Abstract
Background
Computational research had determined that adults with obsessive–compulsive disorder (OCD) display heightened action updating in response to noise in the environment and neglect metacognitive information (such as confidence) when making decisions. These features are proposed to underlie patients’ compulsions despite the knowledge they are irrational. Nonetheless, it is unclear whether this extends to adolescents with OCD as research in this population is lacking. Thus, this study aimed to investigate the interplay between action and confidence in adolescents with OCD.
Methods
Twenty‐seven adolescents with OCD and 46 controls completed a predictive‐inference task, designed to probe how subjects’ actions and confidence ratings fluctuate in response to unexpected outcomes. We investigated how subjects update actions in response to prediction errors (indexing mismatches between expectations and outcomes) and used parameters from a Bayesian model to predict how confidence and action evolve over time. Confidence–action association strength was assessed using a regression model. We also investigated the effects of serotonergic medication.
Results
Adolescents with OCD showed significantly increased learning rates, particularly following small prediction errors. Results were driven primarily by unmedicated patients. Confidence ratings appeared equivalent between groups, although model‐based analysis revealed that patients’ confidence was less affected by prediction errors compared to controls. Patients and controls did not differ in the extent to which they updated actions and confidence in tandem.
Conclusions
Adolescents with OCD showed enhanced action adjustments, especially in the face of small prediction errors, consistent with previous research establishing ‘just‐right’ compulsions, enhanced error‐related negativity, and greater decision uncertainty in paediatric‐OCD. These tendencies were ameliorated in patients receiving serotonergic medication, emphasising the importance of early intervention in preventing disorder‐related cognitive deficits. Confidence ratings were equivalent between young patients and controls, mirroring findings in adult OCD research.
Keywords: Obsessive–compulsive disorder, adolescence, cognition
Introduction
Intrusions and compulsions displayed by individuals with obsessive–compulsive disorder (OCD) are ego‐dystonic in nature (Sasson et al., 1997), as they occur despite being at odds with the core beliefs of the sufferer. Patients repeatedly engage in maladaptive washing and checking behaviour, for instance, despite having the awareness that they are excessive and irrational.
Congruently, adults with OCD do not rely on metacognitive information, such as confidence, to inform decisions (Vaghi et al., 2017). Additionally, adult patients continue to respond to devalued stimuli despite being aware that the stimuli are no longer predictive of outcomes (Apergis‐Schoute et al., 2017; Gillan et al., 2014; Vaghi et al., 2019). One explanation for this disconnect between actions and beliefs is that patients experience elevated uncertainty surrounding how events result from specific actions (Fradkin, Adams, Parr, Roiser, & Hupperts, 2020). They mistrust or place less weight on prior evidence in their decision‐making and hence carry out compulsive behaviours that are at odds with preexisting information to cope with the uncertainty. Supporting this theory, adults with obsessive–compulsive traits are found to rely less on past feedback information when making decisions on a probabilistic learning task, which led them to regard otherwise expected outcomes as ‘surprising’ (Fradkin, Ludwig, Eldar, & Huppert, 2020).
Importantly, Vaghi et al. (2017) used a predictive‐inference task to formally probe the interplay between metacognition (confidence) and action when environments are dynamic or volatile. Adults with OCD were tasked with predicting where a coin would land on a screen and rating how confident they were in their predictions. The task was dynamic/probabilistic in that the coin predominantly landed in the same location with occasional deviations. Patients appropriately updated their confidence ratings based on changes in the coin location, but their actions did not reflect this knowledge. Instead, actions were driven by the most recent observation (where the coin landed most recently) instead of accumulated information (where the coin landed most frequently). The authors concluded that those with OCD can develop an accurate internal model of the task environment but fail to use this knowledge to guide their actions. In addition, computational modelling of the patients’ data revealed that correct confidence updating was uncoupled from excessive actions, further confirming that metacognition does not influence their actions.
No research has assessed whether beliefs and actions are updated abnormally in youths with OCD. Nonetheless, evidence has emerged that paediatric patients show enhanced ‘decision thresholds’ during certain tasks, wherein they continue to sample information prior to making a choice even when sufficient information has already been acquired (Erhan et al., 2017; Hauser, Moutoussis, et al., 2017). This is suggestive of a dissociation between action and knowledge, as young patients continuously seek information even when doing so no longer has value.
Curiously, youths with OCD do not show cognitive impairments to the same extent as their adult counterparts, particularly in the domains of cognitive flexibility and response inhibition even though they are thought to be endophenotypes of the disorder based on a meta‐analysis and systematic review (Abramovitch et al., 2015; Marzuki, Pereira de Souza, Sahakian, & Robbins, 2020). As a result, devising a cognitive model of OCD that can account for both child and adult subtypes is challenging. This is in part due to the limited research conducted on youth‐OCD samples compared to adult samples. Although recent research with larger sample sizes has found elevated action monitoring, inefficient response inhibition, impaired planning, and poor learning to be associated with child OCD (Gottwald et al., 2018; Negreiros et al., 2020; Riesel, 2019; Schachar et al., 2021), indicating older studies employing youth‐OCD samples may be underpowered.
There is also emerging evidence for youths with OCD displaying altered decision‐making particularly when faced with probabilistic tasks. Computational research to date has revealed that young patients show more choice‐switching and more exploration of suboptimal choices during tasks that provide probabilistic feedback (Hauser, Allen, et al., 2017; Marzuki et al., 2021; Norman et al., 2018). The probabilistic tasks may be triggering uncertainty in young patients, which in turn promotes noisy decision‐making (Marzuki et al., 2021).
At present, it is crucial to understand whether young patients’ decision‐making under uncertainty is linked to an action–confidence mismatch, which appears to be a robust finding in adult‐OCD research. Perhaps youths with OCD are aware their decisions are inconsistent with task feedback but persist in making stochastic decisions anyway. If so, dissociated action and confidence could serve as a potentially shared impairment underpinning OCD symptoms in both childhood and adulthood.
Thus, this study aimed to investigate the relationship between confidence and action in a sample of adolescents with OCD using the predictive‐inference task originally devised by McGuire, Nassar, Gold, and Kable (2014) and employed by Vaghi et al. (2017) to test adults with OCD. Based on prior findings in adult‐OCD, we hypothesised that compared to healthy age‐matched controls, adolescent patients’ decisions would be driven by most recent feedback instead of information accumulated across the task, despite appropriate confidence ratings. We also predicted that adolescents with OCD would reveal an action–confidence dissociation on the task. In conjunction, we were interested in investigating the effects of selective‐serotonin reuptake inhibitor (SSRI) treatment on the performance of youths with OCD as previous work has reported cognitive improvements associated with SSRI in both youth and adult OCD populations (Andrés et al., 2008; Lochner et al., 2016; Lochner et al., 2020; Palminteri, Clair, Mallet, & Pessiglione, 2012).
