Abstract
Background
Bile acid diarrhoea (BAD) causes chronic diarrhoea and is primarily treated pharmacologically. This systematic review aimed to evaluate the effectiveness of non‐pharmacological therapies for evidence‐based management of BAD in adults.
Methods
A systematic review of the medical literature was performed from 1975 to 13 July 2021 to identify studies on diet, psychological, and exercise therapies that met diagnostic criteria for BAD in adults with diarrhoea. Effectiveness was judged by responder or improvement in diarrhoea at study endpoint according to each study's definition of diarrhoea. Therapeutic effect on abdominal pain and flatulence was also measured. Risk of bias was assessed using the Risk Of Bias In Non‐Randomised Studies of Interventions tool. A narrative review was conducted using ‘Synthesis Without Meta‐analysis’ guidance. Certainty of the evidence was assessed using Grading of Recommendations Assessment, Development, and Evaluation.
Results
Eight prospective cohort studies were identified on diet therapies from 2 weeks to over 2 years involving 192 patients. No psychological or exercise therapies were found. Carbohydrate modification (one study, n = 2) in primary BAD, and dietary fat intake reductions (five studies, n = 181) and an exclusive elemental diet therapy (two studies, n = 9) in secondary BAD, showed beneficial directions of effect on diarrhoea, abdominal pain, and flatulence. Risks of bias for each study and across studies for each therapy type were serious. Certainty of the evidence was very low for all outcomes.
Conclusions
No conclusions could be drawn on the effectiveness of diet, psychological, or exercise therapies on diarrhoea, abdominal pain, and flatulence for the management of BAD in adults. High‐quality randomised controlled trials are needed.
Keywords: bile acid diarrhoea, bile acid malabsorption, diet, exercise, psychotherapy, systematic review
Key points
We do not know whether non‐pharmacological therapies can improve diarrhoeal symptoms in adults with bile acid diarrhoea.
Available data from cohort studies outlined in this systematic review found that: (i) in primary bile acid diarrhoea, lactose and/or sorbitol and fructose intake reductions provided very low‐certainty evidence of a beneficial effect after optimisation with colestyramine; (ii) in secondary bile acid diarrhoea, dietary fat intake reduction and exclusive oral nutritional supplementation with elemental formula provided very low‐certainty evidence of beneficial effects as sole treatment or after optimisation with bile acid sequestrants; and (iii) there was no evidence on psychological or exercise therapies.
We need high‐quality studies to evaluate the acceptability, feasibility, and effectiveness of diet, psychological, and exercise therapies adjunctive or separate to medication for the management of BAD in adults.
INTRODUCTION
Bile acid diarrhoea (BAD) is a chronic gastrointestinal disorder of bile acid‐induced diarrhoea as a result of dyshomeostasis of enterohepatic bile acid recycling, the symptoms of which usually improve by bile acid sequestrant administration. 1 , 2 The cause of primary BAD (also known as Type 2 BAD) 3 is often idiopathic 4 and BAD was indirectly estimated to affect at least one in 100 of the Western adult population. 5 Secondary BAD, comprising Types 1 and 3, 3 is secondary to inadequate reabsorption of bile acids as a result of disease states affecting the ileum or another organ, respectively. 6 The prevalence is unclear and uncertainties include lack of data on incidence comparisons between countries because of limited screening. 6
Meal ingestion prompts the ejection of conjugated bile acids that constitute two‐thirds of the weight of aqueous bile 7 from the gallbladder into the duodenum. Bile acids are amphiphilic, enteroendocrine hormones that are essential for the mixed micellar solubilisation and absorption of ingested dietary fats, as well as fat‐soluble vitamins A, D2, E, and K, along the small intestine. 8 To complete one enterohepatic cycle, unused bile acids are actively absorbed in the ileum by the apical sodium bile acid transporter and transported back to the liver via portal venous blood, incurring 5% loss of conjugated bile acids to the colon daily. 9 A negative feedback system 10 enables replacement of this loss via further hepatic bile acid synthesis.
In primary BAD, hepatic bile acid synthesis is excessive. 11 Deficiency in serum fibroblast growth factor 19 produced by ileal enterocytes is hypothesised as causative, leading to ileal absorptive capacity saturation, increased colonic spillover, and diarrhoea. 12 Fibroblast growth factor 19 is low during fasting in healthy adults, 13 whereas in primary BAD, fibroblast growth factor 19 was found to fail to increase to inhibit hepatic bile acid synthesis in response to meal ingestion. 12 Excessive prosecretory bile acids in the colon induce watery diarrhoea following their bacterial deconjugation and dihydroxylation. 14 , 15 After cholecystectomy, during both fasting and post‐prandially, negative feedback inhibition becomes continuous to maintain cycling balance with reductions in conjugated bile acid pool sizes and circulation rate increases. 16 After ileal resection of the last 100 cm of the ileum, malabsorption of both conjugated bile acids and dietary fats has been shown to cause steatorrhoea. 17 , 18
Treatment effectiveness requires establishing an accurate diagnosis, which is challenging as a result of low‐quality evidence supporting specific diagnostic tests and varying availability. 1 , 19 , 20 Approximately 30% of adults with primary BAD have been previously diagnosed with diarrhoea‐predominant irritable bowel syndrome, 21 , 22 although BAD and irritable bowel syndrome may co‐exist. 23 , 24 , 25 75Selenium homocholic acid conjugated with taurine (SeHCAT) testing is the current ‘gold standard’ method to diagnose BAD 6 but is based on treatment response from low‐quality evidence. 26 The test involves ingestion of a capsule containing a radiolabelled bile acid analogue, SeHCAT, to calculate the percentage of bile acid retained in the body after seven days and whether diarrhoea is a result of excessive faecal excretion of bile acids. Severe, moderate, and mild BAD are < 5%, < 10%, and < 15% retention of SeHCAT, respectively. 21 Recent observational data using this test showed that primary BAD is also a painful disorder according to Rome IV criteria (abdominal pain frequency at least once a week). From a cohort of 184 patients presenting in secondary care with diarrhoea‐predominant irritable bowel syndrome (76%) or chronic diarrhoea of presumed functional origin (24%), 53 out of 70 (76%) of the patients diagnosed with BAD also had abdominal pain. 27 From data collected via an online survey, abdominal pain (recorded as always, mostly, or fairly often) was present in 77% of 91 respondents with a self‐reported diagnosis of BAD from a BAD support group in the UK of over 1300 members. 28
Although there is an integrated healthcare approach of medication, diet, and behavioural interventions for irritable bowel syndrome, 29 treatment for BAD is limited to life‐long bile acid sequestrants or alternative anti‐diarrhoeal drugs. 19 Medication side effects include poor tolerance, 30 , 31 , 32 constipation, abdominal pain, nausea, and bloating. 33 , 34 , 35 , 36 , 37 The proportion of adults successfully treated pharmacologically was estimated to be 70% (range 63%–100%), as determined from a systematic review performed in 2013, totalling 1223 patients from 18 studies. 38 However, despite medication optimisation, symptoms may persist. 28 , 30 In a Danish retrospective survey, unaltered or worsened diarrhoea was identified amongst 235 out of 377 (64%) respondents. 30 These data were collected over 13 years in BAD diagnosed by SeHCAT. In the online UK cross‐sectional survey, 33% reported persisting diarrhoea, 46% reported abdominal pain, 60% reported flatulence, 71% reported extreme tiredness, and 55% reported reduced activity/exercise levels amongst other symptoms (recorded as always, mostly, or fairly often). 28 Dietary modifications including low‐fat, gluten‐free, low‐carbohydrate, lactose‐free, and wheat‐free diets were also self‐reported, although effectiveness on diarrhoea or individual symptoms was not explored. Clinical practice guidelines developed by an international group of gastroenterologists 19 reported that ‘low‐fat dietary interventions can improve gastrointestinal symptoms for some patients’. This was based on evidence from one cohort study 39 but had no documented appraisal and no dietary recommendations for clinical practice or research. In a systematic review of the management of chronic diarrhoea as a result of BAD, 38 no diet studies were included and one was excluded. 40 An investigation of the effectiveness of non‐pharmacological treatments has not been undertaken to date. Therefore, there is a need to identify all non‐pharmacological interventions and to critically appraise benefits and harms on diarrhoeal symptoms in BAD. The presemt study aimed to evaluate the effectiveness of diet, psychological, and exercise therapies on diarrhoea, abdominal pain, and flatulence in adults with BAD by performing a systematic review.
METHODS
The methods for performing this systematic review were specified in a protocol following Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) for Protocols standards, 41 registered with the International Prospective Register of Systematic Reviews (CRD42020188328). The PRISMA 2020 Checklist 42 was followed in the reporting of this review.
Eligibility criteria
The inclusion and exclusion criteria for the review are specified in Table 1. Studies were included if they examined the effect on diarrhoea of diet, psychological, or exercise therapies in adults (≥ 16 years) with BAD and diarrhoea. The PICO framework 43 was used to structure reporting and includes on study design and reporting of the study. We intended to include all study designs from 1975 onwards in humans, excluding single cases. This was because findings from a prior systematic review showed low‐quality evidence consisting of only one randomised controlled trial (RCT) out of 28 studies examining pharmacotherapies, and did not identify or include two cohort studies on diet. 44 , 45 Additionally, diarrhoea was variably defined and often vaguely described across these studies. Therefore, we included studies in which it was clear from patient or clinician‐reported description that participants had diarrhoea at study start, and an outcome on diarrhoea was reported at study endpoint. In this review, diarrhoea relating to stool consistency was defined according to the validated Bristol Stool Form Scale (BSFS) as stool form Types 6 or 7. 46 , 47 BAD diagnostic tests used in clinical practice and research are described elsewhere. 48 , 49 , 50 , 51 In this review, aside from SeHCAT, three tests were included. The serum C4 test measures fasting serum 7a‐hydroxy‐4‐cholesten‐3‐one (C4), a direct measure of hepatic bile acid synthesis. Faecal bile acid excretion measures total bile acids from stool collected during the last 48 h of dietary modification to a daily fat intake of 100 g for four days. The 14C‐glycocholate stool test (no longer used) measured bile salt excretion via the activity of 14C‐labelled cholic acid in a faecal collection, from when an intravenous saline infusion was started, to 24 h later when carmine red was given orally as a faecal marker to show collection endpoint. 52 An amendment was made to the protocol to include studies in patients with diarrhoea who had had ileal resections or ileal disease in the absence of a test to diagnose BAD because of high certainty (97%) of Type 1 BAD. 36 , 53
Table 1.
