Abstract
Aim
The clinical efficacy of chemoradiotherapy (CRT) is largely dependent on host immune status. The aim of this study was to identify possible markers expressed on circulating mononuclear cells to predict tumour response in patients with locally advanced rectal cancer (LARC).
Methods
Peripheral blood samples were obtained from 47 patients diagnosed with LARC before and after CRT. The numbers of lymphocytes and monocyte subsets were analysed using flow cytometry. Based on clinical and pathological findings, patients were classified as high or low responders.
Results
Lymphocyte counts were markedly decreased after CRT. Total numbers of lymphocytes (p = 0.030) and CD4(+) T cells (p = 0.041) in post‐CRT samples were significantly lower in low responders than in high responders. In contrast, monocyte counts were not reduced and the number of CD14dim(+) CD16(+) nonclassical (patrolling) monocytes were somewhat increased after CRT (p = 0.050). Moreover, the ratios of programmed cell death ligand 1 (PD‐L1) (+) cells on patrolling monocytes before and after CRT were significantly higher in low responders than in high responders (p = 0.0046, p = 0.0006). The same trend was observed for classical and intermediate monocytes. The expression of PD‐L1 on patrolling monocytes before CRT correlated inversely with the number of T cells and natural killer (NK) cells after CRT. PD‐L1(+) ratio in patrolling monocytes was an independent predictor for response to CRT.
Conclusion
Programmed cell death ligand 1 (PD‐L1) expression on patrolling monocytes suppresses cell‐mediated immunity in patients receiving CRT which could be related to tumour response, and may be a useful biomarker for decision‐making in the management of patients with LARC.
Keywords: locally advanced rectal cancer, patrolling monocytes, preoperative chemoradiotherapy, programmed cell death ligand 1 (PD‐L1)
What does this paper add to the literature?
In 47 patients with locally advanced rectal cancer, expression levels of programmed cell death ligand 1 (PD‐L1) on monocytes in peripheral blood before chemoradiotherapy (CRT) are inversely correlated with circulating lymphocyte counts after CRT and histological tumour response. The ratio of PD‐L1(+) cells in CD16(+) patrolling monocytes is an independent predictor for response to CRT.
INTRODUCTION
Preoperative chemoradiotherapy (CRT) is currently considered to be standard initial treatment for patients with locally advanced rectal cancer (LARC), since it can result in downstaging in approximately half of patients with locally advanced RC, resulting in a lower rate of postoperative local recurrence and a higher rate of sphincter‐preserving surgery [1, 2, 3, 4, 5]. However, the response to CRT varies greatly among patients, and CRT may have disadvantages such as delaying surgery or immune suppression in patients who do not have a response. Numerous studies have shown that many radiological findings [6, 7, 8] as well as molecular markers such as gene mutation patterns [8, 9] and protein expression patterns [9, 10] or microRNA expression [9, 11, 12] are related to the therapeutic response. However, the clinical usefulness of these biomarkers remains controversial, and none are currently in routine clinical use.
Approaches to find suitable biomarkers are based on the idea that the sensitivity to CRT is simply associated with a direct effect to induce DNA damage or apoptosis of tumour cells. However, a growing body of evidence suggests that the response of rectal tumours depends not only on direct radiocytotoxity to tumour cells, but also on the tumour microenvironment and host immune status [13, 14]. Many studies have reported that a high density of T cells at the tumour site is strongly associated with a better response to CRT [15, 16, 17, 18]. Other retrospective studies have shown that the total number of lymphocytes and their ratios to neutrophils (neutrophil lymphocyte ratio) or monocytes (lymphocyte monocyte ratio) are significantly correlated with the sensitivity to CRT and outcomes of patients with LARC [19, 20, 21, 22]. Those results strongly suggest that immunologic‐mediated cell death is certainly involved in the process of tumour regression of rectal cancer induced by CRT.
In this study, we prospectively examined the phenotypes of circulating lymphocytes and monocytes of patients with LARC before and after CRT and correlated these values with the observed histological response. We found that the expression levels of programmed cell death ligand 1 (PD‐L1) on monocyte subsets are closely related with circulating lymphocytes after CRT and the tumour response.