Methods
Sample
A sample size calculation was performed using G*Power 3 (Faul, Erdfelder, Lang, & Buchner, 2007) with α = .05 (two‐tailed), power (1‐β) set to.80, and mean and standard deviation based on statistically significant group results from Vaghi et al. (2017). This called for a total sample size of at least approximately n = 6. We recruited 73 youths (12–19 years) to complete the predictive‐inference task (46 controls, 27 patients). Table 1 summarises the demographic and clinical characteristics. Eleven patients from the OCD group were receiving SSRIs at the time of the study while 16 were unmedicated. Eight patients were receiving sertraline (Mean [M] = 118.75 mg; standard deviation [SD] = 53.03) and 3 were receiving fluoxetine (M = 36.67 mg; SD = 15.28). Groups were matched for gender, age, and intelligence quotient (IQ). However, the OCD group had significantly elevated depression, anxiety, and obsessive–compulsive severity scores compared to control participants (CTLs) (Table 1).
Table 1.
Mean scores and standard deviations per group and measure
| CTL (n = 46) | OCD (n = 27) | Statistic | |
|---|---|---|---|
| GENDER(F:M) | 28/18 | 18/9 | χ2(1) = 0.25, p = .62 |
| AGE | 16.59 (1.78) | 16.16 (1.67) | Z = 1.81, p = .07 |
| WASI‐II (IQ)a | 107.61 (11.62) | 107.12 (13.02) | t(70) = 0.17, p = .87 |
| BDI** | 46.46 (5.27) | 59.03 (9.55) | t(35.45) = −6.31, p < .001 |
| BAI** | 45.98 (7.66) | 65.48 (9.37) | Z = −6.88, p < .001 |
| OCI** | 8.13 (6.49) | 31.70 (13.92) | Z = −6.35, p < .001 |
| CY‐BOCS a | N/A | 23.62 (4.84) | N/A |
| CY‐BOCS Obsessions a | N/A | 11.23 (2.18) | N/A |
| CY‐BOCS Compulsions a | N/A | 12.35 (2.99) | N/A |
| Overall LR | 0.61 (0.25) | 0.90 (0.55) | Z = −2.18; p = .029 |
| LR (Small PE) | 0.53 (0.46) | 1.45 (1.62) | Z = −2.78; p = .0055 |
| LR (Medium PE) | 0.53 (0.30) | 0.64 (0.40) | Z = −0.95, p = .34 |
| LR (Large PE) | 0.80 (0.11) | 0.82 (0.13) | Z = −0.29, p = .77 |
| Confidence Ratings (Overall) | 4.50e−17 (2.58e−16) | −6.07e−18 (1.85e−16) | t(71) = 0.90, p = .37 |
| Action–Confidence Association Strength | 0.053 (0.064) | 0.044 (0.079) | t(71) = 0.54, p = .59 |
Details of questionnaires in Supporting Information. Mean (SD) reported. Key: CTL, Control Group; OCD, Obsessive–Compulsive Disorder group; WASI‐II, Wechsler’s Abbreviated Scale of Intelligence – II; IQ, Intelligence Quotient; BDI, Beck’s Depression Inventory(t‐scored); BAI, Beck’s Anxiety Inventory (t‐scored); OCI, Obsessive–Compulsive Inventory; CY‐BOCS, Child Yale‐Brown Obsessive–Compulsive Scale; LR, Learning Rates; PE, Prediction Error; N/A, Not applicable. *p < .05; **p < .01; amissing data from one OCD participant.
Ethical considerations
This study was approved by the East of England – Essex Research Ethics Committee (REC 10/H030149/49). All volunteers gave written informed consent before beginning testing and were compensated at the rate of £8 per hour. Parental consent was also obtained if participants were under 16 years old.
Inclusion and exclusion criteria
Adolescents with OCD were recruited via Child and Adolescent Mental Health Services around the United Kingdom. Healthy controls were recruited via advertisements in state secondary schools and on noticeboards around Cambridgeshire. Patients were screened by an experienced psychiatrist to rule out comorbid psychiatric and neurological conditions in a clinical interview supplemented by the Mini International Neuropsychiatric Interview (MINI, Sheehan et al., 1998, 2010).
To qualify for the study, those in the OCD group had to meet Diagnostic and Statistical Manual of Mental Disorders‐5‐Text Revision diagnostic criteria for OCD, have OCD as their primary diagnosis, and score above 12 on the Children’s Yale‐Brown Obsessive–Compulsive Scale 3, as a score of 12 is reported to be the optimal cut‐off score for predicting remission (Lewin et al., 2011). Apart from OCD, other significant Axis I psychiatric disorders was exclusion criteria. Those with severe physical impairments affecting eyesight or motor performance were also excluded, as they were predicted to affect task performance. CTLs were also screened to ensure they had no history of neurological or psychiatric illness. Details of recruitment and screening for this sample are outlined in Figure S1.
See Appendix S1 for details of the questionnaires.
Predictive‐inference task
Participants were instructed to make predictions about where ‘coins’ emitting from the centre of a circular ring would land by positioning an orange ‘bucket’ on the same circular ring to catch them (Figure 1). After positioning the bucket, participants rated their confidence in their choice on a scale (1–100). Before the task began, participants were informed that coins mostly flew to approximately the same location, but that location could change sometimes. No time limit was imposed but participants were instructed to respond as quickly as possible.
Figure 1.
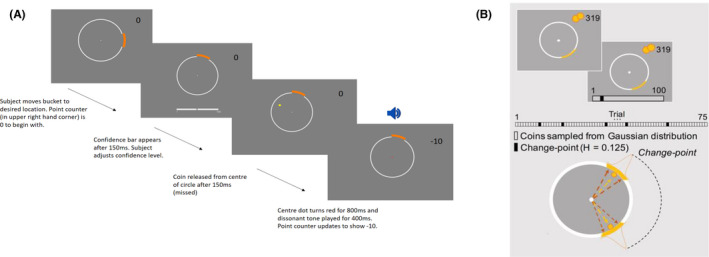
Predictive‐Inference Task. (A) An example of a trial where the subject predicted coin location incorrectly. (B) Coin locations are determined using a Gaussian distribution on most trials. When a change point occurs, the coin location changes drastically according to a uniform distribution. The probability of a change point occurring at any point during the task was 0.125. Black bars in the figure above represent change point trials. Otherwise, coins are sampled from a Gaussian distribution (white bars). Figure B is adapted with permission from Vaghi et al. (2017)
The location the coin would be released to in each trial was mostly determined by sampling a Gaussian distribution. Hence, coins landed in a similar location with small variations driven by noise. The mean of this distribution usually remained stable over a block of trials but changed at random intervals (change‐points, Figure 2) when it was resampled from a uniform distribution. The probability of a change‐point occurring at any point in a block was set to 0.125. Thus, participants were required to form a new belief about the mean of the Gaussian distribution each time a change‐point occurred. Conversely, they had to maintain the same belief about the mean of the Gaussian distribution when small changes in coin position were due to noise. There were 360 possible locations for coins to fly to when a change point occurred. Hence, the task environment is considered to be dynamic or volatile because the location of the coin changes from trial to trial according to probability distributions (McGuire et al., 2014; Nassar, Wilson, Heasly, & Gold, 2010).
