Abstract
During infection with gram-negative bacteria, exposure of immune cells to lipopolysaccharide (LPS) from the bacterial cell membrane induces a rapid cytokine response which is essential for the activation of host defenses against the invading pathogens. Administration of LPS to mice induces a state of hyporesponsiveness, or tolerance, characterized by reduced cytokine production upon subsequent LPS challenge. In the model of experimental Salmonella enterica serovar Typhimurium infection of mice, we assessed the question of whether complete LPS tolerance induced by repetitive doses of LPS interfered with cytokine production and host defense against gram-negative bacteria. Although production of various cytokines in response to serovar Typhimurium was attenuated by LPS pretreatment, LPS-tolerant mice showed improved antibacterial activity, evidenced by a prolongation of survival and a continuously lower bacterial load. We attribute this protective effect to three independent mechanisms. (i) Peritoneal accumulation of leukocytes in the course of LPS pretreatment accounted for enhanced defense against serovar Typhimurium during the first 6 h of infection but not for decreased bacterial load in late-stage infection. (ii) LPS-tolerant mice had an increased capacity to recruit neutrophilic granulocytes during infection. (iii) LPS-tolerant mice showed threefold-increased Kupffer cell numbers, enhanced phagocytic activity of the liver, and strongly improved clearance of blood-borne serovar Typhimurium. These results demonstrate that despite attenuated cytokine response, acquired LPS tolerance is associated with enhanced resistance to infections by gram-negative bacteria and that this effect is mainly mediated by improved effector functions of the innate immune system.
Endotoxin, or lipopolysaccharide (LPS), a glycolipid of the cell membranes of gram-negative bacteria, is one of the most potent stimulators of immune responses known. The immune system responds to LPS with a systemic production of proinflammatory cytokines, which recruit and activate immune cells to eliminate invading pathogens (40). Although these cytokines are indispensible for the efficient control of the growth and dissemination of the pathogen (7, 10, 17), an excessive inflammatory response is potentially autodestructive and may lead to microcirculatory dysfunction, causing tissue damage, septic shock, and eventually death (3, 14). The phenomenon of endotoxin tolerance is known from animal models of “sterile infection” induced by LPS: after an initial low dose of LPS, animals are protected against the detrimental consequences of a subsequent high dose of LPS. This protection is associated with an attenuated cytokine response to LPS (11) due to a downregulation of macrophage responsiveness (12).
The value of endotoxin tolerance induction as a mean of sepsis prophylaxis was studied in animal models of endotoxic shock or polymicrobial sepsis. In these models, protection by tolerance induction was ascribed to the decreased proinflammatory response, resulting in less inflammatory cell infiltration and therefore attenuation of organ damage (15, 19, 38, 49). These models simulate the final phase of sepsis, but they do not entirely reflect the situation of infection with small numbers of virulent pathogens, where activation of host defenses contributes to halt proliferation and dissemination of the pathogen (27, 29). Only if the immune system fails to control the infection does bacterial replication result in overwhelming and finally lethal pathogen numbers. Therefore, it is not surprising that in contrast to models of acute hyperinflammation, neutralization of proinflammatory cytokines worsens the outcome of infection with low numbers of virulent bacteria (8, 41). Moreover, whereas depletion of various leukocyte populations confers protection against endotoxic shock or inflammatory liver damage (20, 22), this treatment renders animals more susceptible to bacterial infection (5, 13).
Considering the obvious differences between models of hyperinflammation and infection with low numbers of virulent bacteria, we were interested to see whether attenuation of cytokine release by induction of endotoxin tolerance would affect the susceptibility of mice to infection with virulent bacteria and which possible consequences could arise from these model experiments for sepsis prophylaxis.
Infection of mice with Salmonella enterica serovar Typhimurium, the equivalent of human typhoid fever, is one of the best-characterized models of systemic and lethal infection (48). This model was chosen for two reasons. (i) The murine pathogen serovar Typhimurium can replicate and cause systemic infection starting from very few inoculated bacteria (21); i.e., host responses can be studied without inducing septic shock. (ii) Efficient host defense against serovar Typhimurium depends on the production of proinflammatory cytokines like tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) and susceptibility is increased by neutralization of these mediators (reviewed in reference 25). If endotoxin tolerance has a negative impact on host defense, e.g., by impairing bacterially induced cytokine release, this should be most obvious during infection with this gram-negative pathogen.
Our study demonstrates that despite impaired systemic release of proinflammatory cytokines in response to viable bacteria, LPS-tolerant mice show increased resistance to serovar Typhimurium infection due to improved antibacterial defense capabilities of the innate immune system.
MATERIALS AND METHODS
Mice.
Male BALB/c mice, 7 to 9 weeks of age, from the breeding facility of the University of Konstanz (Konstanz, Germany) were kept at 24°C and 55% humidity with a 12-h day-night rhythm on a diet of Altromin C 1310 (Altromin Co., Lage, Germany). All animals received humane care in accordance with the National Institutes of Health guidelines and the legal requirements in Germany.
Bacteria.
Serovar Typhimurium LT2 strain ATCC 15277 from the American Type Culture Collection (Manassas, Va.) was cultured overnight in tryptic soy broth (Difco, Detroit, Mich.) at 37°C with gentle rotation. Aliquots of 5 × 108 viable bacteria/ml in 25% glycerol were stored at −80°C. Just before use, the aliquots were thawed and diluted in pyrogen-free saline.
LPS tolerance induction.
For induction of LPS tolerance, mice were injected intraperitoneally (i.p.) or intravenously (i.v.) with a dose of 1 mg of LPS (Salmonella enterica serovar Abortus equi; Metalon, Wustenhofen, Germany) per kg of body weight diluted in pyrogen-free 0.9% NaCl solution (Braun, Melsungen, Germany) at 72, 48, and 24 h prior to challenge with LPS (LPS shock) or serovar Typhimurium (infection).
LPS shock.
Control mice were injected with the same volume of pyrogen-free saline at the same time points. For induction of endotoxic shock, control and LPS-pretreated mice were injected i.p. with 10 mg of LPS/kg, and survival was monitored for 72 h. Blood for determination of plasma TNF-α was obtained from the tail vein 90 min after challenge.
Experimental infection.
Serovar Typhimurium infection was initiated by i.p. inoculation with 107 bacteria per kg of body weight, and survival was monitored for 10 days. Bacterial load, leukocyte counts, myeloperoxidase (MPO) activity, and cytokine levels were analyzed at various time points in parallel experiments.
Polymorphonuclear leukocyte (PMN) depletion.
