Abstract
Purpose
To investigate the effect of polyquaternium‐1 (PQ)‐preserved and benzalkonium chloride (BAK)‐preserved travoprost eye drops on viability of primary human conjunctival goblet cell (GC) cultures and on secretion of mucin and cytokines. Furthermore, to evaluate the physicochemical properties of the branded travoprost eye drop Travatan® and available generics.
Methods
The effect of travoprost eye drops was evaluated on GC cultures. Cell viability was assessed through lactate dehydrogenase (LDH) and tetrazolium dye (MTT) colorimetric assays. Mucin secretion was evaluated by immunohistochemical staining. Secretion of interleukin (IL)‐6 and IL‐8 was measured using BD Cytometric Bead Arrays. pH, viscosity, droplet mass, osmolality and surface tension were measured for all included eye drops.
Results
In the LDH assay, BAK travoprost caused significant GC loss after 2 hrs of incubation compared to the control. PQ travoprost caused no GC loss at any time point. Both PQ‐ and BAK travoprost caused secretion of mucin to the cytoplasma. No difference in IL‐6 and IL‐8 secretion was identified compared to controls. The pH values for the generics were lower (pH 6.0) than the pH value for Travatan (pH 6.7; p < 0.0001). The viscosity was lowest for Travatan, while the mean droplet mass was higher for Travatan (35 mg) than the generics (28–30 mg; p ≤ 0.0318). The osmolality and surface tension did not differ between the eye drops investigated.
Conclusion
BAK travoprost caused GC loss, indicating that PQ preservation may be preferable in treatment of glaucoma. Furthermore, physicochemical properties of branded and generic travoprost eye drops can not be assumed to be identical.
Keywords: benzalkonium chloride, glaucoma, goblet cells, ocular surface, polyquaternium‐1, travoprost
Introduction
Glaucoma is a growing problem worldwide due to the increasing elderly population (Tham et al. 2014; Kolko et al. 2015; Kolko 2017). The disease is associated with high intraocular pressure (IOP), and currently the only medical treatment includes IOP‐reducing agents (Peters et al. 2014; Cvenkel & Kolko 2020). IOP can be reduced with eye drops, and prostaglandin analogues (PGAs) are the first choice because of their high tolerability and effectiveness (European Glaucoma Society 2020). However, treatment is generally associated with low compliance mainly due to side‐effects (Wolfram et al. 2019), the most common being pain, stinging, burning and foreign body sensation (Jaenen et al. 2007). These are the same symptoms seen in Ocular Surface Diseases (OSD).
A healthy ocular surface relies on a stable tear film. The latter consists mainly of an outer lipid layer, an aqueous intermediate layer and an inner mucin layer. The mucin produced on the human ocular surface is mainly MUC5AC and it is secreted by the conjunctival goblet cells (GCs) (Gipson 2016). If the GCs are damaged, the mucin layer will be affected accordingly, thus the tear film will tend to evaporate and OSD may occur (Baudouin et al. 2019; Tiedemann et al. 2019). OSD is much more common in glaucoma patients than in the general population, and it is suspected that the treatment with eye drops is to blame (Schein et al. 1997; Leung et al. 2008). Currently available PGAs include latanoprost, bimatoprost, tafluprost and travoprost. In particular, latanoprost and travoprost formulations are of interest due to a large number of generics. According to the European Medicines Agency (EMA), generic drugs must be identical to the branded drug in terms of indication, active substance and dosage form (EMA 2012). New clinical trials are not required for the generic drugs to keep medicine costs down. This means that additives and physicochemical properties may vary between the PGA products without conducting in vivo evaluation studies of tolerability and efficacy. This has resulted in a substitution of the preservative in generic travoprost eye drops. The branded travoprost eye drop is thus preserved with polyquaternium‐1 (PQ) (EMA 2018), while some of the generics are preserved with benzalkonium chloride (BAK) (Danish Medicines Agency 2019a, 2019b, 2019c, 2019d). BAK‐preserved travoprost (BAK travoprost) has been shown to cause more ocular surface side‐effects than PQ‐preserved travoprost (PQ travoprost) (Kumar et al. 2019), why this substitution may affect patient compliance.
In Danish pharmacies, the medicine price is set by pharmaceutical companies for a 14‐day period, and the patient must always be offered the currently cheapest eye drop. Changes in the price every fortnight mean that patients can receive different eye drops every time they pick up a new prescription (Danish Medicines Agency 2019a, 2019b, 2019c, 2019d). If there are differences among the eye drops, it can result in variations in side effects, compliance and consequently fluctuations in IOP.
This study examines the effect of PQ travoprost and BAK travoprost on cultured primary human conjunctival GCs in terms of viability, mucin and cytokine secretion. Furthermore, the physicochemical properties of the branded and generic travoprost eye drops available in Denmark are measured.
Materials and Methods
Travoprost ophthalmic solutions
Eye drops included the following 40 μg/ml travoprost ophthalmic solutions: Travatan® (Novartis Europharm Limited, Dublin, Ireland, 10 μg/ml PQ), Travoprost Stada (STADA Arzneimittel AG, Bad Vilbel, Germany, 150 μg/ml BAK), Travoprost Teva (Teva Pharmaceutical Industries Ltd., Petah Tikva, Israel, 150 μg/ml BAK) and Bondulc (Actavis Group PTC ehf., Hafnarfjördur, Iceland, 150 μg/ml BAK).
Human conjunctival GC cultivation
Cultivation of GCs from human conjunctival tissue was approved by the Danish National Committee on Health Research (H‐17007902) and the Norwegian Regional Committees for Medical and Health Research Ethics (REK: 2013/803). Cultivation and reseeding were performed as previously described (Hedengran et al. 2021). The cells were cultured for 14 days before reseeding.
Assessment of GC viability
GC viability was determined by lactate dehydrogenase (LDH) assays and tetrazolium dye (MTT) colorimetric assays 3–5 days after reseeding. Viability was measured in triplicate on pure donor cultures from at least three individual donors. A control was included in all assays. Control GCs were incubated with complete culture medium with additives. Survival in the control was set to 100%.