Figure 2.
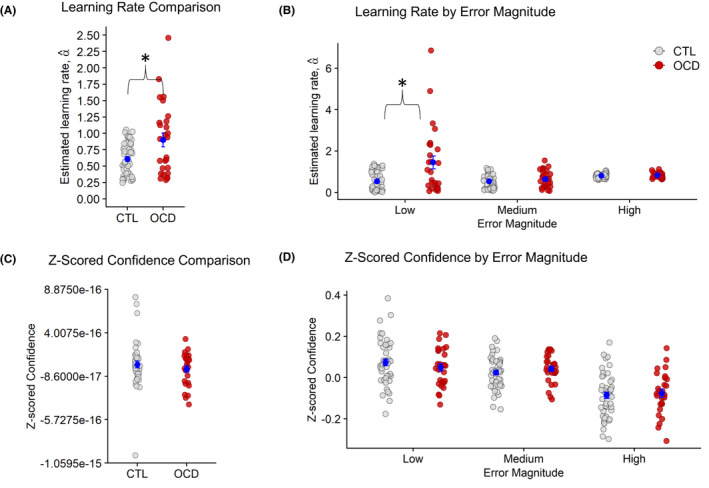
Learning rate and confidence. Note: *p < .05. (A) The OCD group showed significantly higher learning rates compared to CTLs. (B) Learning rates were significantly enhanced in the OCD group only when prediction errors were low. (C and D) There were no significant group differences for mean Z‐scored confidence and when confidence was binned by prediction error
During the task, the bucket could be moved around the ring using a Griffin PowerMate USB rotary controller. Participants confirmed responses by pressing the spacebar on the laptop keyboard. After 150 ms, participants would be prompted to rate their confidence. A coin was then released for 150 ms. If the coin landed within the boundaries of the bucket, participants were awarded 10 points. If the coin landed outside the boundaries, they lost 10 points.
Stimuli were presented to participants using Matlab R2017b and Psychtoolbox v.3. Participants completed one practice block of 20 trials and 4 blocks of 75 trials each in the main task. The task lasted approximately 20 min.
Statistical analysis
Learning rate and confidence analysis
Data manipulation and statistical analysis were conducted in Matlab R2017b and RStudio 3.5.0. Analysis and statistical models were adapted from Vaghi et al.’s (2017).
For each participant, learning rates on every trial (αt) were computed to understand how evidence accumulated in the task’s noisy environment influenced participants’ actions (positioning of the bucket):
| (1) |
| (2) |
In Equations 1 and 2, bt and bt +1 are the chosen bucket positions at trial t and trial t + 1, respectively. δ t , the prediction error, is the difference between the location of the particle (Xt ) at trial t and the position of the bucket at trial t (bt ). Trials where the estimated learning rate (α) exceeded the 95th percentile (calculated separately for each group) or where prediction error = 0 were excluded from the analysis. This type of filtering was employed as extreme values are reported to be due to noisy processes other than error‐driven learning (Nassar et al., 2010). As several trials in our sample revealed extremely high learning rates (α t > 1), our exclusion threshold was more stringent than what was employed by Vaghi et al. (2017) who only excluded trials exceeding the 99th group percentile.
We used the Shapiro–Wilk test to check for normality and Levene’s test to assess homogeneity of variance between groups prior to conducting any group analysis. A two‐sample t‐test was used to confirm that there was no significant difference in the proportion of trials removed between the OCD and CTL groups (Appendix S2). The difference in mean learning rates between groups was analysed using a Wilcoxon rank‐sum test.
Raw confidence ratings were converted to z‐scores (to account for participants having different baseline confidence levels and enable comparison between confidence ratings on a relative scale) and a two‐sample t‐test was used to assess group differences.
Next, we assessed whether the magnitude of prediction error (the difference between expectations and outcomes, Equation 2) had a significant effect on learning rates and confidence. The learning rates and z‐scored confidence ratings were divided into 3 quantiles based on the magnitude of prediction error (small, medium, and large) using the ‘quantile’ function in Matlab. For each prediction error quantile, the mean learning rate and z‐scored confidence were computed separately per group (OCD vs. CTL).
Due to homogeneity of variance and normality violations, the Welch–James test from the ‘welchADF’ package in RStudio (Villacorta, 2017) was used to determine the effects of group on learning rates, depending on the magnitude of prediction error (low, medium, and high). Post‐hoc paired Wilcoxon tests with Bonferroni correction were conducted following the main Welch–James test.
The effects of group and prediction error on confidence were assessed using a mixed‐ANOVA. The Hyun–Feldt correction was applied due to violations of the sphericity assumption.
Influence of model parameters on learning and confidence
We used a quasi‐optimal Bayesian learning model to simulate data. The same model was also fitted to participant data (see Appendix S3). We compared participants’ actions and confidence to that of the Bayesian model by inspecting the average learning rates and confidence ratings 4 trials before and 4 trials after a change point occurred.
Linear regressions were conducted to estimate how much participants updated their actions and confidence over time according to parameters from the Bayesian model. The parameters included absolute prediction error (, the absolute difference between belief [where the bucket was positioned] and location of coin at each trial), change point probability (the relative likelihood that the coin is sampled from a new distribution, that is, a change‐point has occurred), and relative uncertainty (reflects uncertainty regarding the mean of the coin distribution). These parameters were inserted as predictor variables in the regression models. Hit/Miss was also included as a categorical variable in the regressions to assess whether action and confidence were influenced by immediate feedback.
To assess the effects of Bayesian model parameters on participants’ actions, the dependent variable, ‘action’ was calculated by multiplying the learning rate, , by the absolute prediction error, . The predictors in this model were also multiplied by . Within the confidence regression model, z‐scored reported confidence (not multiplied by ) was included as the dependent variable. Predictors associated with t − 1 were inserted in the model, as confidence would be affected by information associated with the immediate previous trial. The last trial of each block per participant was removed before conducting the regressions as learning rates could not be estimated from these trials. Goodness‐of‐fit of each model to participant data was assessed by calculating median R‐squared values.
