality can be impaired with increasing BMI. 14 , 28 , 29 Our findings of lower AMH with increasing BMI are consistent with other studies. There is evidence for an inverse association between AMH and BMI, and AMH and fat mass. 13 , 14 , 15 A recent NHANES study 10 found AMH was inversely associated with waist circumference in obese men and there was an inverse relationship between AMH and diabetic status. A nonsignificant trend for decreasing AMH with BMI was also observed. 10 Our results are concordant with these findings suggesting that lower AMH may be a marker of obesity and metabolic dysfunction.
In expectant fathers, we found the strongest inverse association to be between AMH and FSH. This relationship remained robust after controlling for other factors that reduce AMH, such as increasing age, BMI, and waist circumference. Surprisingly there is little information on the relationship of AMH and FSH in reproductively active men. In men with established gonadal dysfunction, there is an inverse association of AMH with FSH 25 and FSH increases with age. 32 , 33 However, it is unclear if other metabolic factors including BMI influence the relationship between AMH and FSH. In a study of men with maldescended testes, FSH had a negative correlation with AMH but was not associated with AMH in healthy men. 34 In a larger study of healthy men, a negative association between AMH and FSH was thought to indicate an age‐related reduction in testicular function. 26 However, in our data, the inverse relationship between FSH and AMH persisted even when controlling for age and obesity.
An elevated FSH can reflect Sertoli cell dysfunction, with compensatory feedback driving pituitary FSH secretion. 6 A recent study by Waller et al. 35 found that an FSH above age and method‐specific reference intervals conveyed an increased risk of semen abnormality and infertility. FSH regulates Sertoli cell function and subsequent production of AMH and inhibin B. 6 Our findings indicate AMH may provide additional independent information regarding Sertoli cell function in reproductively active men, even when other factors associated with metabolic and cardiovascular health are considered.
Of note, AMH has actions in the HPG axis to increase GnRH neuronal activity and GnRH release. 5 FSH has a role in promoting fat accumulation and there are FSH receptors in adipocytes where FSH promotes lipid biosynthesis. 36 FSH can also induce dose‐dependent increases in leptin and decreases in adiponectin production in cultured human adipocytes. 36 Therefore, our findings of inverse associations of AMH with BMI, waist circumference, and FSH reflect these complex interactions between metabolic and reproductive health.
Obesity is associated with a longitudinal decline in circulating testosterone concentrations. 37 We found a direct association between AMH and testosterone. This was mediated to a large extent by BMI, in contrast to the independent and inverse association of AMH with FSH. Therefore, although AMH is inversely associated 3with both age and BMI, via its associations with FSH and testosterone, it provides further insights into reproductive health in expectant fathers. Androstenedione, progesterone, and 17‐OHP were not correlated with AMH in univariate, age, or age and BMI‐adjusted analyses, suggesting less of a role for the ACTH/adrenal gland axis.
Strengths of the study include the large cohort of expectant fathers, providing a novel opportunity to examine the interactions of AMH with metabolic indices and reproductive hormones in reproductively active men. Limitations of our study include its observational and cross‐sectional nature, preventing the determination of causality. A single blood sample was collected, thus serial measurements of AMH and other hormones were not available. We did not have semen analysis on these men nor were we able to measure inhibin B levels, which may have been further useful markers of reproductive health, and analysis of pregnancy outcomes was outside the scope of this analysis.