The LDH assay was performed as previously described (Hedengran et al. 2021). The GCs were incubated with 1:7 (v/v) culture medium‐diluted eye drops for 30, 60 and 120 min at 37°C, 5% CO2/ 95% air. Absorbance was measured at 490 nm using a SpectraMax i3X multi‐mode microplate reader (Molecular devices, California, USA). Viability was calculated as the ratio between LDH release before membrane permeation and total LDH release. The percentage of living GCs was calculated relative to the control.
For the MTT assay, the GCs were incubated for 30 min at 37°C, 5% CO2/ 95% air with 1:7 (v/v) culture medium‐diluted eye drops. The diluted eye drops were removed, and fresh culture medium was added along with 12.5 mM thiazolyl blue tetrazolium bromide (M5655; Sigma‐Aldrich, Missouri, USA) in 1X Phosphate Buffered Saline (PBS). The GCs were incubated for an additional 60 min at 37°C before the stop solution 0.01% (v/v) HCl in 10% (w/v) sodium dodecyl sulphate (SDS) in PBS was added. The GCs were incubated at room temperature (RT) for 18 hr. Absorbance was measured at 560 nm on the SpectraMax i3X multi‐mode microplate reader. Viabilities for the GCs incubated with eye drops were calculated as fluorescence percentage compared to the control.
Immunocytochemistry for evaluation of mucin secretion
Coverslips with GCs from three individual donors were prepared for immunohistochemical staining. The slides were incubated for 30 min at 37°C, 5% CO2/ 95% air with 1:7 (v/v) culture medium‐diluted Travatan or Travoprost Stada after 14 days of cultivation. Travoprost Stada was included as a representative for the BAK‐preserved generics. A negative control was made by incubating GCs with culture medium. Coverslips were fixated with 4% (w/v) paraformaldehyde and stored at 4°C until staining. TritonX‐100 diluted 0.1% (v/v) in PBS was used to permeate the cell membrane. Nonspecific binding was blocked using 3% (w/v) bovine serum albumin (ab181831; Sigma‐Aldrich, Missouri, USA) in PBS. Coverslips were incubated with antibodies specific to cytokeratin‐7 (CK7; anti‐cytokeratin7, 1:500 v/v; ab181831; Abcam, Cambridge, England) and MUC5AC (anti‐mucin, 1:200 v/v; M5293; Sigma‐Aldrich, Missouri, USA). Washing with PBS was then performed and the slips were incubated with fluorescent secondary antibodies Alexa488 (anti‐rabbit, 1:500 v/v; A11034; Gibco, Life Technologies, Massachusetts, USA) and Texas red (anti‐mouse, 1:200 v/v; T862; Gibco, Life Technologies, Massachusetts, USA). DAPI (4′,6‐diamidino‐2‐phenylindole, 0.3 μM; D3571; Invitrogen, Massachusetts, USA) was used to stain the nuclei. Axioskop 2 (Zeiss; Göttingen, Germany) with an AxioCam MRm camera (Zeiss; Göttingen, Germany) and HXP 120 lighting unit (Zeiss; Göttingen, Germany) was used for imaging and Fiji ImageJ 1.49 for picture optimizing and merging.
Measurement of cytokine/chemokine secretion
Cytokine/chemokine secretion was determined 3–5 days after reseeding. The secretion was measured on GCs from three individual donors. The GCs were incubated with Travatan or Travoprost Stada diluted 1:7 (v/v) in serum‐free culture medium for 30 min at 37°C 5% CO2/ 95% air. Travoprost Stada was included as a representative for the BAK‐preserved generics due to the complexity of the analysis. For each donor, a control of GCs incubated with culture medium was included. The eye drops were removed and the GCs were incubated for 6 hrs with serum‐free medium. After centrifuging for 10 min at 1000 rounds per minute (r.p.m.), the supernatant was removed, placed on ice and stored at −20°C until further analyses. Before the analyses, the supernatant was spun down again for 10 min at 1000 r.p.m. at 4°C. BD Cytometric Bead Arrays, Cytokine XL and Chemokine kits (BD Biosciences, NJ, USA) was prepared according to the manufacturer's protocol. At a ratio of 1:1:1 the supernatant, antibody‐conjugated beads and phycoerythrin (PE) secondary antibodies were incubated for 3 hrs. After washing, beads were detected using a BD Accuri™ C6 personal flow cytometer (Becton Dickinson, NJ, USA). Secretion of the following cytokines and chemokines was determined: interleukin (IL)‐8 (C‐X‐C Motif Chemokine Ligand (CXCL)‐8), IL‐1b, IL‐6, IL‐10, tumour necrosis factor (TNF), IL‐10p70, C‐C Motif Chemokine Ligand (CCL)‐5/Regulated upon Activation, Normal T‐cell Expressed, and Secreted (RANTES), CXCL9/monokine induced by gamma interferon (MIG), CCL2/monocyte chemoattractant protein (MCP)‐1 and CXCL10/Interferon gamma‐induced protein (IP)‐10. These were quantified using a standard curve based on an internal control provided in the kit.
Measurement of physicochemical characterization
All physicochemical properties were measured in triplicate for all eye drops on three containers of each solution. pH value, viscosity, drop mass, drops per container, osmolality and surface tension were measured. pH value was measured at RT on a calibrated 744 pH meter (Metrohm; Nordic ApS, Herisau, Switzerland). To measure viscosity, a TA ARG2 rheometer (TA Instruments, Newcastle, DE) equipped with a 20 mm 1 degree truncated cone was used. After mounting the sample, a conditioning step was initiated including a 10 second preshear with a shear stess of 10 Pa followed by equilibration for 5 min. A shear rate of 5000 1/second was chosen, as this is within the range of shear rates in a human eye blinking (Tiffany 1991). Four measurement points were collected per decade (log mode) with a sample period of 10 seconds. The measurement was set to accept a 5% tolerance for three consecutive measurements, with a maximum of 1 min per time‐point. All measurements were performed at 21°C using soft bearing mode. Drop mass was measured at RT on a XS105 Dual Range analytical balance (Mettler Toledo International, Ohio, USA) measuring 10 drops in the beginning, middle and end of emptying a container. The number of drops in a container was counted manually at RT. Osmolality was measured with freezing point depression (Osmomat 3000; Gonotec, Berlin, Germany). Surface tension was assessed through the Wilhelmy method with a platinum rood probe and force tensiometer K‐100c (Krüss GmbH, Hamburg, Germany) using the software version 3.2.2.3068 (Laboratory Desktop, KRÜSS GmbH). The measurements were performed at RT with an immersion depth of 0.5 mm and detection speed of 10 mm/min accepting a standard deviation of <0.1 mN/m based on 20 values.
