Abstract
Introduction
Homozygous or severe heterozygous familial hypercholesterolemia and elevated lipoprotein(a) levels may be treated with membrane filtration. The MONET system (Fresenius Medical Care, Bad Homburg, Germany) involves plasma separation by centrifugation or filtration.
Methods
Whether the method of plasma separation affects lipoprotein lowering and treatment safety was investigated in a single‐center retrospective study.
Results
The centrifugation‐based plasma separation achieved a higher plasma flow and shorter time to treat 1 L of plasma (46.2 ± 8.6 min), than the filtration‐based system (71.5 ± 40.0 min; p = 0.001). The mean reduction of LDL‐cholesterol was 69% and 67% with centrifugation and filtration and was 75% for lipoprotein(a) with both plasma separation methods. A reduction of IgM by more than 60%, of albumin and total protein by approximately 20% and low frequency of side effects was observed.
Conclusions
The efficacy of lowering atherogenic lipoproteins was comparable with both plasma separation methods. Centrifugation was more time‐efficient compared to filtration.
Keywords: LDL cholesterol, lipoprotein apheresis, Lp(a), plasma separation
1. INTRODUCTION
Homozygous or severe heterozygous familial hypercholesterolemia and elevated levels of lipoprotein(a) (Lp(a)) are serious risk factors for cardiovascular diseases [1, 2, 3]. Besides lifestyle and nutritional measures, primary therapies are of pharmacological nature, among those statins and the recently introduced PCSK‐9 inhibitors [4, 5, 6]. In patients with intolerance to available drugs or in those not sufficiently responding to drug therapy, the use of extracorporeal blood purification techniques is considered to lower blood levels of LDL‐cholesterol and Lp(a) [7, 8, 9, 10]. These include various apheresis techniques, which aim to lower blood levels of atherogenic LDL‐cholesterol and Lp(a) [4]. In addition, pleiotropic effects of lipoprotein apheresis may further contribute to lowered atherogenic risk [4, 11]. Few studies have addressed the association of lipoprotein apheresis with the risk of cardiovascular events. A reduction of cardiovascular risk could be identified [12, 13, 14, 15]; however, further long‐term studies are required on the association of lipoprotein apheresis with the long‐term prognosis of affected patients.
Some lipoprotein apheresis systems eliminate lipoproteins from whole blood, such as the DALI adsorber or the Liposorber D [16, 17]. Others eliminate lipoproteins from plasma and therefore require first plasma separation, either by filtration or centrifugation. Both plasma fractionation methods were established earlier to separate plasma from whole blood for therapeutic purposes and to extract plasma proteins from donor blood and were investigated for efficacy in terms of plasma extraction rate and low platelet contamination of the plasma fraction [18, 19]. In lipoprotein apheresis, a second step removes atherogenic lipoproteins from plasma which can be achieved by different techniques—either adsorption, precipitation, or filtration. The latter uses a specific filter to separate molecules based on their size. This can be realized with the MONET system, where a second filter with high permeability for proteins <100 kDa (90%) and low permeability for proteins >1000 kDa retains the lipoproteins, whereas other plasma proteins pass the filter and are returned to the plasma circuit and then to the patient's circulation [4]. An observational multicenter study revealed a mean reduction rate of 64% for LDL‐cholesterol and of 67% for Lp(a) with the MONET system [20]. Treated plasma volume was the strongest predictor of efficacy of treatments with the MONET system. Therefore, the effectiveness of the plasma separation technique to provide a high plasma yield in a given time could be of importance for the efficacy of lipoprotein reduction. Clinical data comparing the use of centrifugation and filtration for plasma separation in lipoprotein apheresis is scarce. Therefore, this retrospective study was performed to compare specifically both methods of plasma separation in view of the efficacy of lipoprotein reduction and treatment safety.
