Abstract
Borrelia burgdorferi, the spirochetal agent of Lyme disease, stimulated human peripheral blood monocytes to release pro-matrix metalloproteinase-9 (gelatinase B; pro-MMP-9) and active matrix metalloproteinase-1 (collagenase-1; MMP-1). Human neutrophils also released pro-MMP-9 and a 130-kDa protein with gelatinolytic activity in response to live B. burgdorferi. In addition, U937 cells and human keratinocyte cells were also stimulated to release pro-MMP-9 under the same conditions. However, human umbilical vein endothelial cells (HUVECs) released pro-MMP-9 and pro-MMP-2 in a constitutive manner and were not influenced by live spirochetes. MMPs produced by human monocytes also enhanced the penetration of B. burgdorferi through extracellular matrix component barriers in vitro. Plasmin stabilized on the surface of the Lyme disease spirochete was shown to activate pro-MMP-9 to its active form. This active form was also observed in the plasma of mice infected with a relapsing fever borrelia. These results suggest that borreliae can upregulate MMPs and possibly mediate an activation cascade initiated by plasmin bound to the microbial surface. MMPs may play a role in dissemination of the Lyme disease spirochete and in the pathogenesis of Borrelia infection.
Borrelia burgdorferi, the etiologic agent of Lyme disease, is transmitted by hard ticks of the genus Ixodes and causes multisystemic illness in humans and laboratory animals (3, 4, 8, 50). Lyme disease infection is characterized by an expanding skin lesion, erythema migrans, following a tick bite. Arthritis, meningitis, cranial neuropathy, and radiculopathy may persist in patients during the course of infection (23, 49). Other infectious borreliae include the agents of Old World and New World relapsing fever and are transmitted by soft ticks of the family Argasidae. Infection results in a relapsing febrile illness coinciding with periods of spirochetemia followed by periods of remission. Systemic manifestations are also observed following spirochetemic episodes (9).
A key requirement for transmission and survival of these highly motile spirochetes is the need to spread from the blood-feeding arthropod to the host. In some pathogens of low genomic capacity, such as B. burgdorferi (21), host-derived proteases are used by organisms to assist in crossing tissue barriers and thus to disseminate (6, 15). Previous in vivo and in vitro studies have shown that plasmin bound on the surface of both Lyme disease and relapsing fever spirochetes is utilized to disseminate within the tick and the infected host and to degrade extracellular matrix components as well as insoluble matrices (16–18, 26, 29, 33).
Although the role of bound plasmin with spirochetes and other bacteria (6) in infection is important, there are other host proteases that could also be used to enhance dissemination and to mediate tissue damage. Matrix metalloproteinases (MMPs) are a large family of collagenases and gelatinases with broad substrate specificity that are involved in normal physiological processes ranging from developmental morphogenesis and tissue remodeling to neovascularization during wound healing (35, 37, 39, 42, 54). MMPs are generally secreted as latent forms, and their expression is tightly controlled, so that high levels of expression can result in pathological conditions. The plasminogen activation system is thought to mediate activation of some pro-MMPs, such as pro-MMP-9, through an activation cascade involving pro-MMP-3 (43) and may contribute to pathophysiological activation of pro-MMPs in vivo, as suggested by experiments performed with urokinase-deficient mice (10). During abnormal physiological processes, such as metastatic cancer, MMPs have been shown to mediate the spread of tumors through the breakdown of extracellular matrix components and basement membrane (52). Furthermore, metastatic potential is decreased if MMPs are inhibited via overexpression of tissue inhibitor of MMPs. MMPs have also been implicated in the pathogenesis of multiple sclerosis (MS) (2, 19, 38). MMP-9 has been shown to degrade collagen IV and thereby may also have an effect on the vasculature (36, 44, 45). MMP-9 can also break down myelin basic protein, and the generation of myelin peptides via MMP-9 is thought to lead to an autoreactive neuritis (41). In rheumatoid arthritis (RA), MMPs play a similar destructive role (5, 28, 30, 53). Monocyte/macrophage-derived MMP-1 and MMP-9 along with MMPs released from synoviocytes and neutrophils are thought to be responsible for collagen degradation and joint destruction in RA (11, 48, 51, 56). Thus, MMPs may be associated with many of the pathological processes and clinical manifestations that are part of Lyme disease and relapsing fever.
In the present study, Borrelia spirochetes are shown to upregulate and stimulate the activation of MMPs released from human cells in vitro and during experimental infection in mice in vivo. In addition, human peripheral blood monocytes were shown to enhance penetration of B. burgdorferi across extracellular matrix components, suggesting a possible role for MMPs in dissemination of Lyme disease and relapsing fever spirochetes during infection.
MATERIALS AND METHODS
Isolation of monocytes from human donors.
Human peripheral blood monocytes were isolated by sedimentation from whole blood collected from healthy volunteers into 7% EDTA (Sigma, St. Louis, Mo.) in pyrogen-free water. Blood was mixed with 6% Dextran T500 (Pharmacia, Uppsala, Sweden) in pyrogen-free water at a ratio of 4 ml of dextran to 40 ml of blood. The mixture was allowed to stand for 45 min (females) or 60 min (males) at room temperature to sediment erythrocytes and granulocytes. After 1 h, the upper phase containing the leukocyte-rich plasma was centrifuged and the cells were resuspended in one-third of the volume of the original plasma. Aliquots (2 ml) of resuspended cells were underlaid with 5 ml of Accudenze (Accurate Chemical and Scientific, Westbury, N.Y.) followed by centrifugation at 650 × g for 15 min at room temperature. The monocyte-rich band at the plasma-Accudenze interface was collected from each tube. Monocytes were washed once at 400 × g in calcium- and magnesium-free phosphate-buffered saline (PBS) containing 1% low-endotoxin bovine serum albumin (BSA) (Sigma) and 3 mM EDTA (pH 7.4). The monocytes were resuspended in PBS-BSA-EDTA and washed several times to remove platelets. Monocytes were then washed once in serum-free RPMI 1640 (Gibco-BRL, Grand Island, N.Y.) and resuspended at a concentration of 2 × 106 cells per ml, and 0.5 ml was plated into each well of a 24-well plate (Costar, Cambridge, Mass.). Monocytes were allowed to adhere before addition of spirochetes.