Statistics
The software program GraphPad Prism version 9.0.0 for Windows (GraphPad Software, La Jolla, California, USA) was used for statistical analyses and graphics. Three drop mass measurements and one osmolality measurement were eliminated as significant outliers through grubbs' test with a 0.05 significance level, which did not affect the results. Normal distribution of datasets was determined through QQ‐plots. Abnormal distribution was only found in datasets on ratio of IL‐6 secretion and LDH release after 30 min of incubation. One‐way analysis of variance (ANOVA) was used for the datasets with normal distribution. Dunnet's multiple comparison test was used when comparing viability and cytokine release between eye drops and the control, and Tukey's multiple comparison test was used when comparing physicochemical properties. Non‐parametric Kruskal‐Wallis test was applied for the ratio of IL‐6 secretion and LDH release after 30 min of incubation. Mixed effect analyses were applied for statistics on LDH assays, MTT assays and cytokine secretion. Statistical analyses on cytokine secretion were performed on the ratio of cytokine secretion compared to the control. A p‐value of <0.05 was considered statistically significant.
Results
BAK‐preserved travoprost reduces GC viability
The LDH assay revealed a linearly time‐dependent cytotoxicity for all BAK travoprost eye drops. After 120 min of incubation, BAK travoprost caused a significant GC loss compared to the control (p ≤ 0.0187, one‐way ANOVA) (Fig. 1). No differences between the control and PQ travoprost were identified at any time point. The MTT assay showed a significant effect on GCs for all eye drops after 30 min of incubation compared to the control (p ≤ 0.013, one‐way ANOVA) (Fig. 2).
Fig. 1.
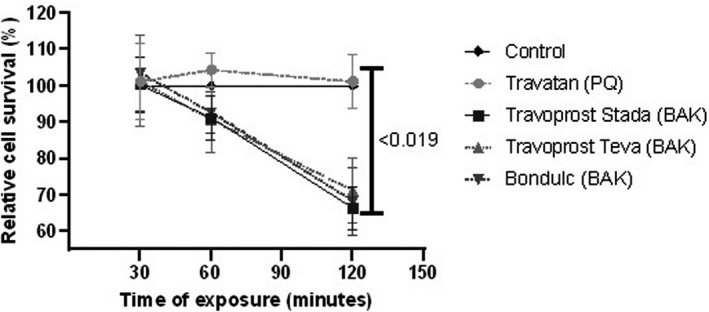
Relative cell survival of primary human conjunctival goblet cell (GC) cultures assessed through lactate dehydrogenase (LDH) assays. Results are presented as mean cell survival relative to a fixed 100 % survival for the control ± standard deviation. Cultures were incubated for 30, 60 and 120 min with polyquarternium‐1 (PQ)‐ or benzalkonium chloride (BAK)‐preserved travoprost eye drops. Cultures from three individual donors were included. The large bar shows significant decrease in GC survival after 2 hrs incubation with the generic BAK‐preserved travoprost eye drops compared to the control. There were no differences in survival between the control and Travatan at any time point. Only p‐values <0.05 are shown.
Fig. 2.
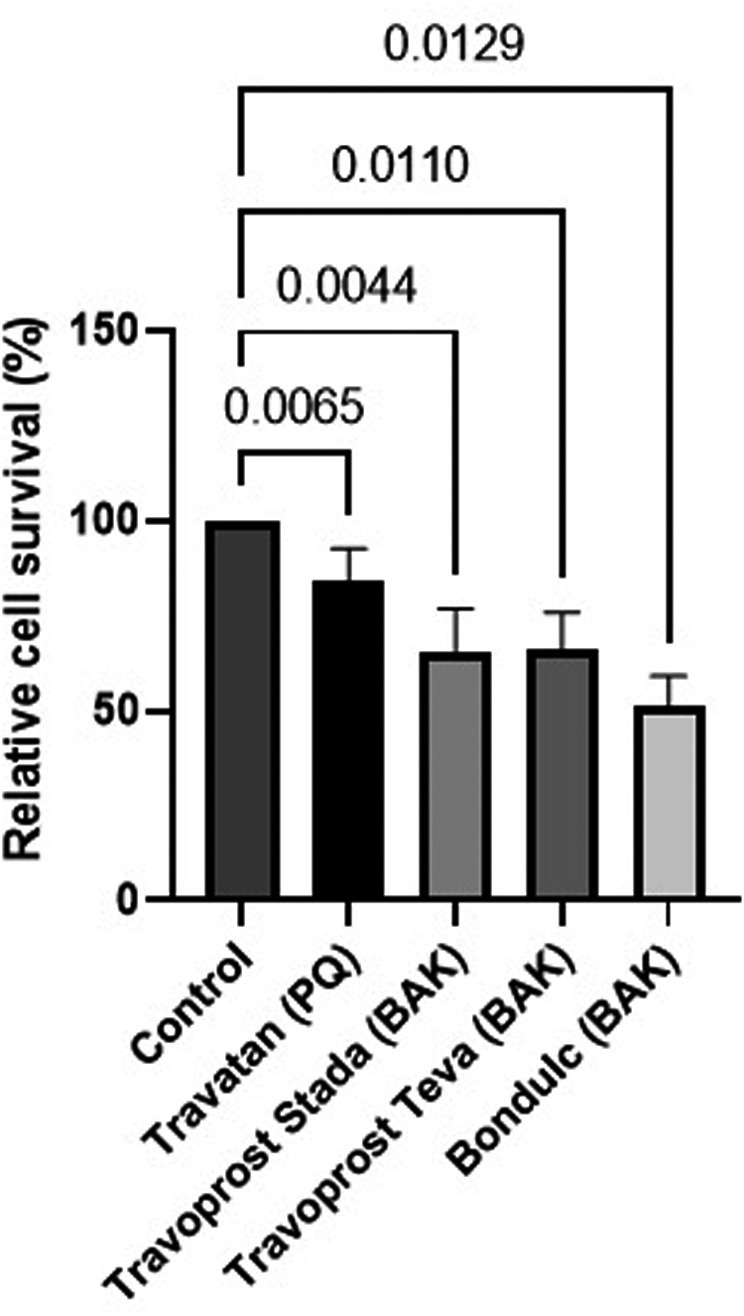
Relative cell survival of primary human conjunctival goblet cell (GC) cultures assessed through tetrazolium dye (MTT) colorimetric assays. Results are presented as mean cell survival relative to a fixed 100 % GC survival for the control ± standard deviation. Cultures were incubated for 30 min with polyquarternium‐1 (PQ)‐ or benzalkonium chloride (BAK)‐preserved travoprost eye drops. Cultures from three individual donors were included. Significant decrease in GC survival was identified for all travoprost eye drops. Only p‐values <0.05 are shown.
