Abstract
Purpose
To analyse the risk of rhegmatogenous retinal detachment (RRD) after cataract surgery, and to identify possible risk factors.
Method
Observational cohort study of patients undergoing cataract surgery in Region Skåne, southern Sweden, during 2015–2017 were retrieved from the Swedish National Cataract Register. These were then cross‐referenced with cases of retinal detachment surgery performed at the Skåne University Hospital in Lund from 2015 to 2020. The main outcome was RRD after cataract surgery. The influence of sex, age, axial length of the eye, rupture of the posterior capsule, patient comorbidity and other cataract complications were analysed.
Results
Among the 58 624 cases of cataract surgery, a total of 298 RRDs (0.51%) were identified up to the end of 2020. The mean time from cataract surgery to RRD was 667 days. The mean age was 65.3 years, compared to 74 years in the control group. A strong correlation was found between RDD and age: <60 years, incidence = 0.50%; 60–75 years, incidence = 0.14%; and >75 years, incidence = 0.04%. The correlation with axial length was also very strong: mean value 23.73 mm in those without RRD, and 25.13 mm in those with RRD (p < 0.001). Sex was also strongly correlated to RDD; 68.8% of cases of RRD being men. Among men younger than 60 years of age, with an axial length ≥25 mm, 9.46% exhibited RRD within the follow‐up period (mean 4.7 years). Rupture of the posterior capsule was found in 2.01% of RRD patients compared to 0.74% in the control group. Diabetes, glaucoma or pseudoexfoliation had no impact on the prevalence of RRD.
Conclusions
The three main risk factors for RRD following cataract surgery were found to be sex, age and axial length. The highest incidence of RRD (9.46%) were identified among men younger than 60 years of age and an axial length ≥25 mm.
Keywords: axial length, cataract surgery, myopia, retinal detachment
Introduction
Cataract is the leading cause of blindness today (Thylefors et al. 1995) and phakoemulsification followed by replacement of the lens is one of the most common forms of surgery. The rate of cataract surgery has increased over recent decades (Behndig et al. 2011) and concern has been expressed that the post‐surgical incidence of rhegmatogenous retinal detachment (RRD) is also increasing, possibly due to the demographics of the patients undergoing surgery. There is a tendency towards operating younger patients (Behndig et al. 2011) who may have a greater risk of RRD as they have a longer life expectancy, and have often not developed posterior vitreous detachment (Mitry et al. 2010). RRD remains one of the most common causes of emergency surgical intervention in ophthalmology, often resulting in significant loss of vision. About 40% do not achieve reading ability in the affected eye, about 10–40% need further surgical intervention, and approximately 5% do not achieve functional or anatomic healing (Barrie 2003). The approximate annual incidence of RRD has been reported to vary from 10.5 per 100 000 (Mitry et al. 2010) to 18.2 per 100 000 (Van de Put et al. 2013). The increased risk of RRD following cataract surgery reported in the literature ranges from 0.2% to 3.6% (Javitt et al. 1991; Lois & Wong 2003; Erie et al. 2006; Clark et al. 2012; Haug & Bhisitkul 2012; Olsen & Jeppesen 2012; Daien et al. 2015; Kim et al. 2019) depending on follow‐up time and patient demographics. The main risk factors described are age, myopia and peroperative complications including rupture of the posterior capsule and vitreous loss (Jakobsson et al. 2009; Olsen & Jeppesen 2012; Daien et al. 2015; Kim et al. 2019). The possible increase in risk of RRD after Nd:YAG laser capsulotomy has been the subject of several studies, but there is no conclusive evidence that it affects the rate of RRD (Grzybowski & Kanclerz 2018).
The purpose of the present study was to investigate the risk of RRD following cataract surgery, and to identify possible risk factors including pre‐operative patient characteristics and complications arising during surgery.
Patients and Methods
All cases of cataract surgery performed from 1 January 2015 to 31 December 2017 in the region of Skåne, southern Sweden, were extracted from the Swedish National Cataract Register. Exclusion criteria were cataract surgery in combination with vitrectomy, corneal or glaucoma surgery, or age <30 years.
The data analysed were age, sex, axial length of the eye (AL), date of surgery and comorbidities (diabetes, glaucoma, macular degeneration and presence of pseudoexfoliations). Complications during surgery, including staining of the anterior capsule, mechanical dilation of the pupil, iris hooks in the capsulorhexis margin, insertion of a capsular tension ring or rupture of the posterior capsule, were also noted.
