1. CASE REPORT
This case illustrates the prominent bone marrow accumulation of crystal‐storing histiocytes (CSH) in the context of a monoclonal light chain gammopathy. Histiocytes contained Immunoglobulin (Ig) fragments derived from neoplastic plasma cells, themselves filled by abnormal inclusions. As recently reported, 1 our case further illustrates informative clues about the peculiar morphological features of CSH.
A 73‐year‐old man presented with axonal polyneuropathy in all limbs. Laboratory investigations showed normal full blood count, renal failure, and serum and urine free kappa (κ) light chains (Table 1). No lytic lesions were seen on X‐ray imaging. The bone marrow aspirates contained 8% atypical anisocytic plasma cells along with innumerable globulin‐laden histiocytes (Figure 1). The histiocytes sometimes aggregated but were also singly distributed. The inclusions showed a spectrum of appearances but mainly included brightly eosinophilic globule‐like forms. Some of the histiocytes had sea‐blue cytoplasm with occasional globules. In addition to the histiocytes, most plasma cells also contained cytoplasmic globulin‐like crystals. Some plasma cells however retained a typical appearance. Plasma cell clonality was verified by flow cytometry as the plasma cells expressed positivity for CD138, CD38, and κ chain, and negativity for CD19 and CD56 monoclonal antibodies. CD68 (KP1) immunostain revealed the numerous macrophages filled by crystalline inclusions. High background was due to heavy or light Ig chain immunostains which were not fully relevant. A diagnosis of CSH along with monoclonal κ light chain gammopathy was made.
TABLE 1.
Laboratory investigations
| Analysis | Units | Values | Reference range | |
|---|---|---|---|---|
| Blood count | Hemoglobin | (g/dl) | 16 | 13–16.5 |
| White blood cells | (×109/L) | 9.23 | 7–11 | |
| Neutrophils | (×109/L) | 6.2 | 1.5–7.5 | |
| Platelets | (×109/L) | 198 | 150–400 | |
| Serum | Urea | (mmol/L) | 7 | 2.8–7.2 |
| Creatinine | (mmol/L) | 143 | 59–104 | |
| Albumine | (g/L) | 45 | 25–52 | |
| Calcium | (mmol/L) | 2.5 | 2.2–2.6 | |
| Gammaglobulinemia | (g/L) | 7.4 | 8–13.5 | |
| C‐reactive protein | (mg/L) | 1 | <5 | |
| Protein | (g/L) | 73 | 60–80 | |
| IgG | (g/L) | 6.94 | 7–16 | |
| IgA | (g/L) | 1.41 | 0.7–4 | |
| IgM | (g/L) | 0.33 | 0.4–2.3 | |
| Protein electrophoresis | No monoclonal band | |||
| Immunofixation | No monoclonal band | |||
| Kappa free light chain | (mg/L) | 248 | 5–15 | |
| Lambda free light chain | (mg/L) | 13 | 8–18 | |
| Ratio Kappa/Lambda | 18.8 | 0.27–1.67 | ||
| Urine | Proteinuria | (g/day) | 3, monoclonal | 0.01–0.14 |
FIGURE 1.