PQ‐ and BAK‐preserved travoprost cause mucin secretion
Secretion of mucin from GCs was evaluated with immunohistochemical stainings after 30 min of incubation with PQ travoprost or BAK travoprost. A negative control incubated with culture medium showed no mucin secretion as the mucin (seen as red) was located in vesicles around the nuclei (seen as blue). GCs incubated with PQ travoprost or BAK travoprost showed mucin dispersed to the cytoplasma. The cell borders are outlined by cytoskeleton staining (seen as green). This indicates a secretagogue effect of both PQ‐ and BAK‐preserved travoprost eye drops (Fig. 3).
Fig. 3.
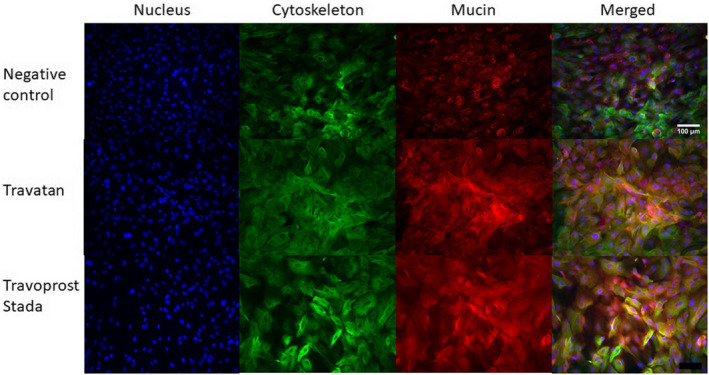
Secretion of mucin from primary human conjunctival goblet cell (GC) cultures assessed through immunohistochemical stainings. GCs were incubated for 30 minutes with culture medium as a negative control, polyquarternium‐1 (PQ)‐preserved Travatan or benzalkonium chloride (BAK)‐preserved Travoprost Stada. Travoprost Stada represents the BAK‐preserved generics. The nucleus was blue with DAPI (4′,6‐diamidino‐2‐phenylindole), the cytoskeleton green with anti‐cytokeratin‐7 and the mucin red with MUC5AC. The three stainings were merged. Dispersion of mucin to the cytoplasma indicates a secretagogue effect for both BAK‐ and PQ‐preserved travoprost. Stainings were performed on cultures from three individual donors. Scale bar = 100 μm. [Colour figure can be viewed at wileyonlinelibrary.com]
PQ‐ and BAK‐preserved travoprost does not increase cytokine release
Cytokine secretion from GCs was assessed after 30 minutes of incubation with PQ travoprost or BAK travoprost. Secretion of IL‐6 and IL‐8 was identified. There were no significant differences in secretion from GCs between PQ travoprost and BAK travoprost compared to the control (Fig. 4). No secretion of IL‐1b, IL‐10, TNF, IL‐10p70, CCL‐5/RANTES, CXCL9/MIG, CCL2/ MCP‐1 or CXCL10/IP‐10 was detected.
Fig. 4.
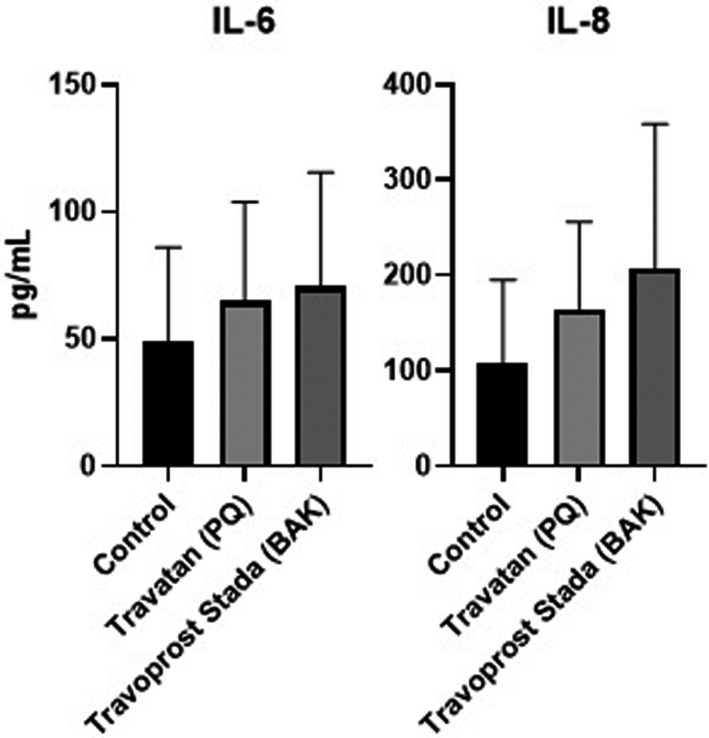
Secretion of interleukin 6 (IL‐6) and interleukin 8 (IL‐8) from primary human conjunctival goblet cell (GC) cultures. Secretion is presented as mean concentration ± standard deviation after 30 min of incubation with polyquarternium‐1 (PQ)‐preserved Travatan and benzalkonium chloride (BAK)‐preserved Travoprost Stada. Travoprost Stada represents the BAK‐preserved generics. As a negative control GCs were incubated with culture medium. Secretion was measured on GCs from three individual donors using BD Cytometric Bead Arrays. No differences in cytokine secretion were identified compared to the control. Only p‐values <0.05 are shown.