The cataract surgery data were then cross‐referenced to cases of retinal detachment surgery performed at the Skåne University Hospital between 1 January 2015 and 31 December 2020, to identify patients who suffered RRD following cataract surgery.
The patients were then divided into three different groups according to their age at the time of cataract surgery, <60 years, 60–75 years and ≥75 years. Patients were also divided into three groups according to AL: <23, 23–25 and ≥25 mm.
The study was approved by the Regional Ethics Committee of Stockholm, Sweden, and was carried out in accordance with the Declaration of Helsinki 2013.
Statistical analysis
Differences in age, sex and AL were analysed using Welch's t‐test. When analysing non‐parametric data the chi‐squared test was used. Age, sex, axial length and rupture of the posterior capsule were also analysed using a multivariate logistics regression model to evaluate variables associated with an increased risk of RRD. Statistical analysis was performed using r (R Core Team (2020), Vienna, Austria. https://www.R‐project.org/) for mac software, and prism 9.1.0 (GraphPad Software, San Diego, CA, USA). A p‐value of <0.05 was considered statistically significant different.
Results
A total of 59 044 phacoemulsification cataract surgeries were performed during the years 2015–2017 in the Region of Skåne, Sweden. The inclusion criteria were met in 58 624 cases, distributed over 37 059 patients, and thus, both eyes were operated on during the follow‐up period in 21 565 cases.
The patient data are presented in Table 1. Among the cases of phacoemulsification cataract surgery, 298 cases of RRD (0.51%) were found, and the mean time from cataract surgery to RRD was 667 days (1.83 years; SD = 525) (Fig. 1). The mean time from cataract surgery until the end of the study, that is, the mean follow‐up time was 1705 days (4.67 years). Seventeen patients (11.4% of those with RRD) developed bilateral RRD during the study period (mean age 61 years, 76.5% male). There was a strong male dominance among those with RRD, 68.8% (n = 205) of all RRD cases, even though slightly more women (58.4%) had undergone cataract surgery. Table 2 gives risk for retinal detachment for the patients with RRD, divided according to age, sex and AL. In the age group <60 years, 2.4% had RRD during the follow‐up period, compared to 0.65% in the group 60–75 years, and 0.17% aged ≥75 years. The mean AL for cataract surgery was 23.72 mm (SD = 1.25) and in the group with RRD, 25.12 mm (SD = 1.51), p‐value <0.001. In Table 3, the AL was divided into three groups: <23, 23–25 and ≥25 mm and for the various age groups.
Table 1.
Patient data and the results of Welch's t‐test and the chi‐squared test.
| All eyes, N = 58 624 (% of N) | RRD N = 298 (% of N) | p‐value according to Welch's t‐test or chi‐squared test | |
|---|---|---|---|
| Mean age | 74.0 years | 65.4 years | <0.001 |
| Female | 34 228 (58.4) | 93 (31.2) | |
| Male | 24 396 (41.6) | 205 (68.8) | |
| Follow‐up time, mean | 1651 days | 1705 days | |
| Axial length, mean | 23.73 mm | 25.13 mm | <0.001 |
| Rupture of posterior capsule | 434 (0.74) | 6 (2.01) | 0.0102 |
| Pseudoexfoliation | 4860 (8.29) | 10 (3.35) | 0.0020* |
| Glaucoma | 3733 (6.36) | 13 (4.36) | 0.1552 |
| Diabetes | 2072 (3.53) | 11 (3.69) | 0.8831 |
| Macular degeneration | 8099 (13.8) | 21 (7.05) | 0.0007* |
| Vision blue colouring | 1411 (2.41) | 8 (2.68) | 0.7538 |
| Mechanical dilation | 1434 (2.40) | 6 (2.01) | 0.6279 |
| Iris hooks in capsulorhexis | 286 (0.48) | 2 (0.67) | 0.6489 |
| Capsular ring | 2533 (4.32) | 17 (5.70) | 0.2421 |
The risk of rhegmatogenous retinal detachment (RRD) is higher in the control group and not in the group with retinal detachment.
Fig. 1.