Inclusion and exclusion criteria for eligible studies
| PICO | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Adults with BAD aged ≥ 16 years and with diarrhoea | Adults with BAD aged ≥ 16 years and with no reporting of diarrhoea prior to undertaking the intervention | |
| Participants | Diagnosis of BAD determined via any of these tests: SeHCAT, serum C4, faecal BA excretion ≥ 48 h collection,14C‐glycocholate stool test | BAD diagnosed by: faecal BA excretion collection < 48 h, diarrhoea defined by faecal weight or BAS trial |
| In Type 1 BAD: a reported method to diagnose BAD was not required | Pregnancy; other medication or food that could influence GI symptoms or motility: metformin; alcohol abuse; other serious morbidities such as active Crohn's disease, microscopic colitis, liver disease, AIDs/HIV, depression | |
| Diarrhoea: as defined or described by study authors or according to the Bristol Stool Form Scale Types 6 or 7 | ||
| Intervention | A therapy, as induction or adjunct treatment for BAD | |
| Induction treatment was defined as a therapy without the use of BAD medication (colestyramine, colesevelam, colestipol or anti‐diarrhoeals) | ||
| Adjunct treatment was defined as a therapy undertaken after BAD medication had been optimised | ||
| Comparator | A placebo, another therapy, or no treatment | |
| Outcomes | Diarrhoea: Number/proportion of observed responders or the reported change using a clear scoring system at study endpoint | Diarrhoea: No reporting by responder or change in diarrhoea at study endpoint |
| Study design | RCT, prospective and retrospective cohort and case series | Single case |
| Study reporting | No language restrictions | Studies reported as an abstract or letter |
Abbreviations: BA, bile acid; BAD, bile acid diarrhoea; BAS, bile acid sequestrant; BSFS, Bristol Stool Form Scale; C4, 7α‐Hydroxy‐4‐cholesten‐3‐one 14 ; C‐glycocholate; GI, gastrointestinal; RCT, randomised controlled trial; SeHCAT, 75selenium homocholic acid taurine.
Information sources, search strategy, and study selection
Cochrane Central Register of Controlled Trials, Embase and MEDLINE through Ovid, and Web of Science were searched from 1975 to 13 July 2021. ClinicalTrials.gov and the EU Clinical Trials Register were checked for ongoing trials or supplementary data for potentially eligible studies. For each search strategy and the development process used, see Supporting information, S1 and S2. Literature search strategies were developed using medical subject headings and free‐text headings. Studies on therapies were identified using diet, including relevant terms relating to fats, carbohydrates including fibre, and protein; psychotherapy including behaviour change; and exercise including yoga. The theme of BAD was then combined with the set operator AND each therapy theme to identify studies. Backward citation searching was conducted in systematic reviews in BAD 19 , 38 , 54 prior to this review and in the included studies on 15 July 2021.
One investigator performed the electronic literature search. The results were uploaded to an EndNote management program (X9; Clarivate), where duplicates were removed. Each title and abstract was screened by two investigators. First, one investigator identified all potentially eligible studies and then a second investigator screened all excluded titles and abstracts to verify exclusion and check retrieved studies against the eligibility criteria. If any titles and abstracts did not provide adequate detail to determine eligibility then full‐text articles were assessed. Two investigators independently screened full‐text articles for inclusion.
Data collection process and data items
Data extraction was undertaken by one investigator, into an Excel spreadsheet (Microsoft Corp.). Extracted data on participant numbers and outcomes were triple checked to minimise mistakes and data selection bias, and all other items were checked twice. Data entries were then checked against each study by a second investigator. We involved a third investigator to resolve disagreement through consensus. We collected data on:
author, year of publication
study design, country of origin, number of centres, diagnostic tool used to diagnose BAD, BAD subtype, BAD severity category
participants: number with diarrhoea; number with diarrhoea who completed the therapy: sex, age, body weight, body mass index
intervention: description including food and nutrient intakes, duration, behaviour change theory or behaviour change techniques, induction or adjunct to BAD medication including dose, use of other medication, tolerance and adherence rates to the intervention with any reported definitions and target rates, using a clear scoring scale
primary outcome, diarrhoea: patient or clinician‐reported definition or description, any measurement tools used, target outcome, stool consistencies and frequencies at baseline and study endpoint, number of responders at study endpoint
secondary outcomes: abdominal pain and flatulence: the measurement tool used, number of participants at baseline and study endpoint, number of responders at study endpoint; adverse effects: the number of clinically relevant reported side or adverse effects, regardless of causality, including constipation (BSFS Types 1 and 2).
Where the mean ± SD could not be collected, median values and interquartile ranges were extracted.
Risk of bias in individual studies and across studies for each therapy
One investigator assessed risk of bias in each included study using the Cochrane ‘Risk Of Bias In Non‐Randomised Studies Of Interventions' (ROBINS‐I) tool). 55 After considering Stage I, a hypothetical pragmatic RCT, the seven domains were addressed: Pre‐intervention: confounding, selection of participants; At intervention: classification of intervention; Post‐intervention: deviation from intended intervention, missing data, selection of the reported result. The ROBINS‐I Guidance (2016) was used to judge risk of bias for each domain (low; moderate; serious; critical; no information). Judgements and justifications were tabulated to support discussion with a second investigator to reach consensus agreement on judgement of risk of bias for each domain and overall in each study and then across studies for each therapy.
Summary measures
We anticipated that performing any meta‐analyses would not be possible as a result of few studies reporting specific dietary interventions with available data. A narrative synthesis of the results was conducted by one investigator using the Synthesis Without Meta‐analysis guideline to guide on reporting and presentation. 56 Presentation of the data was prioritised: primary before secondary BAD, type of diet therapy (whole food‐first approach before artificial nutrition treatment), and outcomes on diarrhoea (first, by stool consistency and frequency, then by stool consistency, and, lastly, by stool frequency, with measurement by responder before change in the scoring scale). Outcomes on diarrhoea in all but one study 39 lacked adequate statistical analyses to estimate effect; therefore, effect was reported as a positive or negative direction using vote counting. 57 The method of vote counting compares the number of observed direction of effects showing ‘benefit’ to the number showing ‘harm’ for a particular outcome to give evidence of effect across studies. A positive/negative direction of effect (benefit/harm) was defined as a patient or clinician‐reported positive/negative response or improvement/worsening in an outcome being assessed.
Certainty assessment
Certainty of the evidence for each therapy on outcomes was judged using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system 58 (see Supporting information, S3). Quality determinants along with risk of bias and direction of effect across studies are presented as a summary of findings with a comments and evidence statement field to aid interpretation and footnotes for explanations. Confidence was assessed as ‘low’ because all study designs were non‐randomised.
RESULTS
Study selection
Searches using all four databases generated, for diet, psychological, and exercise therapies, a total of 2671, 46, and 54 citations, respectively. The PRISMA 2020 flow diagram is shown in Figure 1. From two clinical trial registries, four records of studies were identified, with one an ongoing RCT in secondary BAD (dietary fat intake reduction). Eight full‐text articles were retrieved and backward citation searching of these studies identified one additional study. 59 No RCTs were identified. One retrospective study did not meet the inclusion criteria because outcome data on diarrhoea could not be extracted for 123 out of 143 patients who undertook dietary intervention. 60 Eight studies of diet therapies were included in this review. No studies were identified that examined psychological or exercise therapies.
Figure 1.
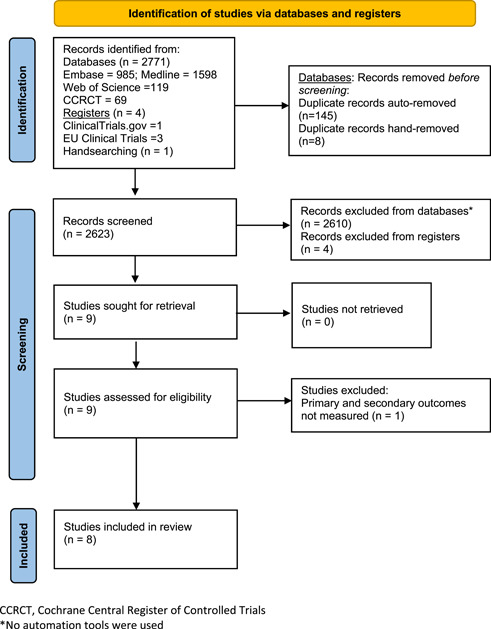
Flow diagram of systematic review (PRISMA 2020).
Study characteristics
All eight included studies were prospective, single‐arm, cohort studies in single centres from the hospital setting, 39 , 44 , 45 , 59 , 61 , 62 , 64 , 68 although one study also included patients from a primary care centre. 62 In two studies the samples were inpatients. 59 , 64 The studies totalled 288 adults, and study sample size ranged from two to 114 participants out of a total of 192 analysed who undertook diet therapy as sole treatment or adjunct to bile acid sequestrants. No data on any psychological or exercise therapies were found within these dietary studies. The study characteristics are shown in Table 1.