Determination of monocyte purity and viability.
Monocyte purity was assayed by fluorescence-activated cell sorting on a FACscan (Becton Dickinson, Mountain View, Calif.) using immunoglobulin G1 (IgG1) and IgG2 murine monoclonal antibodies (MAbs) to human CD45 and CD14, respectively (Becton Dickinson, Franklin Lakes, N.J.). Trypan blue dye exclusion was used at the end of each experiment to determine monocyte viability following B. burgdorferi coculture.
Human cell culture.
U937 cells were maintained in RPMI 1640 with 10% fetal bovine serum (Hyclone, Logan, Utah). Normal human epidermal keratinocytes (NHEKs), obtained at passage 3 from Clonetics (San Diego, Calif.), were grown in keratin growth medium (serum-free basal medium plus supplements; also obtained from Clonetics) and never used in experiments beyond passage 4. Human umbilical vein endothelial cells (HUVECs) were prepared and passaged as described (31) with modifications (47).
Neutrophil isolation and B. burgdorferi coculture.
Neutrophils were collected from human donors using a suspension of blood mixed with PBS and EDTA (3 mM) in a 1:1 ratio. Lymphoprep (Accurate) was warmed, and the blood-PBS-EDTA mixture was layered on top of Lymphoprep in Falcon 50-ml polypropylene conical tubes. Generally, 1 part Lymphoprep to 1.5 to 2 parts blood was layered and centrifuged at 400 × g for 30 min at 25°C. The pellet was saved, resuspended in 25 ml of cold PBS, and centrifuged again at 400 × g for 10 min at 10°C. The pellet was now resuspended in 20 ml of 0.2% NaCl for 45 s and then 20 ml of 1.6% NaCl for erythrocyte lysis. The mixture was then centrifuged at 400 × g for 10 min at 10°C. This step was repeated two to three times until a clean pellet was obtained. Neutrophils were counted and resuspended at a concentration of 106 neutrophils per ml in serum-free RPMI 1640. Three milliliters was plated in each well of a six-well plate. These cells were then coincubated with live B. burgdorferi for 30, 60, and 90 min at 25°C. Neutrophils were gently rotated with concentrations of spirochetes ranging from 0.3 to 8 per neutrophil.
Coculture of B. burgdorferi with peripheral blood monocytes and other human cells.
B. burgdorferi strain N40 (3), Borrelia hermsii low-passage DAH strain (a gift from John Leong, University of Massachusetts and Rocky Mountain Laboratory, Mo.), and infectious Borrelia crocidurae serotype C2 (a gift from Sven Bergstrom, University of Umea, Umea, Sweden) were cultured in serum-free BSK medium (Sigma). All lots were tested by the Limulus ameobocyte assay (Sigma) for the presence of lipopolysaccharide (LPS). All spirochetes were grown to logarithmic phase and harvested by centrifugation. In some experiments, B. burgdorferi cells were bound with human plasminogen and human urokinase as previously described (18). Borreliae were resuspended in RPMI 1640, and 10 μl of this suspension was added to each well of a 24-well plate containing 106 monocytes, U937 cells, HUVECs, or NHEKs. Borreliae were added at concentrations of 1, 10, 50, 100, or 500 spirochetes per monocyte, U937 cell, HUVEC, or NHEK in a final volume of 500 μl. All coculture experiments were carried out in each of the cells, normal culture medium but under serum-free conditions. Sham controls (uninoculated BSK medium that was treated identically to logarithmic cultures) were included with monocytes to control for exogenous LPS. Some experiments used polymyxin B sulfate (Sigma) at 35 μg/ml to control for exogenous LPS. LPS (100 ng/ml) (Difco, Detroit, Mich.) was also used with NHEKs as a positive control for pro-MMP-9 upregulation. All infected human cell cultures were then incubated at 37°C and 5% CO2.
Zymography.
Zymography was carried out by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in gels of 10% polyacrylamide (0.75-mm thickness) containing 0.1% gelatin. Conditioned medium (90 μl) was subjected to electrophoresis (20 mV) under nonreducing conditions. Supernatants were centrifuged first at low speed to remove eukaryotic cells and then at high speed to remove spirochetes prior to eletrophoresis. Gels were washed twice for 30 min in 200 ml of 2.5% Triton X-100 and then incubated at 37°C for 20 h in a calcium assay buffer as described (14). In the presence of SDS, the proenzymes can become enzymatically active and hence enable detection of pro-MMPs by zymography. Gels were stained with Coomassie brilliant blue R-250 (0.25% Coomassie brilliant blue in 10% acetic acid and 20% methanol) for 1 h, followed by destaining in 10% acetic acid and 20% methanol.
SDS-PAGE and immunoblot.