Physicochemical properties differ between branded and generic travoprost eye drops
All physicochemical properties were measured in triplicate for each eye drops on three containers of each travoprost ophthalmic solution (Fig. 5). The pH value varied between the branded Travatan (pH 6.7) and the generic travoprost eye drops (pH 6.0; p < 0.0001, one‐way ANOVA). Differences in viscosity were identified with Travatan having the lowest viscosity (0.76 mPa.s) and Travoprost Teva the highest (0.93 mPa.s; p < 0.0001, one‐way ANOVA). The mean drop mass was higher for Travatan (35 mg) compared to generics (28–30 mg; p ≤ 0.0318, one‐way ANOVA), and there were fewer drops in a bottle of Travatan (69 drops) compared to the generics (87–92 drops; p ≤ 0.007, one‐way ANOVA). The osmolality and surface tension did not differ between the eye drops.
Fig. 5.
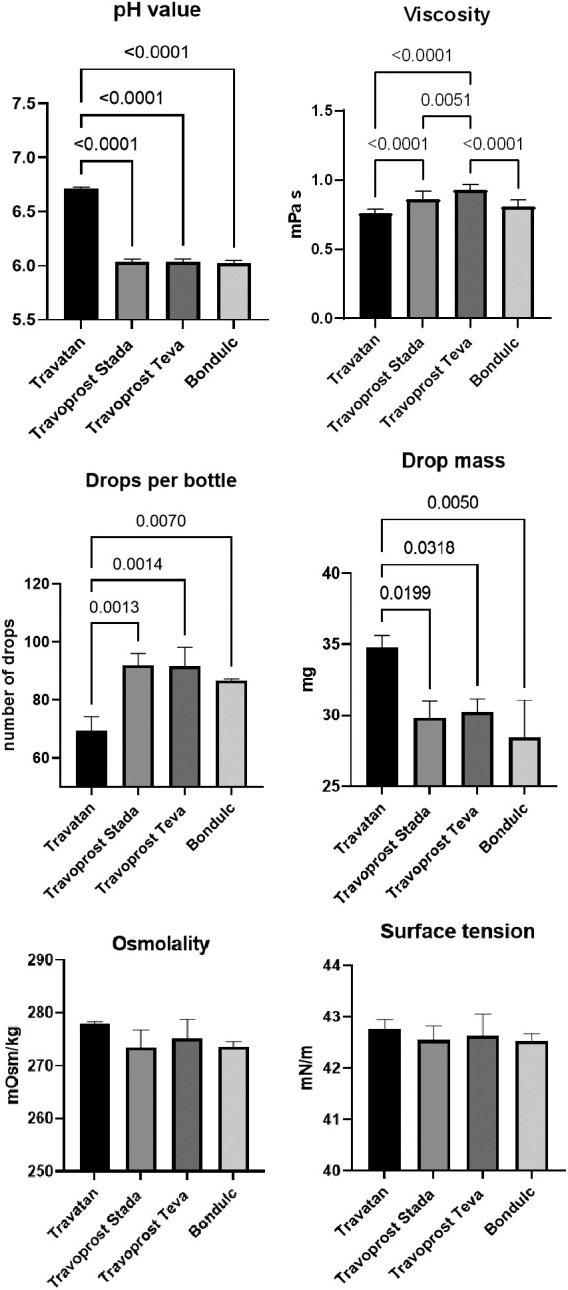
Physicochemical properties of the branded travoprost eye drop Travatan and the generics Travoprost Stada, Travoprost Teva and Bondulc. pH value, viscosity (mPa.s), drop mass (mg), number of drops per bottle, osmolality (mOsm/kg) and surface tension (mN/m) were measured. Values are shown as mean ± standard deviation. Significant differences in pH value, viscosity, drop mass and number of drop per bottle were identified. Only p‐values ≤0.05 are shown.
Discussion
The current study showed that generic BAK travoprost eye drops were more cytotoxic than PQ travoprost. Significant differences in pH value, viscosity, droplet mass and droplets per bottle between the branded and generic travoprost eye drops were identified. Immunohistochemical staining showed mucin secretion in GCs incubated with BAK‐ or PQ travoprost compared to control GCs. No increased secretion of IL‐6 and IL‐8 was detected after incubation with BAK‐ or PQ travoprost.
Cell viability was affected by BAK travoprost in a linearly time‐dependent manner assessed through LDH assays, whereas PQ travoprost was not cytotoxic at any time point. According to MTT assays, PQ travoprost caused 16% GC loss, while BAK travoprost caused 34–42% GC loss after 30 min. The LDH assay is a sensitive cytotoxicity assay that allows detection of low levels of cell death through membrane damage. The MTT assay detects inhibited proliferation. When comparing the two assays, one gains insight into a possible mechanism behind BAKs toxic effect. The difference between the results from the LDH and MTT assays could be due to an inhibited proliferation, which ultimately leads to cell death for the BAK‐preserved eye drops after 120 minutes incubation. BAK has previously been thought to be toxic due to an inhibition of the mitochondrial function, which supports the current theory (Datta et al. 2017).