Intervention
Intervention descriptions are shown in Table 2. In primary BAD, a ‘malabsorbed sugar‐free diet’ was administered depending on the results of hydrogen breath testing using standardised challenges of 20 g of lactose and 20 g of fructose plus 3.5 g of sorbitol in one study. 61 The carbohydrate modification consisted of either a lactose‐free diet or reductions in sorbitol and fructose in excess of glucose, or both, as described elsewhere. 65 , 66 However, the carbohydrate modification the two participants with moderate or severe BAD undertook is unclear. Nutritional intakes were not measured and no nutritional data were reported.
Table 2.
Study characteristics of included studies.
| First study author Year | Country Study design | Diagnostic tool used to diagnose BAD | Type of BAD % patients, BAD severity category | Number of patients with diarrhoea (% female) | Number that completed diet therapy (% female) | Age, years mean (SD) range | Body weight, kg Baseline mean (SD), range /Endpoint mean (SD), range | BMI, kg/m2 Baseline mean (SD), range /Endpoint mean (SD), range |
|---|---|---|---|---|---|---|---|---|
| Carbohydrate intake modification: reduction in lactose and/or fructose and sorbitol | ||||||||
|
Fernandez‐Banares 2007 |
Spain Cohort, prospective |
SeHCATa |
Primary 100% severe to moderate (<11%) |
2 (–) | 2 (–) | – | – | – |
| Fat intake reduction | ||||||||
|
Bosaeus 1979 |
Sweden Cohort, prospective |
14C stool test |
Secondaryb – |
9 (100) | 9 (100) |
58 (7.6) 48–75 |
– | – |
|
Danielsson 1991 |
Sweden Cohort, prospective |
SeHCATc |
Secondaryb 100% severe |
4 (100) | 4 (100) | – | – | – |
|
Jackson 2017 |
England Cohort, prospective |
SeHCATa |
Secondaryd 29% severe 17% moderate (0%–5%) 25% mild (10%–15%) 28% borderline (>15% to 20%) |
188 (–) | 114 (57) |
Median: 64 (IQR: 55–71) 23–86 |
Median: 77 (IQR: 64–90) 39–127 /– |
Median: 27 (IQR: 23–31) 16–45 /– |
|
Larsen 2019 |
Denmark Cohort, prospective |
SeHCATa |
Secondarye 100% severe to mild (0% to ≤15%) |
14 (–) | 14 (–) | – | – | – |
|
Watson 2015 |
England Cohort, prospective |
SeHCATa |
Secondaryf 50% severe 17% moderate (0%–5%) 25% mild (10%–15%) 8% borderline (>15% to 20%) |
62 (–) | 40 (50) |
61 (12) 22–90 |
71.2 (18.3) 32.7–109.3 /– |
25.5 (5.2) 12.8–38.1 /– |
| Exclusive elemental (Vivonex) | ||||||||
|
Nelson 1977 |
Scotland Cohort, prospective |
Clinician judgment |
Secondaryg – |
6 (–) | 6 (–) | – | – | – |
|
Russell 1979 |
Scotland Cohort, prospective |
Clinician judgment |
Secondaryh – |
3 (–) | 3 (–) | – | – (stable) | – |
Abbreviations: BAD, bile acid diarrhoea; BMI, body mass index; IQR, interquartile range; SD, standard deviation, SeHCAT, 75selenium homocholic acid taurine; WBR, whole body retention.
SeHCAT measured by 7‐day whole body retention.
Cancer survivors, gynaecological post radiation treatment.
SeHCAT measured daily for up to 72 h to calculate the biological half‐life of SeHCAT to determine diagnosis of BAD: severe, <40 h.
Cancer survivors, 51 (84%) with small intestinal bacterial overgrowth, 21 (34%) with pancreatic insufficiency.
Cancer survivors, colon and pelvic organ.
Four cancer survivors, 13 (33%) with small intestinal bacterial overgrowth, five (13%) with pancreatic insufficiency.
Ileal resections: four Crohn's disease, one carcinoma of the caecum, one mesenteric thrombosis.
Crohn's disease: ‘extensive damage to the terminal ileum’ (no resections).
In secondary BAD, five studies were on fat intake reduction in cancer survivors from four European centres. 39 , 44 , 45 , 62 , 68 All included patients with severe BAD, although severity was not measured in one study conducted before SeHCAT was used to diagnose BAD. 45 The two studies with the largest sample populations included patients with mild and borderline severity defined by SeHCAT. 39 , 62 Quantified dietary intervention targets were defined in three studies in two ways. In one study, the target fat intake was 40 g day−1. 45 In two studies, ‘20% of total energy’ was used, 39 , 62 although how the fat intake target was calculated was not stated. In the other two studies, the interventions of ‘low‐fat’ 63 and ‘reduced‐fat’ 44 were not described or quantified. One study described what ‘low‐fat’ consisted of by food group and preparation methods. 45 One out of these five studies provided quantitative nutritional data. 39 Fat and fibre intakes only were measured from 7‐day food diaries analysed using an electronic nutritional analysis programme (Dietplan6; ForestField Software Ltd). 39 Baseline (habitual) dietary fat intake was a mean of 62.3 g day−1 (median 58.9 g, range 34.5–100.8). Intake decreased to a mean of 42.2 g day−1, ranging widely (median 39.1 g, range 24.5–80.8) (p < 0.01). Mean dietary fibre intake did not change from a habitual low quantity of 14.8 g−1 day (median 13.8 g, range 10–32) at baseline to 14.4 g (median 13.6 g, range 4.4–34.7) at follow up. However, how dietary fibre was defined was not stated. It may have be an underestimation if measurement was for non‐starch polysaccharides only. In one study, seven‐day food diaries and dietary recall data were collected but no nutrient intakes were reported. 62 Nutritional supplementation (Forceval, Alliance; and Calcichew‐D3 Forte, Takeda) was reported to be prescribed in severe and moderate BAD in one study. 39 Two years later in the same centre, in the study published in 2017, Forceval only was reported to be prescribed after checking nutritional adequacy of trace elements and fat soluble vitamins. 62
In secondary BAD, for hospitalised ileal patients, two studies reported on an exclusive elemental diet given orally (Vivonex; Eaton Laboratories). 59 , 64 Key treatment purpose was to provide short‐term bowel rest by reducing stool volume, feacal bile acid excretion, and stool frequency at the same time as ensuring nutritional intake adequacy in patients at risk of fat malabsorption in bile acid malabsorption. Prescribed as an orange‐flavoured powder mixed with tap water, data sources showed its nutrient composition as: fat, 1.4% 67 or 0.43 g per sachet (as safflower oil) 64 ; carbohydrate, 90.5% (glucose and oligosaccharides) 67 ; mixed amino acids, 8.1%. 67 Both studies reported on nutritional intake, as assessed by the number of sachets used per day 64 or as the energy intake range per day over the study period, 59 although procedures to verify either were not described. Energy intakes were clinician‐reported as less than the energy intake aim for each study (12,552 kJ day−1): a mean of 10,251 kJ day−1 (4.9 sachets day−1, range 3–6) for up to 15 days in one study 64 and 10,460 to 12,552 kJ day−1 for 2–3 weeks in the other. 59
No studies reported on behaviour change theory or use of behaviour change techniques to improve dietary adherence or clinical outcomes.
Delivery of the interventions by a dietitian was reported in four studies but without reference to specialist expertise in gastroenterology. 39 , 45 , 62 , 68 Missed reporting of dietitian‐delivery is likely in all others, 44 , 59 , 61 , 64 including dietetic monitoring in the two inpatient studies. 59 , 64 Planned dietetic follow up was 6–8 weeks in two studies 39 , 62 and not stated in any others. Study endpoints varied within each study. In the carbohydrate modification study, the duration was at least 12 months. 61 Across studies investigating fat intake, the reduction duration ranged from 2 weeks 63 to after 2 years. 44 Patients undertook the exclusive elemental diet therapy for 8–21 days, specified in one study as dependent on each patient's treatment effect for which 2–3 weeks sufficed. 59
In primary, moderate to severe BAD, bile acid sequestrants were offered before diet therapy. 61 In secondary BAD, two out of five studies also used them before fat intake reduction. 44 , 68 In one study, colestyramine was used after dietary intervention for persisting diarrhoea. 45 In two studies, a SeHCAT screening algorithm stratified treatment according to BAD severity defined by SeHCAT. This mild to borderline BAD to fat intake reduction was offered before colesevelam. For moderate BAD severity, patient choice was offered, whereas, for severe BAD, the dose of colesevelam was optimised first.
The use of other medication in the investigation of carbohydrate modification was not reported. 61 In secondary BAD, the study design permitted the use of other anti‐diarrhoeals in three studies, 59 , 62 , 68 and was not reported in the other four. 39 , 44 , 45 , 64 Use of laxatives was reported in two out of the seven studies. 62 , 68 In studies of fat intake reduction, antibiotics reported because of the severity of their BAD were given to all patients before initiating dietary change in one study 44 and for prior coexisting small intestinal bacterial overgrowth in three studies in unclear proportions of patients. 39 , 62 , 68
Tolerance to the intervention was not defined or measured in any of the studies.
An adherence rate target was given in one study. 39 A high rate of 90% was achieved in 28 out of 40 (70%) patients obtained from patient‐recorded 7‐day food diaries completed before the intervention and again prior to study endpoint analysed electronically (Dietplan6). In another study, necessary adherence to a fat intake of 40 g day−1 to control diarrhoea was verified via fat intake re‐challenge. 45 ‘Palatability problems’ were noted with the exclusive elemental diet therapy in one study, 59 but, as an adherence marker, the proportion who maintained body weight was unclear. No definitions or data on adherence were reported in any of the other studies on carbohydrate modification, 61 fat intake reduction, 44 , 62 , 68 and exclusive elemental diet therapy. 64
Outcomes
Diarrhoea
Diarrhoea was defined by stool consistency in five of eight studies, as measured at baseline via patient self‐reporting using the BSFS 47 in two studies. 62 , 68 However, outcomes using this validated tool were reported in only one study. 62 Normalisation of stool consistency by the proportion of responders at study endpoint was clinician‐reported in one study, 45 whereas improvement without further quantification was clinician‐reported in three studies. 59 , 61 , 68
Three studies reported on changes in stool frequency without accounting for improvements in loose stool consistencies to measure response to therapy. 39 , 44 , 64 In one study, an unvalidated, verbally administered 11‐point numerical rating scale, NRS‐10 (0, no symptoms to 10, severe symptom affecting daily life) 68 was used to measure frequency. 39 In one study, the number of bowel movements per week was reported having measured stool frequency by using patient‐reported daily counting on a diary card. 44 In another study, frequency was reported per day, but how this was measured was not reported. 64 In the three studies that reported on both stool consistencies and frequencies, 61 , 62 , 68 a normal frequency was described as ≤ 3 63 or ≤ 2 61 bowel movements day−1.