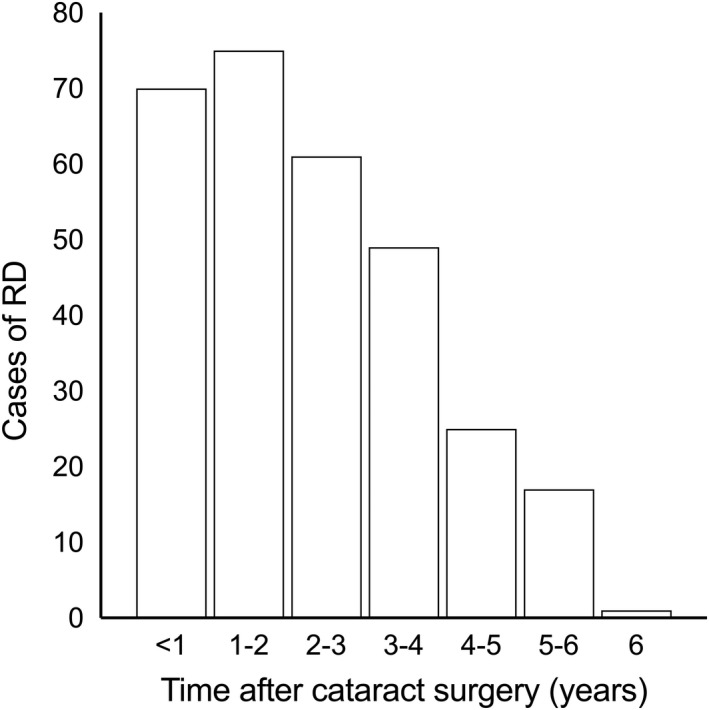
Time from cataract surgery until retinal detachment.
Table 2.
Incidence of RRD according to sex, age and axial length during the study period (mean 4.67 years).
| All eyesN = 58 624 (% of N) | RRDAge < 60 yearsN = 3281 (% of N) | RRDAge 60–75 yearsN = 26 320 (% of N) | RRDAge ≥ 75 yearsN = 29 023 (% of N) | |
|---|---|---|---|---|
| RRD | 298 (0.51) | 80 (2.40) | 170 (0.65) | 48 (0.17) |
| Female | 93 | 20 | 50 | 23 |
| Male | 205 | 60 | 120 | 25 |
| Follow‐up time, mean | 1651 days | 1754 days | 1643 days | 1658 days |
| Axial length, mean | 25.13 mm | 26.04 mm | 25.00 mm | 24.05 mm |
| Axial length ≥25 mm | 140 (1.86) | 57 (6.40) | 76 (1.84) | 7 (0.28) |
| Axial length <25 mm | 158 (0.31) | 23 (0.94) | 94 (0.42) | 41 (0.15) |
| Axial length <25 mm female:male | 60 (0.20):98 (0.48) | 7 (0.49): 16 (1.63) | 31 (0.23): 63 (0.74) | 22 (0.14):19 (0.17) |
| Axial length ≥25 mm female:male | 33 (0.92):107 (2.69) | 13 (3.13): 44 (9.46) | 19 (0.94):57 (2.70) | 1 (0.09):6 (0.44) |
Table 3.
Incidence rhegmatogenous retinal detachment (RRD) depending on age and axial length during the study period (mean 4.67 years).
| All eyesN = 58 624 (% RRD) | RRDAge < 60 yearsN = 3281 (% RRD) | RRDAge 60–75 yearsN = 26 320 (% RRD) | RRDAge ≥ 75 yearsN = 29 023 (% RRD) | |
|---|---|---|---|---|
| Axial length <23 mm | 18/15 948 (0.11) | 2/704 (0.28) | 12/6755 (0.18) | 5/8725 (0.06) |
| Axial length 23–25 mm | 140/35 138 (0.39) | 21/1698 (1.24) | 82/15 433 (0.53) | 36/17 839 (0.20) |
| Axial length ≥25 mm | 140/7538 (1.86) | 57/880 (6.48) | 76/4132 (1.84) | 7/2459 (0.28) |
When the effects of age, sex and AL were combined, a significant increase in the incidence RRD was seen (Fig. 2). Age <60 years combined with an AL ≥25 mm resulted in an incidence of 6.40%, and when male sex was also included, the incidence of RRD increased to 9.46% during the follow‐up period.
Fig. 2.