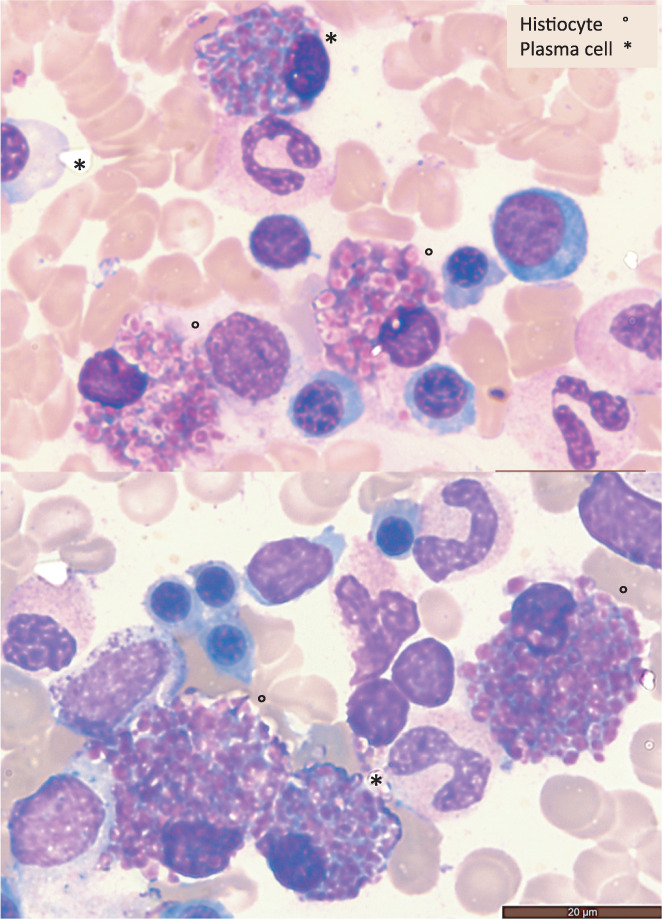
Crystal‐storing‐histiocytes and atypical plasma cells (2 fields, bone marrow, May Grunwald Giemsa stain, ×1000)
2. DISCUSSION
CSH is a very uncommon phenomenon characterized by the abnormal intra‐cytoplasmic accumulation of crystallized Ig in histiocytes. 2 It is typically associated with disorders that express monoclonal Ig, particularly B‐lymphoproliferative disorders with plasmocytic differentiation, such as myeloma, marginal zone lymphoma, lymphoplasmacytic lymphoma, and monoclonal gammopathy of unknown significance. 3 A majority of cases show serum and/or urine paraprotein. Preferential association with κ light chains has been reported. 1 In the present case, numerous histiocytes with abundant cytoplasm filled with globular eosinophilic material were identified among plasma cells. The accumulation of crystallized κ light chain molecules within the cytoplasm of non‐neoplastic histiocytes likely results from a conformational alteration in the Ig molecules—itself induced by amino‐acids exchanges—conferring abnormal crystallization properties. CSH is typically observed on bone marrow aspirates but has also been seen in other tissues including the kidneys, spleen, lymph nodes, skin, thyroid, lungs, liver, and gastrointestinal tract. 4 There is no intervention targeted to CSH and therapy is directed against the underlying malignancy.
Our case raised some valuable educational points: (i) abnormal inclusions shapes were globular, never elongated, crystals as occasionally previously reported. 2 , 5 Some inclusions roughly mimicked the Gasser anomaly seen in lymphocytes with lysosomal metabolic disorders; (ii) the extensive filling of the histiocytes, sometimes aggregated, favored the theory of an accumulation disorder; (iii) the increase in the free κ light chains in serum and urine confirmed the relationship between k chains and the presence of CSH; (iv) the atypical plasma cells signaled the initial step of the process, leading to the secondary development of CSH.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Lesesve J‐F, Thomas B. Crystal‐storing histiocytosis associated with monoclonal kappa light chain gammopathy. Int J Lab Hematol. 2022;44(6):978‐979. doi: 10.1111/ijlh.13848
DATA AVAILABILITY STATEMENT
All data available on request.
REFERENCES
- 1. Wiese‐Hansen H, Leh F, Lodvir Hemsing A, Reikvam H. Immunoglobulin‐storing histiocytosis: a case based systemic review. J Clin Med. 2021;10(9):1834‐1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kilic I, Picken MM, Velankar MM, Pambuccian SE. Bone marrow imprints of crystal‐storing histiocytosis. Diagn Cytopathol. 2020;48(3):244‐252. [DOI] [PubMed] [Google Scholar]
- 3. Lebeau A, Zeindl‐Eberhart E, Müller EC, et al. Generalized crystal‐storing histiocytosis associated with monoclonal gammopathy: molecular analysis of a disorder with rapid clinical course and review of the literature. Blood. 2002;100(5):1817‐1827. [PubMed] [Google Scholar]
- 4. Fang H, Chiu A, Reichard KK. Crystal‐storing histiocytosis in bone marrow: a clinicopathologic study of eight cases and review of the literature. Am J Clin Pathol. 2018;149(2):148‐163. [DOI] [PubMed] [Google Scholar]
- 5. Lesesve JF, Bronowicki JP, Galed‐Placed I. Crystal‐storing histiocytosis in ascites from a patient with IgM kappa lymphoplasmacytic lymphoma. Cytopathology. 2011;22(3):207‐208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data available on request.