To formally examine whether confidence and action are dissociated in adolescent patients, a third linear regression model was conducted with absolute confidence update (absolute difference between z‐scored confidence scores on trial t and t − 1) as the independent variable and absolute action update (absolute difference between where the bucket was positioned at trial t and t − 1) as the dependent variable. If confidence and action were linked, increased adjusting of the bucket position would correspond to a similar magnitude of change in confidence ratings.
To ascertain the presence of group differences in performance, beta coefficients associated with each predictor across the three regressions were extracted and compared between patients and controls. Independent sample t‐tests were employed for these comparative analyses. Wherever the homogeneity of variance assumption was violated Welch’s independent t‐test was used instead. If the normality assumption was violated the Wilcoxon rank‐sum test was used.
Effect sizes
For t‐tests, Cohen’s d was calculated as a measure of effect size whenever a significant effect was detected (small: <= 0.2 to <0.5, moderate: 0.5 > to < 0.8, and large: >= 0.8) (Cohen, 1988).
For Wilcoxon rank‐sum tests, the effect size was determined via the Wilcoxon effect size (Wilcoxon r) calculated by dividing the test z‐statistic by the square root of the sample size (Z/√N). The following interpretations of effect size values were used, small: 0.1 to <0.3, moderate: 0.3 to <0.5, and large: >= 0.5 (Tomczak & Tomczak, 2014).
Medication analysis
The analyses described above were repeated subdividing the OCD group into unmedicated patients (MED−), and patients medicated with SSRIs (MED+). These exploratory analyses were conducted to investigate whether patients’ actions and confidence were modulated by SSRIs.
Results
CTL versus OCD Analysis
The OCD group showed increased learning rates (Figure 2A) compared to CTLs (Z = −2.18; p = .029, Wilcoxon’s r = .26; see Table 1 for the statistical summary). Next, our analysis of the effects of prediction error on learning rates revealed a significant main effect of prediction error magnitude, T wj(2,34.87) = 73.73, p < .001. Learning rates overall were greater at large (M = 0.81; SD = 0.11) compared to medium‐sized (M = 0.57, SD = 0.34) prediction errors (Z = −5.61; p < .001; Wilcoxon’s r = .44).
Importantly, a significant group‐by‐prediction error interaction was detected (Figure 2B), T wj(2,34.87) = 5.78, p = .0068. Post‐hoc Wilcoxon tests revealed that OCD displayed enhanced learning rates compared to CTLs in response to small prediction errors (Z = −2.79; p = .0055; Wilcoxon’s r = .32). There were no group differences in learning rates at medium (p = .34) and large (p = .77) prediction errors.
In contrast, Z‐scored confidence ratings (Figure 2C) did not differ significantly between groups (p = .37). When analysing effects of prediction error on confidence, we found a significant main effect of prediction error (F[2,142] = 32.11, p < .001). Participants predictably, reduced their confidence when prediction errors were large (M = −0.08, SD = 0.11) but increased in confidence when prediction errors were medium (M = 0.03, SD = 0.07) and small (M = 0.06, SD = 0.10), (large vs. medium: t[72] = 5.97, p < .001; large vs. small: t[72] = 6.61, p < .001; and small vs. medium: p > .05). There was no significant effect of group (F[1,71] = 0.05, p = .83) nor a significant group‐by‐prediction error interaction (F[2,142] = 32.11; p = .52; Figure 2D).
Regression models
The median r‐squared values for the action regression model were CTL: 0.87 and OCD: 0.80, while the median r‐squared values for the confidence regression model were considerably lower (CTL: 0.085, OCD: 0.0643) indicating poor model fit (Appendix S4).
There were no group differences in any of the beta values corresponding to parameters in the action model (pairwise comparisons: p > .05). In the confidence model, CTLs’ confidence was more influenced by prediction errors (beta coefficients: M = −0.086, SD = 0.13) compared to that of OCD participants (beta coefficients: M = −0.013, SD = 0.16), t[71] = −2.12; p = .037; Cohen’s d = 0.51. There were no other group differences in parameter values in the confidence model (pairwise comparisons: p > .05). The action and confidence regression results are summarised in Tables S1 and S2, respectively.
Finally, the confidence–action regression model revealed no group differences in degree of action–confidence coupling, t[71] = 0.54, p = .59.
As learning rates were abnormally increased in the OCD group specifically during small prediction errors, we also applied the regression analyses to low prediction error magnitude trials only. However, no significant group differences were detected for model parameters (Tables S3 and S4).
Analysis of medication effects
There were no significant differences in gender, age, and IQ between CTL, MED−, and MED+ groups (see Table S5 for the statistical summary).
We excluded trials with learning rates that were greater than the 95th percentile for each of the 3 groups, identical to what was done for the OCD versus CTL analysis. A Kruskal–Wallis test on learning rates showed a significant group effect, χ2(2) = 8.44, p = .015 (Figure 3A). MED− displayed higher learning rates than CTL (Z = −3.09, p = .007, Wilcoxon’s r = .38) but not MED+ (MED+ = MED−, p = .69; MED+ = CTL, p = 1.00). Z‐scored confidence ratings were comparable across the 3 groups (χ2[2] = 4.34, p = .11).
Figure 3.
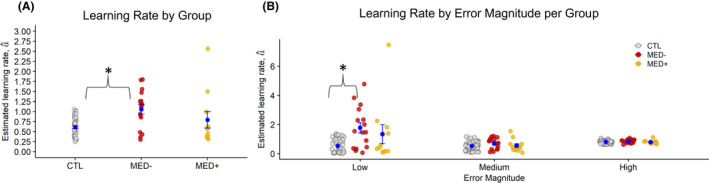
Learning rate by medication. (A) The MED− group showed significantly increased learning rates compared to CTLs. There were no significant differences between MED− and MED+, and MED+ and CTLs. (B) MED− showed significantly higher learning rates following low prediction errors compared to CTLs, but not MED+ patients. No group differences were observed when considering medium and high prediction error trials
After dividing learning rates by prediction error and conducting a Welch–James test, we found a significant effect of group (T wj[2,17.22] = 5.12, p = .018), prediction error (T wj[2,20.06] = 66.23, p < .001), and a significant group‐by‐prediction error interaction (T wj[4,19.81] = 4.22, p = .012). MED− showed higher learning rates associated with small prediction errors compared to CTLs (Z = −3.69, p < .001, Wilcoxon’s r = .45). Learning rates at small prediction errors were equivalent between MED+ and CTLs (p = .70), as well as between MED− and MED+ (p = .40) (Figure 3B).
Action and confidence models revealed no significant group effects (Appendix S5 and Tables S6 and S7).