2. MATERIAL AND METHODS
2.1. Study objectives and procedures
The objective of this retrospective, single‐center study has been the efficacy and safety of lipoprotein apheresis with the MONET filter employing plasma separation either with membrane filtration or centrifugation. Plasma separation was performed either by filtration with the Plasmaflux P2 dry filter using the Art Universal machine and Art tubing sets (all Fresenius Medical Care, Bad Homburg, Germany) or by centrifugation using the COM.TEC system including the P1R tubing system (both Fresenius Kabi, Bad Homburg, Germany). The COM.TEC system was connected to the MONET filter (Fresenius Medical Care, Bad Homburg, Germany) using the BioRet tubing system (Aries s.r.l., Mirandola, Italy). Anticoagulation of the extracorporeal circuit in both systems was achieved by using citrate (acid‐citrate‐dextrose, ACD‐A solution), which is continuously infused in the extracorporeal circuit before centrifugation or before the plasma filtration filter in case of centrifugation or filtration for plasma separation, respectively. All patients received apheresis via peripheral veno‐venous access.
Plasma derived from either plasma separation technique enters the MONET filter, the membrane of which has low permeability for proteins >1000 kDa, with a sieving coefficient for LDL‐cholesterol of ≤0.09. This should result in retaining lipoprotein complexes, whereas smaller plasma proteins can pass the membrane and are returned to the patient.
Patient demographic, medical, treatment‐related, and laboratory data were obtained from routine documentation in patient records.
2.2. Patients
Patients with a very high cardiovascular risk consisting of a manifest cardiovascular disease and severe dyslipidemia with elevated LDL‐cholesterol and/or Lp(a) beyond the secondary prevention limits [21, 22] and treated between May 2013 and January 2020 with MONET lipoprotein apheresis were included into this study on performance (i) if plasma separation was performed by centrifugation with the COM.TEC system; (ii) if plasma separation was performed with filtration in these patients as well; and (iii) if pre‐ and posttreatment laboratory data on lipids and lipoproteins were available. Safety data routinely collected before and after apheresis using both plasma separation methods during this time period were analyzed, too. Frequency of side effects was based on all treatments performed during this time period.
All patients had given their informed consent to use their clinical data for secondary data analysis for research purposes at time of enrolment into the apheresis program. All data used for analysis were pseudonymized. No ethics committee was consulted due to the retrospective nature of the study.
2.3. Analysis
Results on continuous variables are given as mean and standard deviation (mean ± SD). Data were tested for normality (Kolmogorov–Smirnov test), in case of nonnormality, differences between the results were tested using the Wilcoxon signed‐rank test for dependent samples and Mann–Whitney U test for independent samples. A p value <0.05 was considered statistically significant. Calculated p values serve as descriptive measures only. If possible (given start and end values), plasma concentrations were corrected using the formula [23]: Ccorr = c* (Hct0/Hctn)*(1‐Hctn)/(1‐Hct0) (Hct0 = pretreatment hematocrit; Hctn = hematocrit value at sampling time n). Whole blood measures (blood cell counts) were corrected using the formula [24]: Ccorr = C*Hct0/Hctn. Analyses of reduction rates were performed comparing the means of patient individual means of all treatments.
All statistical analyses were performed with the IBM SPSS for Windows software, version 22.
3. RESULTS
Between May 2013 and January 2020, 2562 treatments with filtration for plasma separation and 1497 treatments with centrifugation for plasma separation were performed. Fourteen patients, who fulfilled the inclusion criteria for this retrospective study on performance, underwent in total 476 lipoprotein apheresis treatments with centrifugation using the COM.TEC system as plasma separation method. Twelve of these fourteen patients received also lipoprotein apheresis treatments with filtration as the plasma separation technique during this period. Patient demographic data and plasma lipoprotein profiles at patient individual start of lipoprotein apheresis are displayed in Table 1.
TABLE 1.