Anti-Ly-6G (RB6-8C5) immunoglobulin G (IgG) or control rat IgG (Biotrend, Cologne, Germany) was administered 16 h (0.6 mg/mouse i.p.) prior to and 6 and 30 h (0.3 mg/mouse i.p.) after infection of LPS-tolerant (LPS i.v.) and control mice with serovar Typhimurium (106/kg i.p.). Twenty-four hours after the injection of bacteria, blood was obtained from the tail vein for determination of total and differential leukocyte counts, and survival was monitored for 10 days. Anti-Ly-6G rat IgG2b was purified from supernatants of the RB6-8C5 clone (provided by R. Coffman, DNAX, Palo Alto, Calif.) grown in 350-ml culture flasks (CL 350; Integra Biosciences, Fernwald, Germany).
Determination of bacterial clearance.
Control and LPS-tolerant mice were infected i.v. with 108 serovar Typhimurium cells/kg. Recovery of injected bacteria from the blood, liver, and spleen was determined 5, 20, 40, and 90 min after infection. To study the role of macrophages, liver and spleen macrophages were depleted by treatment with liposomes containing dichloromethylene biphosphonate (Cl2MBP) (Roche Diagnostics GmbH, Mannheim, Germany) (47) prior to the injection of bacteria: Cl2MBP liposomes were injected i.v. in 0.2 ml at 24, 48, and 71 h after the third injection of saline or LPS. At 72 h, serovar Typhimurium (108 bacteria/kg) was injected i.v., and recovery of bacteria was determined 10 min after infection in blood and various organs.
Leukocyte counts.
Cells obtained by peritoneal lavage with 10 ml of ice-cold phosphate-buffered saline under terminal pentobarbital anesthesia (Narcoren; Merial, Hallbergmoos, Germany) were counted in a Neubauer chamber. Differential cell counts were performed microscopically after May-Grünwald–Giemsa staining (Merck, Darmstadt, Germany) of cytospin preparations. Blood was obtained by cardiac puncture with heparinized syringes. White blood cell counts were determined microscopically in a Neubauer chamber after erythrocyte lysis with Türk's solution (Merck). Leukocyte differential counts were done on May-Grünwald–Giemsa-stained smears.
Determination of CFU.
CFU were determined from serial dilutions of organ homogenates, blood, or peritoneal lavage fluid plated on Columbia blood agar plates (Heipha, Heidelberg, Germany) and incubated at 37°C for 24 h before colonies were counted.
Immunohistological staining of macrophages.
Liver samples were fixed for 24 h in 10% neutral buffered formalin (Sigma, Deisenhofen, Germany), dehydrated, and embedded in paraplast (Sherwood Medical Co., St. Louis, Mo.). Slices (3 μm thick) were cut with a microtome (Microm International, Walldorf, Germany). After rehydration, the slices were incubated with 1% (wt/vol) trypsin (Sigma) for 20 min at 37°C to retrieve antigen followed by inactivation of endogenous peroxidase activity by treatment with 1% (vol/vol) H2O2 in methanol for 10 min at room temperature. Nonspecific binding was blocked by incubation with 0.2 mg of goat IgG (Biotrend)/ml in 5% (wt/vol) milk powder in Tris-buffered saline (TBS) for 1 h at 37°C. Then, the slices were incubated overnight at 4°C with the primary monoclonal rat anti-mouse F4/80 IgG2b antibody (Serotec, Oxford, United Kingdom) in a 1:50 dilution. The secondary polyclonal goat anti-rat IgG antibody coupled to horseradish peroxidase (Biotrend) was diluted 1:50 in TBS (final protein concentration, 40 μg/ml), and the slices were incubated at 37°C for 30 min. After being washed in TBS, the 3′-3′ diaminobenzidine peroxidase substrate (Sigma) was added, and the reaction was stopped after 20 to 30 min by washing. Nuclei were counterstained with Mayer's hemalaun solution (Merck) for 30 s. Control samples without primary or secondary antibody confirmed the specificity of the reaction. F4/80-positive nucleated cells were counted in 20 representative ×630 magnification fields per sample.
Cytokine determination.
Aliquots of organ homogenates, blood, and peritoneal lavage fluid were centrifuged at 14,000 × g for 7 min, and the supernatants were used for the determination of cytokines in a sandwich enzyme-linked immunosorbent assay. Flat-bottom high-binding polystyrene microtiter plates (Greiner, Nürtingen, Germany) were coated with a sheep anti-mouse TNF-α capture polyclonal antibody (protein solution, 20 mg/ml; in-house preparation). Recombinant murine TNF-α served as the standard (a gift of G. Adolf, Bender & Co, Vienna, Austria). The biotinylated anti-TNF-α tracer antibody was purchased from Pharmingen (Hamburg, Germany). For measurement of interleukin-6 (IL-6) and IFN-γ, matched antibody pairs and standards were purchased from Pharmingen. The quantity of tracer antibody bound was determined using streptavidin-peroxidase (Jackson ImmunoResearch, West Grove, Pa.) and TMB liquid substrate solution (Sigma). The detection limits were 10 (TNF-α and IFN-γ) and 25 (IL-6) pg.
Determination of MPO activity.
Samples from liver and spleen were excised, weighed, frozen in liquid nitrogen, and stored at −70°C. Tissues were homogenized with a polytron homogenizer (PT 1200; Kinematica, Lucerne, Switzerland) in 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% (wt/vol) hexadexylammonium bromide (Sigma). The homogenates were shock frozen in liquid nitrogen, thawed rapidly, and centrifuged at 14,000 × g for 7 min. Serial dilutions of the supernatants were added to TMB liquid substrate for determination of MPO activity. The reaction was stopped by the addition of H2SO4, and the absorption was determined at 450 nm. MPO from human leukocytes (Sigma) served as the standard.
Statistics.
Data in the tables are given as means ± standard deviation (SD), and data in the figures are given as means ± standard error of the mean (SEM). Analysis of pretreatment effects was done with the two-sided, unpaired Student's t test or the two-sided Welch test for two groups. In case of unequal variances, the data were first transformed by log (X + 1). For experiments with three groups, one-way analysis of variance (ANOVA) (P < 0.05) was performed, followed by the two-sided, unpaired Student's t test according to the method of Shaffer (39) or Dunnett's test for comparison with the control group. For more than three groups, Bonferroni's multiple-comparison test for selected groups was used. The survival curves were created by the method of Kaplan and Meier. For statistical comparison, survival curves were analyzed using the log rank test. All tests were done with Prism version 3.0 for Windows (GraphPad Software, San Diego, Calif.). Although some experiments using a group size (n) of three animals do not meet statistical requirements, the statistical analysis is provided in order to allow estimation of significance.
RESULTS
Effect of LPS pretreatment on cytokine production and sensitivity to endotoxic shock.