The effects of diet therapies on diarrhoea are shown in Table 3. In all studies, vote counting was positive, indicating that all three diet therapies provided a beneficial direction of effect on diarrhoea.
Table 3.
Diet therapy characteristics and outcomes of included studies: diarrhoea
| First study author Year | Intervention Description Duration | Induction or adjunct to BAS BAS used, dose prescribed | Definition of diarrhoea used Measurement tools used | Diarrhoea description/Measurement | Evidence of an effect (%) | |
|---|---|---|---|---|---|---|
| Baseline | Endpoint | Vote count: Positive/Negative | ||||
| Carbohydrate intake modification | ||||||
| Outcome by stool consistency and frequency, responder (%) | ||||||
|
Fernandez‐Banares 2007 |
Lactose‐free and/or reduced fructose plus sorbitol intakesa Other components: undefined 12 months |
Adjunct COL started at 8 g/day, 2–12 g/day |
Three loose or liquid bowel movements/day for ≥4 weeks and a stool weight >200 g/day Aim after 12 months: 2 or fewer formed or semi‐formed stools/day and no clinical relapse at >12 months PR |
Loose or liquid | – |
Improved: ‘good response’ in 2/2 patients (100), unquantified ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| Vote count: 1/0 | ||||||
| Fat intake reduction | ||||||
| Outcome by stool consistency and frequency, responder (%) | ||||||
| Larsen 2019 |
Low‐fat: undefined Other dietary components: undefined 2–4 weeks |
Induction and adjunct COL, COV, COS |
Loose stools, BSFS types 6 and 7 Frequent, >3 movements per day BSFS PR |
– | – |
Improved bowel function in 14/14 patients (100), unquantified ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| Jackson 2017 |
Low‐fat: 20% total daily energy, all types of dietary fat Other dietary components: personalized, undefined 4–12 weeks |
Induction and adjunct COVb |
BSFS type 6 or 7 even intermittently Modified GSRS, BSFS PR |
T7: 47% = 51/114 T6: 53% = 61/114 ≥7×/day: 37% = 42/114 ≥4–6×/day: 42% = 48/114 |
Reduction in proportionc by: Consistency: T7, 40% T6, 17% Frequency: ≥7×/day: 32% ≥4–6×/day: 30% |
Improved in no. of patients by Consistency: 20/51 (39) 10/61 (16) Frequency: 13/42 (31) 14/48 (29) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| Outcome by stool consistency, responder (%) | ||||||
| Bosaeus 1979 |
Low‐fat: 40g/day Defined by food items and preparation methods 3–6 months |
Induction |
Watery CR |
Watery | Formed |
Normalized in 8/9 (88) patients Unchanged in 1/9 (12) patients (cholecystectomized) until COL added to the low‐fat diet Watery diarrhoea returned in all 9 patients when fat intake was temporarily increased ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| Outcome by stool frequency, responder (%) | ||||||
| Danielsson 1991 |
Low‐fat: undefined Other dietary components: undefined 2 years |
Adjunctd COL, 4–8 g/day |
Severe chronic or intermittent diarrhoea interfering with daily activities 7‐day symptom and stool frequency diary card PR |
Stool frequency per daye: 7.4, 5.7, 4, 3.4 |
Stool frequency per daye: 5.1, 5.0, 2.6, 2 |
Normalized in 2/4 (50) patients Improved in 4/4 (100) patients, unquantified ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| Outcome by change in stool frequency, scoring scale | ||||||
| Watson 2015 |
Low‐fat: 20% total energy from fat Other dietary components: tailored, undefined 7.9 weeks (3–20) |
Induction or adjunct COVb |
– Bowel frequency, NRS‐10 PR |
Median score, 8/10 | Median score, 5/10 |
Improved by median score, 3/10 (37.5), p < 0.01 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| Vote count: 5/0 | ||||||
| Exclusive elemental | ||||||
| By stool consistency, responder (%) | ||||||
| Russell 1979 |
Vivonex Up to 6 sachets/day given orally Water allowed 2–3 weeks |
Induction |
Watery, unformed CR |
Watery, unformed | Less watery and better formed |
Improved in 3/3 (100) patients, unquantified ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| By stool frequency, responder (%) | ||||||
| Nelson 1977 |
Vivonex only Up to 6 sachets/day given orallyf 8–15 days |
Induction |
Urgent, watery CR |
2–10×/day | 0–7×/day |
Normalized in 2/6 (33) patients Improved in 6/6 (100) patients, unquantified ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Positive |
| Vote count: 2/0 | ||||||
Abbreviations: BAS, bile acid sequestrant; BSFS, Bristol Stool Form Scale; COL, colestyramine; COS, colestipol; COV, colesevelam; CR, clinician‐reported; GSRS, Gastrointestinal Symptoms Rating Scale; NRS‐10, Numeric Rating Scale‐10; PMH, past medical history; PR, patient‐reported.
Data on sorbitol, fructose, and glucose in Fernandez‐Bañares 66 (pp. 829–830, Appendix A).
An algorithm based on bile acid diarrhoea severity determined by SeHCAT was used to determine medication treatment before starting diet therapy.
Data extracted from spider diagram, in Jackson et al. 62 (figs. 2 and 3, p. 416).
Given metronidazole and doxycycline for 7–10 days before starting BAS.
Stool frequency data in Danielsson et al. 44 (fig. 6, p. 1185) was given per week, not per day and therefore divided by 7. Patient 7 did not have a high stool frequency: excluded from analysis.
One patient may have been fed via a nasogastric tube.
Abdominal pain and flatulence
Two out of five studies on fat intake reduction from the same centre measured abdominal pain and flatulence but in different ways. 39 , 62 In one study, to measure proportions of responders, the validated Gastrointestinal Symptom Rating Scale 69 was modified from a seven‐point rating scale to two outcome categories of ‘frequent and causes major changes in life’ or ‘never or occasional’. 62 In the other study, NRS‐10 68 was used to measure patient‐reported symptom change. 39
Abdominal pain improved, as measured by responder 62 and by reduction in NSR‐10 score. 39 For flatulence, there was an improvement according to NSR‐10 scoring in one study 39 and a trend for improvement by responder in the other. 62 Both therapies were given positive vote counts for each symptom. The data are provided in the Supporting information (Table S1).
Adverse effects
Adverse effects were not reported in any studies.
Risk of bias and certainty of the evidence
Overall risk of bias for each included study was judged to be serious (see Supporting information, Table S2). Risk of bias justification of assessments across studies for each therapy using the ROBINS‐I tool is provided in the Supporting information (Table S3). Certainty of the evidence using GRADE was assessed as very low quality for each outcome (Table 4). Very low‐quality evidence was in particular a result of limitations of study design, impresicion due to very small population sizes, and inconsistency in the definition of diarrhoea and its measurement across studies.
Table 4.
GRADE Summary of findings on diet therapies
| What is the effectiveness of diet therapies on diarrhoea, abdominal pain, and flatulence? | ||||
|---|---|---|---|---|
| Patients or population: adults with bile acid diarrhoea and chronic diarrhoea | ||||
| Interventions: Carbohydrate intake modification, fat intake reduction optimised on medication; exclusive elemental (Vivonex) | ||||
| Setting: Hospital outpatients for carbohydrate intake modification, fat intake reduction; inpatients for exclusive elemental | ||||
| Outcome | Direction of effect | Number of participants | Certainty of the evidence | Comments |
| ROB across studies | (studies) | GRADE | ||
| Carbohydrate intake modification on diarrhoea |
Positive Serious |
2 (1 cohort study) |
⊕◯◯◯ |
Data in primary BAD only. Long‐term evaluation made (one study, clinician‐reported) but in two patients only. No validated tool used to assess diarrhoea |
| Fat intake reduction on diarrhoea |
Positive Serious |
181 (5 cohort studies) |
⊕◯◯◯ |
Data in secondary BAD, cancer survivors only. Large sample size (one study). Data on sex, age and weight/BMI (two studies). Validated method used to assess diarrhoea (two studies). Longer‐term evaluation made (one study, patient‐reported). Long‐term evaluation made (one study, clinician‐reported) |
| Exclusive elemental diet therapy on diarrhoea |
Positive Serious |
9 (2 cohort studies) |
⊕◯◯◯ |
Data in secondary BAD, Type 1 BAD/BAM only. No validated tool used to assess diarrhoea |
| Fat intake reduction on abdominal pain |
Positive Serious |
122 (2 cohort studies) |
⊕◯◯◯ |
Data in secondary BAD, cancer survivors only. No validated tool used to assess abdominal pain |
| Fat intake reduction on flatulence |
Positive Serious |
122 (2 cohort studies) |
⊕◯◯◯ |
Data in secondary BAD, cancer survivors only. No validated tool used to assess flatulence |
| Adverse effects |
‐ Serious |
192 (8 cohort studies) |
⊕◯◯◯ Very Lowe |
Laxatives: allowed (2 studies), unclear (6 studies). Anti‐diarrhoeals: allowed (3 studies), unclear (5 studies) |
Abbreviations: BAD, bile acid diarrhoea; BAM, bile acid malabsorption; BMI, body mass index; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; ROB, risk of bias.