The conjunctiva consists of epithelial cells and GCs. While epithelial cells make up the vast majority of the conjunctiva, GCs are important in keeping the surface lubricated through mucin secretion. If the GCs are damaged, mucin secretion will decrease, the tear film will be disrupted and OSD may occur (Baudouin et al. 2019). On cultured human conjunctival epithelial cells, BAK travoprost has been found to be more cytotoxic than PQ travoprost (Brignole‐Baudouin et al. 2011). Therefore, all conjunctival cells are at risk when applying BAK‐preserved eye drops, as many glaucoma patients do daily. The deleterious effect of BAK compared to PQ has previously been seen in clinical trials evaluating Ocular Surface Disease Index (OSDI) scores, quality of life and impression cytology grades, where PQ travoprost was significantly better than BAK travoprost (Sezgin Akçay et al. 2014; Kumar et al. 2019; Kumar et al. 2020). However, the IOP‐lowering effects of PQ‐ and BAK travoprost have been shown to be comparable (Gandolfi et al. 2012; Peace et al. 2015). In rabbits, instillation with BAK travoprost induced hyperemia, abnormalities in the ocular surface, damaged epithelial cells, inflammatory cell infiltration, and decreased GC density (Liang et al. 2012). This was not seen for PQ travoprost. Of note, the current laboratory‐based study provides information on a single acute exposure of PQ‐ and BAK travoprost to cultured GCs. Clinical trials, however, provide information on multiple instillations in the eye over a period of time. Both types of studies are key in getting the full picture of how a drug affects the ocular tissue and patients. Both the laboratory studies and clinical studies indicate that PQ travoprost has a better safety profile and tolerability than BAK travoprost in terms of OSD.
While a decrease in mucin can cause OSD, increased mucin secretion is seen in various conditions with chronic inflammation and allergy as a way to protect the ocular surface against inflammatory agents and allergens (Dartt & Masli 2014). The mucin secretion induced by acute exposure to PQ‐ and BAK travoprost in GC cultures may theoretically indicate an irritant effect when applying PQ‐ or BAK‐preserved eye drops. The exact mechanism behind the secretion, however, is unknown. Furthermore, the current study does not show what causes the mucin secretion. It could be due to the preservatives but also the active compound. Most importantly, there is no difference in secretion between the generic BAK‐preserved eye drop and the branded PQ‐preserved eye drop. A difference in tolerability based on mucin secretion is, therefore, unlikely.
IL‐6 is a proinflammatory cytokine, whereas IL‐8 is both proinflammatory and proangiogenic (Li et al. 2003; Ghasemi et al. 2011; Zahir‐Jouzdani et al. 2017). IL‐6 concentration has been found to increase in tear film from patients treated with BAK‐preserved latanoprost, PQ travoprost or BAK‐preserved bimatoprost compared to controls (Lopilly Park et al. 2012), and both IL‐6 and IL‐8 have been found to be increased in patients treated with BAK‐preserved eye drops compared to preservative‐free (PF) eye drops (Mohammed et al. 2020). While the present study did not show a significant increase in cytokine secretion, IL‐6 and IL‐8 secretions were 1.47‐ and 1.95‐fold higher for GC incubated with BAK travoprost and 1.4‐ and 1.74‐fold higher for GC incubated with PQ travoprost compared to the control. An eye drop‐induced increase in cytokine secretion can therefore not be excluded and may be part of the damage to the ocular surface, loss of GCs and development of OSD (Na et al. 2012; Baudouin et al. 2019).
Significant differences were found between the branded travoprost eye drop Travatan and the generics in terms of pH value, viscosity, droplet mass and number of drops per bottle. The observed variation in the pH values of the eye drops may raise concern, as a pH value of 6.7 was measured for Travatan, whereas all the generics had a pH value of 6.0. By comparison, the pH of the tear film is 7.6 (Fischer & Wiederholt 1982). In a previous study by Wadhwani et al. (2016), the pH values of Travatan and three generics hereof were determined to be 5.8 and 4.7–5.9 respectively. A similar difference has been identified between the branded latanoprost eye drop Xalatan (pH 6.0) and the latanoprost generics (pH 6.7–6.8), proving that this issue goes beyond travoprost eye drops (Kolko & Koch Jensen 2017). Formulation variations across continents may occur. As Wadhwani et al. (2016) conducted their research in Asia, where the proclaimed pH value of Travatan is 6.0 (Monthly Index of Medical Specialities 2021), differences from our results may be due to this fact. In Europe, the pH value of Travatan is not declared in the Summary of Product Characteristics (SmPC). It should be noted that the observed variations in pH are within the pH range given in the SmPC of Travoprost Stada (5.5–7.0). To our knowledge, the effect of pH value on the development of OSD has not been investigated. However, exposure to highly acidic or alkaline fluids can cause an ophthalmic emergency and permanent damage the ocular surface. Hence, variations in the pH value of eye drops will very likely affect tolerability.
The viscosity of the tear film is lower in patients with dry eyes (6.9 mPa.s) than in controls (11 mPa.s) (McDonnell et al. 2019). The measured viscosities in the current study and in the study by Wadhwani et al. (2016) were notably smaller than this (≤1 mPa.s), posing a potential risk factor for developing dry eyes when using travoprost eye drops. The differences identified between the branded and generic travoprost eye drops were small and are unlikely to have clinical relevance.
The droplet mass varied with only 5 mg from approximately 30 mg for the generics (on average) to 35 mg for Travatan. The effect of this on efficacy is debateable. A smaller drop will result in less wash out, which will increase contact time between active substance and the ocular surface – but also less applied active substance. Travatan contained 69 drops, and the generics contained approximately 90 drops (on average) as expected with the differences in drop mass as all bottles contained 2.5 ml. Droplet mass is not expected to have impact on tolerability nor development of OSD.Little variation in osmolalities of the eye drops was identified (273–278 mOsm/kg) and all were close to the osmolality of the tear film (303.2 mOsm/kg) (Murube 2006). Wadhwani et al. (2016) identified larger variations (262–313 mOsm/kg), with three out of four eye drops being hyperosmolal compared to the tear film. In patients with OSD, hyperosmolality is most often the case (Murube 2006). Hyperosmolality have been shown to cause tear film evaporation, epithelial cell inflammatory response and GC loss (Dry Eye WorkShop 2007). However, all measured osmolalities were close to the tear film and are unlikely to have clinical relevance.