Conditioned medium (95 μl) was electrophoresed on 10% polyacrylamide gels at 20 mA. Prestained molecular weight standards were obtained from Gibco-BRL. Immunoblotting was carried out following protein transfer to nitrocellulose using methods previously described (16). Mouse MAbs raised against human pro-MMP-9 and MMP-1 were purchased from Oncogene Research Products (Cambridge, Mass.). MAbs to MMP-1 bound both latent and active forms of the enzyme (IgG2ak isotype) and recognized amino acids 332 to 350 (numbered from the propeptide) of human MMP-1. MAbs to pro-MMP-9 (isotype IgG1k) recognized an undetermined epitope of pro-MMP-9, not the active form of the enzyme. Nitrocellulose membranes with transferred proteins were incubated with the MAbs at 0.4 mg/ml. This was followed by incubation with goat alkaline phosphatase-conjugated anti-mouse IgG as the secondary antibody (Kirkegaard and Perry, Gaithersburg, Md.). Proteins recognized by MAbs were visualized by addition of nitro blue tetrazolium–5-bromo-4-chloro-3-indolylphosphate substrate (Kirkegaard and Perry).
RT-PCR of MMP-9 and β-actin.
U937 cells were were cocultured with 0, 10, and 100 B. burgdorferi organisms per cell. Total RNA was extracted at 48 h of coincubation with Trizol reagent (Gibco-BRL), following the manufacturer's guidelines. Phorbol-12-myristate-13-acetate (PMA), an agent known to activate macrophages and U937 cells (27, 55), was used as a positive control at a concentration of 150 nM. The Titan One Tube reverse transcription (RT)-PCR kit (Boehringer Mannheim) was used to amplify mRNA following the manufacturer's guidelines for a 9600 Perkin Elmer GeneAmp Thermocycler. Specific primers to mmp-9 were generated to target an internal region of the transcript. The mmp-9 forward primer utilized was 5′-GCCTGCACCACGGACGGTCGCTCC-3′ and the reverse primer utilized was 5′-GAGGTGCCGGATGCCATTCACGTC-3′. An internal region of the β-actin gene was also targeted utilizing the forward primer 5′-CCAAGGCCAACCGCGAGAAGATGAC-3′ and the reverse primer 5′-AGGGTACATGGTGGTGCCGCCAGAC-3′. Amplimers were separated by electrophoresis in 2.0% agarose gels and stained as previously described (25).
In vitro penetration assay using B. burgdorferi and human monocytes.
Biocoat cell culture inserts (for 24-well plates) were purchased from Becton Dickinson Labware (Bedford, Mass.) containing polyethylene tetraphalate membranes (0.45 or 1 μm), coated by the manufacturer with either collagen type I, collagen type IV, or laminin. When inserted into a well in a 24-well plate, this membrane formed a barrier, making an upper and lower chamber. Monocytes (3 × 105) in 250 μl of RPMI 1640 were pipetted into the upper cell culture insert chamber, and the cells were allowed to adhere for 30 min, followed by the addition of 108 B. burgdorferi organisms in 20 μl of RPMI 1640, which was also added to the top chamber. Before placing the cell culture insert containing the monocytes and B. burgdorferi into the well, 300 μl of RPMI 1640 was added to the lower chamber. B. burgdorferi penetration was quantified by direct enumeration of spirochetes that had migrated across the barrier into the lower chamber at 24 h by dark-field microscopy. In some experiments, MMP inhibitor I (Oncogene Research Products) was used at 300 μM final concentration in both the upper and lower chambers. This inhibitor did not affect the growth of log-phase cultures of B. burgdorferi at this concentration or the viability of monocytes, as indicated by Trypan blue dye exclusion at 24 h.
Infection of mice with relapsing fever spirochetes.
Six C3H/Hen mice were infected by subcutaneous inoculation with 104 uncultivable relapsing fever spirochetes previously isolated from humans and known to invade the brain and cause meningitis (1, 24, 26). Mice were sacrificed at peak spirochetemia (2 × 107/ml), and 15 μl of plasma was analyzed by gelatin zymography.
RESULTS
Borrelia spirochetes induce release of pro-MMP-9 from U937 cells.
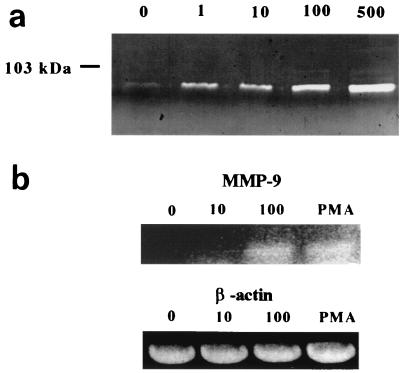
U937 cells were used first to investigate MMP-9 release into culture supernatant in response to stimulation with B. burgdorferi. U937 cells cocultured with B. burgdorferi for 24 h revealed elevated levels of MMP-9 in culture supernatant (Fig. 1a), as detected by zymography using gelatin as a substrate. These results were confirmed by Western blot (not shown). This release was not affected by 35 μg of polymyxin B, an inhibitor of LPS activation, per ml (data not shown). The induction of MMP-9 in U937 cells occurs in a dose-dependent manner in response to increasing numbers of B. burgdorferi. Upregulation and release of pro-MMP-9 occurred at the transcriptional level, as shown by RT-PCR using specific primers to MMP-9 (Fig. 1b). Transcription of β-actin was shown to be constitutive in U937 cells, and this expression was not influenced during B. burgdorferi coculture. The same results were also observed when either New World or Old World relapsing fever borreliae (B. hermsii and B. crocidurae, respectively) were cocultured under the same conditions with U937 cells and the supernatant was analyzed by gelatin zymography (data not shown).
FIG. 1.