In conclusion, the inverse association of AMH with FSH levels supports the role of AMH as a biomarker of reproductive health that is independent of age and BMI. Taken together with the finding that BMI mediates the association of AMH with testosterone, our results indicate that in expectant fathers, AMH provides potential insight into Sertoli rather than Leydig cell function. Further research is warranted to determine whether AMH may be associated with reproductive as well as cardiometabolic outcomes in men as they transition from middle to older ages.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
The ORIGINS Project has received core funding support from the Telethon Perth Children's Hospital Research Fund, Joondalup Health Campus (JHC), the Paul Ramsay Foundation, and the Commonwealth Government of Australia through Channel 7 Telethon Trust. Substantial in‐kind support has been provided by Telethon Kids Institute and JHC. The work presented in this manuscript was funded in part by a philanthropic donation by Mr Roland Soo and Ms Dawn Chow to the University of Western Australia. Roche Diagnostics International, Switzerland, provided assay kits for anti‐Mullerian hormone, luteinising hormone, follicle‐stimulating hormone, estradiol and sex hormone‐binding globulin without charge for the purposes of this study. The authors would like to thank all the participants and staff involved in the ORIGINS Project, and in the Cardiovascular Risk Evaluation in Expectant Fathers (CARE‐Dads) substudy of ORIGINS. The ORIGINS Project is only possible because of the commitment of the families in ORIGINS. They are grateful to all the participants, health professionals, and researchers, including Lucy Giggs and Luke Cummins, who supported the project. They would also like to acknowledge and thank the following teams and individuals who have made The ORIGINS Project possible: The ORIGINS Project team; CEO Dr Kempton Cowan, executive staff, and obstetric, neonatal, and paediatric teams, JHC; Director Professor Jonathan Carapetis and executive staff, Telethon Kids Institute; Mayor Tracey Roberts, City of Wanneroo; Mayor Albert Jacobs, City of Joondalup; Professor Fiona Stanley, patron of ORIGINS; members of ORIGINS Community Reference and Participant Reference Groups; Research Interest Groups; and the ORIGINS Scientific Committee. This study is a subproject of the ORIGINS Project. Open access publishing facilitated by The University of Western Australia, as part of the Wiley ‐ The University of Western Australia agreement via the Council of Australian University Librarians.
Hadlow NC, Brown SJ, Lim EM, et al. Anti‐Müllerian hormone concentration is associated with central adiposity and reproductive hormones in expectant fathers Clin Endocrinol (Oxf). 2022;97:634‐642. 10.1111/cen.14725
DATA AVAILABILITY STATEMENT
Data are not publicly available due to privacy considerations.
REFERENCES
- 1. Ramlau‐Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22(6):1634‐1637. [DOI] [PubMed] [Google Scholar]
- 2. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahn BE, Brannigan RE. Obesity and male infertility. Curr Opin Urol. 2017;27(5):441‐445. [DOI] [PubMed] [Google Scholar]
- 4. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45(5):685‐698. [DOI] [PubMed] [Google Scholar]
- 5. Silva MSB, Giacobini P. New insights into anti‐Mullerian hormone role in the hypothalamic‐pituitary‐gonadal axis and neuroendocrine development. Cell Mol Life Sci. 2021;78(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grinspon RP, Rey RA. Anti‐Mullerian hormone and Sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr. 2010;73(2):81‐92. [DOI] [PubMed] [Google Scholar]
- 7. Sansone A, Isidori AM, Kliesch S, Schlatt S. Immunohistochemical characterization of the anti‐Mullerian hormone receptor type 2 (AMHR‐2) in human testes. Endocrine. 2020;68(1):215‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dennis NA, Jones GT, Chong YH, van Rij AM, McLennan IS. Serum anti‐Mullerian hormone (AMH) levels correlate with infrarenal aortic diameter in healthy older men: is AMH a cardiovascular hormone? J Endocrinol. 2013;219(1):13‐20. [DOI] [PubMed] [Google Scholar]
- 9. Ricci M, Mohapatra B, Urbiztondo A, et al. Differential changes in TGF‐beta/BMP signaling pathway in the right ventricular myocardium of newborns with hypoplastic left heart syndrome. J Card Fail. 2010;16(8):628‐634. [DOI] [PubMed] [Google Scholar]
- 10. Beydoun HA, Hossain S, Beydoun MA, Weiss J, Zonderman AB, Eid SM. Anti‐Mullerian hormone levels and cardiometabolic disturbances by weight status among men in the 1999 to 2004 National Health and Nutrition Examination Survey. J Endocr Soc. 2019;3(5):921‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qayyum R, Akbar S. Serum anti‐Mullerian hormone and all‐cause mortality in men. Endocrine. 2016;54(1):225‐231. [DOI] [PubMed] [Google Scholar]