Patient description at start of first lipoprotein apheresis
| Parameter | Mean ± SD |
|---|---|
| N = 14 | |
| Age | 57.6 ± 16.2 |
| Sex (male/female) | 6/8 |
| Lipoprotein parameters | |
| Total cholesterol (mmol/l) | 7.1 ± 3.1 |
| LDL cholesterol (mmol/l) | 4.5 ± 2.7 (N = 13) |
| HDL cholesterol (mmol/l) | 1.8 ± 1.0 |
| Triglycerides (mmol/l) | 3.7 ± 5.4 |
| Lp(a) (mg/dL) | 95.2 ± 38.1 (N = 10) |
In total, 476 treatments with centrifugation and 410 treatments with filtration for plasma separation were included in the analysis for performance. Patients received between 1 and 93 treatments with centrifugation and between 1 and 128 treatments with filtration as plasma separation method. The mean treated plasma volume was with centrifugation significantly higher than with filtration for plasma separation. Mean treatment time was significantly shorter for the centrifugation‐based system than with treatments employing plasma filtration; in consequence, the required time to treat 1 L of plasma with the centrifugation‐based system was significantly lower than with the filtration‐based system (Table 2). Hence, the centrifugation‐based procedure yields a higher plasma flow rate than the filtration‐based procedure.
TABLE 2.
Achieved treatment parameters with both methods of plasma separation
| Parameter | Centrifugation (COM.TEC) | Plasma filtration (Art Universal) | p |
|---|---|---|---|
| No. of patients | 14 | 12 | |
| No. of treatments | 476 | 410 | |
| Treated plasma volume, mL | 3863 ± 451 | 3506 ± 459 | <0.001 |
| Treatment time, min | 161.7 ± 18.2 | 229.8 ± 57.6 | 0.001 |
| Time to treat 1 L of plasma, min | 46.2 ± 8.6 | 71.5 ± 40.0 | 0.001 |
Note: Mean ± SD.
There were no significant differences in the reduction rates for any lipoprotein between the plasma separation procedures comparing the patient means of the treatments intraindividually (Figure 1). The patient mean LDL‐cholesterol reduction was 69% and 67% in treatments with centrifugation and filtration for plasma separation, respectively. Ninety‐three percent and ninety percent of treatments with plasma separation by centrifugation and filtration, respectively, achieved an LDL‐cholesterol reduction by ≥60%. High‐density lipoprotein (HDL)‐cholesterol was reduced by 29% and 26% in treatments with centrifugation and filtration for plasma separation, respectively. The mean reduction of Lp(a) based on patient means amounted to 75% with both plasma separation methods (Figure 1).
FIGURE 1.
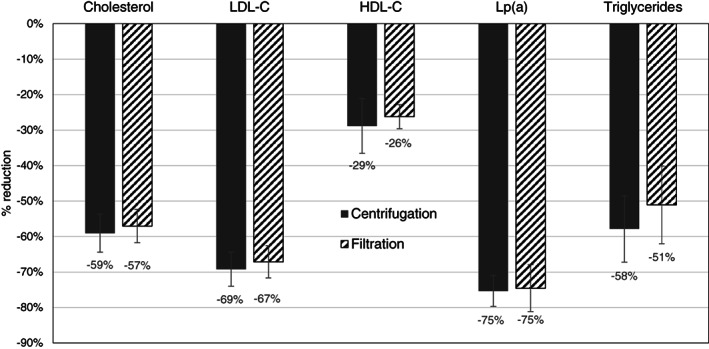
Reduction rates of lipids after lipoprotein apheresis with plasma centrifugation (COM.TEC) (n = 14, Lp(a): n = 11) and plasma filtration (Art Universal) (n = 12, Lp(a): n = 9)
In view of safety, posttreatment laboratory parameters are displayed as (hematocrit‐corrected) percentage of pretreatment value (Figure 2). Whereas blood cells did not change, a considerable reduction of immunoglobulins, particularly of IgM by more than 60% irrespective of the plasma separation method, and an approximately 20% reduction of albumin and total protein was observed. Despite this, the long‐term pretreatment values of these parameters did not decrease over up to 7 years, as could be documented in patients having been treated for 4–10 years with centrifugation (n = 5) or filtration (n = 4) as plasma separation method. Fibrinogen was not measured posttreatment; however, the mean pretreatment values of 360 and 335 mg/dL with centrifugation and filtration, respectively, as plasma separation method indicate a posttreatment recovery of fibrinogen within normal ranges.