Gelatin zymogram of pro-MMP-9 release into culture supernatant in response to B. burgdorferi by U937 cells. (a) U937 cells were cultured with either 0 (no spirochetes), 1, 10, 100, or 500 B. burgdorferi (strain N40) per cell. (b) Increasing levels of mmp-9 mRNA were detected in response to 0, 10, and 100 B. burgdorferi per U937 cell by RT-PCR using mmp-9-specific primers. PMA was used a positive control. Levels of β-actin mRNA were not influenced by increasing numbers of B. burgdorferi.
B. burgdorferi spirochetes induce release of pro-MMP-9 from human peripheral blood monocytes.
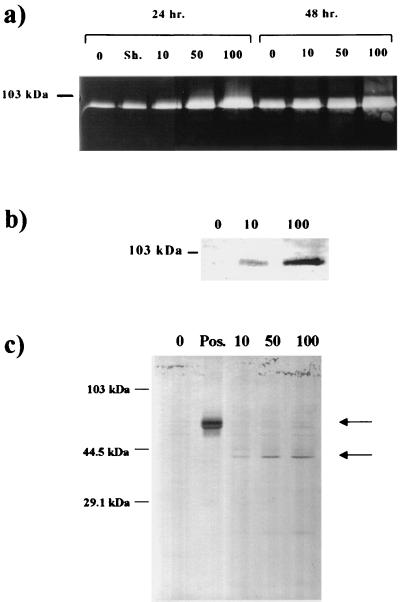
Gelatin zymography revealed elevated levels of MMP-9 in conditioned medium when human monocytes were cocultured with 10, 50, and 100 B. burgdorferi per cell for 24 and 48 h(Fig. 2a). A sham-infected control was used to control for the possibility of LPS in the bacterial media. Western blots of 48-h culture supernatants, using MAbs to MMP-9, confirmed that the gelatinolytic activity observed at 92 kDa by zymography was in fact MMP-9 (Fig. 2b). In addition to MMP-9, increasing levels of active MMP-1 were also detected in culture supernatant when human peripheral blood monocytes were cocultured with increasing numbers of B. burgdorferi for 48 h as detected by Western blot (Fig. 2c). Viability of monocytes at 48 h was determined by Trypan blue dye exclusion to ensure experimental validity. Wells treated with 100 spirochetes per monocyte ranged in viability from 84 to 89% at 48 h, compared to control wells (no addition of spirochetes) with a 48-h range of 93 to 96%.
FIG. 2.
B. burgdorferi upregulates release of MMP-9 and MMP-1 from human peripheral blood monocytes. (a) Gelatin zymography of human peripheral blood monocytes, showing increasing levels of pro-MMP-9 released following coculture with 0 (no spirochetes), 10, 50, or 100 B. burgdorferi per cell. Sh., sham-infected control. (b) Western blot analysis showing that the gelatinolytic activity observed at 92 kDa in panel is pro-MMP-9. (c) Western blot of pro- and active MMP-1 from human peripheral blood monocytes following coculture with 0 (no spirochetes), 10, 50, or 100 B. burgdorferi per cell. Pos., positive control (bovine serum). Top arrow, latent forms of MMP-1 (57 or 52 kDa); bottom arrow, active forms (46 or 42 kDa).
Human keratinocytes but not human endothelial cells release pro-MMP-9 in response to B. burgdorferi.
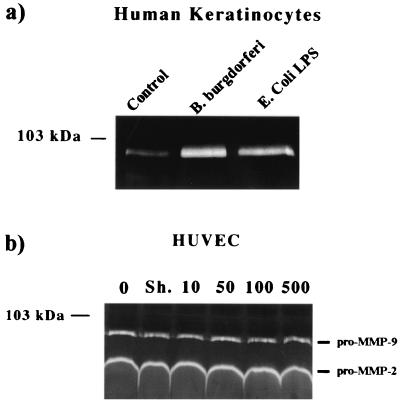
Primary human keratinocytes (NHEKs), when cocultured with B. burgdorferi or LPS, revealed elevated levels of pro-MMP-9 in the culture supernatant (Fig. 3a). This was in contrast to HUVECs, which released both pro-MMP-9 and pro-MMP-2 constitutively into the culture supernatant (Fig. 3b). Levels as high as 100 and 500 B. burgdorferi per endothelial cell failed to influence release of pro-MMP-9 or pro-MMP-2 into the culture supernatant as detected by zymography (Fig. 3a).
FIG. 3.
B. burgdorferi upregulates the release of pro-MMP-9 in human keratinocytes but not endothelial cells. (a) Gelatin zymography of culture supernatant from primary human keratinocytes following coculture with 50 B. burgdorferi per cell and with 100 ng of LPS, showing release of pro-MMP-9. Control received no spirochetes or LPS. (b) Gelatin zymography of culture supernatant from primary human endothelial cells following coculture with 0 (no spirochetes), 10, 50, 100, and 500 B. burgdorferi, showing constitutive release of both pro-MMP-9 and pro-MMP-2. Sh., sham-infected control.
Neutrophils release MMP-9 and 130-kDa protein with gelatinolytic activity in response to B. burgdorferi.