Comparing model and human behaviour
The learning rate and confidence trajectories displayed by the quasi‐optimal Bayesian model matched those observed in human participants (Figure 4). After change points occurred (signalling the need to discard old beliefs in favour of new information), participants suitably reacted by increasing their learning rates and decreasing their confidence ratings, hence demonstrating a similar behaviour to that of the Bayesian model. However, the OCD group revealed higher learning rates than CTLs before a change point occurred (Figure 4A). MED− also showed increased learning rates before and after change points occurred compared to CTLs. Confidence patterns were equivalent between all participant groups (Figure 4B).
Figure 4.
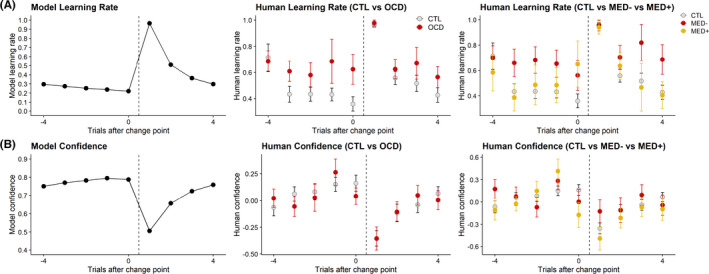
Comparing behaviour between the quasi‐optimal Bayesian model and human participants following change points. (A) Both the Bayesian model and participants displayed increased learning rates following change points. However, the OCD group revealed elevated learning rates prior to change points compared to CTLs. The MED− group also showed higher learning rates than CTLs on trials preceding and following change points. (B) The Bayesian model and human participants both decreased confidence ratings following change points. There was no observable difference between groups
Correlations
Correlations between task measures and clinical/intelligence scores were quantified using Pearson’s correlations (Table S8). In all subjects, anxiety scores were found to correlate with learning rates overall (r = .24, p = .041) and learning rates at low prediction errors (r = .23, p = .049). However, correlations between these measures were not significant when analysing the OCD and CTL groups separately (Appendix S6).
We also checked for correlations between task measures and symptom dimensions within the OCD group (washing, checking, hoarding, obsessing, ordering, and neutralising) quantified using the OCI‐R (Foa et al, 1998), but no significant associations were detected (all p > .05).
Discussion
This study investigated the relationship between action and confidence in adolescents with OCD compared to healthy adolescents. The paradigm used was previously implemented by Vaghi et al. (2017) who uncovered increased action updating following recent feedback and a novel dissociation between action and confidence in adults with OCD. As predicted, adolescent patients in our study displayed increased action updating following recent feedback, most prominently when prediction errors were low. This was driven primarily by unmedicated patients. By contrast, there was no difference in confidence updating between patients and controls, although, in our model‐based analysis, the adolescent OCD group’s confidence ratings were significantly less influenced by prediction errors. Contrary to our prediction, adolescents with OCD did not display a significant dissociation between action and confidence.
Increased learning rates in OCD
While prior findings in adults with OCD indicated increased learning rates regardless of prediction error magnitude (Vaghi et al., 2017), current results showed that adolescents with OCD primarily updated actions excessively following low prediction errors. Enhanced updating at low prediction errors might indicate that patients are ‘tracking’ the location of the coin by moving the bucket every time the coin makes a small deviation from its last location. Perhaps the adolescents with OCD are preoccupied with ensuring the coin lands with high certainty in the bucket, which could be evocative of not‐just‐right obsessions (where a sufferer feels that their environment is not as it should be) often associated with OCD. The majority of paediatric OCD patients describe having not‐just‐right‐related obsessions (Nissen & Parner, 2018). Trials where the coin just barely lands in the bucket may have triggered a not‐just‐right perception in patients, leading to the urge to rearrange the bucket. Indeed, ordering/symmetry‐related compulsions are strongly associated with not‐just‐right perceptions (Coles, Frost, Heimberg, & Rhéaume, 2003). Nonetheless, this is just speculation at present and should be researched further in future work using appropriate ‘not‐just right experiences’ scales (Coles, Heimberg, Frost, & Steketee, 2005).
Additionally, frequent choice corrections observed in adolescents with OCD are consistent with research reporting increased error‐related negativity (ERN) in paediatric OCD patients (Marzuki et al., 2020 for review). Importantly, error signals are generated in the absence of feedback and are triggered by a person’s own awareness that an error has indeed occurred (Gehring, Goss, Coles, Meyer, & Donchin, 1993). Heightened ERN in OCD could be likened to an internal ‘alarm bell’ that frequently sounds regardless of the degree of volatility in the external environment. In line with this, Fradkin, Adams, et al. (2020) computational model of OCD proposes that feeling excessively uncertain about the otherwise stable environment leads to patients perceiving that their rituals are not performed adequately, culminating in a tendency to repeat actions.
Moreover, excessive updating could be a form of atypical information gathering, which has been observed prior in youths with OCD on information sampling (Hauser, Moutoussis, et al., 2017) and perceptual decision‐making tasks (Erhan et al., 2017). Increased perceptual uncertainty could be driving this need to gather more information and could be linked to compulsive checking (although learning rates did not correlate with checking scores in our study). Alternatively, patients may be taking a longer time than controls to learn the exact optimal location to place the bucket, which would be consistent with research suggesting that youths with OCD have learning deficits (Gottwald et al., 2018).
Stress experienced during the task could also be promoting heightened action updating by the young patients. Indeed, anxious individuals have been found to update their behaviour quicker, albeit following negative outcomes, on probabilistic tasks (Aylward et al., 2019). This possible role of stress is supported by the OCD group having elevated anxiety compared to controls (despite not qualifying for an anxiety disorder diagnosis) and trait anxiety scores correlating with learning rates in all participants. However, the correlations were insignificant when considering OCD and control groups separately, and thus the role of anxiety in promoting altered action updating in this clinical population is inconclusive and needs to be researched further.
Confidence results
Normal confidence updating suggests that adolescent patients can construct a relatively accurate internal model of the task, but do not appear to use this information to guide their actions, mirroring adults with OCD (Vaghi et al., 2017).
However, the model‐based analysis revealed that the OCD group’s confidence ratings were insensitive to the influence of prediction errors, while controls appropriately decreased their confidence ratings when predictions errors were high. We caution that these results should be interpreted carefully given that (a) the confidence model fit was subpar, and (b) our model‐free results showed comparable confidence ratings between patients and controls.