METHODS
Monoclonal antibodies and reagents
PE‐conjugated anti‐CD4 (RPA‐T4), anti‐CD16 (B73.1), APC‐conjugated anti‐CD8a (HIT8a), anti‐CD274 (PD‐L1) (29E.2A3), BV421‐conjugated anti‐CD3 (OKT3), anti‐CD14 (M5E2) and FITC‐conjugated anti‐CD279 (PD‐1) (EH12.2H7) were purchased from BioLegend Inc. FITC‐conjugated anti‐CD3 (HIT3a), BV421‐conjugated anti‐PD‐1 (EH12.1), BUV395‐conjugated anti‐CD56 (NCAM16.2) and FVS‐780 were purchased from BD Biosciences. FcR blocking reagent was purchased from Miltenyi Biotec B.V.& Co. KG.
Patients
A total of 50 consecutive patients diagnosed with primary rectal cancer with clinical stage T2‐4 M0, were treated with a total dose of 50.4 Gy of radiation therapy and concomitant oral capecitabine (1650 mg/m2/day) at the Department of Surgery, Jichi Medical University between May 2017 and June 2020. Of these, 47 completed the CRT regimen without notable toxicities, and were prospectively enrolled in the study. The study protocol was approved by the Ethics Committee of Jichi Medical University (RIN‐A20‐098), and written informed consent was obtained from all patients. Most patients underwent standard total mesorectal excision, after an interval of 8–10 weeks following CRT. All resected specimens were examined pathologically, and the findings were recorded in accordance with the TNM classification. The histological regression of the primary rectal lesion in response to CRT was evaluated and classified as high or low, based on the amount of residual cancer according to the Japanese Classification of Colorectal Carcinoma, that is, lesions in which no tumour remained (grade 3) and more than two‐thirds of the cancer had degraded, necrotized or disappeared (grade 2) were classified as high responders, while those with less than two‐thirds reduction (grades 1a and 1b) were classified as low responders.
Blood sampling and analysis of leucocyte phenotypes
Peripheral venous blood samples were obtained before neoadjuvant CRT and 8 weeks after completion of CRT, prior to surgery. Peripheral blood mononuclear cells were collected before and after CRT using mononuclear cell preparation tube‐sodium heparin (Becton Dickinson) and cell counts in the samples analysed using an automated haematology analyser (XE‐5000, Sysmex). Lymphocyte and monocyte subsets were also analysed using flow cytometry. Briefly, whole blood was treated with FACS lysing solution (Becton Dickinson) to lyse red blood cells and fixed with 1% formaldehyde. The cells (1 × 106) were suspended in PBS containing 0.02% EDTA and incubated for 15 min to label dead cells with FVS‐780. After washing with PBS, the cells were incubated with 5 μl FcR blocking reagent for 10 min and immunostained with relevant mAbs for 30 min according to the manufacturer's recommendations. After washing with PBS, the cells suspension was applied to BD LSRFortessa X‐20 (Becton‐Dickinson) and antigen expression analysed using Flow Jo software (Becton‐Dickinson). In phenotype analyses, more than 10,000 events in regions gated for lymphocytes or monocytes were performed.
Statistical analysis
Cell numbers were analysed using a Student's t‐test. Correlation was examined with Pearson's simple linear regression analysis. All analyses were performed with Graph Pad Prism 8 Software, and p‐values <0.05 were statistically significant.
RESULTS
Patient characteristics
Forty‐six of 47 enrolled patients underwent resection after receiving CRT. Among them, 20 and five patients had a pathological response of grades 2 and 3, respectively, categorized as high responders, while 21 patients with grades 1a or 1b were classified as low responders. One patient who had a macroscopic complete response after CRT with no detectable tumour despite multiple biopsies refused surgical resection and survived more than 2 years without recurrence, and is included among the high responders. The characteristics of the 47 patients in the high and low responder groups are summarized in Table 1. Among high responders, there were significantly more males than females. High responders tended to be more in patients with tumours confined to the rectal wall (cT < 4) on computed tomography (CT) imaging (p = 0.058). No other clinical factor before CRT was different between the two groups. Pathological characteristics of resected tumours were less advanced among high responders due to antitumour effects of CRT.