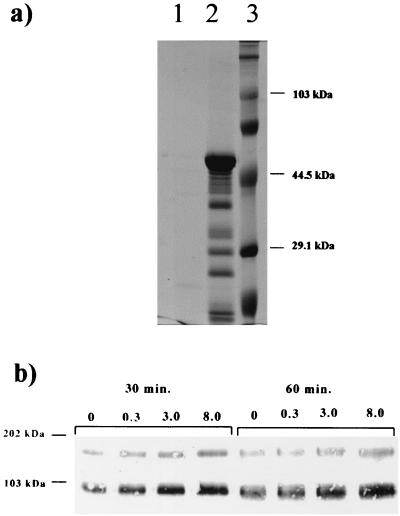
Human neutrophils cocultured with 8 B. burgdorferi per neutrophil released a broad range of peptides into the culture medium relative to unstimulated neutrophils, as detected by SDS-PAGE (Fig. 4a). Pro-MMP-9 and a 130-kDa protein were also both detected in the culture supernatant at 30 and 60 min, as demonstrated by Western blot (Fig. 4b) using the same MAb to MMP-9 used in the previous monocyte experiments. This response appears to be dose dependent (Fig. 4b), increasing with greater numbers of B. burgdorferi. Both of these proteins also exhibited gelatinolytic activity at identical molecular weights, as determined by zymography (data not shown). A 130-kDa protein with gelatinolytic activity and MMP-9 have recently been detected in the cerebrospinal fluid of human patients with Lyme disease (32, 40).
FIG. 4.
Human neutrophils upregulate the release of pro-MMP-9 and cross-reactive complexes in response to B. burgdorferi. (a) SDS-PAGE of culture supernatant of human neutrophils cocultured with 8 live B. burgdorferi per neutrophil. Lane 1, no spirochetes; lane 2, with spirochetes; lane 3, molecular size standards. (b) Western blot of culture supernatant from human neutrophils following coculture with 0, 0.3, 3.0, or 8.0 B. burgdorferi per cell for either 30 or 60 min, showing the presence of pro-MMP-9 and a cross-reactive 130-kDa protein.
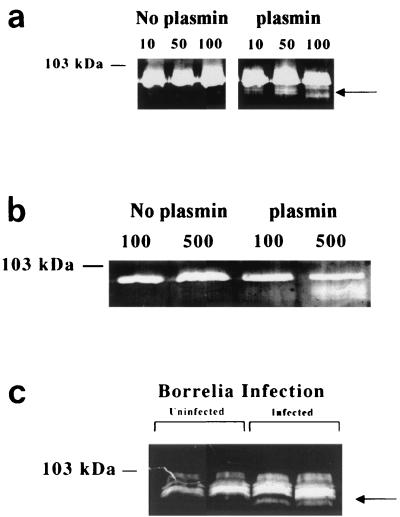
Plasmin on the surface of Borrelia spirochetes activates pro-MMP-9 released from human peripheral blood monocytes and U937 cells.
B. burgdorferi organisms were incubated with human plasminogen and urokinase and washed as previously described to determine if activation of pro-MMP-9 was influenced by spirochetes bound with plasmin. These spirochetes when cocultured with monocytes, induced the activation of pro-MMP-9 (92 kDa) to its active form (82 to 84 kDa) at 48 h with 10 organisms per cell (Fig. 5a). At 24 h, activation was noted with 100 B. burgdorferi per monocyte (data not shown). These results were also observed in the human monocyte-like U937 cells at 48 h (Fig. 5b), although detectable activation was not observed with less than 100 B. burgdorferi spirochetes per U937 cell. Active forms of MMP-9 were also not observed in sham-infected controls (to control for unbound plasmin) or when spirochetes were coated alone with either urokinase or plasminogen (data not shown).
FIG. 5.
Plasminogen and urokinase bound to spirochetes activate MMPs. (a) Gelatin zymography of culture supernatant from human peripheral blood monocytes cocultured with 10, 50, and 100 B. burgdorferi per cell with or without bound plasmin. Borreliae with bound plasmin resulted in elevated levels of active MMP-9 (arrow). (b) Gelatin zymography of culture supernatant from U937 cells cocultured with 100 and 500 B. burgdorferi per cell with or without bound plasmin. Borreliae with bound plasmin resulted in elevated levels of active MMP-9. (c) Mice infected with a relapsing fever borrelia exhibited elevated plasma levels of active MMP-9 (arrow) at peak spirochetemia.
Experimentally infected mice have elevated levels of active MMP-9 in plasma.
Previous reports indicated that patients with gram-negative sepsis have elevated levels of pro-MMP-9 as well as the active form in the plasma. Although infection with B. burgdorferi in humans does not generally lead to overt spirochetemia, the relapsing fever borreliae reach high numbers in the blood of humans as well as animals and have also been shown to interact with the plasminogen activation system (26). In Fig. 5c, mice infected with a relapsing fever borrelia exhibited elevated plasma levels of active MMP-9 (arrow) relative to uninfected, litter mate control mice in 15 μl of plasma, as detected by zymography.
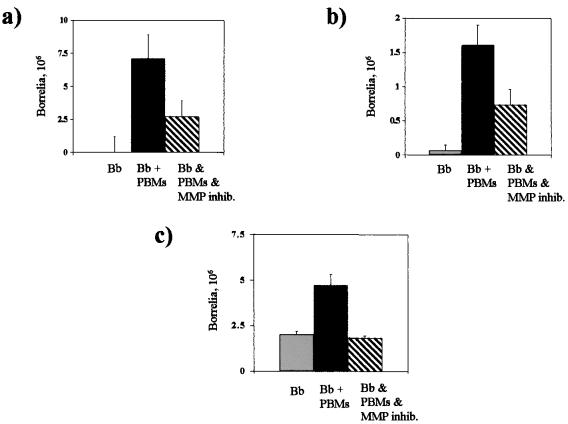
Human peripheral blood monocytes enhance penetration of B. burgdorferi across extracellular matrix components.