Inconsistency: narrative synthesis conducted, no estimates of the therapy effect possible.
Indirectness: diarrhoea or abdominal pain or flatulence was measured differently across studies, unvalidated tools were used.
Imprecision: population samples were too small to generate precise results.
Inconsistency: differing end‐points across studies to measure therapy effect.
No studies reported on adverse effects.
DISCUSSION
This first systematic review of studies of diet, psychological, and exercise therapies meeting diagnostic criteria for BAD in adults with diarrhoea identified eight prospective cohort studies from Denmark, Spain, Sweden, and the UK, published between 1977 and 2019. No RCTs were identified. None investigated psychological or exercise therapies. Three types of diet therapy showed beneficial directions of effect on diarrhoea, abdominal pain, and flatulence. Data on any adverse effects were lacking. From very low‐quality evidence, no conclusions can be drawn on the effectiveness of diet, psychological, or exercise therapies on diarrhoea, abdominal pain, and flatulence for the management of BAD in adults. Therefore, no recommendations for clinical and dietetic practice can be made.
In primary BAD, the beneficial effect on diarrhoea of removing lactose, sorbitol and fructose after optimisation with colestyramine 61 is suggestive of co‐existing diarrhoea‐predominant irritable bowel syndrome with underpinning gastrointestinal hypersensitivity, 70 rather than defective carbohydrate malabsorption. 71 Hydrogen breath testing using 20 g of the test carbohydrate is not adequately diagnostic for either lactose or fructose intolerance, 72 indicating that, in the study published by Fernandez‐Banares in 2007, 61 there was inadequate rationale for dietary exclusion of either carbohydrate. Indeed, lactose is well tolerated at a dose of 12 g, as shown in a randomised, double‐blind, cross‐over study amongst adults with lactose malabsorption who were otherwise healthy, given as 240 ml of of cow's milk (2% fat) twice daily for 7 days. 73 This demonstrates the importance of food challenge to verify benefit on diarrhoea, which was not reported even though the study duration was 12 months. Diarrhoea and symptoms including abdominal pain and flatulence may present in susceptible individuals when carbohydrates are poorly absorbed in the small intestine and ferment in the large intestine in healthy adults, 74 , 75 as well as in gastrointestinal disorders. 76 From accumulated evidence on these carbohydrates, a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) 77 , 78 , 79 evolved, with efficacy on global symptoms in irritable bowel syndrome supported by the findings of a network meta‐analysis. 80 Compared to the limited data on sorbitol and fructose contents in foods used in this study, 61 our current knowledge on FODMAP composition of foods across food groups is far greater. 81 , 82 , 83 , 84 This suggests that a superior version of the carbohydrate modification therapy is the low FODMAP dietary intervention and that the intervention in this study did not truly treat diarrhoea as a result of BAD. 61
Perhaps surprisingly, there is a paucity of evidence demonstrating diarrhoea induction as a result of dietary components. Food transit through the small intestine may be important to the rate of bile salt absorption, a faster ileocolonic inflow increasing potential for colonic spillover and diarrhoea. Physical aspects such as food ingestion timing, food particle size, and osmotic actions were not considered factors reported in the study design of the interventions of the included studies. For example, coarse wheat bran (15 g) but not fine psyllium (Fybogel, 7 g) significantly accelerates small intestinal transit compared to control (cooked pudding rice, 25 g) in healthy volunteers. 85 In the large intestine, absorptive capacity of water is enhanced by short‐chain fatty acids derived from non‐digestible carbohydrates, 86 although excess (e.g., when given as lactulose) leads to osmotic diarrhoea once absorptive capacity is reached. 87 It might be speculated that amylase‐resistant starches, found in cooked and cooled rice and used in oral‐rehydration solution for acute diarrhoea, 88 could potentially increase net colonic absorption of fluid in BAD and reduce faecal flow rate. Dietary fibre intakes are often unnecessarily low amongst people with diarrhoeal disorders and diseases, 89 , 90 as evidenced in one study 39 in this review. However, BAD and diarrhoea‐predominant irritable bowel syndrome exhibit dysbiosis and, in comparison, reduced bacterial diversity was shown in BAD. 24 Although the exclusive elemental diet therapy was designed for short‐term use, our current understanding supports inclusion of carbohydrate substrates as an important variable to modulate the colonic microbiota 91 via their physicochemical properties. 89 Psyllium, for example, appears to be safe in the long‐term treatment of BAD based on patient‐reported survey data. 30 Containing arabinoxylan, it is a viscous fibre and recommended as a food supplement to treat symptoms of irritable bowel syndrome. 92 Viscous fibres also include alginates, beta‐glucans, and pectins 89 found naturally in seaweed, oats, and chickpea husks. Interestingly, in a synthetic form, hydroxypropyl cellulose (European 463, used as a food thickener) was mistakenly used as the inactive comparator against colestyramine in an RCT in primary BAD and rapidly reduced the number of watery stools. 93 New insight into understanding the mechanisms of action that improve bowel habit may lie with microbiome‐manipulation therapies. 94
In the management of secondary BAD, fat intake reduction was found to have a beneficial effect on diarrhoea, abdominal pain, and flatulence in cancer survivors at 2 weeks and up to 2 years as induction treatment in borderline to moderate BAD or adjunct to BAS. Fat intakes ranged from 25 to 81 g day−1, likely reflecting the broad variation in body weight and low and very high body mass indices. 39 However, none of the studies described behaviour change techniques used to support dietary adherence and internal validity, nor any underpinning behaviour change theories to potentially improve intervention effectiveness. 95 Psychological factors that hinder or aid adherence to fat intake reductions in this patient population are as yet unknown.
The strongest stimulators for bile acid release are fatty acids with a chain length of at least 12 carbons, 96 indicating that intake per day is not a suitable outcome measure. In lean, healthy adults, as little as 1.5 g fat (Intralipid) stimulated one‐quarter of the gallbladder bile volume to be ejected, 97 whereas 25 g was found to expel 85% over 75 min. 98 The exclusive elemental diet therapy provided less than 0.5 g of fat per sachet. However, inadequate gallbladder volume evacuation (e.g., when ‘nil by mouth’ or in very low calorie dieting) initiates gallbladder dysmotility and stasis, which is reversible but can precipitate to gallstones. 99 This is relevant in primary BAD because obesity management may be a treatment option to consider. 27 One study conducted in obese, weight‐reducing adults showed protection against biliary sludge when the fat intake was 12.2 g day−1 compared to 3 g day−1. 100 This suggests that, when managing bile salt output, lower and upper thresholds of fat intake may be applicable. To prevent gallbladder stasis, 10 g per meal, irrespective of the level of obesity, has been proposed. 101 In studies of fat intake reduction excluding those for body weight reduction, fat intake was calculated based on a proportion of an individual's energy intake, 102 , 103 , 104 , 105 However, when considering that bile acids are recycled four to 12 times daily 106 with 0.2 to 0.6 g day−1 lost into the colon, 11 we hypothesise that, for further research studies, the quantity of fat intake per eating session rather than per day is appropriate for BAD. Furthermore, with bile acid pool doubling in primary BAD, 11 data are lacking to justify any differences in fat intake goal by age, weight, height or body mass index. 107
Protein but not starch, 108 coffee (regular and decaffeinated), 109 and colestyramine taken with a meal 110 also stimulate gallbladder bile acid excretion. The amino acids in the exclusive elemental diet therapy have been proposed as the reason why gallbladder contractions were no different compared to 60 ml of corn oil in 300 ml of water consumed within 5 min, although the small study was in healthy adults. 67 This further indicates complexity in the role of macronutrients and other dietary components when considering the entire enterohepatic pathway and also that dietary intervention in primary BAD may be different from that in secondary BAD.
Caution should be exercised when interpreting these findings for multiple reasons. The small number of studies, although prospective, included no RCTs and no control groups. Five of the eight studies had very small sample sizes of less than 10 patients, 44 , 45 , 59 , 61 , 64 whereas 59% of the total of 192 patients analysed were from one study. 62 The findings cannot be generalised to outside of Europe, and data are lacking from outside of hospital settings.
Demographic data on sex, age, and body mass index were missing in primary BAD and in secondary BAD in those who were not cancer survivors, particularly after cholecystectomy, which may be an under‐recognised group. Data from a multicentre survey conducted in 38 UK hospitals (1036 patients) showed the mean ± SD age was 50 ± 17 years across all BAD subtypes. 111 In a cross‐sectional study conducted in a single‐centre hospital in 70 patients with primary BAD, the mean ± SD age was very similar, at 48 ± 15 years. 27 The older ages of the patients in the studies of fat intake reduction 39 , 45 , 62 may have implications for aids used to support dietary adherence that were not reported on in any of the studies, such as digital technology. The inclusion of low body weights was a major confounder for two studies of fat intake reduction. For these patients, relief of diarrhoeal symptoms may not have been a priority compared to other important goals such health‐related quality of life. 112 Indeed, fat intake reduction to a very low level may have been an inappropriate treatment goal for those with a body mass index of less than 18.5 kg m–2, an indicator of undernutrition as defined by the World Health Organisation's weight classification system.
Searches were conducted to include as many studies as possible because non‐pharmacological therapies have not been previously systematically reviewed. The broad inclusion criteria allowed inconsistency in definitions for the diagnosis of BAD. However, this was unavoidable as a result of the availability and advancement of valid, accurate tests over the last 50 years. Historically, diarrhoea has been variably and poorly defined, and, by accepting all author definitions in the eligibility criteria, no studies were rejected. Had the definition of diarrhoea been restricted to stool consistency, defined using the BSFS or descriptively, then three out of the eight studies would have been excluded. Eligibility criteria also allowed reporting of outcomes on diarrhoea, abdominal pain, and flatulence, which were variably measured with variable duration to study endpoint and without statistical analysis in six of the eight studies.