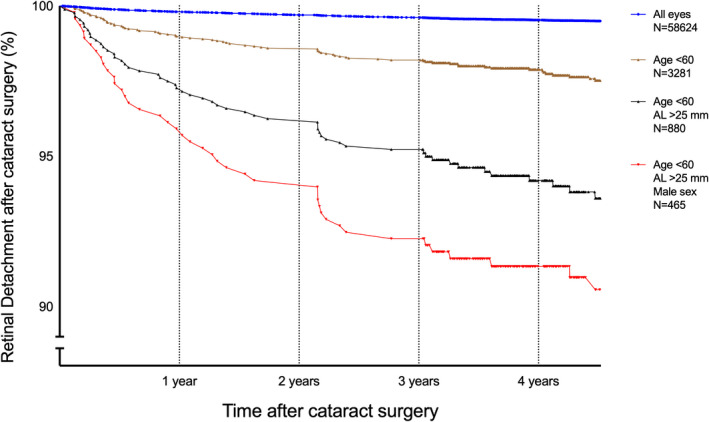
Kaplan–Meier curves showing RRD as a function of time after cataract surgery for different subgroups of patients. The whole study group consisted of 58 624 eyes undergoing cataract surgery. Of these, 298 had RRD (0.51%). The other curves are for subgroups: age < 60 years, n = 3281 with 80 RRD (2.40%); age <60 years and AL ≥25 mm, n = 880 with 57 RRD (6.40%), and age <60 years, AL ≥25 mm and male sex, n = 465 with 44 RRD (9.46%).
The variables age at cataract surgery, sex, AL and rupture of the posterior capsule, were analysed using multiple logistics regression analysis, and the results presented in Table 4. The goodness of fit obtained with the Hosmer–Lemeshow test was positive, with a p‐value of 0.0429.
Table 4.
Multiple logistics regression for risk of developing rhegmatogenous retinal detachment.
| Odds ratios | 95% confidence interval | |
|---|---|---|
| Age at cataract surgery | 0.927 | 0.917–0.937 |
| Axial length | 1.476 | 1.392–1.561 |
| Sex (male) | 2.666 | 2.086–3.432 |
| Rupture of posterior capsule | 2.382 | 0.9128–5.090 |
Rupture of posterior capsule and sex are binary variables, the others are linear and not grouped.
The only peroperative surgical event that was significantly correlated to an increased risk of RRD was rupture of the posterior capsule. In the RRD group, 2.01% of the surgeries were complicated by capsular rupture, compared to 0.74% in the non‐RRD group (p‐value 0.01). Mechanical pupil dilation, staining of the anterior capsule, iris hooks in the capsulorhexis margin and insertion of a capsular tension ring had no impact on the incidence of RRD.
Comorbidity such as glaucoma, pseudoexfoliation, macular degeneration and diabetic retinopathy had no significant impact on the incidens of RRD (Table 1). The incidence of pseudoexfoliation and macular degeneration were significantly less in the RRD group than in the non‐RRD group, and therefore, had no impact on the risk of retinal detachment.
Discussion
Our study further analyse the high risk increase for RRD seen in some subgroups of cataract patients. It is notable that although 58.4% of the patients undergoing cataract surgery in our study were women, only 31.2% of those with RRD were female. Male sex has previously been reported to be a strong predisposing factor for RRD in studies from Denmark (Olsen & Jeppesen 2012) (58.3%) and France (Daien et al. 2015) (64.8%), but slightly less in Korea (Kim et al. 2019) (54.6%). The reason for this is not known, but anatomical differences have been suggested, as men have longer AL (Olsen et al. 2007) and posterior vitreous detachment seems to occur later (Hayreh & Jonas 2004). It has also been suggested that male eyes more often have a history of ocular trauma, although this was not confirmed in larger studies (Hayreh & Jonas 2004). The incidence of RRD in males in the present study (68.8%) is similar to that found in a previous study on retinal detachment by our group (Thylefors et al. 2020) (66%), despite the fact that this earlier study comprises all forms of RRD, and not only pseudophakic RRD. Similar result were found in a review by Mitry et al. (2010), where male to female ratios of 1.3:1 to 2.3:1 were reported. Male sex thus appears to be an independent risk factor for RRD, and not linked to cataract surgery.