First, we established an LPS pretreatment regimen that induced profound tolerance to subsequent LPS injections. Mice were injected one to three times with 1 mg of LPS/kg of body weight at 24-h intervals. Groups of three mice were sacrificed 90 min after the single, double, or triple LPS injection regimen, and samples from the liver, spleen, and blood were taken for determination of cytokine levels. High levels of TNF-α, IFN-γ, and IL-6 were detected in plasma and homogenates of liver and spleen after a single LPS injection. Cytokine production was attenuated upon the second LPS treatment and strongly reduced or even completely suppressed after the third LPS injection (Table 1). Confirming the well-known state of LPS tolerance, LPS-pretreated mice were protected from an otherwise-lethal dose of LPS (10 mg/kg i.p.; 100% survival of LPS-tolerant mice versus 0% survival of control mice within 72 h; n = 7; peak TNF-α in plasma, 0.3 ± 0.2 ng/ml in LPS-tolerant mice versus 10.1 ± 2.2 ng/ml in controls; P < 0.001).
TABLE 1.
Effect of repeated LPS injections on cytokine production in vivoa
| Treatmentb | Amt
|
|||||
|---|---|---|---|---|---|---|
| TNF-α
|
IL-6
|
IFN-γ
|
||||
| Plasma | Liver | Plasma | Liver | Plasma | Spleen | |
| Saline | <20 | 610 ± 150 | <50 | 1,150 ± 90 | <20 | 2,120 ± 980 |
| 1 × LPS | 1,980 ± 550 | 4,150 ± 290 | 44,700 ± 17,400 | 6,500 ± 500 | 8,010 ± 2,670 | 18,540 ± 12,480 |
| 2 × LPS | <20c | 360 ± 130c | 17,000 ± 5,800 | 3,300 ± 550d | <20c | 2,200 ± 1,250c |
| 3 × LPS | <20c | 20 ± 30c | 2,800 ± 2,250c | 1,330 ± 390c | <20c | 1,500 ± 170c |
Mice were treated with serovar Abortus equi LPS (1 mg/kg i.p.) one to three times at 24-h intervals. Ninety minutes (TNF-α) or 6 h (IL-6 and IFN-γ) after the last LPS injection, cytokines were determined in the plasma and liver (TNF-α and IL-6) or spleen (IFN-γ). Saline-injected mice served as controls. Data are expressed as means ± SD (n = 3) and are given in picograms per milliliter for plasma and picograms per gram for the liver and spleen.
1 ×, 2 ×, and 3 × LPS indicate one-, two-, and three-injection regimens.
Significantly different from 1 × LPS (P < 0.01; Dunnett's test after one-way ANOVA).
Significantly different from 1 × LPS (P < 0.05).
Attenuation of cytokine production in response to serovar Typhimurium infection in LPS-tolerant mice.
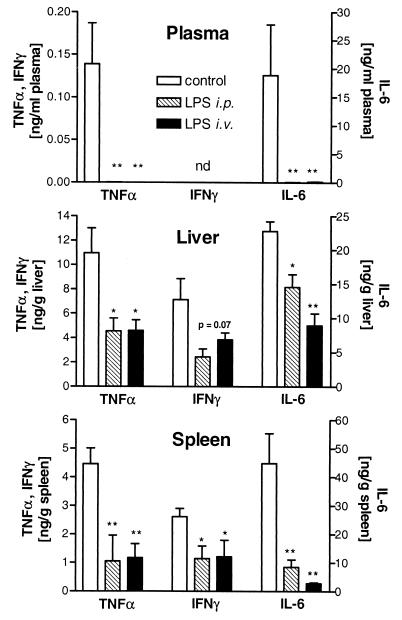
As immune cells isolated from LPS-tolerant mice displayed impaired cytokine release upon ex vivo stimulation with heat-killed serovar Typhimurium (data not shown), we were interested to see whether this would hold true for infection in vivo. Control mice inoculated i.p. with 107 serovar Typhimurium organisms/kg responded to infection with an early release of various cytokines, such as TNF-α, IL-6, and IFN-γ, with maximal concentrations 3 h postinfection. This increase in cytokine levels in plasma, liver, and spleen in the initial phase of infection was strongly attenuated in LPS-tolerant mice (Fig. 1). Since proinflammatory cytokines were shown to be essential for activation of host defenses against serovar Typhimurium (25), we were interested to see whether the impaired cytokine response of LPS-tolerant mice to live serovar Typhimurium affected their susceptibility to infection with these gram-negative bacteria.
FIG. 1.
LPS-tolerant mice show reduced peak levels of TNF-α, IFN-γ, and IL-6 in plasma, liver, and spleen after serovar Typhimurium infection. BALB/c mice were rendered tolerant by daily i.p. or i.v. injections of 1 mg of serovar Abortus equi LPS/kg for 3 days. Twenty-four hours after the last LPS injection, control (n = 9) and LPS-tolerant (n = 6) mice were infected i.p. with serovar Typhimurium (107 bacteria/kg) and killed 3 h after infection for determination of cytokines. Data are expressed as means ± SEM. Dunnett's test was performed after one-way ANOVA with P of <0.05 (∗) and <0.01 (∗∗) versus control. Plasma IFN-γ was below the detection limit (nd).
Prolonged survival of LPS-tolerant mice after lethal infection with serovar Typhimurium.
Mice were pretreated with saline or LPS as described above and infected with serovar Typhimurium (107 bacteria/kg i.p.) 24 h after the last LPS injection. LPS-tolerant mice survived significantly longer than nontolerant control mice (154 ± 13 h versus 76 ± 8 h; n = 12; P < 0.001). The onset of weight loss observed in control mice as early as 1 day after the injection of bacteria was delayed by 2 days in LPS-tolerant mice. In addition, LPS-tolerant mice showed no symptoms of disease, i.e., no piloerection and apathy, during the first 3 days.
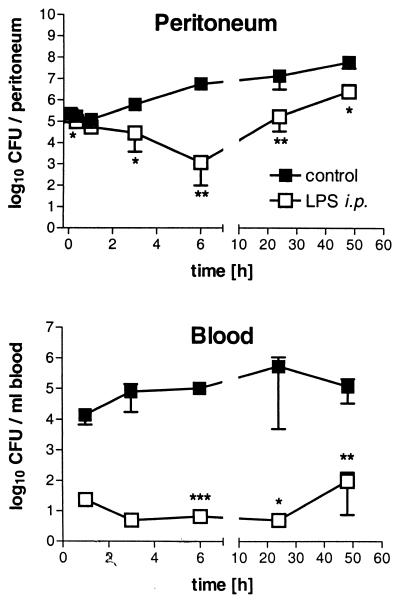
Reduction of bacterial load in LPS-tolerant mice.