TABLE 1.
Clinical and pathological features of the patients with rectal cancer who underwent preoperative chemoradiotherapy (CRT)
| Variables | Low response (n = 21) | High response (n = 26) | p‐value | |
|---|---|---|---|---|
| Clinical variables before CRT | ||||
| Age | 61 (38–73) | 63 (48–76) | 0.720 | |
| Gender | (M/F) | 13/8 | 23/3 | 0.043 |
| Macroscopic appearance | (1/2/3) | 0/20/1 | 2/22/2 | 0.369 |
| Location | (Ra,Rab/Rb,RbP) | 1/20 | 1/25 | 1.000 |
| Tumour length | (cm) | 7.1 (3.1–10.2) | 5.8 (2.8–14.6) | 0.454 |
| Circumferential rate | (%) | 70% (33%–100%) | 67% (20%–100%) | 0.578 |
| Depth of invasion | (cT: ≤3/4) | 14/7 | 24/2 | 0.058 |
| Nodal metastasis | (cN: 0/1≤) | 7/14 | 6/20 | 0.520 |
| Clinical stage | (cStage: 2/3) | 7/14 | 6/20 | 0.520 |
| Histology | (tub, pap/por) | 20/1 | 25/1 | 1.000 |
| Serum CEA | (ng/ml) | 4.5 (0.8–42.4) | 3.7 (0.8–23.0) | 0.336 |
| Serum CA19‐9 | (U/ml) | 14.0 (1.0–205) | 11.5 (2.0–1650) | 0.822 |
| Surgery a | (LAR/APR/ISR/TPE) | 10/10/0/1 | 11/13/1/0 | 0.931 |
| Pathological variables after CRT a | ||||
| Depth of invasion | (pt: ≤1/2≤) | 0/21 | 9/16 | 0.002 |
| Nodal metastasis | (pn: 0/1≤) | 10/11 | 18/7 | 0.137 |
| Lymphatic invasion | (Negative/positive) | 10/11 | 23/2 | 0.001 |
| Venous invasion | (Negative/positive) | 3/18 | 19/6 | <0.001 |
| Stage | (pStage: ≤1/2≤) | 1/20 | 15/10 | <0.001 |
Note: All the patients completed neoadjuvant CRT with 50.5 Gy irradiation and oral capecitabine 1650 mg/m2/days. p‐values were evaluated with Fisher's exact test or Mann–Whitney's test.
Abbreviations: APR, abdominoperineal resection; ISR, intersphincteric resection; LAR, low anterior resection; TPE, total pelvic exenteration.
Surgery was performed in 46 patients and their pathological data are presented.
Leucocyte subpopulations in circulating blood before and after chemoradiotherapy
The numbers of white blood cells and their subpopulations in peripheral blood were investigated from the medical records and flowcytometric analysis. As shown in Figure 1, the numbers of total leucocytes as well as neutrophils and lymphocytes were significantly reduced after CRT. The reduction rates were more prominent for lymphocytes than neutrophils (neutrophils; 4072 ± 197 to 3207 ± 197, p = 0.0027, lymphocytes; 1536 ± 69.2 to 791 ± 70.0, p < 0.0001). In contrast, the number of circulating monocytes was not significantly decreased after CRT (Figure 1C). As a result, the neutrophil‐lymphocyte and lymphocyte‐monocyte ratios were increased and decreased after CRT, respectively (Figure S1). Among the lymphocyte subpopulations, the reduction rate was most remarkable in CD4(+) T cells (511 ± 30.2 to 199 ± 30.9, p < 0.0001), while the number of CD3(−) CD16(+) NK cells was not changed by CRT (Figures 1E–H). As shown in Figure 2A, circulating monocytes were phenotypically divided into three subtypes, CD14high(+) CD16(−) classical, CD14med(+) CD16(+) intermediate and CD14dim(+)CD16(+) nonclassical (patrolling) monocytes. Although the numbers of classical and intermediate monocytes were not changed, the numbers of patrolling monocytes were increased with marginal significance (61 ± 5.4 to74 ± 5.4, p = 0.050).