Myopia is another risk factor for RRD, as described in several studies (Javitt et al. 1991; Mitry et al. 2010; Olsen & Jeppesen 2012; Van de Put et al. 2013; Daien et al. 2015; Kim et al. 2019). The patients who developed RRD had a significantly longer axial length than those who did not develop RRD. The association between AL and RRD has been studied previously but the studies have used different limits of AL in the dividing of the groups. We found two studies showing an increasing risk with AL >26 mm (Sheu et al. 2010; Lin et al. 2013) but we used slightly different ranges of AL also used by Laube et al. (2017): <23, 23–25 and ≥25 mm. In our study, the incidence of RRD increased substantially at AL ≥25 mm with a 1.86% incidence during the follow‐up period. This can be compared to 0.39% in the group with AL of 23–25 mm, and 0.11% for those with an AL of <23 mm. Axial length ≥25 mm was found in 7538 eyes which corresponds to 12.8% of the total number of eyes undergoing cataract surgery. An AL of >26 mm was found in 2859 eyes, or 4.9%. Thus, if an axial length of ≥25 mm is used to define myopia, almost three times more cases are included in the myopic group, compared to studies using an axial length exceeding 26 mm.
The AL ≥25 mm as an additional risk factor for RRD in men remains up to the age of 75 years (Table 2) where the RRD cumulative incidence was 2.7%, while for women, it was only visible in the group aged <60 years. Above 75 years, the risk of RRD appears to be very low for both sexes, regardless of AL. In patients with an AL, <23 mm showed no increase in the incidence of RRD, regardless of age, and only the group <60 years of age with AL of 23–25 mm showed an increase in the incidence of RRD (Table 3). The odds ratio is increased by 1.47 for each mm increase in the AL (Table 4).
It is known from previous studies that the incidence of RRD is higher in those of lower ages (Olsen & Jeppesen 2012; Daien et al. 2015; Kim et al. 2019). Various age intervals have been used previously, but we used <60 years (3281 eyes), 60–75 years (26 320 eyes) and ≥75 years (29 023 eyes). The incidence of RRD was 2.4% in the <60 years group, 0.64% for those aged 60–75 years and 0.035% in those aged ≥75 years, over the follow‐up period. These findings indicate that cataract surgery should be carefully considered in those aged less than 60 years, as this could lead to an increased risk of RRD.
The effect of combining age, sex and AL is quite striking, as can be seen in the Kaplan–Meier curves in Fig. 2. The top curve shows the result for the whole cataract surgery population where the risk of RRD was 0.51% during the follow‐up period. This corresponds to 109 cases of RRD per 100 000 per year, which is approximately 10 times the normal rate of 10.5 RRD per 100 000 per year (Mitry et al. 2010) in the whole population. The lower curves give the results when age <60, AL ≥25 mm, and male sex are successively added. The final group, including all three variables, comprised 465 eyes (0.79% of all eyes), among which 44 cases of RRD were identified (14.7% of all RRD cases), that is, a cumulative incidence of 9.46% (Table 2). This is an incidence of 2012 cases of RRD per 100 000 per year, approximately 200 times the normal rate.
This is similar to the incidence reported by Laube et al. (2017) of 10.2% RRD in the group with AL >25 mm in patients younger than 61 years. Daien et al. (2015) gave a Kaplan–Meier figure where less than 4% of patients aged 40–54 years had RRD during a 46‐month period, and high myopia approximately 9% RRD in 49 months. In their study, only severe myopia was identified by the diagnosis code H52.1, and it is thus unclear whether milder forms of myopia were also included. Furthermore, myopia is not only related to the AL, as the refraction of the eye also depends on the refractive power of the cornea and lens (Olsen et al. 2007). In our opinion, increased AL is a more reliable and accurate risk factor.
The importance of age, AL and sex are underlined by the multiple logistics regression analyses, showing that older age decreases the risk of RRD while sex and AL increase it. The highest risk, with an odds ratio 2.66, was associated with being male but, as mentioned above, this is an independent variable not linked to cataract surgery. Rupture of the posterior capsule had an odds ratio of 2.38 but the confidence interval is quite broad, ranging from 0.91 to 5.09. The odds ratio increases by 1.47 with each mm increase in AL, implying a greater risk when performing cataract surgery on myopic eyes.
The strength of our study is the large group of 58 624 eyes, obtained from the Swedish National Cataract Register, from the Region of Skåne with 1.4 million inhabitants. This register also includes data such as the AL, patient comorbidity and other peroperative cataract complications. All cases of RRD in this region are treated at the Skåne University Hospital in Lund. Another strength is that the number of retinal detachments (298) is quite large, which allowed us to perform subgroup analysis.