FIGURE 2.
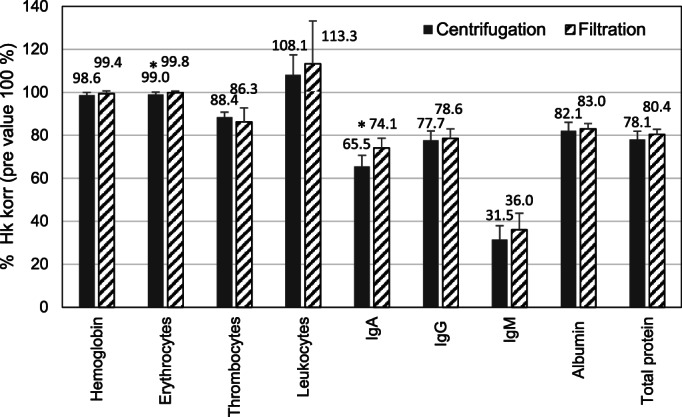
Safety laboratory parameters after lipoprotein apheresis with plasma centrifugation (COM.TEC) and plasma filtration (Art Universal) (hemoglobin, erythrocytes, thrombocytes, leukocytes: n = 14/12 [COM.TEC/Art Universal], IgA, IgG, albumin, total protein: n = 10/11, IgM: n = 9/11), given as hematocrit (Hct) corrected value relative to the pretreatment value; *p < 0.05 COM.TEC vs. Art Universal system, Mann–Whitney U test
Side effects as documented in the patient record are given in Table 3. The most frequent side effects observed with centrifugation for plasma separation was hypotension and tingling, with filtration, nausea was most frequently observed. Overall, the frequency was below 1% of all treatments for any category of side effect. Technical issues were mostly related to the puncture for vascular access which had to be repeated in 8.2% and 6.9% of treatments applying centrifugation or filtration, respectively. Flushing of the MONET filter turned out to be necessary, likely due to increased transmembrane pressure (TMP) during the course of the treatment. This was documented from the year 2016 onwards. During the years 2016–2020, filter flushing was required in 41.1% of the treatments applying centrifugation, and in 3.6% of treatments applying filtration as plasma separation method. Apart from flushing, any technical issue occurred in 12.1% and 8.6% of treatments with centrifugation or filtration as plasma separation method, respectively.
TABLE 3.
Safety of lipoprotein apheresis with plasma centrifugation (COM.TEC) and plasma filtration (Art Universal) as frequency of patient‐related side effects, a given as percentage of all treatments in the observation period (COM.TEC: n = 1497; Art Universal: n = 2562)
| Side effect | Centrifugation (COM.TEC) | Plasma filtration (Art Universal) |
|---|---|---|
| Patient‐related side effects | ||
| Hypotension | 0.43% | 0.13% |
| Tingling (Ca) | 0.39% | 0.07% |
| Dizziness | 0.31% | 0.20% |
| Hypertension | 0.23% | 0.00% |
| Malaise | 0.16% | 0.07% |
| Headache | 0.12% | 0.20% |
| Nausea | 0.12% | 0.53% |
| Cramps (legs) | 0.08% | 0.20% |
| Technical issues | ||
| New puncture | 8.2% | 6.9% |
| Filter flushing | 23.0% | 1.6% |
All other categories of side effects occurred in less than 0.1% of treatments in both groups.