FIGURE 1.
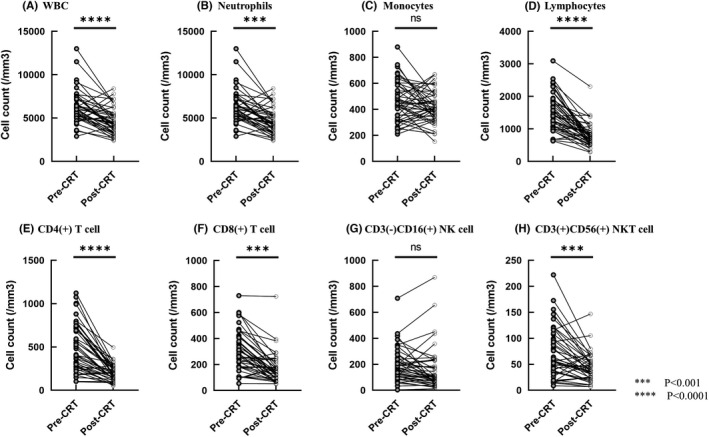
The number of peripheral white blood cells (A), neutrophils (B), monocytes (C), lymphocytes (D) and lymphocyte subsets (E–H) obtained before and after chemoradiotherapy (CRT). ***p < 0.001, ****p < 0.0001 by student t‐test
FIGURE 2.
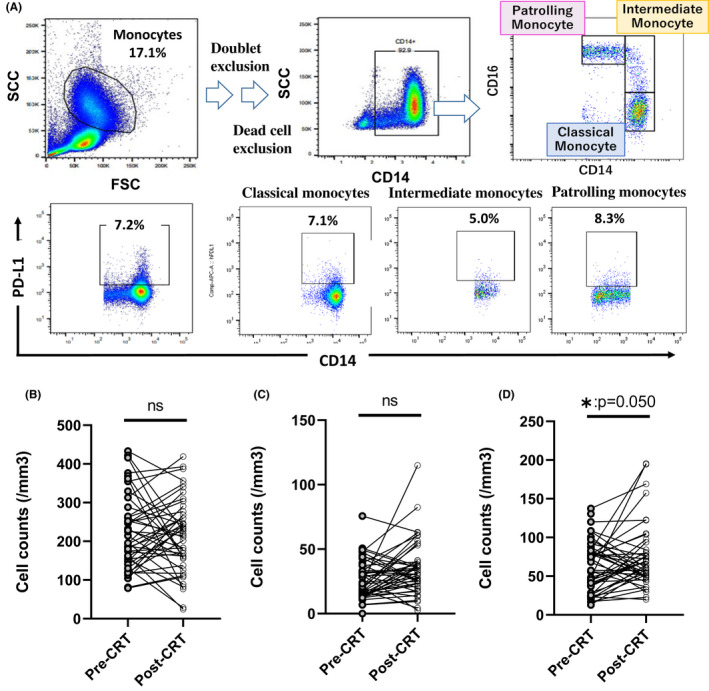
Circulating monocytes were divided into three subsets by these gating strategy (A). The number of classical (B), intermediate (C) and patrolling (D) monocytes in peripheral blood obtained before and after chemoradiotherapy (CRT). *p < 0.05 by Student's t‐test
Leucocyte subsets and tumour response against chemoradiotherapy
The numbers of leucocyte subsets were compared with tumour response to CRT. As shown in Table 2, neutrophils, monocytes, lymphocytes and their subpopulations in blood sampled before CRT did not show any significant differences between the high and low responder groups. After CRT, however, total lymphocyte counts and the lymphocyte‐monocyte ratio in 27 patients classified as high responders were significantly higher than in low responders (685 ± 74.9 vs. 881 ± 69.2, p = 0.030; 1.7 ± 0.19 vs. 2.2 ± 0.18, p = 0.048). Among the lymphocyte subsets, the number of CD4(+) T cell was significantly higher in high responders (222 ± 18.4 vs. 173 ± 69.2, p = 0.041). The same trend was observed regarding CD8(+) T cells and CD3(−) CD16(+) NK cells, although the differences did not reach statistical significance. In contrast, the numbers of each monocyte subset before and after CRT were not different between the two groups.