In migration assays using cell culture inserts for 24-well plates, human monocytes freshly isolated from donors enhanced the penetration of B. burgdorferi through the extracellular matrix components collagen type I (Fig. 6a), laminin (Fig. 6b), and collagen type IV (Fig. 6c). At 24 h, cell culture inserts containing both human monocytes and spirochetes revealed that the number of spirochetes that were counted in the lower chambers of all barriers tested (Fig. 6a, b, and c) was significantly greater than that in control wells just containing borreliae. The level of inhibition observed with this MMP inhibitor at 300 μM (Fig. 6) suggests that other factors, such as the release of non-MMP proteases from monocytes or possible chemotaxis of the borreliae away from the monocytes, may also be involved in addition to the MMP-mediated extracellular matrix degradation that may enhance penetration of the Lyme disease spirochete. The above results were also shown with U937 cells using conditions identical to those described for human peripheral blood monocytes (data not shown).
FIG. 6.
Human peripheral blood monocytes enhance penetration of B. burgdorferi (Bb) across extracellular matrix components. Monocytes (PBMs) enhanced penetration of B. burgdorferi at 24 h across (a) collagen type I, (b) laminin, and (c) collagen type IV. Inhibition of penetration was observed at 24 h across (a) collagen type I, (b) laminin, and (c) collagen type IV using an MMP inhibitor (inhib.).
DISCUSSION
Previous work in our laboratory has shown that plasminogen, which is the zymogen of plasmin, is an important host factor that contributes to dissemination of borreliae in the tick and vertebrate host during infection (16). Plasminogen-deficient mice challenged with Lyme disease spirochetes generally undergo a reduced infection, as evidenced by culture and PCR of infected tissues as well as xenodiagnosis using uninfected ticks (16). Plasminogen-deficient mice infected with relapsing fever spirochetes have less severe meningitis, as determined by reduced mononuclear cell infiltration (26). Mononuclear cells and neutrophils are known to express many different MMPs (34) that are often involved in host tissue destruction in various inflammatory diseases and during infection processes such as tuberculosis (13) and periodontal disease (20). Monocyte/macrophage infiltration is prominently observed in tissue specimens infected with borreliae in both humans and experimentally infected animals, and it is known that lipopeptides that are surrogates for outer surface lipoproteins can activate monocytes (46).
Although B. burgdorferi possesses two zinc proteases according to its genomic sequence (21), we were unable to detect endogenous collagenolytic activity using various conditions (17). It is known that plasmin is able to activate procollagenases in vivo (10), although this is likely to occur through a cascade involving MMP-3. This prompted the speculation that plasmin on the surface of the Borrelia spirochetes could be involved in activation of the same host molecules that mediate joint destruction in RA and neuronal degeneration in MS (12).
The results presented in this study demonstrate that borreliae are capable of upregulating and activating human inflammatory cell MMPs. In this regard, the pathophysiological process observed in Lyme disease patients is similar to that in persons with chronic inflammatory diseases such as RA and MS.
Although active MMP-9 was only observed in the presence of B. burgdorferi with surface-bound plasmin (Fig. 4a and b), the presence of active MMP-1 in the medium indicated (Fig. 2c) that human monocytes have the capacity to generate an active form of this enzyme in response to B. burgdorferi in the absence of plasmin. The presence of the active form of MMP-1 in the absence of plasmin suggests two different activation cascades for MMP-1 and MMP-9. Release of collagenases such as MMP-1 by inflammatory cells in response to Lyme disease borreliae could also explain the arthritis and joint destruction observed in Lyme disease patients.
Interestingly, the upregulation and activation of MMPs may work, at least initially, in favor of dissemination of the spirochetes. In this regard, utilization of MMPs may not be unique to spirochetes but could be a broadly deployed mechanism of many pathogenic microorganisms. These molecules could be used to penetrate various host barriers that would otherwise preclude infection and transmission to new hosts, as suggested by enhanced penetration across collagen I, laminin, and collagen IV (Fig. 6). Inhibition of B. burgdorferi penetration across these extracellular matrix components using an MMP inhibitor (Fig. 6) suggests that active MMPs released in response to the presence of the spirochetes may play a role in invasion by B. burgdorferi. In the skin, MMPs released specifically from human keratinocytes and inflammatory cells in response to B. burgdorferi could be activated by a cascade involving plasmin stabilized on the microbial surface. In a synergistic manner, these broad-spectrum proteases could facilitate the dissemination of the spirochete from the site of a tick bite through the skin to distant organ sites, thereby explaining a possible mechanism of bacterial migration in the expanding skin lesion that is often observed in Lyme disease patients.
Mononuclear cell infiltration is the prominent finding in tissues affected by borreliae. In light of the data presented in this study, it may be of interest to test the hypothesis that mononuclear cells enhance the spread of microorganisms through release of potent enzymes, such as MMPs, and possibly other enzymes normally engaged in tissue remodeling and immune cell extravasation (22). These enzymes, and the activation cascade mediated by the spirochete, could enable the organism to penetrate the tight, selectively impervious endothelial junctions of the blood-brain barrier. Collagen type IV, which is degraded by MMP-9, is an important constituent of the blood-brain barrier along with laminin, and neither is efficiently degraded by plasmin on the surface of borreliae (17). Degradation of collagen type IV along with laminin, via upregulation and activation of MMPs, may be a critical step in the process of bacterial brain invasion and resultant meningitis. This could explain the role of elevated levels of active MMP-9 observed in mouse plasma at peak spirochetemia (Fig. 5c). Active MMP-9 in the plasma of infected mice at peak spirochetemia may be generated by a cascade initiated by microbially bound plasmin in vivo as suggested by activation of pro-MMP-9 by B. burgdorferi with surface-bound plasmin in vitro (Fig. 5a and b). This would explain, perhaps in part, why plasminogen-deficient mice are protected during borrelia infection (16, 26).