4. DISCUSSION
This retrospective study, which includes real‐world data, provides clinical insights into the previously scarcely studied comparison of plasma separation by centrifugation and filtration for lipoprotein apheresis and its impact on performance and safety. It confirmed a comparable efficacy of LDL‐cholesterol reduction by 69% and 67% with MONET lipoprotein apheresis treatments employing centrifugation or filtration for plasma separation. This was achieved at shorter treatment times with centrifugation than with filtration. The extent of lowering of LDL‐cholesterol met the requirements to LDL‐cholesterol apheresis efficacy of >60% LDL‐cholesterol reduction as formulated by the Federal Joint Committee (Gemeinsamer Bundesausschuss) in Germany [25], and was in the range also found in previous studies [20, 26, 27]. No minimal reduction rate for Lp(a) has been formulated so far, but the achieved reduction rate by in average 75% is at the upper end and above the published performances of a broad range of apheresis techniques [28, 29], and as reported previously with the same MONET system [20, 26, 27]. This reduction rate in our study cohort suggests a lowering from a pre‐treatment Lp(a) level of approximately 100 mg/dL to below 50 mg/dL, a level above which an elevated risk of cardiovascular events has been estimated [30].
In the plasma filtration procedure employed in this cohort using the Art Universal system, the blood/plasma flow was fixed to ≥3:1, whereas the centrifugation method using the COM.TEC system allowed a blood/plasma flow ratio of 2:1, thus delivering a reasonable plasma flow even if the blood flow is limited, for example, due to difficult vascular conditions. The advantages of the centrifugation method are, therefore, the resulting shorter treatment times compared with the filtration methods at equal efficacy. Similar findings on shorter treatment times and higher plasma removal efficacy with centrifugation‐based plasma separation have been published for total plasma exchange (TPE) [31, 32]. Centrifugation‐based TPE could, in comparison to filtration‐based TPE, reduce treatment time without compromising treatment efficacy. This can be viewed as an advantage in supporting patient acceptance and is particularly relevant in centers that perform many interventions per year. In addition to the actual treatment time, the time for preparation of the devices and the patients is relevant. These were reported as 10–15 min for centrifugation with the COM.TEC device and 15–20 min with the Art Universal device. However, a thorough time and cost analysis, as already published for other devices [32], could provide further evidence for the acceptability of the method.
The target population for centrifugation‐based plasma separation therefore includes patients with vascular conditions that might limit blood flow and, in consequence, plasma flow. This may avoid the need for an arteriovenous shunt as vascular access.
Since the method of lipoprotein filtration is rather size than molecule specific, also other proteins in the respective size range may decrease [11]. The anti‐atherogenic HDL decreased by 29% and 26% with the centrifugation‐ and filtration‐based plasma separation, respectively, slightly more than previously reported [20]. Albumin and total protein were only moderately lowered by approximately 20%, slightly more than reported by Kozik‐Jaromin et al. [33] and by Julius et al. [34]. Most prominent was the lowering of IgM levels by more than 60%, which exceeded that reported by Julius et al. [34], and which was comparable to that reported by Kozik‐Jaromin et al. [33]. Obviously, the immunoglobulins recover after treatment, likely by redistribution from nonplasmatic compartments and de novo synthesis, which was supported also by long‐term follow‐up of pretreatment immunoglobulin values in single patients, which rather increased than decreased (data not shown). The elimination capacity of lipoprotein filtration for immunoglobulins has been therapeutically acknowledged in treating AB0 incompatible transplant recipients [35]. Unfortunately, we could not assess the lowering of fibrinogen by membrane filtration due to lack of posttreatment values in patient records. Earlier studies have shown fibrinogen reduction of 58% [33] and 50% [34] with the MONET system. These studies and our data on side effects do not support a long‐term reduction of plasma fibrinogen that would put patients at increased bleeding risk. With mean values of 360 and 335 mg/dL fibrinogen at start of treatment with centrifugation and filtration as plasma separation method, respectively, and a presumed reduction rate of 50%–60%, posttreatment values should rarely be less than 100 mg/dL, a minimum level associated to achieving normal hemostasis [36]. The pretreatment values were comparable to those described by Julius et al. [26], who reported mean posttreatment values of approximately 200 mg/dL with the MONET lipoprotein apheresis system. The protein‐lowering effect of plasma filtration can also be used therapeutically, for example, in rheopheresis for indications that require a reduction in plasma viscosity, such as in age‐related macular degeneration or sudden hearing loss [7].