We next examined whether the prolongation of survival resulted from improved bacterial killing or from tolerance to higher numbers of serovar Typhimurium. Therefore, we determined the time course of the bacterial load in different organs of control and LPS-tolerant mice infected i.p. with serovar Typhimurium (107 bacteria/kg). In control mice, after a negligible early reduction of CFU in the peritoneum, bacterial numbers increased and after 6 h strongly outnumbered the primary inoculum. In contrast, in LPS-tolerant mice we found a continuous decrease in bacterial numbers in the peritoneal cavities during the first 6 h of infection. At the end of this period, the peritonea of LPS-tolerant animals contained about 104 times fewer CFU than those of control mice. In addition, dissemination of bacteria into the blood, liver, and spleen, which became apparent 30 min after the induction of infection, was strongly reduced in LPS-tolerant mice (Fig. 2). The reduction of bacterial load was associated with up to five times the number of leukocytes in the peritoneal cavities of LPS-tolerant mice than in those of controls (Fig. 3). As the elevated numbers of peritoneal leukocytes had already been observed at the onset of infection, we studied the interrelationship between the LPS pretreatment and the leukocytes in more detail.
FIG. 2.
Time course of bacterial load in peritoneal lavage fluid and blood of control and LPS-tolerant mice during serovar Typhimurium infection (107 bacteria/kg i.p.). Data are shown as means ± SEM (n = 3). For statistical analysis, the unpaired two-sided Student's t test was performed for each time point with log-transformed data. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (all versus control).
FIG. 3.
Time course of peritoneal cell numbers in control and LPS-tolerant mice infected with serovar Typhimurium (107 bacteria/kg i.p.). Total peritoneal cell numbers are expressed as means ± SEM (n = 3). For statistical analysis, log-transformed data were tested by the unpaired two-sided Student's t test. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (all versus control).
i.p. LPS pretreatment induces local accumulation of neutrophils.
Analysis of the number and composition of peritoneal leukocytes prior and subsequent to the LPS injections revealed a steady accumulation of leukocytes during the pretreatment phase, which was mainly due to an influx of PMNs. At the time of induction of infection, LPS-pretreated mice had total numbers of peritoneal leukocytes that were about fivefold higher than control values. In contrast to control mice, where less than 2% of total peritoneal leukocytes were PMNs, the locally elicited cells in LPS-pretreated animals were 80% PMNs (Table 2). It is conceivable that the i.p. administration of LPS during tolerance induction, the local accumulation of PMNs in the peritoneal cavity, and the early inactivation of bacteria represent a causal sequence of events in this model.
TABLE 2.
Effect of repeated LPS injections on peritoneal leukocyte countsa
| Treatmentb | Total leukocytes (106) | No. of PMN (106) | No. of mononuclear cells (106) |
|---|---|---|---|
| Naive | 1.7 ± 0.2 | <0.02 | 1.7 ± 0.2 |
| 1 × LPS | 4.5 ± 1.0c | 3.7 ± 0.9d | 0.8 ± 0.1 |
| 2 × LPS | 7.9 ± 4.3d | 6.5 ± 3.7d | 1.5 ± 0.6 |
| 3 × LPS | 23.3 ± 2.4d | 17.4 ± 3.2d | 5.9 ± 1.0d |
Mice were treated with serovar Abortus equi LPS (1 mg/kg i.p.) one to three times at 24-h intervals. Twenty-four hours after the last LPS injection, total and differential counts of peritoneal cells were performed. Saline injection had no effect on peritoneal cell numbers compared to those of naive mice. Data are expressed as means ± SD (n = 3).
1 ×, 2 ×, and 3 × LPS indicate one-, two-, and three-injection regimens.
Significantly different from control (P < 0.05).
Significantly different from control (P < 0.01).
Effect of i.v. LPS administration.
In order to test the hypothesis that i.p. leukocyte accumulation represents the protective mechanism of tolerance induction, we changed the route of LPS administration: instead of i.p. administration, LPS was injected via the tail vein, thus circumventing local accumulation of leukocytes prior to i.p. serovar Typhimurium infection. In contrast to i.p. LPS pretreatment, the total number and composition of peritoneal leukocytes was not increased after the i.v. LPS injections. In parallel, the reduction of the bacterial load in the peritoneal cavity, blood, liver, and spleen was much less pronounced in the i.v. LPS-pretreated mice than in the i.p. LPS-pretreated animals. Six hours after the injection of bacteria, i.v. LPS-pretreated mice contained 102 to 103 times more CFU than i.p. LPS-pretreated animals. However, at 24 and 48 h after infection, comparable numbers of serovar Typhimurium cells were recovered from blood and peritoneal lavage fluid of i.v. and i.p. LPS-pretreated mice (Fig. 4). Taken together, accumulation of leukocytes due to i.p. administration of LPS seems to be a prerequisite for improved inactivation of serovar Typhimurium in the peritoneum during the early course of infection but does not explain the systemic reduction of bacteria in i.v. LPS-pretreated mice at late stages of infection.
FIG. 4.
Effect of different LPS administration routes for tolerance induction on time course of bacterial load. Control and LPS-tolerant mice were infected i.p. with serovar Typhimurium (107 bacteria/kg). The data are means ± SEM (n = 9 for controls and n = 6 for the LPS groups). For statistical analysis, an unpaired two-tailed Student's t test was done after one-way ANOVA of log-transformed data to compare the three groups at each time point individually. ∗, P < 0.05 for LPS i.p. versus control; ∗∗, P < 0.01 for LPS i.p. versus control; ∗∗∗, P < 0.001 for LPS i.p. versus control; †, P < 0.05 for LPS i.v. versus control; ††, P < 0.01 for LPS i.v. versus control; †††, P < 0.001 for LPS i.v. versus control; ‡, P < 0.05 for LPS i.v. versus LPS i.p.; ††, P < 0.01 for LPS i.v. versus LPS i.p.; ‡‡‡, P < 0.001 for LPS i.v. versus LPS i.p.
Increased emergency recruitment of leukocytes in LPS-tolerant mice.