ACKNOWLEDGMENTS
We thank Charles Thill for expert technical assistance throughout this study. We also thank Wayne Bellucci, Elizebeth Scotto-Lavino, and Sanford Simon for assistance with neutrophil isolation and keratinocyte culture. In addition, we are grateful for help from Edna Gergel and Martha Furie with monocyte and HUVEC isolation and culture.
This study was supported by grant AR40445 from the National Institutes of Health and by a grant from the Mathers Foundation to J.L.B.
REFERENCES
- 1.Anda P, Sanchez-Yebra W, del Mar Vitutia M, Perez Pastrana E, Rodriguez I, Miller N S, Backenson P B, Benach J L. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet. 1996;348:162–165. doi: 10.1016/s0140-6736(96)02332-x. [DOI] [PubMed] [Google Scholar]
- 2.Anthony D C, Ferguson B, Matyzak M K, Miller K M, Esiri M M, Perry V H. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- 3.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease following intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 4.Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 5.Blaser J, Triebel S, Maasjosthusmann U, Romisch J, Krahl-Mateblowski U, Freudenberg W, Fricke R, Tschesche H. Determination of metalloproteinases, plasminogen-activators and their inhibitors in the synovial fluids of patients with rheumatoid arthritis during chemical synoviorthesis. Clin Chim Acta. 1996;244:17–33. doi: 10.1016/0009-8981(95)06172-x. [DOI] [PubMed] [Google Scholar]
- 6.Boyle M D, Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb Haemostasis. 1997;77:1–10. [PubMed] [Google Scholar]
- 7.Bugge T H, Flick M J, Daugherty C C, Degen J L. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 9.Cadavid D, Barbour A G. Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin Infect Dis. 1998;26:151–164. doi: 10.1086/516276. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 11.Cawston T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol Med Today. 1998;4:130–137. doi: 10.1016/s1357-4310(97)01192-1. [DOI] [PubMed] [Google Scholar]
- 12.Chandler S, Miller K M, Clements J M, Lury J, Corkill D, Anthony D C, Adams S E, Gearing A J. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 13.Chang J C, Wysocki A, Tchou-Wong K M, Moskowitz N, Zhang Y, Rom W N. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51:306–311. doi: 10.1136/thx.51.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J M, Aimes R T, Ward G R, Youngleib G L, Quigley J P. Isolation and characterization of a 70-kDa metalloprotease (gelatinase) that is elevated in Rous sarcoma virus-transformed chicken embryo fibroblasts. J Biol Chem. 1991;266:5113–5121. [PubMed] [Google Scholar]
- 15.Coleman J L, Benach J L. Use of the plasminogen activation system by microorganisms. J Lab Clin Med. 1999;134:567–576. doi: 10.1016/s0022-2143(99)90095-1. [DOI] [PubMed] [Google Scholar]
- 16.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of Borrelia burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 17.Coleman J L, Roemer E J, Benach J L. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect Immun. 1999;68:3929–3936. doi: 10.1128/iai.67.8.3929-3936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman J L, Sellati T J, Testa J E, Kew R R, Furie M B, Benach J L. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cossins J A, Clements J M, Ford J, Miller K M, Pigott R, Vos W, Van der Valk P, De Groot C J. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol (Berlin) 1997;94:590–598. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- 20.Ding Y, Uitto V J, Haapasalo M, Lounatmaa K, Konttinen Y T, Salo T, Grenier D, Sorsa T. Membrane components of Treponema denticola trigger proteinase release from human polymorphonuclear leukocytes. J Dent Res. 1996;75:1986–1993. doi: 10.1177/00220345960750121101. [DOI] [PubMed] [Google Scholar]
- 21.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs H, Simon M M, Wallich R, Bechtel M, Kramer M D. Borrelia burgdorferi induces secretion of pro-urokinase-type plasminogen activator by human monocytes. Infect Immun. 1996;64:4307–4312. doi: 10.1128/iai.64.10.4307-4312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Monco J C, Benach J L. Lyme neuroborreliosis. Ann Neurol. 1995;37:691–702. doi: 10.1002/ana.410370602. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Monco J C, Miller N S, Backenson P B, Anda P, Benach J L. A mouse model of Borrelia meningitis after intradermal injection. J Infect Dis. 1997;175:1243–1245. doi: 10.1086/593681. [DOI] [PubMed] [Google Scholar]
- 25.Gebbia J A, Backenson P B, Coleman J L, Anda P, Benach J L. Glycolytic enzyme operon of Borrelia burgdorferi: characterization and evolutionary implications. Gene. 1997;188:221–228. doi: 10.1016/s0378-1119(96)00811-6. [DOI] [PubMed] [Google Scholar]
- 26.Gebbia J A, Monco J C, Degen J L, Bugge T H, Benach J L. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J Clin Investig. 1999;103:81–87. doi: 10.1172/JCI5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genton C, Kruithof E K, Schleuning W D. Phorbol ester induces the biosynthesis of glycosylated and nonglycosylated plasminogen activator inhibitor 2 in high excess over urokinase-type plasminogen activator in human U-937 lymphoma cells. J Cell Biol. 1987;104:705–712. doi: 10.1083/jcb.104.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruber B L, Sorbi D, French D L, Marchese M J, Nuovo G J, Kew R R, Arbeit L A. Markedly elevated serum MMP-9 (gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker. Clin Immunol Immunopathol. 1996;78:161–171. doi: 10.1006/clin.1996.0025. [DOI] [PubMed] [Google Scholar]