Confidence updating that is insensitive to prediction errors supports the proposal by Fradkin, Adams, et al. (2020) that individuals with OCD do not use external evidence to inform their beliefs. The finding is also reminiscent of recent research revealing that healthy adults with compulsive traits and intrusive thoughts display inflated confidence (Rouault, Seow, Gillan, & Fleming, 2018; Seow & Gillan, 2020). Although, Seow and Gillan (2020) reported that compulsive participants’ confidence ratings were not strongly influenced by feedback (hit/miss), change point probability, and relative uncertainty, while our model analysis showed a reduced effect of only prediction error on patients' confidence.
No group differences in confidence–action coupling
Despite observing elevated learning rates but relatively normal confidence ratings in adolescents with OCD, and previous studies reporting a mismatch between action and confidence in obsessive–compulsive adults (Hauser, Allen, et al., 2017; Rouault et al., 2018; Seow & Gillan, 2020; Vaghi et al., 2017, 2019) we found no group differences when formally testing the strength of association between action and confidence.
The results suggest that perhaps confidence and action become dissociated with age or disorder duration in OCD. An alternative explanation is that metacognition could still be developing in healthy adolescents, resulting in a lack of noticeable group differences and the deficit only apparent during adulthood. This is supported by research demonstrating that accurate metacognition only emerges in early adolescence but strengthens over time (Fandakova et al., 2017; Moses‐Payne, Habicht, Bowler, Steinbeis, & Hauser, 2020; Weil et al., 2013). There is also computational evidence showing healthy adolescents do not use confidence to inform their decision‐making (Jepma, Schaaf, Visser, & Huizenga, 2020). Hence, perhaps detecting an effect of OCD in our study is difficult as the adolescent control group is also updating action and confidence independently.
Medication effects
We found that excessive updating was driven by unmedicated patients with OCD, while patients medicated with SSRIs did not differ significantly from controls. SSRIs are thought to reengage regions involved in goal‐directed frontostriatal circuits, enabling greater resistance against obsessions and compulsions (Palminteri et al., 2012). Thus, perhaps, SSRIs are diminishing adolescent patients’ compulsive action updates. SSRIs may also promote improved learning from prediction errors as animal studies have shown that serotonergic activity is tied to prediction errors when mice are foraging in dynamic or uncertain environments (Grossman, Bari, & Cohen, 2021; Matias, Lottem, Dugué, & Mainen, 2017).
Our results are consistent with research reporting that medicated adult patients show superior performance to medication‐naïve patients on various learning and planning tasks (Lochner et al., 2020; Palminteri et al., 2012). Importantly, SSRIs administered to adolescents and children with OCD are associated with significant improvements in verbal memory, processing speed, inhibition, and cognitive flexibility (Andrés et al., 2008).
Limitations
First, although our sample size was adequate (based on a sample size calculation), the effect size we obtained from Vaghi et al.’s adult data may not be a perfect estimate for our developmental sample. Additionally, the medication results should be considered preliminary at present given that the sample sizes in MED− and MED+ groups were small.
Another limitation is that we, unfortunately, did not collect data on several clinical measures including state anxiety, intolerance of uncertainty, and just‐right symptomatology which would have improved our interpretation of the learning rate variability within the OCD group. Future work should employ a more comprehensive battery of clinical questionnaires to improve the understanding of cognitive results garnered.
Finally, our adolescent sample revealed markedly high learning rates on the task indicative of noisy/random decisions (Nassar et al., 2010). Trials with these high learning rates were treated as outliers and removed, and the proportion of such trials did not differ significantly between OCD and control groups. However, they suggest that some participants were responding randomly in certain trials, perhaps due to a lack of focus. Future work looking to administer this task in child samples should strive to improve focus by making the task more suited to young people.
Conclusions
Prompted by previous work detailing a novel action–outcome dissociation in adults with OCD, we demonstrate that adolescents with OCD deviated most from healthy adolescent behaviour when experiencing small prediction errors where they made frequent action updates. Computational modelling revealed patients’ confidence ratings were not as influenced by prediction errors as those of CTLs. We posit that youths with OCD update their actions according to internal factors that need to be researched further, rather than following observable changes in the task environment. This is consistent with prior research reporting uncertainty‐driven information sampling and error‐related negativity in this clinical population. We also provide preliminary evidence for aberrant action‐updating to be remediated by SSRI treatment in youths with OCD, emphasising the importance of early intervention in improving disorder‐related decision‐making deficits.
Supporting information
Figure S1. Screening and recruitment details for sample. Key‐ CTL: Control Group; OCD: Patient Group; ADHD: Attention Deficit Hyperactivity Disorder; CY‐BOCS: Children’s Yale‐Brown Obsessive–Compulsive Scale.
Appendix S1. Questionnaires.
Appendix S2. Overall data checks.
Appendix S3. Computational model.
Appendix S4. Regression results for CTL vs. OCD groups (low error magnitude trials only).
Appendix S5. Regression results for CTL vs. MED− vs, MED+ groups.
Appendix S6. Correlations.
Table S1. Summary of parameters for CTL and OCD obtained from the regression model on action.
Table S2. Summary of parameters for CTL and OCD obtained from the regression model on confidence.
Table S3. Summary of parameters for CTL and OCD obtained from Action regression model for low error magnitude trials only.
Table S4. Summary of parameters for CTL and OCD obtained from Confidence regression model for low error magnitude trials only.
Table S5. Mean scores and standard deviations per group and statistical test.
Table S6. Summary of parameters for CTL MED‐ and MED+ obtained from Action regression model.
Table S7. Summary of parameters for CTL, MED‐, and MED+ obtained from Confidence regression model.
Table S8. Correlations between demographic/clinical measures and task outcome measures – all subjects.
Acknowledgements
This research was funded by the Wellcome Trust (Grant 104631/Z/14/Z/ to T.W.R.). For open access purposes, the author has applied a CC‐BY public copyright licence to any Author Accepted Manuscript version arising from this submission. M.K. reported receiving Clinical Lectureship funding from the National Institute of Health Research during the conduct of the study. B.J.S. reported consulting for Cambridge Cognition and for Greenfield Bioventures outside the submitted work. T.W.R. reported receiving grants from Wellcome Trust that supported this research during the conduct of the study; and receiving personal fees from Cambridge Cognition outside the submitted work. The remaining authors have declared that they have no competing or potential conflicts of interest.
Key points.
Adults with OCD are known to show a dissociation between action and confidence, consistent with OCD being an ego‐dystonic disorder.
We found, for the first time, that adolescents with OCD update their actions excessively when changes in the environment are small.
Patients’ confidence was updated similarly to control subjects.
There is a possible positive effect of SSRI medication on action updating.
Patients’ actions may be driven by internal uncertainty rather than following observable changes in the task, reminiscent of real‐life obsessions and compulsions which are at odds with reality.
Conflict of interest statement: See Acknowledgements for full disclosures.