TABLE 2.
The number of leucocyte subsets in circulating blood before and after preoperative chemoradiotherapy (CRT) in 47 patients with locally advanced rectal cancer
| Cell counts of lymphocyte or monocyte subsets (/mm3) | Before CRT | After CRT | ||||
|---|---|---|---|---|---|---|
| Low response (n = 21) | High response (n = 26) | p‐value | Low response (n = 21) | High response (n = 26) | p‐value | |
| Neutrophils | 4128 ± 325 | 4026 ± 292 | 0.827 | 3250 ± 268 | 3171 ± 246 | 0.829 |
| Lymphocytes | 1497 ± 6125 | 1568 ± 113 | 0.678 | 685 ± 74.9 | 881 ± 68.6 | 0.030 |
| CD3(+)CD4(+) T cell | 519 ± 60.8 | 505 ± 54.7 | 0.868 | 173 ± 19.8 | 222 ± 18.5 | 0.041 |
| CD3(+)CD8(+) T cell | 313 ± 34.5 | 323 ± 31.1 | 0.833 | 146 ± 25.5 | 186 ± 23.9 | 0.260 |
| CD3(+)CD56(+) NKT cell | 73 ± 10.7 | 72 ± 9.7 | 0.94 | 46 ± 5.9 | 41 ± 5.5 | 0.467 |
| CD3(−)CD16(+) NK cell | 210 ± 25.3 | 176 ± 28.1 | 0.362 | 118 ± 36.2 | 194 ± 33.8 | 0.131 |
| Monocytes | 467 ± 35.5 | 470 ± 31.9 | 0.948 | 408 ± 26.7 | 423 ± 26.8 | 0.684 |
| Classical monocyte | 224 ± 21.7 | 212 ± 19.6 | 0.692 | 214 ± 20.9 | 215 ± 19.6 | 0.940 |
| Intermediate monocyte | 28 ± 3.3 | 26 ± 3.3 | 0.628 | 33 ± 4.6 | 36 ± 4.3 | 0.648 |
| Patrolling monocyte | 62 ± 7.3 | 60 ± 6.5 | 0.851 | 76 ± 9.0 | 72 ± 8.4 | 0.706 |
| NLR | 3.21 ± 0.31 | 2.80 ± 0.28 | 0.327 | 5.21 ± 0.50 | 4.10 ± 0.46 | 0.108 |
| LMR | 3.40 ± 0.26 | 3.50 ± 0.23 | 0.761 | 1.77 ± 0.19 | 2.11 ± 0.18 | 0.048 |
Note: Data show mean ± SEM, p‐values calculated with Student's t‐test. Classical, intermediate and patrolling monocytes were determined as CD14high(+) CD16(−), CD14mid(+) CD16(+) and CD14low(+) CD16(+) phenotype, respectively.
Abbreviations: LMR, lymphocyte monocyte ratio; NLR, neutrophil lymphocyte ratio.
Programmed cell death ligand 1 expression on monocyte subsets and tumour response after chemoradiotherapy
We next examined the expression of PD‐L1 on each of the monocyte subsets (Figure 2A). The expression levels of PD‐L1 were not different among monocyte subpopulations which were not significantly altered by CRT. However, the PD‐L1 expression on monocytes did correlate with tumour response to CRT (Figure 3). The ratios of PD‐L1(+) cells among classical monocytes were significantly higher in the low response group than among high responders both before and after CRT (before CRT: 5.2% ± 0.48% vs. 3.7% ± 0.45%, p = 0.030, after CRT: 5.3% ± 0.56% vs. 3.7% ± 0.52%, p = 0.050; Figure 3A). The differences in PD‐L1(+) cell ratios were more pronounced in patrolling monocytes (before CRT: 6.7% ± 0.66% and 4.1% ± 0.61% p = 0.0046, after CRT: 5.8% ± 0.53% and 3.1% ± 0.49%, p = 0.0006) (Figure 3C). The same trend was shown for intermediate monocytes (Figure 3B).