However, one weakness of this study is that we did not compare the rates of RRD in age‐matched groups of pseudophakic myopic eyes with phakic myopic eyes, but only compared the rate of RRD in the whole population versus that in pseudophakic patients. Also, as we have not analysed each chart for all the patients there may be a risk that they have been previously vitrectomized, then done the cataract surgery and subsequent developed the RRD later on. However, in Region Skåne we have for more than 10 years almost always done a combined phaco and vitrectomy for all the patients doing vitrectomy who are phacic. This means that there are practically no such patients so it cannot be a problem when analysing the result for this study.
Other patient comorbidities showed no significant correlation with RRD (Table 1). Age‐related macular degeneration (AMD) was almost twice as common in the population without RRD as in those with RRD. This reflects the fact that AMD becomes more common in the elderly population in contrast to RRD. Some of these AMD patients must have received treatment for wet AMD with repeated anti‐VEGF injections, which could be a risk factor for RRD, but this was not investigated in this study. Storey et al. (2019)) found the risk of RRD after anti‐VEGF injections to be one in 530 patients (one RRD per 7692 injections). Diabetes mellitus has previously been reported by Daien et al. (2015) to be more common in patients with pseudophakic RRD, but was not significantly more common in the present study, nor in studies by Kim et al. (2019) or (Sheu et al. (2010). Complications during surgery in terms of the use of mechanical pupil dilation, staining of the anterior capsule, iris hooks in the capsulorhexis margin and insertion of a capsular ring, were not found to be risk factors for RRD, whereas rupture of the posterior capsule was. Capsular rupture is a well‐known risk factor (Javitt et al. 1991; Jakobsson et al. 2009; Quek et al. 2012; Tuft et al. 2012), and in the present study, 2.01% of the patients with RRD had capsular ruptures, compared to 0.74% in the whole study group. The fact that the other markers of complicated cataract surgery were not significantly correlated to RRD is also of importance to the cataract surgeon, since it indicates that these surgical tools can be used to reduce the risk of capsular rupture.
The risk of developing pseudophakic RRD was found to decrease gradually with time in the present study, on 6 years, but Erie et al. (2006) reported that the risk continued for up to 25 years after surgery. Another study has reported an increase in the incidence of RRD after 4 years of follow‐up (Sheu et al. 2010). This means that the high risk reported by us and others is only during follow‐up time, but the risk is maintained for several more years. For cataract patients aged over 75 years, we found no indications of an increased risk of RRD following cataract surgery, regardless of myopia and sex. Patients aged 60–75 years, especially men with an AL ≥25 mm, should be informed of the higher risk of RRD (2.7%) following cataract surgery. In those younger than 60 years old, only those with an axial length <23 mm and females with an AL <25 have no risk increase of RRD. All other patients should thus also be informed of the increased risk of RRD, especially men with an AL ≥25 mm. In our opinion, the risk of a vision‐threatening condition such as RRD in some groups means that the benefit of cataract surgery should be carefully considered and the patient thoroughly informed. Also, when cataract surgery is necessary, the lens chosen should not cause anisometropia that force the patient to operate the other eye as well. We suggest that high‐risk patients be advised to postpone their cataract surgery if possible, and particularly if the indication is only refractive error correction.
RRD = rhegmatogenous retinal detachment.
References
- Barrie T (2003): Debate overview. Repair of a primary rhegmatogenous retinal detachment. Br J Ophthalmol 87: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behndig A, Montan P, Stenevi U, Kugelberg M & Lundstrom M (2011): One million cataract surgeries: Swedish National Cataract Register 1992‐2009. J Cataract Refract Surg 37: 1539–1545. [DOI] [PubMed] [Google Scholar]