A further limitation in this review process is that the screening of records and data collection were not independently conducted by at least two investigators. Although records and data were checked by another investigator, this is not compatible with best practice to reduce risk of error. In our opinion, it is unlikely that these results would have altered had we employed independent review by two investigators.
On the basis of the lack of evidence, no conclusions could be drawn on the effectiveness of diet, psychological, or exercise therapies for the management of BAD. High‐quality RCTs in diet and as yet unexplored therapies compared to usual care are needed, which assess their acceptability, feasibility, and effectiveness in treating symptoms of BAD. Because BAD is a lifelong condition and therapies may be adjunctive to medication, any study design should carefully consider optimal treatment adherence by incorporating behaviour change techniques underpinned by behaviour change theory. 97 The outcome measures most important to people with BAD should be prioritised and, if possible, validated methods should be used to assess them. Prioritisation should be given to studies in primary BAD that could have the greatest positive impact on those living with this often debilitating condition.
AUTHOR CONTRIBUTIONS
Yvonne A. McKenzie was responsible for the conception, protocol development, literature searches and screening, data collection, qualitative data synthesis, and writing of the manuscript. Active contributions were made by: Jana Sremanakova on literature screening, data extraction review, and qualitative data synthesis review; Sorrel Burden on protocol development, data extraction review, critical review of all drafts; Chris Todd on study design. All authors critically reviewed its content and approved the final version submitted for publication.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
Not applicable.
TRANSPARENCY DECLARATION
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study reported. The reporting of this work is compliant with the PRISMA 2020 statement and Checklist. The lead author affirms that no important aspects of the study have been omitted and that any discrepancies from this study as planned were explained.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
This article is dedicated to Juanita Rothman (1925–2019).
Biographies
Yvonne McKenzie is a clinical dietitian specialising in gastroenterology. She worked with Oxford University Hospitals NHS Trust for 10 years and, since 2007, has her own private practice, Digestible Nutrition, seeing adult patients at Nuffield Health, The Manor Hospital, Oxford. Since the Covid‐19 pandemic, she has embraced solving people's gut and food problems via video consultations, which has the important advantage of no travel for those with urgent, diarrhoeal symptoms and supports environmentally sustainable diets and living. She is a co‐investigator for the MODULATE trial: a multi‐arm multi‐stage trial of low FODMAP diet, amitriptyline, ondansetron or loperamide, sponsored by the University of Leeds, and funded by the National Institute for Health Research. Alongside this, she is undertaking a PhD on diet and bile acid diarrhoea at The University of Manchester (2019–2026). She qualified as a dietitian in 1998 via a Diploma in Dietetics (1997) and an MSc in Nutrition (1996) at King's College London. She also has a Post Graduate Certificate in Sport and Exercise Nutrition at Leeds Beckett University (2017). She is a member of the British Dietetic Association and for the Gastroenterology Specialist Group was a committee member and then deputy chair (2006–2012) and clinical lead in irritable bowel syndrome (2007–2022). She has three publications in the Journal of Human Nutrition & Dietetics. Yvonne was involved in the development of clinical guidance by the National Institute for Health and Clinical Excellence in bile acid diarrhoea [DG44] (2021), and irritable bowel syndrome: [QS114] (2016) and [CG61] (2015). She is proud to continue voluntary work by being in the Steering Group of the Irritable Bowel Syndrome Priority Setting Partnership (2021–2023) facilitated by the James Lind Alliance to identify and prioritise research gaps in the diagnosis, treatment, and management of irritable bowel syndrome, funded by Guts UK Charity and the British Society of Gastroenterology.
Jana Sremanakova is a PhD student in Clinical Nutrition and Nutrition Research Assistant at the University of Manchester. Jana has a background in Genetics, Molecular Biology, Nutrition and Exercise Sciences and primarily focuses on cancer research. Over the last 5 years, Jana worked extensively with Dr Sorrel Burden and Prof Chris Todd on projects related to preoperative nutritional support, nutrition in oncology, malnutrition and physical activity for the prevention of falls. Jana has gained methodological expertise in undertaking systematic reviews, mixed methods research, and developing lifestyle resources embedded in behaviour change theory for cancer prevention. Jana is the first author and co‐author of over 20 peer‐reviewed publications. Jana has been awarded Research Impact Scholarship from The University of Manchester to conduct PhD research titled Healthy Eating and Active Lifestyle After Bowel Cancer Workbook: Development and feasibility trial.
Chris Todd is Director of the UK National Institute for Health Research (NIHR) Policy Research Unit Older People and Frailty, and Lead for Healthy Ageing, NIHR Applied Research Collaboration‐Greater Manchester. Until April 2022 he led the Healthy Ageing Research Group at the University of Manchester, a research group comprising some 40+ staff and postgraduates. Chris is NIHR Senior Investigator and Fellow of the Royal College of Physicians of Edinburgh. Chris's work is broadly Health Services Research related to fall prevention, frailty and activity promotion amongst older people, including the use of technologies in support of interventions with older people. Over the last 30 years Chris has been PI or CI on more than 100 grants and fellowships from funders including NIHR, MRC, NHS, EC, CRUK and various charities. Chris led the EC projects ProFaNE Prevention of Falls Network Europe; and ProFouND Prevention of Falls Network for Dissemination. He has some 300 peer reviewed research publications and been invited to speak at more than 100 international/national conferences. He has sat on numerous advisory and funding panels. Chris is an experienced PhD supervisor with 34 former PhD student, and seven ongoing including Yvonne.
Dr Sorrel Burden is a clinical academic in dietetics. She has worked extensively in the NHS as a clinical dietitian in gastroenterology and nutritional support. Currently, Sorrel is a Reader in Nutrition and Dietetics at the University of Manchester and works as a dietitian leading research in home parenteral nutrition on the Intestinal Failure Unit at Salford Royal Foundation Trust. Her current research interests include nutrition in intestinal failure, preoperative nutritional support and nutrition in oncology. Sorrel has gained methodological expertise in undertaking systematic reviews, mixed methods research and using big data to answer research questions in nutrition and dietetics. Sorrel has over 90 peer‐reviewed publications and has been awarded numerous research grants from the National Institute for Health Research, Medical Research Council, charities and industry.
McKenzie YA, Sremanakova J, Todd C and Burden S. Effectiveness of diet, psychological, and exercise therapies for the management of bile acid diarrhoea in adults: A systematic review. J Hum Nutr Diet. 2022;35:1087–1104. 10.1111/jhn.13005
REFERENCES
- 1. Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut. 2018;67(8):1380–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–407. [DOI] [PubMed] [Google Scholar]
- 3. Fromm H, Malavolti M. Bile acid‐induced diarrhoea. Clin Gastroenterol. 1986;15(3):567–82. [PubMed] [Google Scholar]
- 4. Thaysen EH, Pedersen L. Idiopathic bile acid catharsis. Gut. 1976;17(12):965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walters JRF, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol. 2010;3(6):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camilleri M, Vijayvargiya P. The role of bile acids in chronic diarrhea. Am J Gastroenterol. 2020;115(10):1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Ciaula A, Garruti G, Lunardi Baccetto R, Molina‐Molina E, Bonfrate L, Wang DQH, et al. Bile acid physiology. Ann. Hepatol. 2017;16(Suppl 1):S4–14. [DOI] [PubMed] [Google Scholar]
- 8. Wang TY, Liu M, Portincasa P, Wang DQ. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest. 2013;43(11):1203–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brunner H, Northfield TC, Hofmann AF, Go VL, Summerskill WH. Gastric emptying and secretion of bile acids, cholesterol, and pancreatic enzymes during digestion. Duodenal perfusion studies in healthy subjects. Mayo Clin Proc. 1974;49(11):851–60. [PubMed] [Google Scholar]
- 10. Hofmann AF, Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984;25(13):1477–89. [PubMed] [Google Scholar]
- 11. van Tilburg AJ, de Rooij FW, van den Berg JW, van Blankenstein M. Primary bile acid malabsorption: a pathophysiologic and clinical entity? Scand J Gastroenterol Suppl. 1992;194:66–70. [DOI] [PubMed] [Google Scholar]
- 12. Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7(11):1189–94. [DOI] [PubMed] [Google Scholar]
- 13. LundÅSen T, GÄLman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260(6):530–6. [DOI] [PubMed] [Google Scholar]
- 14. Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid‐induced secretion in polarized monolayers of T84 colonic epithelial cells: structure‐activity relationships. Am J Physiol ‐ Gastrointest Liver Physiol. 2007;292(1):G290–7. [DOI] [PubMed] [Google Scholar]
- 15. Mekhjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50(8):1569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pomare E, Heaton K. The effect of cholecystectomy on bile salt metabolism. Gut. 1973;14(10):753–62. [PMC free article] [PubMed] [Google Scholar]