The measured surface tensions of the eye drops were all similar (approximately 42 mN/m) and close to the surface tension of the tear film (43.6 ± 2.7 mN/m; Tiffany et al. 1989). In patients with dry eyes, a high surface tension is most often seen, and it is associated with a short tear break‐up time (Tiffany et al. 1989). The surface tension of the travoprost eye drops is probably not relevant in the development of OSD.
The differences in preservation and pH value between branded and generic travoprost eye drops will likely cause the branded travoprost to have better tolerability. Furthermore, efficacy can be affected. This could be due to a difference in drug uptake but more likely due to compliance. If the generic eye drops have worse tolerability, compliance and with it efficacy will decrease.
There are limitations to the current study. When performing analyses on GCs, the eye drops were diluted to mimic the dilution in the tear film upon instillation. The design of this study is of course static. Upon instillation in the eye, the concentration of the eye drops will gradually decrease, which is not tested in our setup. Of note, the dilution caused the difference in pH values to diminish to 7.6–7.7. The differences in the eye drops' cytotoxicity are, therefore, likely not due to differences in pH value. The studies were performed on GC cultures, and the cells may not act entirely the same in cultures and in vivo. Nevertheless, BAK travoprost presented more cytotoxic profile compared to PQ travoprost. Furthermore, the sample size of cultures from three donors when investigating mucin and interleukin secretion is rather small. The physicochemical properties were measured on newly opened bottles of eye drops. The eye drops have a durability of 1 month after breakage and the properties may change during this month. Upon instillation the physicochemical properties will further change as the eye drops are diluted in the tear film. The significance of differences in physicochemical properties is, therefore, speculative. To further investigate the impact of differences between branded and generic travoprost eye drops on safety, tolerability and efficacy, clinical trials with evaluations of GC density and OSD would be of interest.
Conclusion
The introduction of generics has caused drug prices to fall, making treatment more accessible to patients. However, with the very few requirements set by medical authorities, eye drops can vary on various parameters. We identified significant differences in both preservation and physicochemical properties between branded and generic travoprost eye drops. In addition, we found that BAK‐preserved travoprost eye drops are more cytotoxic to GCs than PQ‐preserved travoprost eye drops. The inappropriate addition of BAK in generic travoprost eye drops could potentially give patients more adverse effects and consequently decrease compliance. In the long run, such differences can cause patients to lose sight. In order to increase compliance and decrease risk of glaucomatous progression, more regulations are needed regarding production of generics.
Acknowledgement: Special thanks to Laboratory Technician Charlotte Taul.
Funding: The study is funded by the Synoptik‐Foundation, Fight for Sight, Denmark, the Danish Eye Research Foundation, Bagenkop Nielsen's Eye Foundation and the A.P Møller Foundation for Promotion of Medical Science.
Conflict of interests: The authors declare no conflict of interests with relevance to the present study.
Contribution: The study was planned by AH, GP, SH and MK. Concept and design were performed in collaboration with AH, GP, MK, JJ, SWL, KF and SHH. Analyses were conducted by AH, JCF, PMH, SHH and GBL. Interpretation and analysis of data were performed by AH and MK. The manuscript was written by AH and MK. The manuscript was revised and approved by all authors.
Contributor Information
Anne Hedengran, Email: anne.hedengran.nagstrup@regionh.dk.
Miriam Kolko, Email: miriamk@sund.ku.dk.
References
- Baudouin C, Rolando M, Benitez Del Castillo JM et al. (2019): Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog Retin Eye Res 71: 68–87. [DOI] [PubMed] [Google Scholar]
- Brignole‐Baudouin F, Riancho L, Liang H & Baudouin C (2011): Comparative in vitro toxicology study of travoprost polyquad‐preserved, travoprost BAK‐preserved, and latanoprost BAK‐preserved ophthalmic solutions on human conjunctival epithelial cells. Curr Eye Res 36: 979–988. [DOI] [PubMed] [Google Scholar]
- Cvenkel B & Kolko M (2020): Current medical therapy and future trends in the management of glaucoma treatment. J Ophthalmol 6(138): 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Medicines Agency (2019a): Substitution https://laegemiddelstyrelsen.dk/da/apoteker/substitution/. (Accessed 28 sep 2021).
- Danish Medicines Agency (2019b): Product summary for Bondulc.
- Danish Medicines Agency (2019c): Product summary for Travoprost Stada.
- Danish Medicines Agency (2019d): Product summary for Travoprost Teva.
- Dartt DA & Masli S (2014): Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr Opin Allergy Clin Immunol 14: 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Baudouin C, Brignole‐Baudouin F, Denoyer A & Cortopassi GA (2017): The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Invest Ophthalmol Vis Sci 58: 2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry Eye WorkShop (2007): The definition and classification of dry eye disease: report of the definition and classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 75–92. [DOI] [PubMed] [Google Scholar]
- European Glaucoma Society (2020): Terminology and guidelines for glaucoma, 5th edition. [DOI] [PubMed]
- European Medicines Agency (2012): Generic and hybrid medicines https://www.ema.europa.eu/en/human‐regulatory/marketing‐authorisation/generic‐hybrid‐medicines. (Accessed 28 sep 2021).
- European Medicines Agency (2018): Summary of product characteristics Travatan https://www.medicines.org.uk/emc/product/1556/smpc#gref.