References
- Abramovitch, A. , Abramowitz, J.S. , Mittelman, A. , Stark, A. , Ramsey, K. , & Geller, D.A. (2015). Research Review: Neuropsychological test performance in pediatric obsessive‐compulsive disorder – A meta‐analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56, 837–847. [DOI] [PubMed] [Google Scholar]
- Andrés, S. , Lázaro, L. , Salamero, M. , Boget, T. , Penadés, R. , & Castro‐Fornieles, J. (2008). Changes in cognitive dysfunction in children and adolescents with obsessive‐compulsive disorder after treatment. Journal of Psychiatric Research, 42, 507–514. [DOI] [PubMed] [Google Scholar]
- Apergis‐Schoute, A.M. , Gillan, C.M. , Fineberg, N.A. , Fernandez‐Egea, E. , Sahakian, B.J. , & Robbins, T.W. (2017). Neural basis of impaired safety signaling in Obsessive Compulsive Disorder. Proceedings of the National Academy of Sciences of the United States of America, 114, 3216–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward, J. , Valton, V. , Ahn, W.Y. , Bond, R.L. , Dayan, P. , Roiser, J.P. , & Robinson, O.J. (2019). Altered learning under uncertainty in unmedicated mood and anxiety disorders. Nature Human Behaviour, 3, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1988). The effect size. Statistical power analysis for the behavioral sciences, 77–83. [Google Scholar]
- Coles, M.E. , Frost, R.O. , Heimberg, R.G. , & Rhéaume, J. (2003). “Not just right experiences”: Perfectionism, obsessive‐compulsive features and general psychopathology. Behaviour Research and Therapy, 41, 681–700. [DOI] [PubMed] [Google Scholar]
- Coles, M.E. , Heimberg, R.G. , Frost, R.O. , & Steketee, G. (2005). Not just right experiences and obsessive–compulsive features: Experimental and self‐monitoring perspectives. Behaviour Research and Therapy, 43, 153–167. [DOI] [PubMed] [Google Scholar]
- Erhan, C. , Bulut, G.Ç. , Gökçe, S. , Ozbas, D. , Turkakin, E. , Dursun, O.B. , … & Balcı, F. (2017). Disrupted latent decision processes in medication‐free pediatric OCD patients. Journal of Affective Disorders, 207, 32–37. [DOI] [PubMed] [Google Scholar]
- Fandakova, Y. , Selmeczy, D. , Leckey, S. , Grimm, K.J. , Wendelken, C. , Bunge, S.A. , & Ghetti, S. (2017). Changes in ventromedial prefrontal and insular cortex support the development of metamemory from childhood into adolescence. Proceedings of the National Academy of Sciences of the United States of America, 114, 7582–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Lang, A.G. , & Buchner, A. (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Foa, E.B. , Kozak, M.J. , Salkovskis, P.M. , Coles, M.E. , & Amir, N. (1998). The validation of a new obsessive‐compulsive disorder scale: The obsessive‐compulsive inventory. Psychological Assessment, 10, 206–214. [Google Scholar]
- Fradkin, I. , Adams, R.A. , Parr, T. , Roiser, J.P. , & Huppert, J.D. (2020). Searching for an anchor in an unpredictable world: A computational model of obsessive compulsive disorder. Psychological Review, 127, 672–699. [DOI] [PubMed] [Google Scholar]
- Fradkin, I. , Ludwig, C. , Eldar, E. , & Huppert, J.D. (2020). Doubting what you already know: Uncertainty regarding state transitions is associated with obsessive compulsive symptoms. PLoS Computational Biology, 16, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring, W.J. , Goss, B. , Coles, M.G. , Meyer, D.E. , & Donchin, E. (1993). A neural system for error detection and compensation. Psychological Science, 4, 385–390. [Google Scholar]
- Gillan, C.M. , Morein‐Zamir, S. , Urcelay, G.P. , Sule, A. , Voon, V. , Apergis‐Schoute, A.M. , … & Robbins, T.W. (2014). Enhanced avoidance habits in obsessive‐compulsive disorder. Biological Psychiatry, 75, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald, J. , de Wit, S. , Apergis‐Schoute, A.M. , Morein‐Zamir, S. , Kaser, M. , Cormack, F. , … & Sahakian, B.J. (2018). Impaired cognitive plasticity and goal‐directed control in adolescent obsessive–compulsive disorder. Psychological Medicine, 48, 1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, C.D. , Bari, B.A. , & Cohen, J.Y. (2021). Serotonin neurons modulate learning rate through uncertainty. Current Biology, 32, 586–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, T.U. , Allen, M. , Rees, G. , Dolan, R.J. , Bullmore, E.T. , Goodyer, I. , … & Pantaleone, S. (2017). Metacognitive impairments extend perceptual decision making weaknesses in compulsivity. Scientific Reports, 7, 6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, T.U. , Moutoussis, M. , Iannaccone, R. , Brem, S. , Walitza, S. , Drechsler, R. , … & Dolan, R.J. (2017). Increased decision thresholds enhance information gathering performance in juvenile Obsessive‐Compulsive Disorder (OCD). PLoS Computational Biology, 13, e1005440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepma, M. , Schaaf, J.V. , Visser, I. , & Huizenga, H.M. (2020). Uncertainty‐driven regulation of learning and exploration in adolescents: A computational account. PLoS Computational Biology, 16, e1008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin, A.B. , De Nadai, A.S. , Park, J. , Goodman, W.K. , Murphy, T.K. , & Storch, E.A. (2011). Refining clinical judgment of treatment outcome in obsessive–compulsive disorder. Psychiatry Research, 185, 394–401. [DOI] [PubMed] [Google Scholar]
- Lochner, C. , Chamberlain, S.R. , Kidd, M. , Taljaard, L. , Fineberg, N.A. , Robbins, T.W. , & Stein, D.J. (2020). The effects of acute serotonin challenge on executive planning in patients with obsessive–compulsive disorder (OCD), their first‐degree relatives, and healthy controls. Psychopharmacology (Berl), 237, 3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner, C. , Chamberlain, S.R. , Kidd, M. , Taljaard, L. , Fineberg, N.A. , & Stein, D.J. (2016). Altered cognitive response to serotonin challenge as a candidate endophenotype for obsessive‐compulsive disorder. Psychopharmacology, 233(5), 883–891. [DOI] [PubMed] [Google Scholar]
- Marzuki, A.A. , Pereira de Souza, A.M.F.L. , Sahakian, B.J. , & Robbins, T.W. (2020). Are candidate neurocognitive endophenotypes of OCD present in paediatric patients? A systematic review. Neuroscience and Biobehavioral Reviews, 108, 617–645. [DOI] [PubMed] [Google Scholar]