We determined the numbers of circulating leukocytes in either tolerant or control mice during lethal Salmonella infection (107 bacteria/kg i.p.). We found sustained leukocytosis with an increased proportion of neutrophilic granulocytes in LPS-pretreated mice throughout the course of infection. The difference between the white blood cell counts of tolerant and control mice was most prominent at late phases of infection: 48 h postinfection, blood leukocytes were up to fourfold higher in LPS-tolerant mice (P < 0.01). The percentages of PMNs steadily increased in all groups during the course of infection but were consistently higher in LPS-pretreated animals (Table 3). Additionally, determination of MPO activity suggested significantly increased tissue PMN numbers in the livers (sixfold increase by 3 h; P < 0.01) and spleens (twofold increase by 24 h [P < 0.001] and threefold increase by 48 h [P < 0.05]) of LPS-pretreated mice during infection. Thus, it seemed probable that the enhanced capacity to recruit phagocytes to the major sites of bacterial proliferation contributed to a reduction of the bacterial load in LPS-tolerant mice. To assess the contribution of PMNs to the prolonged survival of LPS-tolerant mice, we depleted neutrophils by administration of anti-PMN antibodies. Injection of anti-Ly-6G rat IgG2b (clone RB6-8C5) (5) at −16, +6, and +30 h efficiently depleted circulating PMN numbers by >90% (P < 0.001 for saline and LPS pretreatment plus RB6-8C5 vs. the respective control IgG) and partially reversed the beneficial effect of LPS pretreatment on survival time (Table 4). Although the survival benefit to LPS-tolerant mice was decreased by approximately 60% (26-h survival prolongation versus 68 h) by the depletion of neutrophils, tolerant animals still survived significantly longer than PMN-depleted controls (P < 0.001). We concluded that increased PMN numbers only partially account for the prolonged survival of LPS-tolerant mice.
TABLE 3.
Effect of LPS pretreatment on blood leukocytes during serovar Typhimurium infectiona
| Time (h) | Pretreatment | Total leukocytes (106/ml) | % PMN | % Lymphocytes | % Monocytes |
|---|---|---|---|---|---|
| 1 | Control | 2.3 ± 1.6 | 18 ± 8 | 70 ± 11 | 9 ± 5 |
| LPS i.p. | 5.2 ± 1.9b | 76 ± 6b | 14 ± 6c | 10 ± 2 | |
| LPS i.v. | 2.9 ± 1.0 | 51 ± 8b | 35 ± 6c | 14 ± 4 | |
| 48 | Control | 1.2 ± 1.0 | 51 ± 14 | 34 ± 15 | 12 ± 8 |
| LPS i.p. | 4.9 ± 2.4c | 61 ± 11 | 27 ± 7 | 10 ± 7 | |
| LPS i.v. | 3.3 ± 1.3c | 68 ± 3 | 20 ± 4 | 12 ± 2 |
Mice were treated i.p. or i.v. with serovar Abortus equi LPS (1 mg/kg) three times at 24-h intervals. Twenty-four hours after the last LPS injection, LPS-tolerant mice and saline-pretreated controls were infected with serovar Typhimurium (107 bacteria/kg i.p.). Total and differential counts of blood leukocytes were performed 1 and 48 h after infection. Data are expressed as means ± SD (n = 9 for saline controls and n = 6 for the LPS groups).
Significantly different from saline control (P < 0.05).
Significantly different from control (P < 0.01).
TABLE 4.
Effect of PMN depletion on survival timea
| Treatment | Survival time (h)
|
|
|---|---|---|
| Mean ± SD | Median | |
| Saline + control IgG | 123 ± 22 | 115 |
| Saline + anti-Ly-6G IgG | 47 ± 3 | 47 |
| LPS + control IgG | 191 ± 60 | 168b |
| LPS + anti-Ly-6G IgG | 73 ± 3 | 74c |
Mice were pretreated i.v. with saline or serovar Abortus equi LPS (1 mg/kg) three times at 24-h intervals. Twenty-four hours after the last LPS injection, LPS-tolerant mice and saline-pretreated controls were infected with serovar Typhimurium (106 bacteria/kg i.p.), and survival time was determined. To study the role of PMNs, the mice received anti-Ly-6G IgG (RB6-8C5) or normal rat IgG i.p. 16 h prior to (0.6 mg) and 8 and 32 h after (0.3 mg) initiation of infection. The data are from one of two experiments (n = 6).
Significantly different from saline plus control IgG (P < 0.05).
Significantly different from saline plus anti-Ly-6G (P < 0.001).
Increased hepatic uptake of serovar Typhimurium in LPS-tolerant mice.
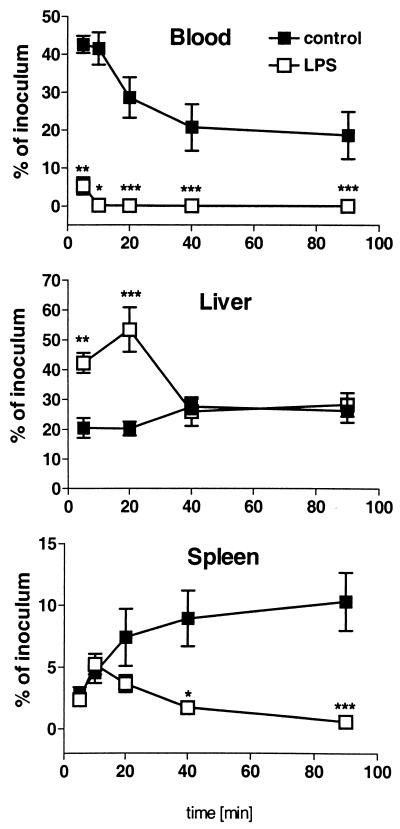
Besides PMNs, the macrophages of the reticuloendothelial system (RES) are involved in the elimination of bacteria. To test whether the activity of the RES was altered by LPS pretreatment, we determined the clearance of systemically injected serovar Typhimurium (108 cells/kg) in LPS-tolerant and control mice. Serovar Typhimurium was cleared much more rapidly in LPS-tolerant mice (0.1 ± 0.1% of inoculum recovered from the blood of LPS-tolerant mice 20 min after i.v. administration versus 28.6 ± 13.5% in controls; n = 6; P < 0.0001) (Fig. 5). Simultaneously, the livers of LPS-pretreated mice contained approximately two to three times more bacteria than the livers of control mice after the first 20 min (Fig. 5). At later time points, similar numbers of bacteria were found in the livers of both treatment groups. In contrast, although splenic uptake of bacteria was comparable during the first 10 min, the numbers of bacteria continuously increased in the spleens of controls but not those of LPS-pretreated mice (Fig. 5).
FIG. 5.
Effect of LPS tolerance on blood clearance of bacteria and phagocytic activity in liver and spleen. Control and LPS-tolerant mice were infected i.v. with serovar Typhimurium (108 bacteria/kg). The data are calculated as percent recovery of the inoculum and expressed as means ± SEM (n = 6). For statistical analysis, the unpaired Student's t test with log-transformed data was performed. ∗, P < 0.05 versus control; ∗∗, P < 0.01 versus control; ∗∗∗, P < 0.001 versus control).
We next assessed whether the increased early hepatic uptake of bacteria reflected numerical changes in Kupffer cells, i.e., liver macrophages, resulting from LPS pretreatment. Immunohistological examination demonstrated an approximately threefold increase of cells positive for the macrophage antigen F4/80 in the livers of LPS-tolerant mice compared to those of controls (15.1 ± 6.3/×630 field in LPS-tolerant mice versus 4.5 ± 0.7 in controls; n = 4; P < 0.05).