- Fischer FH & Wiederholt M (1982): Human precorneal tear film pH measured by microelectrodes. Graefes Arch Clin Exp Ophthalmol 218: 168–170. [DOI] [PubMed] [Google Scholar]
- Gandolfi S, Paredes T, Goldberg I et al. (2012): Comparison of a travoprost BAK‐free formulation preserved with polyquaternium‐1 with BAK‐preserved travoprost in ocular hypertension or open‐angle glaucoma. Eur J Ophthalmol 22: 34–44. [DOI] [PubMed] [Google Scholar]
- Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S & Hassan ZM (2011): Roles of IL‐8 in ocular inflammations: a review. Ocul Immunol Inflamm 19: 401–412. [DOI] [PubMed] [Google Scholar]
- Gipson IK (2016): Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res 54: 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedengran A, Beguna X, Müllertz O et al. (2021): Benzalkonium chloride preserved anti‐glaucomatous eye drops and their effect on human conjunctival goblet cells in vitro. Biomed Hub 6: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A & Zeyen T (2007): Ocular symptoms and signs with preserved and preservative‐free glaucoma medications. Eur J Ophthalmol 17: 341–349. [DOI] [PubMed] [Google Scholar]
- Kolko M (2017): Detection and prevention of blindness in patients with glaucoma is a socio‐economical challenge. Ugeskr Laeger 179: V06160444. [PubMed] [Google Scholar]
- Kolko M, Horwitz A, Thygesen J, Jeppesen J & Torp‐Pedersen C (2015): The prevalence and incidence of glaucoma in Denmark in a fifteen year period: a Nationwide study. PLoS One 10: e0132048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolko M & Koch Jensen P (2017): The physical properties of generic latanoprost ophthalmic solutions are not identical. Acta Ophthalmol 95: 370–373. [DOI] [PubMed] [Google Scholar]
- Kumar S, Singh T, Ichhpujani P & Vohra S (2019): Ocular surface disease with BAK preserved Travoprost and polyquaternium 1(Polyquad) preserved Travoprost. Rom J Ophthalmol 63: 249–256. [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Singh T, Ichhpujani P, Vohra S & Thakur S (2020): Correlation of ocular surface disease and quality of life in Indian glaucoma patients: BAC‐preserved versus BAC‐free Travoprost. Turk J Ophthalmol 50: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung EW, Medeiros FA & Weinreb RN (2008): Prevalence of ocular surface disease in glaucoma patients. J Glaucoma 17: 350–355. [DOI] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ & Singh RK (2003): IL‐8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170(6): 3369–3376. [DOI] [PubMed] [Google Scholar]
- Liang H, Brignole‐Baudouin F, Riancho L & Baudouin C (2012): Reduced in vivo ocular surface toxicity with polyquad‐preserved travoprost versus benzalkonium‐preserved travoprost or latanoprost ophthalmic solutions. Ophthalmic Res 48: 89–101. [DOI] [PubMed] [Google Scholar]
- Lopilly Park HY, Kim JH, Lee KM & Park CK (2012): Effect of prostaglandin analogues on tear proteomics and expression of cytokines and matrix metalloproteinases in the conjunctiva and cornea. Exp Eye Res 94: 13–21. [DOI] [PubMed] [Google Scholar]
- McDonnell A, Lee JH, Makrai E, Yeo LY & Downie LE (2019): Tear film extensional viscosity is a novel potential biomarker of dry eye disease. Ophthalmology 126: 1196–1198. [DOI] [PubMed] [Google Scholar]
- Mohammed I, Kulkarni B, Faraj LA, Abbas A, Dua HS & King AJ (2020): Profiling ocular surface responses to preserved and non‐preserved topical glaucoma medications: a 2‐year randomized evaluation study. Clin Exp Ophthalmol 48: 973–982. [DOI] [PubMed] [Google Scholar]
- Monthly Index of Medical Specialities (2021): Travatan https://www.mims.com/philippines/drug/info/travatan?type=full. (Accessed 28 sep 2021).
- Murube J (2006): Tear osmolarity. Ocul Surf 4: 62–73. [DOI] [PubMed] [Google Scholar]
- Na KS, Mok JW, Kim JY, Rho CR & Joo CK (2012): Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Invest Ophthalmol Vis Sci 53: 5443–5450. [DOI] [PubMed] [Google Scholar]
- Peace JH, Ahlberg P, Wagner M, Lim JM, Wirta D & Branch JD (2015): Polyquaternium‐1‐preserved Travoprost 0.003% or benzalkonium chloride‐preserved Travoprost 0.004% for glaucoma and ocular hypertension. Am J Ophthalmol 160: 266–274.e1. [DOI] [PubMed] [Google Scholar]
- Peters D, Bengtsson B & Heijl A (2014): Factors associated with lifetime risk of open‐angle glaucoma blindness. Acta Ophthalmol 92: 421–425. [DOI] [PubMed] [Google Scholar]
- Schein OD, Munoz B, Tielsch JM, Bandeen‐Roche K & West S (1997): Prevalence of dry eye among the elderly. Am J Ophthalmol 124: 723–728. [DOI] [PubMed] [Google Scholar]
- Sezgin Akçay B, Güney E, Bozkurt TK, Topal CS, Akkan JC & Ünlü C (2014): Effects of polyquaternium‐ and benzalkonium‐chloride‐preserved travoprost on ocular surfaces: an impression cytology study. J Ocul Pharmacol Ther 30(7): 548–553. [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T & Cheng CY (2014): Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta‐analysis. Ophthalmology 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- Tiedemann D, Mouhammad ZA, Utheim TP, Dartt DA, Heegaard S, Petrovski G & Kolko M (2019): Conjunctival goblet cells, the overlooked cells in glaucoma treatment. J Glaucoma 28: 325–333. [DOI] [PubMed] [Google Scholar]
- Tiffany JM (1991): The viscosity of human tears. Int Ophthalmol 15: 371–376. [DOI] [PubMed] [Google Scholar]
- Tiffany JM, Winter N & Bliss G (1989): Tear film stability and tear surface tension. Curr Eye Res 8: 507–515. [DOI] [PubMed] [Google Scholar]
- Wadhwani M, Mishra SK, Angmo D et al. (2016): Evaluation of physical properties of generic and branded Travoprost formulations. J Curr Glaucoma Pract 10: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram C, Stahlberg E & Pfeiffer N (2019): Patient‐Reported Nonadherence with Glaucoma Therapy. J Ocul Pharmacol Ther 35: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir‐Jouzdani F, Atyabi F & Mojtabavi N (2017): Interleukin‐6 participation in pathology of ocular diseases. Pathophysiology 24: 123–131. [DOI] [PubMed] [Google Scholar]