- Clark A, Morlet N, Ng JQ, Preen DB & Semmens JB (2012): Risk for retinal detachment after phacoemulsification: a whole‐population study of cataract surgery outcomes. Arch Ophthalmol 130: 882–888. [DOI] [PubMed] [Google Scholar]
- Daien V, Le Pape A, Heve D, Carriere I & Villain M (2015): Incidence, risk factors, and impact of age on retinal detachment after cataract surgery in France: a national population study. Ophthalmology 122: 2179–2185. [DOI] [PubMed] [Google Scholar]
- Erie JC, Raecker ME, Baratz KH, Schleck CD & Robertson DM (2006): Risk of retinal detachment after cataract extraction, 1980‐2004: a population‐based study. Trans Am Ophthalmol Soc 104: 167–175. [PMC free article] [PubMed] [Google Scholar]
- Grzybowski A & Kanclerz P (2018): Does Nd:YAG capsulotomy increase the risk of retinal detachment? Asia Pac J Ophthalmol (Phila) 7: 339–344. [DOI] [PubMed] [Google Scholar]
- Haug SJ & Bhisitkul RB (2012): Risk factors for retinal detachment following cataract surgery. Curr Opin Ophthalmol 23: 7–11. [DOI] [PubMed] [Google Scholar]
- Hayreh SS & Jonas JB (2004): Posterior vitreous detachment: clinical correlations. Ophthalmologica 218: 333–343. [DOI] [PubMed] [Google Scholar]
- Jakobsson G, Montan P, Zetterberg M, Stenevi U, Behndig A & Lundstrom M (2009): Capsule complication during cataract surgery: retinal detachment after cataract surgery with capsule complication: Swedish Capsule Rupture Study Group report 4. J Cataract Refract Surg 35: 1699–1705. [DOI] [PubMed] [Google Scholar]
- Javitt JC, Vitale S, Canner JK, Krakauer H, McBean AM & Sommer A (1991): National outcomes of cataract extraction. I. Retinal detachment after inpatient surgery. Ophthalmology 98: 895–902. [DOI] [PubMed] [Google Scholar]
- Kim J, Ryu SY, Hong JH & Chung EJ (2019): Incidence and risk factors for retinal detachment after cataract surgery in Korea: a nationwide population‐based study from 2011 to 2015. Graefes Arch Clin Exp Ophthalmol 257: 2193–2202. [DOI] [PubMed] [Google Scholar]
- Laube T, Brockmann C, Lehmann N & Bornfeld N (2017): Pseudophakic retinal detachment in young‐aged patients. PLoS One 12: e0184187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Ho WL, Ger LP & Sheu SJ (2013): Analysis of factors correlated with the development of pseudophakic retinal detachment‐‐a long‐term study in a single medical center. Graefes Arch Clin Exp Ophthalmol 251: 459–465. [DOI] [PubMed] [Google Scholar]
- Lois N & Wong D (2003): Pseudophakic retinal detachment. Surv Ophthalmol 48: 467–487. [DOI] [PubMed] [Google Scholar]
- Mitry D, Charteris DG, Fleck BW, Campbell H & Singh J (2010): The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol 94: 678–684. [DOI] [PubMed] [Google Scholar]
- Olsen T, Arnarsson A, Sasaki H, Sasaki K & Jonasson F (2007): On the ocular refractive components: the Reykjavik Eye Study. Acta Ophthalmol Scand 85: 361–366. [DOI] [PubMed] [Google Scholar]
- Olsen T & Jeppesen P (2012): The incidence of retinal detachment after cataract surgery. Open Ophthalmol J 6: 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek DT, Lee SY, Htoon HM & Ang CL (2012): Pseudophakic rhegmatogenous retinal detachment in a large Asian tertiary eye centre: a cohort study. Clin Experiment Ophthalmol 40: e1–e7. [DOI] [PubMed] [Google Scholar]
- Sheu SJ, Ger LP & Ho WL (2010): Late increased risk of retinal detachment after cataract extraction. Am J Ophthalmol 149: 113–119. [DOI] [PubMed] [Google Scholar]
- Storey PP, Pancholy M, Wibbelsman TD, Obeid A, Su D, Borkar D, Garg S & Gupta O (2019): Rhegmatogenous retinal detachment after intravitreal injection of anti‐vascular endothelial growth factor. Ophthalmology 126: 1424–1431. [DOI] [PubMed] [Google Scholar]
- Thylefors B, Negrel AD, Pararajasegaram R & Dadzie KY (1995): Global data on blindness. Bull World Health Organ 73: 115–121. [PMC free article] [PubMed] [Google Scholar]
- Thylefors J, Zetterberg M & Jakobsson G (2020): Anatomical outcome of retinal detachment surgery comparing different surgical approach. Acta Ophthalmol 99: e908–e913. [DOI] [PubMed] [Google Scholar]
- Tuft SJ, Gore DM, Bunce C, Sullivan PM & Minassian DC (2012): Outcomes of pseudophakic retinal detachment. Acta Ophthalmol 90: 639–644. [DOI] [PubMed] [Google Scholar]
- Van de Put MAJ, Hooymans JMM, Los LI & G Dutch Rhegmatogenous Retinal Detachment Study (2013): The incidence of rhegmatogenous retinal detachment in The Netherlands. Ophthalmology 120: 616–622. [DOI] [PubMed] [Google Scholar]