With centrifugation‐based plasma separation, we observed a more frequent need for flushing the MONET plasma fractionator than with filtration‐based plasma separation. This flushing is triggered by an elevated TMP. Plasma separated by centrifugation may have a higher viscosity or different composition such as higher residual thrombocyte count as also described earlier [19], which may lead to depositions on the membrane surface, which in turn leads to a rise in TMP. Nevertheless, the increased flush rate had no negative effect on long‐term pretreatment protein levels.
The low frequency of patient‐related side effects demonstrated that both methods of plasma separation used for MONET lipoprotein apheresis result in safely performed treatments. Technical issues affected mainly the vascular cannulation, which was independent from the plasma separation system.
Our study has certain limitations. Due to its retrospective nature, we had to rely on available data, without control of treatment order, allocation criteria, and documented parameters. Therefore, only about 25% of treatments performed in the center could be included in the performance analysis. The single‐center setting, small number of patients, in most cases an imbalance in the number of treatments with either system within a patient cannot rule out potential bias. However, the real‐world setting is also a strength of the study as it reflects current practice and provides new data since as yet clinical data on filtration‐based lipoprotein apheresis with centrifugation for plasma separation are scarce. To investigate the impact of lipoprotein apheresis on cardiovascular risk was beyond the scope of this study, but long‐term, multicenter, and adequately powered studies for this objective would be warranted.
5. CONCLUSION
Lipoprotein apheresis employing membrane filtration showed comparable efficacy of lowering atherogenic lipoproteins with both filtration or centrifugation as plasma separation methods. Centrifugation is more time efficient, requiring less time to treat the same plasma volume. This can be an advantage in patients with a difficult vascular situation, allowing the use of a peripheral vascular access at low blood flow rates and avoiding an arteriovenous shunt as vascular access, eventually facilitating lipoprotein apheresis with its potential lowering of atherogenic risk.
CONFLICTS OF INTEREST
Heinrich Prophet and Grit Waitz are/were full‐time employees at Nephrocare Rostock and Apheresis Center Rostock, Germany. Saynab Atiye and Adelheid Gauly are full‐time employees of Fresenius Medical Care, Bad Homburg, Germany.
AUTHOR CONTRIBUTIONS
Grit Waitz, Heinrich Prophet, and Saynab Atiye contributed to the concept and design of the project. Grit Waitz contributed to data analysis. Adelheid Gauly drafted the article. All authors reviewed and approved the publication.
ETHICS STATEMENT
All patients had given their informed consent to use their clinical data for secondary data analysis for research purposes at time of enrolment into the apheresis program.
ACKNOWLEDGMENTS
This study was funded by Fresenius Medical Care Deutschland GmbH. The authors would like to thank the nursing team of the Apheresis Center Rostock for excellent technical support. They would also like to thank Dr Wolfgang Ramlow for initiating and supervising the project.