FIGURE 3.
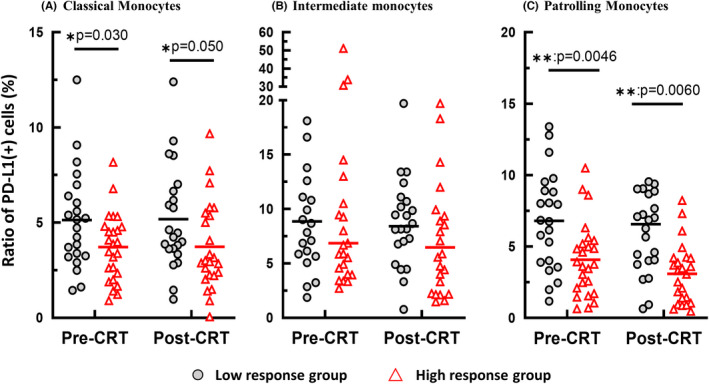
Ratio of programmed cell death ligand 1 (PD‐L1) (+) cells classical (A), intermediate (B) and patrolling (C) monocytes in peripheral blood of low and high responding patients before and after chemoradiotherapy (CRT). *p < 0.05, **p < 0.001 by student t‐test
Programmed cell death ligand 1 expression on patrolling monocytes before chemoradiotherapy correlates with circulating lymphocyte counts after chemoradiotherapy
As shown in Figure 4A, the ratio of PD‐L1(+) cells in patrolling monocytes before CRT showed a significant inverse correlation with lymphocyte counts in peripheral blood obtained after CRT (r = −0.29, p = 0.0075). The same correlation was observed with CD4(+) or CD8(+) T cells as well as CD3(−) CD16(+) NK cells with similar correlation coefficients (Figures 4B–D). PD‐L1 expression on classical monocytes had a similar, but weaker, correlation with post‐CRT lymphocytes counts (Figures 4E–H).
FIGURE 4.
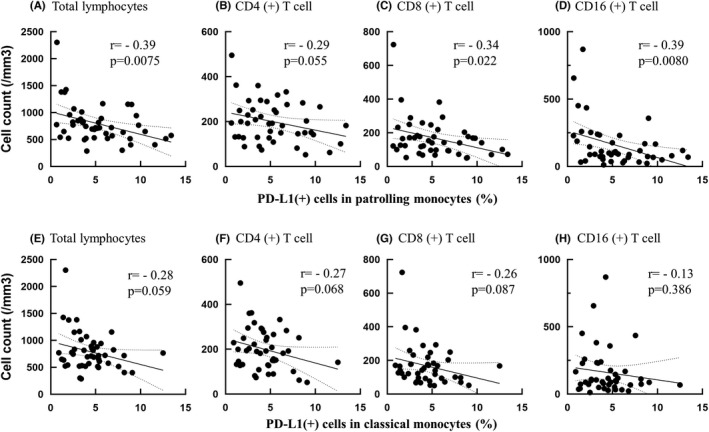
Correlation between programmed cell death ligand 1 (PD‐L1) (+) ratio in patrolling (AD) and classical (EH) monocytes in prechemoradiotherapy (CRT) blood specimens and the number of total lymphocytes and their subsets in post‐CRT specimen. Correlation efficiencies and p‐values were calculated with Pearson's simple linear regression analysis
Predictive impact of programmed cell death ligand 1 on patrolling monocytes on tumour response
Then, we divided the patients with the expression levels of PD‐L1 on pre‐CRT patrolling monocytes. With ROC analysis (Figure 5), the optimal cutoff value was determined as 5.2% corresponding to maximum sensitivity and specificity (76.0% and 66.7%, respectively) for predicting response against CRT. As shown in Table 3, PD‐L1(+) ratio in patrolling monocytes was selected as possible predictor for response to CRT together with gender, longitudinal tumour size, cT4, serum CEA level, neutrophil lymphocyte ratio. Multivariate analysis showed PD‐L1 (+) ratio in patrolling monocytes less than 5.2% was an independent predictor for good response to CRT.