In order to examine the possible causal relationship of increased numbers of Kupffer cells and improved clearance of blood-borne serovar Typhimurium, we depleted macrophages by injection of Cl2MBP-containing liposomes prior to the injection of bacteria. In line with the efficient elimination of F4/80-positive liver macrophages, which was controlled by immunohistology, administration of liposomes strongly decreased the hepatic uptake of bacteria in control and tolerant mice, resulting in complete ablation of the improved clearance of serovar Typhimurium observed in nondepleted LPS-tolerant mice (Fig. 6). These results suggest that, besides leukocyte accumulation in the peritoneum and accelerated neutrophil recruitment, improved activity of the RES due to increased numbers of Kupffer cells contributes to the systemic reduction of serovar Typhimurium numbers in LPS-tolerant mice.
FIG. 6.
Clearance of serovar Typhimurium in control (co) and LPS-tolerant (LPS) mice after macrophage depletion with Cl2MBP liposomes. LPS tolerance was induced by daily i.p. administration of 1 mg of serovar Abortus equi LPS/kg for 3 days. Twenty-four, 48, and 71 h after the last LPS injection, liposomes (+) or pyrogen-free saline (−) was injected i.v. One hour after the last injection of liposomes, mice were infected i.v. with serovar Typhimurium (108 bacteria/kg). Ten minutes after injection of serovar Typhimurium, viable bacteria were determined in blood and liver homogenates and calculated as percent recovery of the inoculum. Pooled data from three experiments are expressed as means + SEM, with 7 to 14 mice per group. For statistical analysis, the Bonferroni test for selected groups was done after one-way ANOVA. ∗, P < 0.05; ∗∗∗, P < 0.001 versus saline control (co −); †††, P < 0.001 versus LPS control (LPS −).
DISCUSSION
Endotoxin tolerance is known to protect prophylactically against mortality and morbidity in endotoxic shock, LPS- and TNF-α-mediated liver damage, and various models of fulminant infection with high numbers of bacteria. In these models, the crucial role of the proinflammatory cytokines TNF-α, IL-1, and IFN-γ as distal mediators of LPS toxicity leading to shock and death is well documented (4, 31, 42). It was therefore logical to ascribe protection due to tolerance induction to an attenuated response of effector cells, diminished sensitivity of target cells, and a general limitation of tissue damage by infiltrating leukocytes (1, 38). On the other hand, the pivotal role of an intact cytokine response, in particular the release of TNF-α, IL-1, IFN-γ, and IL-6, for host defense against bacterial infections has been unequivocally shown in different infection models (8, 9, 33, 41, 46). These studies clearly demonstrate that in contrast to the models of hyperinflammatory damage, a successful immune defense against infectious diseases, which normally start with low numbers of virulent bacteria, requires a vigorous inflammatory response.
These experimental differences prompted us to carry out an LPS tolerance and infection study where we created a more drastic situation of hyporesponsiveness to endotoxin by giving repeated injections of a nearly lethal LPS dose (0.3 times the 50% lethal dose). For the infection, we chose a lethal dose of virulent serovar Typhimurium, a gram-negative bacterium that causes systemic reactions in mice and symptoms resembling human typhoid fever (21). In contrast to the commonly used single low-dose injection of LPS 24 h prior to high-dose LPS challenge, our LPS tolerance induction regimen not only blunted the release of TNF-α but also inhibited or reduced the production of other cytokines, i.e., IFN-γ and IL-6, in response to subsequent LPS challenge (Table 1) or i.p. serovar Typhimurium infection (Fig. 1). In contrast to LPS challenge, cytokine release was not abrogated completely after infection with viable serovar Typhimurium, suggesting that immune stimuli other than LPS, e.g., peptidoglycan, porins, or flagellins, are also transmitted by these gram-negative bacteria. Experimental induction of LPS hyporesponsiveness did not cause increased susceptibility of mice to serovar Typhimurium infection, as observed in innately LPS-unresponsive (lpsd) mice (30), but instead improved survival. Since this was associated with a decrease in the bacterial load in the peritoneal lavage fluid, blood, liver, and spleen, the prolongation of survival is unlikely to stem from the known dampening of the proinflammatory immune response in LPS tolerance. This view is supported by our observation (unpublished) that immunosuppression by dexamethasone, which protects against LPS shock by blocking the proinflammatory response, failed to increase the survival time of serovar Typhimurium-infected mice. Moreover, although LPS-tolerant mice survived significantly longer than control animals, the bacterial loads of various organs at the time of death did not differ substantially among the different treatment groups. This indicates that prolonged survival was not the result of an improvement in the immune system's capacity to deal with high numbers of bacteria.
This led us to the assumption that improved early inactivation of serovar Typhimurium might be responsible for the increase in mean survival time, raising the question of possible mechanisms contributing to enhanced host defense. A comparison of the time course of bacterial proliferation in control and LPS-tolerant mice showed that enhanced inactivation of bacteria in tolerant mice was observed as early as 1 h after inoculation. Consequently, 6 h postinfection LPS-tolerant mice carried approximately 4 orders of magnitude fewer CFU in the peritoneal cavity than control animals. Dissemination of bacteria to the blood and subsequently to the liver and spleen was also diminished. Therefore, we related the accumulation of professional phagocytes in the peritoneal cavity, the later site of injection of bacteria, to the enhanced inactivation of serovar Typhimurium observed in LPS-tolerant mice immediately after infection. The experiments with i.v. instead of i.p. LPS injections support this interpretation (Fig. 4). Others have also pointed out the importance of localized therapy in the prevention of lethal sepsis by tolerance induction. In their experimental setting, i.p. injection of monophosphoryl lipid A was much more efficient in decreasing mortality after otherwise-lethal cecal ligation and puncture than i.v. administration (1). Surprisingly, mice made tolerant by i.v. LPS injection also showed an extended survival time compared to control mice. Moreover, similar decreases in bacterial load in the blood and peritoneal lavage fluid 48 h after i.p. Salmonella infection were found in i.p. and i.v. LPS-pretreated animals. This suggests an additional mechanism for fighting the bacteria at later stages of infection.