Waitz G, Atiye S, Gauly A, Prophet H. Comparison of plasma separation using centrifugation or filtration for MONET lipoprotein apheresis in patients with cardiovascular disease and severe dyslipidemia. Ther Apher Dial. 2022;26:1281–1288. 10.1111/1744-9987.13840
Funding information Fresenius Medical Care Deutschland GmbH
REFERENCES
- 1. Hu P, Dharmayat KI, Stevens CAT, Sharabiani MTA, Jones RS, Watts GF, et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta‐analysis. Circulation. 2020;141:1742–59. [DOI] [PubMed] [Google Scholar]
- 2. Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 2019;74:2982–94. [DOI] [PubMed] [Google Scholar]
- 3. Jang AY, Han SH, Sohn IS, Oh PC, Koh KK. Lipoprotein(a) and cardiovascular diseases‐ revisited. Circ J. 2020;84:867–74. [DOI] [PubMed] [Google Scholar]
- 4. Julius U. Lipoprotein apheresis in the management of severe hypercholesterolemia and of elevation of lipoprotein(a): current perspectives and patient selection. Med Devices. 2016;9:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weingärtner O, Lütjohann D, Plösch T, Elsässer A. Individualized lipid‐lowering therapy to further reduce residual cardiovascular risk. J Steroid Biochem Mol Biol. 2017;169:198–201. [DOI] [PubMed] [Google Scholar]
- 6. Warden BA, Fazio S, Shapiro MD. The PCSK9 revolution: current status, controversies, and future directions. Trends Cardiovasc Med. 2020;30:179–85. [DOI] [PubMed] [Google Scholar]
- 7. Padmanabhan A, Connelly‐Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice ‐ evidence‐based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34:171–354. [DOI] [PubMed] [Google Scholar]
- 8. Thompson GR, Group H‐ULAW . Recommendations for the use of LDL apheresis. Atherosclerosis. 2008;198:247–55. [DOI] [PubMed] [Google Scholar]
- 9. Kayikcioglu M. LDL apheresis and Lp (a) apheresis: a clinician's perspective. Curr Atheroscler Rep. 2021;23:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson GR. The scientific basis and future of lipoprotein apheresis. Ther Apher Dial. 2022;26:32–6. [DOI] [PubMed] [Google Scholar]
- 11. Makino H, Koezuka R, Tamanaha T, Ogura M, Matsuki K, Hosoda K, et al. Familial hypercholesterolemia and lipoprotein apheresis. J Atheroscler Thromb. 2019;26:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang A, Richhariya A, Gandra SR, Calimlim B, Kim L, Quek RGW, et al. Systematic review of low‐density lipoprotein cholesterol apheresis for the treatment of familial hypercholesterolemia. J Am Heart Assoc. 2016;5:e003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, et al. Lipoprotein apheresis for lipoprotein(a)‐associated cardiovascular disease: prospective 5 years of follow‐up and Apolipoprotein(a) characterization. Arterioscler Thromb Vasc Biol. 2016;36:2019–27. [DOI] [PubMed] [Google Scholar]
- 14. Jaeger BR, Richter Y, Nagel D, Heigl F, Vogt A, Roeseler E, et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med. 2009;6:229–39. [DOI] [PubMed] [Google Scholar]
- 15. Moriarty PM, Gray JV, Gorby LK. Lipoprotein apheresis for lipoprotein(a) and cardiovascular disease. J Clin Lipidol. 2019;13:894–900. [DOI] [PubMed] [Google Scholar]
- 16. Bosch T. Practical aspects of direct adsorption of lipoproteins from whole blood by DALI LDL‐apheresis. Transfus Apher Sci. 2004;31:83–8. [DOI] [PubMed] [Google Scholar]
- 17. Otto C, Berster J, Otto B, Parhofer KG. Effects of two whole blood systems (DALI and Liposorber D) for LDL apheresis on lipids and cardiovascular risk markers in severe hypercholesterolemia. J Clin Apher. 2007;22:301–5. [DOI] [PubMed] [Google Scholar]
- 18. Gurland HJ, Lysaght MJ, Samtleben W, Schmidt B. A comparison of centrifugal and membrane‐based apheresis formats. Int J Artif Organs. 1984;7:35–8. [PubMed] [Google Scholar]
- 19. Vezon G, Piquet Y, Manier C, Schooneman F, Mesnier F, Moulinier J. Technical aspects of different donor plasmapheresis systems and biological results obtained in collected plasma. Vox Sang. 1986;51(Suppl 1):40–4. [DOI] [PubMed] [Google Scholar]