FIGURE 5.
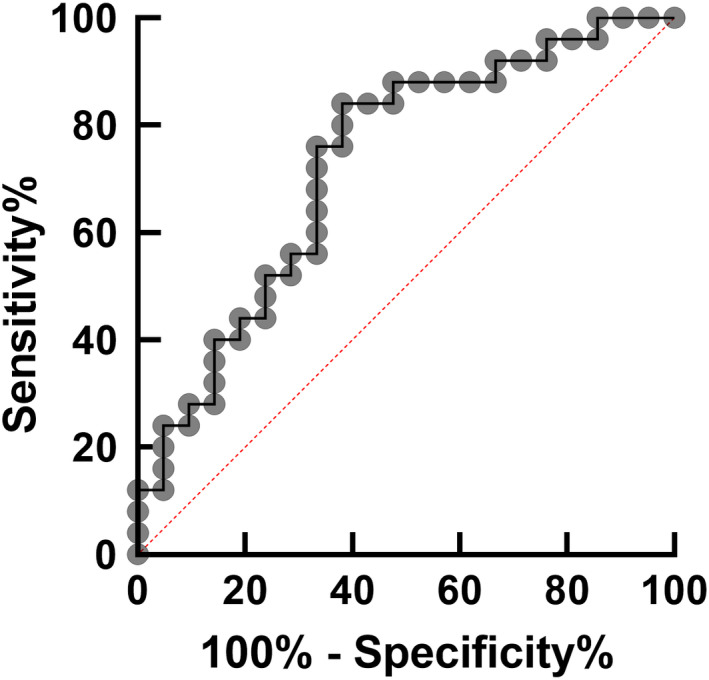
ROC curve for determining the cutoff point of programmed cell death ligand 1 (PD‐L1) (+) ratio in prechemoradiotherapy (CRT) patrolling monocytes for high response against CRT
TABLE 3.
Univariate and multivariate analysis of clinical factors to predict high response to preoperative chemoradiotherapy (CRT) for patients with locally advanced rectal cancer
| Co variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐values | OR | 95% CI | p‐values | |
| Female | 0.221 | 0.048–0.941 | 0.043 | 0.122 | 0.017–0.864 | 0.023 |
| Longitudinal tumour size >5.6 cm | 0.313 | 0.094–1.040 | 0.080 | 0.367 | 0.070–1.924 | 0.227 |
| Rab/Rb, RbP | 0.275 | 0.028–2.671 | 0.362 | |||
| cT = 4 | 0.167 | 0.230–0.916 | 0.058 | 0.264 | 0.0429–32.375 | 0.213 |
| Nodal metastasis (+) | 1.667 | 0.460–6.034 | 0.520 | |||
| Circumferential tumour extent: 80%< | 0.387 | 0.104–1.404 | 0.197 | |||
| Poorly differentiated histology | 1.250 | 0.074–21.256 | 1.000 | |||
| CEA:10 ng/ml< | 0.253 | 0.064–1.004 | 0.052 | |||
| NLR: 4.0< | 0.208 | 0.037–1.169 | 0.115 | 0.179 | 0.016–1.971 | 0.153 |
| LMR: <2.7 | 0.386 | 0.104–1.440 | 0.197 | 0.328 | 0.041–2.597 | 0.286 |
| PD‐L1% in patrolling monocyte: 5.2%< | 0.158 | 0.043–0.474 | 0.007 | 0.116 | 0.021–0.637 | 0.006 |
Note: Univariate and multivariate logistic regression analyses were performed to evaluate the impact of factors before CRT.
Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; LMR, lymphocyte monocyte ratio; NLR, neutrophil lymphocyte ratio; OR: odds ratio.