- 17. Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection: I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62(5):918–34. [PubMed] [Google Scholar]
- 18. Poley JR, Hofmann AF. Role of fat maldigestion in pathogenesis of steatorrhea in ileal resection. Fat digestion after two sequential test meals with and without cholestyramine. Gastroenterology. 1976;71(1):38–44. [PubMed] [Google Scholar]
- 19. Sadowski DC, Camilleri M, Chey WD, Leontiadis GI, Marshall JK, Shaffer EA, et al. Canadian Association of Gastroenterology Clinical Practice Guideline on the management of bile acid diarrhea. J Can Ass Gastroenterol. 2020;3(1):e10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smalley W, Falck‐Ytter C, Carrasco‐Labra A, Wani S, Lytvyn L, Falck‐Ytter Y. Spotlight: laboratory evaluation of functional diarrhea and diarrhea‐predominant irritable bowel syndrome in adults (IBS‐D). Gastroenterology. 2019;157(3):858. [DOI] [PubMed] [Google Scholar]
- 21. Wedlake L, A'Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30(7):707–17. [DOI] [PubMed] [Google Scholar]
- 22. Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta‐analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42(1):3–11. [DOI] [PubMed] [Google Scholar]
- 23. Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10(9):1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sagar NM, Duboc H, Kay GL, Alam MT, Wicaksono AN, Covington JA, et al. The pathophysiology of bile acid diarrhoea: differences in the colonic microbiome, metabolome and bile acids. Sci Rep. 2020;10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao L, Yang W, Chen Y, Huang F, Lu L, Lin C, et al. A Clostridia‐rich microbiota enhances bile acid excretion in diarrhea‐predominant irritable bowel syndrome. J Clin Invest. 2020;130(1):438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Institute for Health and Care Excellence . SeHCAT (tauroselcholic [75 selenium] acid) for diagnosing bile acid diarrhoea. Diagnostics guidance [DG44]. 2021 [cited 2022 Jan 21]. Available from: https://www.nice.org.uk/guidance/dg44
- 27. Shiha MG, Ashgar Z, Fraser EM, Kurien M, Aziz I. High prevalence of primary bile acid diarrhoea in patients with functional diarrhoea and irritable bowel syndrome‐diarrhoea, based on Rome III and Rome IV criteria. EClin Med. 2020;25:100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bannaga A, Kelman L, O'Connor M, Pitchford C, Walters JRF, Arasaradnam RP. How bad is bile acid diarrhoea: an online survey of patient‐reported symptoms and outcomes. BMJ Open Gastroenterol. 2017;4(1):e000116‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Black CJ, Ford AC. Best management of irritable bowel syndrome. Frontline Gastroenterol. 2021;12(4):303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Damsgaard B, Dalby HR, Krogh K, Jørgensen S, Arveschough AK, Agnholt J, et al. Long‐term effect of medical treatment of diarrhoea in 377 patients with SeHCAT scan diagnosed bile acid malabsorption from 2003 to 2016; a retrospective study. Aliment Pharmacol Ther. 2018;47(7):951–7. [DOI] [PubMed] [Google Scholar]
- 31. Orekoya O, McLaughlin J, Leitao E, Johns W, Lal S, Paine P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med. 2015;15(3):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin S, Sanders DS, Gleeson JT, Osborne C, Messham L, Kurien M. Long‐term outcomes in patients diagnosed with bile‐acid diarrhoea. Eur J Gastroenterol Hepatol. 2016;28(2):240–5. [DOI] [PubMed] [Google Scholar]
- 33. Borghede MK, Schlütter JM, Agnholt JS, Christensen LA, Gormsen LC, Dahlerup JF. Bile acid malabsorption investigated by selenium‐75‐homocholic acid taurine (75SeHCAT) scans: causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med. 2011;22(6):e137–e40. [DOI] [PubMed] [Google Scholar]
- 34. Ford GA, Preece JD, Davies IH, Wilkinson SP. Use of the SeHCAT test in the investigation of diarrhoea. Postgrad Med J. 1992;68(798):272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rössel P, Sortsøe Jensen H, Qvist P, Arveschoug A. Prognosis of adult‐onset idiopathic bile acid malabsorption. Scand J Gastroenterol. 1999;34(6):587–90. [DOI] [PubMed] [Google Scholar]
- 36. Smith MJ, Cherian P, Raju GS, Dawson BF, Mahon S, Bardhan KD. Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond. 2000;34(5):448–51. [PMC free article] [PubMed] [Google Scholar]
- 37. Wildt S, Nørby Rasmussen S, Lysgård M, Rumessen JJ. Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT test. Scand J Gastroenterol. 2003;38(8):826–30. [DOI] [PubMed] [Google Scholar]
- 38. Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39(9):923–39. [DOI] [PubMed] [Google Scholar]
- 39. Watson L, Lalji A, Bodla S, Muls A, Andreyev HJ, Shaw C. Management of bile acid malabsorption using low‐fat dietary interventions: a useful strategy applicable to some patients with diarrhoea‐predominant irritable bowel syndrome? Clin Med. 2015;15(6):536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koga T, Nishida T, Miwa H, Yamamoto M, Kaku K, Yao T, et al. Effects of dietary butter fat on fecal bile acid excretion in patients with Crohn's disease on elemental diet. Dig Dis Sci. 1984;29(11):994–9. [DOI] [PubMed] [Google Scholar]
- 41. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;349:349–g7647. [DOI] [PubMed] [Google Scholar]
- 42. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. [DOI] [PMC free article] [PubMed]
- 43. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well‐built clinical question: a key to evidence‐based decisions. ACP J Club. 1995;123(3):A12–A3. [PubMed] [Google Scholar]
- 44. Danielsson A, Nyhlin H, Persson H, Stendahl U, Stenling R, Suhr O. Chronic diarrhoea after radiotherapy for gynaecological cancer: occurrence and aetiology. Gut. 1991;32(10):1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bosaeus I, Andersson H, Nyström C. Effect of a low‐fat diet on bile salt excretion and diarrhoea in the gastrointestinal radiation syndrome. Acta Radiol Oncol Radiat Phys Biol. 1979;18(5):460–4. [DOI] [PubMed] [Google Scholar]
- 46. Blake M, Raker J, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(7):693–703. [DOI] [PubMed] [Google Scholar]
- 47. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–4. [DOI] [PubMed] [Google Scholar]
- 48. Hughes LE, Ford C, Brookes MJ, Gama R. Bile acid diarrhoea: current and potential methods of diagnosis. Ann Clin Biochem. 2021;58(1):22–8. [DOI] [PubMed] [Google Scholar]
- 49. Fani B, Bertani L, Paglianiti I, Fantechi L, De Bortoli N, Costa F, et al. Pros and cons of the SeHCAT test in bile acid diarrhea: a more appropriate use of an old nuclear medicine technique. Gastroenterol Res Pract. 2018;2018:2097359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11(10):1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suhr O, Danielsson A, Nyhlin H, Truedsson H. Bile acid malabsorption demonstrated by SeHCAT in chronic diarrhoea, with special reference to the impact of cholecystectomy. Scand J Gastroenterol. 1988;23(10):1187–94. [DOI] [PubMed] [Google Scholar]
- 52. Valentin N, Acosta A, Camilleri M. Early investigational therapeutics for gastrointestinal motility disorders: from animal studies to Phase II trials. Expert Opin Invest Drugs. 2015;24(6):769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Borghede MK, Schlütter JM, Agnholt JS, Christensen LA, Gormsen LC, Dahlerup JF. Bile acid malabsorption investigated by selenium‐75‐homocholic acid taurine ((75)SeHCAT) scans: causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med. 2011;22(6):e137–40. [DOI] [PubMed] [Google Scholar]
- 54. Ruiz‐Campos L, Gisbert JP, Ysamat M, Arau B, Loras C, Esteve M, et al. Systematic review with meta‐analysis: the prevalence of bile acid malabsorption and response to colestyramine in patients with chronic watery diarrhoea and previous cholecystectomy. Aliment Pharmacol Ther. 2019;49(3):242–50. [DOI] [PubMed] [Google Scholar]
- 55. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta‐analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McKenzie JE, Brennan SE. Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, editors. Cochrane handbook for systematic reviews of interventions.Wiley; 2019. p. 321–47. [Google Scholar]
- 58. Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, editors. Cochrane handbook for systematic reviews of interventions.Wiley; 2019. p. 375–402. [Google Scholar]
- 59. Russell RI, Hall MJ. Elemental diet therapy in the management of complicated Crohn's disease. Scott Med J. 1979;24(4):291–5. [DOI] [PubMed] [Google Scholar]
- 60. Gupta A, Muls AC, Lalji A, Thomas K, Watson L, Shaw C, et al. Outcomes from treating bile acid malabsorption using a multidisciplinary approach. Supp Care Cancer. 2015;23(10):2881–90. [DOI] [PubMed] [Google Scholar]
- 61. Fernández‐Bañares F, Esteve M, Salas A, Alsina M, Farré C, González C, et al. Systematic evaluation of the causes of chronic watery diarrhea with functional characteristics. Am J Gastroenterol. 2007;102(11):2520–8. [DOI] [PubMed] [Google Scholar]
- 62. Jackson A, Lalji A, Kabir M, Muls A, Gee C, Vyoral S, et al. The efficacy of a low‐fat diet to manage the symptoms of bile acid malabsorption ‐ outcomes in patients previously treated for cancer. Clin Med. 2017;17(5):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Larsen HM, Borre M, Christensen P, Mohr Drewes A, Laurberg S, Krogh K, et al. Clinical evaluation and treatment of chronic bowel symptoms following cancer in the colon and pelvic organs. Acta Oncol. 2019;58(5):776–81. [DOI] [PubMed] [Google Scholar]
- 64. Nelson LM, Carmichael HA, Russell RI, Atherton ST. Use of an elemental diet (Vivonex) in the management of bile acid‐induced diarrhoea. Gut. 1977;18(10):792–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fernández‐Bañares F, Rosinach M, Esteve M, Forné M, Espinós JC, Maria Viver J. Sugar malabsorption in functional abdominal bloating: a pilot study on the long‐term effect of dietary treatment. Clin Nutr. 2006;25(5):824–31. [DOI] [PubMed] [Google Scholar]
- 66. Fernández‐Bañares F. Dieta controlada en lactosa. In: Salas J, Bonada A, Trallero R, Saló ME, editors. Nutrición y dietética clínica. Barcelona: Doyma Ediciones; 2000. p. 203–8.