- Marzuki, A.A. , Tomić, I. , Ip, S.H.Y. , Gottwald, J. , Kanen, J.W. , Kaser, M. , … & Robbins, T.W. (2021). Association of environmental uncertainty with altered decision‐making and learning mechanisms in youths with obsessive‐compulsive disorder. JAMA Network Open, 4, e2136195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias, S. , Lottem, E. , Dugué, G.P. , & Mainen, Z.F. (2017). Activity patterns of serotonin neurons underlying cognitive flexibility. eLife, 6, e20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, J.T. , Nassar, M.R. , Gold, J.I. , & Kable, J.W. (2014). Functionally dissociable influences on learning rate in a dynamic environment. Neuron, 84, 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses‐Payne, M.E. , Habicht, J. , Bowler, A. , Steinbeis, N. , & Hauser, T.U. (2020). I know better! Emerging metacognition allows adolescents to ignore false advice. Developmental Science, 24, e13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar, M.R. , Wilson, R.C. , Heasly, B. , & Gold, J.I. (2010). An approximately Bayesian delta‐rule model explains the dynamics of belief updating in a changing environment. Journal of Neuroscience, 30, 12366–12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negreiros, J. , Belschner, L. , Best, J.R. , Lin, S. , Franco Yamin, D. , Joffres, Y. , … & Stewart, S.E. (2020). Neurocognitive risk markers in pediatric obsessive–compulsive disorder. Journal of Child Psychology and Psychiatry, 61, 605–613. [DOI] [PubMed] [Google Scholar]
- Nissen, J.B. , & Parner, E. (2018). The importance of insight, avoidance behavior, not‐just‐right perception and personality traits in pediatric obsessive‐compulsive disorder (OCD): A naturalistic clinical study. Nordic Journal of Psychiatry, 72, 489–496. [DOI] [PubMed] [Google Scholar]
- Norman, L.J. , Carlisi, C.O. , Christakou, A. , Murphy, C.M. , Chantiluke, K. , Giampietro, V. , … & Rubia, K. (2018). Frontostriatal dysfunction during decision making in attention‐deficit/hyperactivity disorder and obsessive‐compulsive disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(8), 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palminteri, S. , Clair, A.H. , Mallet, L. , & Pessiglione, M. (2012). Similar improvement of reward and punishment learning by serotonin reuptake inhibitors in obsessive‐compulsive disorder. Biological Psychiatry, 72, 244–250. [DOI] [PubMed] [Google Scholar]
- Riesel, A. (2019). The erring brain: Error‐related negativity as an endophenotype for OCD—A review and meta‐analysis. Psychophysiology, 56(4), e13348. [DOI] [PubMed] [Google Scholar]
- Rouault, M. , Seow, T. , Gillan, C.M. , & Fleming, S.M. (2018). Psychiatric symptom dimensions are associated with dissociable shifts in metacognition but not task performance. Biological Psychiatry, 84, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson, Y. , Zohar, J. , Chopra, M. , Lustig, M. , Iancu, I. , & Hendler, T. (1997). Epidemiology of obsessive‐compulsive disorder: a world view. Journal of Clinical Psychiatry, 58(Suppl 12), 7–10. [PubMed] [Google Scholar]
- Schachar, R.J. , Dupuis, A. , Anagnostou, E. , Georgiades, S. , Soreni, N. , Arnold, P.D. , … & Crosbie, J. (2021). Obsessive‐compulsive disorder in children and youth: Neurocognitive function in clinic and community samples. Journal of Child Psychology and Psychiatry. [DOI] [PubMed] [Google Scholar]
- Seow, T.X.F. , & Gillan, C.M. (2020). Transdiagnostic phenotyping reveals a host of metacognitive deficits implicated in compulsivity. Scientific Reports, 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D.V. , Lecrubier, Y. , Sheehan, K.H. , Amorim, P. , Janavs, J. , Weiller, E. , … & Dunbar, G.C. (1998). The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. Journal of Clinical Psychiatry, 59(Suppl. 20), 22–33. [PubMed] [Google Scholar]
- Sheehan, D.V. , Sheehan, K.H. , Shytle, R.D. , Janavs, J. , Bannon, Y. , Rogers, J.E. , … & Wilkinson, B. (2010). Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI‐KID). Journal of Clinical Psychiatry, 71, 313–326. [DOI] [PubMed] [Google Scholar]
- Tomczak, M. , & Tomczak, E. (2014). The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends in Sport Sciences, 1, 19–25. [Google Scholar]
- Vaghi, M.M. , Cardinal, R.N. , Apergis‐Schoute, A.M. , Fineberg, N.A. , Sule, A. , & Robbins, T.W. (2019). Action‐outcome knowledge dissociates from behavior in obsessive‐compulsive disorder following contingency degradation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghi, M.M. , Luyckx, F. , Sule, A. , Fineberg, N.A. , Robbins, T.W. , & De Martino, B. (2017). Compulsivity reveals a novel dissociation between action and confidence. Neuron, 96, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacorta, P.J. (2017). The welchADF package for robust hypothesis testing in unbalanced multivariate mixed models with heteroscedastic and non‐normal data. R Journal, 9, 309. [Google Scholar]
- Weil, L.G. , Fleming, S.M. , Dumontheil, I. , Kilford, E.J. , Weil, R.S. , Rees, G. , … & Blakemore, S.‐J. (2013). The development of metacognitive ability in adolescence. Consciousness and Cognition, 22, 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Screening and recruitment details for sample. Key‐ CTL: Control Group; OCD: Patient Group; ADHD: Attention Deficit Hyperactivity Disorder; CY‐BOCS: Children’s Yale‐Brown Obsessive–Compulsive Scale.
Appendix S1. Questionnaires.
Appendix S2. Overall data checks.
Appendix S3. Computational model.
Appendix S4. Regression results for CTL vs. OCD groups (low error magnitude trials only).
Appendix S5. Regression results for CTL vs. MED− vs, MED+ groups.
Appendix S6. Correlations.
Table S1. Summary of parameters for CTL and OCD obtained from the regression model on action.
Table S2. Summary of parameters for CTL and OCD obtained from the regression model on confidence.
Table S3. Summary of parameters for CTL and OCD obtained from Action regression model for low error magnitude trials only.
Table S4. Summary of parameters for CTL and OCD obtained from Confidence regression model for low error magnitude trials only.
Table S5. Mean scores and standard deviations per group and statistical test.
Table S6. Summary of parameters for CTL MED‐ and MED+ obtained from Action regression model.
Table S7. Summary of parameters for CTL, MED‐, and MED+ obtained from Confidence regression model.
Table S8. Correlations between demographic/clinical measures and task outcome measures – all subjects.