- 12. Kadariya D, Kurbanova N, Qayyum R. Association of anti‐Mullerian hormone with C‐reactive protein in men. Sci Rep. 2019;9(1):13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pietilainen KH, Kaprio J, Vaaralahti K, Rissanen A, Raivio T. Circulating anti‐Mullerian hormone levels in adult men are under a strong genetic influence. J Clin Endocrinol Metab. 2012;97(1):E161‐E164. [DOI] [PubMed] [Google Scholar]
- 14. Andersen JM, Herning H, Aschim EL, et al. Body mass index is associated with impaired semen characteristics and reduced levels of anti‐Mullerian hormone across a wide weight range. PLoS One. 2015;10(6):e0130210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robeva R, Tomova A, Kirilov G, Kumanov P. Anti‐Mullerian hormone and inhibin B levels reflect altered Sertoli cell function in men with metabolic syndrome. Andrologia. 2012;44(Suppl 1):329‐334. [DOI] [PubMed] [Google Scholar]
- 16. Martins AD, Majzoub A, Agawal A. Metabolic syndrome and male fertility. World J Men's Health. 2019;37(2):113‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silva DT, Hagemann E, Davis JA, et al. Introducing the ORIGINS project: a community‐based interventional birth cohort. Rev Environ Health. 2020;35(3):281‐293. [DOI] [PubMed] [Google Scholar]
- 18. Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, Handelsman DJ. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab. 2005;90(11):5928‐5936. [DOI] [PubMed] [Google Scholar]
- 19. Travison TG, Vesper HW, Orwoll E, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aksglaede L, Sørensen K, Boas M, et al. Changes in anti‐Mullerian hormone (AMH) throughout the life span: a population‐based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab. 2010;95(12):5357‐5364. [DOI] [PubMed] [Google Scholar]
- 21. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2020. Accessed October, 2021. https://www.R-project.org/
- 22. Tingley DYT, Hirose K, Keele L, Imai K. R package for causal mediation analysis. J Stat Softw. 2014;59(5):1‐38. http://www.jstatsoft.org/v59/i05/ 26917999 [Google Scholar]
- 23. Chong YH, Dennis NA, Connolly MJ, et al. Elderly men have low levels of anti‐Mullerian hormone and inhibin B, but with high interpersonal variation: a cross‐sectional study of the Sertoli cell hormones in 615 community‐dwelling men. PLoS One. 2013;8(8):e70967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yeap BB, Manning L, Chubb SAP, et al. Progressive impairment of testicular endocrine function in ageing men: testosterone and dihydrotestosterone decrease, and luteinizing hormone increases, in men transitioning from the 8th to 9th decades of life. Clin Endocrinol. 2018;88(1):88‐95. [DOI] [PubMed] [Google Scholar]
- 25. Xu HY, Zhang HX, Xiao Z, Qiao J, Li R. Regulation of anti‐Mullerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl. 2019;21(2):109‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramezani Tehrani F, Mansournia MA, Solaymani‐Dodaran M, Minooee S, Azizi F. Serum variations of anti‐Mullerian hormone and total testosterone with aging in healthy adult Iranian men: a population‐based study. PLoS One. 2017;12(7):e0179634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aksglaede L, Olesen IA, Carlsen E, Petersen JH, Juul A, Jorgensen N. Serum concentration of anti‐Mullerian hormone is not associated with semen quality. Andrology. 2018;6(2):286‐292. [DOI] [PubMed] [Google Scholar]
- 28. Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93(7):2222‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Håkonsen LB, Thulstrup AM, Aggerholm AS, et al. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod Health. 2011;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drøyvold WB, Nilsen TIL, Krüger Ø, et al. Change in height, weight and body mass index: longitudinal data from the HUNT Study in Norway. Int J Obes. 2006;30(6):935‐939. [DOI] [PubMed] [Google Scholar]
- 31. Wilson R, Abbott JH. Age, period and cohort effects on body mass index in New Zealand, 1997‐2038. Aust N Z J Public Health. 2018;42(4):396‐402. [DOI] [PubMed] [Google Scholar]
- 32. Nahoul K, Roger M. Age‐related decline of plasma bioavailable testosterone in adult men. J Steroid Biochem. 1990;35(2):293‐299. [DOI] [PubMed] [Google Scholar]
- 33. Bjørnerem Å, Straume B, Midtby M, et al. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso Study. J Clin Endocrinol Metab. 2004;89(12):6039‐6047. [DOI] [PubMed] [Google Scholar]
- 34. Tuttelmann F, Dykstra N, Themmen AP, Visser JA, Nieschlag E, Simoni M. Anti‐Mullerian hormone in men with normal and reduced sperm concentration and men with maldescended testes. Fertil Steril. 2009;91(5):1812‐1819. [DOI] [PubMed] [Google Scholar]
- 35. Waller E‐J, Conceicao J, Matson P, Yovich JL. Proposed age‐stratified reference intervals of FSH derived from normozoospermic men. Asian Pac J Reprod. 2021;10:162‐167. [Google Scholar]
- 36. Liu XM, Chan HC, Ding GL, et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca2+/CREB pathway. Aging Cell. 2015;14(3):409‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community‐dwelling men. J Clin Endocrinol Metab. 2013;98(8):3289‐3297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data are not publicly available due to privacy considerations.