LPS is a potent stimulator of hematopoiesis, and administration of LPS or derivatives is associated with the production of various colony-stimulating factors (28, 34), increased total numbers of circulating leukocytes (18), neutrophila (23), and augmented numbers of monocyte/macrophage precursors in the bone marrow (26). Early reports ascribed increased resistance against infection and lethal irradiation after pretreatment with endotoxin to the leukopoietic properties of endotoxin (43). During infection with serovar Typhimurium, PMNs are able to limit bacterial growth within host cells by lysis of infected hepatocytes and subsequent phagocytosis of extracellular bacteria, e.g., in the sinusoids of the liver (6). Since we actually found higher numbers of circulating neutrophils in the blood as well as increased tissue infiltration of PMNs indicated by enhanced MPO activity in LPS-tolerant mice during the course of infection, it is conceivable that this mechanism contributes to bacteriostasis in the liver and spleen, which are the major sites of replication of serovar Typhimurium. Indeed, PMN depletion reduced the increase in survival time associated with LPS tolerance (i.v.) by approximately 60%.
The enhanced clearance of bacteria from the blood of LPS-tolerant mice, on the other hand, is due to a more efficient phagocytic activity of the RES, as shown by our i.v. inoculation experiments. This interpretation is in line with previous findings that showed enhanced phagocytosis of bacteria or latex particles by Kupffer cells of LPS-tolerant animals in vivo or in the perfused liver ex vivo (16, 37). By immunohistological examination, we demonstrated approximately threefold-augmented numbers of F4/80-positive cells in the livers of LPS-tolerant mice. The antigen recognized by the F4/80 clone is expressed by several macrophage populations, including Kupffer cells in the liver (2). This suggests that the enhancement of RES activity associated with LPS tolerance induction originates at least partly from an increase in the numbers of liver macrophages. Independent evidence for this conclusion derives from our macrophage depletion experiments using Cl2MBP liposomes that selectively accumulate in macrophages, which are subsequently driven into apoptosis (47).
Our results showing that it is primarily cells of the innate immune system that are involved in increased resistance of LPS-pretreated mice against serovar Typhimurium infection are corroborated by the finding that athymic BALB/c mice, which lack functional T cells, and their wild-type littermates benefit equally from LPS tolerance induction prior to bacterial infection (our unpublished data and reference 32).
Besides antibody-mediated phagocytosis, opsonization of bacteria by complement components facilitates receptor-mediated uptake of bacteria by phagocytes. Published data are conflicting as to the activity of the complement system in endotoxin tolerance (24, 36). Since it is feasible that an increase in complement activity in the course of an acute-phase response elicited by endotoxin administration could account for improved phagocytosis in our model, we determined complement activity (50% hemolytic complement values) of sera from LPS-tolerant and control mice. In a modified rabbit erythrocyte lysis assay (45), no difference in total (classical plus alternative) complement activity was detectable after LPS pretreatment. Moreover, depletion of complement component C3 by administration of cobra venom factor (44), which efficiently abrogated complement-mediated erythrocyte lysis, did not ablate improved reduction of bacteria in serovar Typhimurium-infected i.v. LPS-tolerant mice (unpublished results). Therefore, we consider this possibility unlikely.
In conclusion, this study provides evidence that induction of profound LPS tolerance, despite reducing cytokine production, improves host defense against infection with virulent serovar Typhimurium. Several independent mechanisms contribute to enhanced resistance of LPS-pretreated mice by decreasing the bacterial load at different stages of infection, as shown by assessing the immunomodulation and blocking the respective alterations. Namely, local accumulation of leukocytes in the peritoneal cavity, improved recruitment of PMNs during the course of infection, and an increase in liver macrophage numbers account for the improved host defense. Although the data shown here derive from experiments with a gram-negative facultative intracellular bacterium, the protective effect of LPS tolerance induction also applies for other models using extracellular or gram-positive bacteria as infectious agents. We could show that our pretreatment to induce LPS tolerance increased the survival rates of mice lethally infected with Staphylococcus aureus, Listeria monocytogenes, or a human stool suspension to induce a multigerm peritonitis, which more closely mimics the physiological situation of the septic patient. Similar findings were reported recently for infection of LPS-tolerant mice with Cryptococcus neoformans (35).
The combination of two desirable effects, i.e., attenuation of systemic inflammatory responses and a concomitant fortification of host defense against infections, makes LPS tolerance a valuable model for sepsis prophylaxis.
ACKNOWLEDGMENTS
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (HA 2567/3-1). M. D. Lehner received a stipend from the Landesgraduiertenförderung Baden-Wüttemberg.
We are indebted to Hans van Dijk and Piet Aerts (Eijkman-Winkler Institute, University of Utrecht, The Netherlands) for providing purified cobra venom factor, to G. Adolf for the recombinant murine TNF-α, and to Robert Coffman for the gift of the RB6-8C5 hybridoma. We also thank Burkhard Helpap (Pathology, Hospital Singen, Singen, Germany) for advice about histological analysis. The excellent technical assistance of Margarete Kreuer-Ullmann, Ulla Gebert, Ina Seuffert, Elisabeth Schmidt, and Leonardo Cobianchi is greatly appreciated.