- 29.Hu L T, Pratt S D, Perides G, Katz L, Rogers R A, Klempner M S. Isolation, cloning, and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi Infect. Immun. 1997;65:4989–4995. doi: 10.1128/iai.65.12.4989-4995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson C J, Arkell J, Nguyen M. Rheumatoid synovial endothelial cells secrete decreased levels of tissue inhibitor of MMP (TIMP1) Ann Rheum Dis. 1998;57:158–161. doi: 10.1136/ard.57.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchner A, Koedel U, Fingerle V, Paul R, Wilske B, Pfister H W. Upregulation of matrix metalloproteinase-9 in the cerebrospinal fluid of patients with acute lyme neuroborreliosis. J Neurol Neurosurg Psychiatry. 2000;68:368–371. doi: 10.1136/jnnp.68.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klempner M S, Noring R, Epstein M P, McCloud B, Rogers R A. Binding of human urokinase type plasminogen activator and plasminogen to Borrelia species. J Infect Dis. 1996;174:97–104. doi: 10.1093/infdis/174.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Machein U, Conca W. Expression of several matrix metalloproteinase genes in human monocytic cells. Adv Exp Med Biol. 1997;421:247–251. doi: 10.1007/978-1-4757-9613-1_32. [DOI] [PubMed] [Google Scholar]
- 35.Matrisian L M. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 36.Mun-Bryce S, Rosenberg G A. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274:R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- 37.Nagase H, Woessner J F., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 38.Ozenci V, Rinaldi L, Teleshova N, Matusevicius D, Kivisakk P, Kouwenhoven M, Link H. Metalloproteinases and their tissue inhibitors in multiple sclerosis. J Autoimmun. 1999;12:297–303. doi: 10.1006/jaut.1999.0285. [DOI] [PubMed] [Google Scholar]
- 39.Pagenstecher A, Stalder A K, Kincaid C L, Shapiro S D, Campbell I L. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- 40.Perides G, Charness M E, Tanner L M, Peter O, Satz N, Steere A C, Klempner M S. Matrix metalloproteinases in the cerebrospinal fluid of patients with Lyme neuroborreliosis. J Infect Dis. 1998;177:401–408. doi: 10.1086/514198. [DOI] [PubMed] [Google Scholar]
- 41.Proost P, Van Damme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun. 1993;192:1175–1181. doi: 10.1006/bbrc.1993.1540. [DOI] [PubMed] [Google Scholar]
- 42.Quigley J P, Braithwaite R S, Armstrong P B. Matrix metalloproteases of the developing sea urchin embryo. Differentiation. 1993;54:19–23. doi: 10.1111/j.1432-0436.1993.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 43.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French D L, Quigley J P. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg G A. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995;12:833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg G A, Estrada E Y, Dencoff J E. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 46.Sellati T J, Bouis D A, Kitchens R L, Darveau R P, Pugin J, Ulevitch R J, Gangloff S C, Goyert S M, Norgard M V, Radolf J D. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- 47.Sellati T J, Burns M J, Ficazzola M A, Furie M B. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect Immun. 1995;63:4439–4447. doi: 10.1128/iai.63.11.4439-4447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sopata I, Wize J, Filipowicz-Sosnowska A, Stanislawska-Biernat E, Brzezinska B, Maslinski S. Neutrophil gelatinase levels in plasma and synovial fluid of patients with rheumatic diseases. Rheumatol Int. 1995;15:9–14. doi: 10.1007/BF00286763. [DOI] [PubMed] [Google Scholar]
- 49.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 50.Steere A C, Malawista S E, Hardin J A, Ruddy S, Askenase W, Andiman W A. Erythema chronicum migrans and Lyme arthritis: the enlarging clinical spectrum. Ann Intern Med. 1977;86:685–698. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- 51.Tetlow L C, Lees M, Ogata Y, Nagase H, Woolley D E. Differential expression of gelatinase B (MMP-9) and stromelysin-1 (MMP-3) by rheumatoid synovial cells in vitro and in vivo. Rheumatol Int. 1993;13:53–59. doi: 10.1007/BF00307734. [DOI] [PubMed] [Google Scholar]
- 52.Tonn J C, Kerkau S, Hanke A, Bouterfa H, Mueller J G, Wagner S, Vince G H, Roosen K. Effect of synthetic matrix-metalloproteinase inhibitors on invasive capacity and proliferation of human malignant gliomas in vitro. Int J Cancer. 1999;80:764–772. doi: 10.1002/(sici)1097-0215(19990301)80:5<764::aid-ijc22>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 53.Tsuchiya K, Maloney W J, Vu T, Hoffman A R, Schurman D J, Smith R L. RT-PCR analysis of MMP-9 expression in human articular cartilage chondrocytes and synovial fluid cells. Biotechnol Histochem. 1996;71:208–213. doi: 10.3109/10520299609117161. [DOI] [PubMed] [Google Scholar]
- 54.Uhm J H, Dooley N P, Oh L Y, Yong V W. Oligodendrocytes utilize a matrix metalloproteinase, MMP-9, to extend processes along an astrocyte extracellular matrix. Glia. 1998;22:53–63. doi: 10.1002/(sici)1098-1136(199801)22:1<53::aid-glia5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 55.Vassalli J D, Hamilton J, Reich E. Macrophage plasminogen activator: induction by concanavalin A and phorbol myristate acetate. Cell. 1977;11:695–705. doi: 10.1016/0092-8674(77)90086-1. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe Y, Shimamori Y, Fujii N, Yamaguchi R, Fujimoto Y, Matsuno H. Correlation between the appearance of gelatinases in the synovial fluid of patients with rheumatoid arthritis and polymorphonuclear elastase, stromelysin-1, and the tissue inhibitor of metalloproteinase-1. Clin Exp Rheumatol. 1997;15:255–261. [PubMed] [Google Scholar]