- 20. Ramlow W, Roseler E, Heigl F, Spitthöver R, Ringel J, Schmitz G, et al. Efficacy of lipid reduction with DALI and MONET. Atheroscler Suppl. 2017;30:217–24. [DOI] [PubMed] [Google Scholar]
- 21. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the Management of Dyslipidaemias: the task force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;253:281–344. [DOI] [PubMed] [Google Scholar]
- 22. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. [DOI] [PubMed] [Google Scholar]
- 23. Van Beaumont W. Evaluation of hemoconcentration from hematocrit measurements. J Appl Physiol. 1972;32:712–3. [DOI] [PubMed] [Google Scholar]
- 24. Hovland A, Hardersen R, Enebakk T, Mollnes TE, Lappegård KT. Patient tolerance regarding different low‐density lipoprotein apheresis columns: frequent minor side effects and high patient satisfaction. J Clin Lipidol. 2011;5:45–9. [DOI] [PubMed] [Google Scholar]
- 25. Bundesministerium für Gesundheit (German Ministry of Health) . Bekanntmachung des Bundesausschusses der Ärzte und Krankenkassen über eine Änderung der Richtlinien über die Bewertung ärztlicher Untersuchungs‐ und Behandlungsmethoden gemäß § 135 Abs. 1 des Fünften Buches Sozialgesetzbuch (SGB V) (BUB‐Richtlinien). BAnz. 2003;123:14486. [Google Scholar]
- 26. Julius U, Fischer S, Schatz U, Passauer J, Bornstein S. Why an apheresis center should offer more than one lipoprotein apheresis method. Ther Apher Dial. 2013;17:179–84. [DOI] [PubMed] [Google Scholar]
- 27. Mickiewicz A, Borowiec‐Wolna J, Bachorski W, Gilis‐Malinowska N, Gałąska R, Raczak G, et al. Long‐term lipoprotein apheresis in the treatment of severe familial hypercholesterolemia refractory to high intensity statin therapy: three year experience at a lipoprotein apheresis centre. Cardiol J. 2019;26:669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klingel R, Fassbender T, Fassbender C, Gohlen B. From membrane differential filtration to lipidfiltration: technological progress in low‐density lipoprotein apheresis. Ther Apher Dial. 2003;7:350–8. [DOI] [PubMed] [Google Scholar]
- 29. Schettler VJJ, Neumann CL, Peter C, Zimmermann T, Julius U, Roeseler E, et al. Current insights into the German Lipoprotein Apheresis Registry (GLAR) ‐ almost 5 years on. Atheroscler Suppl. 2017;30:50–5. [DOI] [PubMed] [Google Scholar]
- 30. Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hafer C, Golla P, Gericke M, Eden G, Beutel G, Schmidt JJ, et al. Membrane versus centrifuge‐based therapeutic plasma exchange: a randomized prospective crossover study. Int Urol Nephrol. 2016;48:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kes P, Janssens ME, Basic‐Jukic N, Kljak M. A randomized crossover study comparing membrane and centrifugal therapeutic plasma exchange procedures. Transfusion. 2016;56:3065–72. [DOI] [PubMed] [Google Scholar]
- 33. Kozik‐Jaromin J, Roseler E, Heigl F, Spitthöver R, Ringel J, Schmitz G, et al. Safety aspects of lipidapheresis using DALI and MONET ‐ multicenter observational study. Atheroscler Suppl. 2017;30:225–31. [DOI] [PubMed] [Google Scholar]
- 34. Julius U, Siegert G, Kostka H, Schatz U, Hohenstein B. Effects of different lipoprotein apheresis methods on serum protein levels. Atheroscler Suppl. 2015;18:95–102. [DOI] [PubMed] [Google Scholar]
- 35. Eskandary F, Wahrmann M, Biesenbach P, Sandurkov C, Konig F, Schwaiger E, et al. ABO antibody and complement depletion by immunoadsorption combined with membrane filtration–a randomized, controlled, cross‐over trial. Nephrol Dial Transplant. 2014;29:706–14. [DOI] [PubMed] [Google Scholar]
- 36. Zöllner S, Pablik E, Druml W, Derfler K, Rees A, Biesenbach P. Fibrinogen reduction and bleeding complications in plasma exchange, immunoadsorption and a combination of the two. Blood Purif. 2014;38:160–6. [DOI] [PubMed] [Google Scholar]