REFERENCES
- 1.Astiz M E, Saha D C, Carpati C M, Rackow E C. Induction of endotoxin tolerance with monophosphoryl lipid A in peritonitis: importance of localized therapy. J Lab Clin Med. 1994;123:89–93. [PubMed] [Google Scholar]
- 2.Austyn J M, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 4.Car B D, Eng V M, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Aguet M, Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan W J. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conlan W J. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect Immun. 1996;64:1043–1047. doi: 10.1128/iai.64.3.1043-1047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross A, Asher L, Seguin M, Yuan L, Kelly N, Hammack C, Sadoff J, Gemski P., Jr The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J Clin Investig. 1995;96:676–686. doi: 10.1172/JCI118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai W J, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 9.Dalrymple S A, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eden C S, Shahin R, Briles D. Host resistance to mucosal gram-negative infection. Susceptibility of lipopolysaccharide nonresponder mice. J Immunol. 1988;140:3180–3185. [PubMed] [Google Scholar]
- 11.Erroi A, Fantuzzi G, Mengozzi M, Sironi M, Orencole S F, Clark B D, Dinarello C A, Isetta A, Gnocchi P E. Differential regulation of cytokine production in lipopolysaccharide tolerance in mice. Infect Immun. 1993;61:4356–4359. doi: 10.1128/iai.61.10.4356-4359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahmi H, Chaby R. Selective refractoriness of macrophages to endotoxin-induced production of tumor necrosis factor, elicited by an autocrine mechanism. J Leukoc Biol. 1993;53:45–52. doi: 10.1002/jlb.53.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Friedman R L, Moon R J. Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun. 1977;16:1005–1012. doi: 10.1128/iai.16.3.1005-1012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galanos C, Freudenberg M A. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993;187:346–356. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson G L, Rhodes M J, Hegel T. Monophosphoryl lipid A as a prophylactic for sepsis and septic shock. Prog Clin Biol Res. 1995;392:567–579. [PubMed] [Google Scholar]
- 16.Hafenrichter D G, Roland C R, Mangino M J, Flye M W. The Kupffer cell in endotoxin tolerance: mechanisms of protection against lethal endotoxemia. Shock. 1994;2:251–256. [PubMed] [Google Scholar]
- 17.Hagberg L, Hull R, Hull S, McGhee J R, Michalek S M, Svanborg Eden C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984;46:839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He W, Fong Y, Marano M A, Gershenwald J E, Yurt R W, Moldawer L L, Lowry S F. Tolerance to endotoxin prevents mortality in infected thermal injury: association with attenuated cytokine response. J Infect Dis. 1992;165:859–864. doi: 10.1093/infdis/165.5.859. [DOI] [PubMed] [Google Scholar]
- 19.Henricson B E, Benjamin W R, Vogel S N. Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect Immun. 1990;58:2429–2437. doi: 10.1128/iai.58.8.2429-2437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewett J A, Schultze A E, VanCise S, Roth R A. Neutrophil depletion protects against liver injury from bacterial endotoxin. Lab Investig. 1992;66:347–361. [PubMed] [Google Scholar]
- 21.Hsu H S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989;53:390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iimuro Y, Yamamoto M, Kohno H, Itakura J, Fujii H, Matsumoto Y. Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats—analysis of mechanisms of lethality in endotoxemia. J Leukoc Biol. 1994;55:723–728. doi: 10.1002/jlb.55.6.723. [DOI] [PubMed] [Google Scholar]
- 23.Kiani A, Tschiersch A, Gaboriau E, Otto F, Seiz A, Knopf H P, Stutz P, Farber L, Haus U, Galanos C, Mertelsmann R, Engelhardt R. Downregulation of the proinflammatory cytokine response to endotoxin by pretreatment with the nontoxic lipid A analog SDZ MRL 953 in cancer patients. Blood. 1997;90:1673–1683. [PubMed] [Google Scholar]
- 24.Landy M, Pillemer L. Increased resistance to infection and accompanying alteration in properdin levels following administration of bacterial lipopolysaccharides. J Exp Med. 1956;104:383–409. doi: 10.1084/jem.104.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langermans J A M, van Furth R. Cytokines and the host defense against Listeria monocytogenes and Salmonella typhimurium. Biotherapy. 1994;7:169–178. doi: 10.1007/978-94-011-0233-9_4. [DOI] [PubMed] [Google Scholar]
- 26.Madonna G S, Vogel S N. Early endotoxin tolerance is associated with alterations in bone marrow-derived macrophage precursor pools. J Immunol. 1985;135:3763–3771. [PubMed] [Google Scholar]
- 27.Mastroeni P, Clare S, Khan S, Harrison J A, Hormaeche C E, Okamura H, Kurimoto M, Dougan G. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CSF) levels. Immunology. 1971;21:427–436. [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano M, Onozuka K, Yamasu H, Zhong W F, Nakano Y. Protective effects of cytokines in murine Salmonellosis. In: Friedman H E A, editor. Microbial infections. New York, N.Y: Plenum Press; 1992. pp. 89–95. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien A D, Rosenstreich D L, Scher I, Campbell G H, MacDermott R P, Formal S B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980;124:20–24. [PubMed] [Google Scholar]
- 31.Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson R C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348:550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- 32.Parant M, Galelli A, Parant F, Chedid L. Role of B-lymphocytes in nonspecific resistance to Klebsiella pneumoniae infection of endotoxin-treated mice. J Infect Dis. 1976;134:531–539. doi: 10.1093/infdis/134.6.531. [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 34.Quesenberry P, Halperin J, Ryan M, Stohlman F. Tolerance to the granulocyte-releasing and colony-stimulating factor elevating effects of endotoxin. Blood. 1975;45:789–800. [PubMed] [Google Scholar]
- 35.Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon J M. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect Immun. 2000;68:3748–3753. doi: 10.1128/iai.68.6.3748-3753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowley D. Stimulation of natural immunity to Escherichia coli infections. Lancet. 1955;i:232. doi: 10.1016/s0140-6736(55)90163-x. [DOI] [PubMed] [Google Scholar]
- 37.Ruggiero G, Andreana A, Utili R, Galante D. Enhanced phagocytosis and bactericidal activity of hepatic reticuloendothelial system during endotoxin tolerance. Infect Immun. 1980;27:798–803. doi: 10.1128/iai.27.3.798-803.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salkowski C A, Detore G, Franks A, Falk M C, Vogel S N. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaffer J P. Modified sequentially rejective multiple test procedures. J Am Stat Assoc. 1986;81:826. [Google Scholar]
- 40.Shahin R D, Engberg I, Hagberg L, Svanborg Eden C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- 41.Tite J P, Dougan G, Chatfield S N. The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol. 1991;147:3161–3164. [PubMed] [Google Scholar]
- 42.Tracy K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 43.Urbaschek R, Urbaschek B. Induction of nonspecific resistance and stimulation of granulopoiesis by endotoxins and nontoxic bacterial cell wall components and their passive transfer. Ann NY Acad Sci. 1985;459:97–110. doi: 10.1111/j.1749-6632.1985.tb20819.x. [DOI] [PubMed] [Google Scholar]
- 44.Van den Berg C W, Aerts P C, Van Dijk H. In vivo anti-complementary activities of the cobra venom factors from Naja naja and Naja haje. J Immunol Methods. 1991;136:287–294. doi: 10.1016/0022-1759(91)90015-8. [DOI] [PubMed] [Google Scholar]
- 45.van Dijk H, Rademaker P M, Willers J M. Estimation of classical pathway of mouse complement activity by use of sensitized rabbit erythrocytes. J Immunol Methods. 1980;39:257–268. doi: 10.1016/0022-1759(80)90060-5. [DOI] [PubMed] [Google Scholar]
- 46.van Furth R, van Zwet T L, Buisman A M, van Dissel J T. Anti-tumor necrosis factor antibodies inhibit the influx of granulocytes and monocytes into an inflammatory exudate and enhance the growth of Listeria monocytogenes in various organs. J Infect Dis. 1994;170:234–237. doi: 10.1093/infdis/170.1.234. [DOI] [PubMed] [Google Scholar]
- 47.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 48.Xu H R, Hsu H S. Dissemination and proliferation of Salmonella typhimurium in genetically resistant and susceptible mice. J Med Microbiol. 1992;36:377–381. doi: 10.1099/00222615-36-6-377. [DOI] [PubMed] [Google Scholar]
- 49.Yao Z, Foster P A, Gross G J. Monophosphoryl lipid A protects against endotoxic shock via inhibiting neutrophil infiltration and preventing disseminated intravascular coagulation. Circ Shock. 1994;43:107–114. [PubMed] [Google Scholar]