- 67. Hopman WP, de Jong AJ, Rosenbusch G, Jansen JB, Lamers CB. Elemental diet stimulates gallbaldder contraction and secretion of cholecystokinin and pancreatic polypeptide in man. Dig Dis Sci. 1987;32(1):45–9. [DOI] [PubMed] [Google Scholar]
- 68. Zubek J, White R. PMO‐031 An audit investigating the efficacy of the low FODMAP diet in improving symptoms in patients with functional gastro‐intestinal symptoms. Gut. 2012;61(Suppl 2):A86. [Google Scholar]
- 69. Svedlund J, Sjödin I, Dotevall G. GSRS ‐ a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33(2):129–34. [DOI] [PubMed] [Google Scholar]
- 70. Misselwitz B, Butter M, Verbeke K, Fox MR. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut. 2019;68(11):2080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Helwig U, Koch AK, Koppka N, Holtmann S, Langhorst J. The predictive value of the hydrogen breath test in the diagnosis of fructose malabsorption. Digestion. 2019;99(2):140–7. [DOI] [PubMed] [Google Scholar]
- 72. Lomer M. The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015;41(3):262–75. [DOI] [PubMed] [Google Scholar]
- 73. Suarez FL, Savaiano D, Arbisi P, Levitt MD. Tolerance to the daily ingestion of two cups of milk by individuals claiming lactose intolerance. Am J Clin Nutr. 1997;65(5):1502–6. [DOI] [PubMed] [Google Scholar]
- 74. Madsen JL, Linnet J, Rumessen JJ. Effect of nonabsorbed amounts of a fructose–sorbitol mixture on small intestinal transit in healthy volunteers. Dig Dis Sci. 2006;51(1):147–53. [DOI] [PubMed] [Google Scholar]
- 75. Hyams JS. Sorbitol intolerance: an unappreciated cause of functional gastrointestinal complaints. Gastroenterology. 1983;84(1):30–3. [PubMed] [Google Scholar]
- 76. Shepherd SJ, Lomer MC, Gibson PR. Short‐chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):707–17. [DOI] [PubMed] [Google Scholar]
- 77. Gibson PR. History of the low FODMAP diet. J Gastroenterol Hepatol. 2017;32:5–7. [DOI] [PubMed] [Google Scholar]
- 78. Gibson PR, Shepherd SJ. Personal view: food for thought–western lifestyle and susceptibility to Crohn's disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21(12):1399–409. [DOI] [PubMed] [Google Scholar]
- 79. Whelan K, Martin LD, Staudacher HM, Lomer M. The low FODMAP diet in the management of irritable bowel syndrome: an evidence‐based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018;31(2):239–55. [DOI] [PubMed] [Google Scholar]
- 80. Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta‐analysis. Gut. 2021. Aug 10;gutjnl‐2021‐325214. 10.1136/gutjnl-2021-325214. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 81. Tuck C, Ly E, Bogatyrev A, Costetsou I, Gibson P, Barrett J, et al. Fermentable short chain carbohydrate (FODMAP) content of common plant‐based foods and processed foods suitable for vegetarian‐ and vegan‐based eating patterns. J Hum Nutr Diet. 2018;31(3):422–35. [DOI] [PubMed] [Google Scholar]
- 82. Muir JG, Shepherd SJ, Rosella O, Rose R, Barrett JS, Gibson PR. Fructan and free fructose content of common Australian vegetables and fruit. J Agricult Food Chem. 2007;55(16):6619–27. [DOI] [PubMed] [Google Scholar]
- 83. Muir JG, Rose R, Rosella O, Liels K, Barrett JS, Shepherd SJ, et al. Measurement of short‐chain carbohydrates in common Australian vegetables and fruits by high‐performance liquid chromatography (HPLC). J Agricult Food Chem. 2009;57(2):554–65. [DOI] [PubMed] [Google Scholar]
- 84. Biesiekierski JR, Rosella O, Rose R, Liels K, Barrett JS, Shepherd SJ, et al. Quantification of fructans, galacto‐oligosacharides and other short‐chain carbohydrates in processed grains and cereals. J Hum Nutr Diet. 2011;24(2):154–76. [DOI] [PubMed] [Google Scholar]
- 85. McIntyre A, Vincent RM, Perkins AC, Spiller RC. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut. 1997;40(2):223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mortensen PB, Hegnhøj J, Rannem T, Rasmussen HS, Holtug K. Short‐chain fatty acids in bowel contents after intestinal surgery. Gastroenterology. 1989;97(5):1090–6. [DOI] [PubMed] [Google Scholar]
- 87. Binder HJ. Role of colonic short‐chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297–313. [DOI] [PubMed] [Google Scholar]
- 88. Rao MC. Oral rehydration therapy: new explanations for an old remedy. Annu Rev Physiol. 2004;66:385–417. [DOI] [PubMed] [Google Scholar]
- 89. Gill SK, Rossi M, Bajka B, Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. 2021;18(2):101–16. [DOI] [PubMed] [Google Scholar]
- 90. Davis R, Day A, Barrett J, Vanlint A, Andrews JM, Costello SP, et al. Habitual dietary fibre and prebiotic intake is inadequate in patients with inflammatory bowel disease: findings from a multicentre cross‐sectional study. J Hum Nutr Diet. 2021;34(2):420–8. [DOI] [PubMed] [Google Scholar]
- 91. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Moayyedi P, Andrews CN, MacQueen G, Korownyk C, Marsiglio M, Graff L, et al. Canadian Association of Gastroenterology clinical practice guideline for the management of irritable bowel syndrome (IBS). J Can Assoc Gastroenterol. 2019;2(1):6–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fernández‐Bañares F, Rosinach M, Piqueras M, Ruiz‐Cerulla A, Modolell I, Zabana Y, et al. Randomised clinical trial: colestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment Pharmacol Ther. 2015;41(11):1132–40. [DOI] [PubMed] [Google Scholar]
- 94. Vanner S, Whelan K. Fermentable carbohydrates in functional bowel disorders: new insights. J Hum Nutr Diet: Off J Br Diet Assoc. 2019;32(4):411–2. [DOI] [PubMed] [Google Scholar]
- 95. Rigby RR, Mitchell LJ, Hamilton K, Williams LT. The use of behavior change theories in dietetics practice in primary health care: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2020;120(7):1172–97. [DOI] [PubMed] [Google Scholar]
- 96. McLaughlin J, Grazia Lucà M, Jones MN, D'Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116(1):46–53. [DOI] [PubMed] [Google Scholar]
- 97. Marciani L, Cox EF, Hoad CL, Pritchard S, Totman JJ, Foley S, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology. 2010;138(2):469–77. [DOI] [PubMed] [Google Scholar]
- 98. Froehlich F, Gonvers J, Fried M. Role of nutrient fat and cholecystokinin in regulation of gallbladder emptying in man. Dig Dis Sci. 1995;40(3):529–33. [DOI] [PubMed] [Google Scholar]
- 99. Pazzi P, Gamberini S, Buldrini P, Gullini S. Biliary sludge: the sluggish gallbladder. Dig Liver Dis. 2003;35:39–45. [DOI] [PubMed] [Google Scholar]
- 100. Festi D, Colecchia A, Orsini M, Sangermano A, Sottili S, Simoni P, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes. 1998;22(6):592–600. [DOI] [PubMed] [Google Scholar]
- 101. Stone BG, Ansel HJ, Peterson FJ, Gebhard RL. Gallbladder emptying stimuli in obese and normal‐weight subjects. Hepatology. 1992;15(5):795–8. [DOI] [PubMed] [Google Scholar]
- 102. Djuric Z, Poore KM, Depper JB, Uhley VE, Lababidi S, Covington C, et al. Methods to increase fruit and vegetable intake with and without a decrease in fat intake: compliance and effects on body weight in the nutrition and breast health study. Nutr Cancer. 2002;43(2):141–51. [DOI] [PubMed] [Google Scholar]
- 103. Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–76. [DOI] [PubMed] [Google Scholar]
- 104. Nasser R, Cook SL, Dorsch KD, Haennel RG. Comparison of two nutrition education approaches to reduce dietary fat intake and serum lipids reveals registered dietitians are effective at disseminating information regardless of the educational approach. J Am Diet Assoc. 2006;106(6):850–9. [DOI] [PubMed] [Google Scholar]
- 105. Hebert JR, Ebbeling CB, Olendzki BC, Hurley TG, Ma Y, Saal N, et al. Change in women's diet and body mass following intensive intervention for early‐stage breast cancer. J Am Diet Assoc. 2001;101(4):421–31. [DOI] [PubMed] [Google Scholar]
- 106. Hofmann AF, Molino G, Milanese M, Belforte G. Description and simulation of a physiological pharmacokinetic model for the metabolism and enterohepatic circulation of bile acids in man. Cholic acid in healthy man. J Clin Invest. 1983;71(4):1003–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Marciani L, Cox EF, Hoad CL, Totman JJ, Costigan C, Singh G, et al. Effects of various food ingredients on gall bladder emptying. Eur J Clin Nutr. 2013;67(11):1182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hopman W, Jansen J, Lamers C. Comparative study of the effects of equal amounts of fat, protein, and starch on plasma cholecystokinin in man. Scand J Gastroenterol. 1985;20(7):843–7. [DOI] [PubMed] [Google Scholar]
- 109. Douglas BR, Jansen JB, Tham RT, Lamers CB. Coffee stimulation of cholecystokinin release and gallbladder contraction in humans. Am J Clin Nutr. 1990;52(3):553–6. [DOI] [PubMed] [Google Scholar]
- 110. Thimister PW, Hopman WP, Loualidi A, Rosenbusch G, Willems HL, Trijbels FJ, et al. Cholestyramine influences meal‐stimulated pancreaticobiliary function and plasma cholecystokinin independent of gastric emptying and food digestion. Scand J Gastroenterol. 1997;32(8):778–84. [DOI] [PubMed] [Google Scholar]
- 111. Summers JA, Peacock J, Coker B, McMillan V, Ofuya M, Lewis C, et al. Multicentre prospective survey of SeHCAT provision and practice in the UK. BMJ Open Gastroenterol. 2016;3(1):e000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Irvine EJ, Tack J, Crowell MD, Gwee KA, Ke M, Schmulson MJ, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1469–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