DISCUSSION
It was believed that both radiotherapy and chemotherapy inhibit the immune response in patients. However, previous studies have suggested that radiation therapy can induce the generation of tumour‐specific effector T cells which can promote tumour regression [23, 24] and that tumour shrinkage is largely dependent on the systemic immune response especially on T cell‐mediated cellular immunity [13, 14]. However, the number of circulating lymphocytes is generally decreased by radiation therapy and/or chemotherapy which may critically impair the anti‐tumour effects of CRT. Consistently, many retrospective studies have demonstrated that circulating lymphocyte counts correlate with tumour response in patients with LARC who received neoadjuvant CRT [19, 20, 21, 22].
In this prospective study, we confirmed that peripheral blood lymphocyte counts obtained after CRT are markedly reduced and a decreased lymphocyte count, especially CD4(+) T cells, was associated with a poor histological response. This is consistent with the results of previous studies [19, 25, 26], suggesting that CD4(+) T cells in circulating blood may play a central role to evoke an immune response against tumour‐specific antigens induced by CRT.
In contrast, the number of circulating monocytes and their subsets were not decreased after CRT. Monocytes are phenotypically divided into classical, intermediate and nonclassical (patrolling) monocytes with different functions [27]. CD14high(+) CD16(−) classical monocytes are the primary cells recruited in inflammatory tissue, differentiate into macrophages and play critical roles to regulate the local immune response. CD14dim(+) CD16(+) nonclassical monocytes migrate in vascular beds to search for harmful micro particles or damaged endothelial cells and promote their removal to maintain vascular homeostasis and are referred to as patrolling monocytes [28]. Although patrolling monocytes are generally considered to be the first line of defence for the recognition and clearance of pathogens, their roles in tumour biology remain unclear. Recent preclinical studies have suggested that patrolling monocytes can prevent tumour metastases through the activation of NK cells [29, 30, 31]. In contrast, Jung et al. showed that Ly6Clo patrolling monocytes can exert immunosuppressive roles to reduce the effects of anti‐VEGFR2 therapy [32]. These results suggest that patrolling monocytes may play divergent roles in different phases of tumour progression.
In this study, it was shown that the number of patrolling monocytes, but not other types of monocytes, increased after CRT. However, the number of these monocytes did not significantly correlate with tumour response. We then examined the expression of PD‐L1 on these monocyte subsets, since the PD‐1/PD‐L1 axis is believed to play a critical role in suppressing the immune system and mediating evasion of host immune surveillance in malignant tumours [33]. Interestingly, expression of PD‐L1 on circulating monocytes had a strong inverse correlation with tumour response to CRT. The ratio of PD‐L1 (+) cells among classical and patrolling monocytes were significantly higher in patients with low response group than counterparts. The difference was more prominent in patrolling monocytes, and the same trend was observed regardless of the timing of blood sampling.
Programmed cell death ligand 1 is known to be expressed not only on tumour cells but also on stromal and haematopoietic cells and recent preclinical studies have suggested that expression of PD‐L1 on host myeloid cells has a stronger impact on antitumour immunity than PD‐L1 present on tumour cells [34, 35, 36]. Previous studies have shown that the level of PD‐L1 expression on circulating monocytes is strongly related to the progression of infectious diseases [37, 38, 39]. More recently, de Coana et al. [40] have shown that high expression of PD‐L1 on monocyte subpopulations correlates with shorter survival in patients with melanoma who received PD‐1 blockade therapy, suggesting that expression of PD‐L1 on monocytes may affect tumour response to cytotoxic therapies.
The roles of PD‐L1 expression on monocytes in tumour immunity are largely unknown. This is the first report to show a correlation between PD‐L1 expression on circulating monocytes and tumour response to CRT. In this study, the ratio of PD‐L1(+) cells among monocytes, especially patrolling monocytes, showed an inverse correlation with the number of circulating T cells and NK cells after CRT. Moreover, PD‐L1 on patrolling monocytes was an independent predictor for response to CRT with multivariate analysis. These facts suggest the possibility that PD‐L1 expression on monocytes has a suppressive effect on lymphoid haematogenesis during CRT, which may result in impaired cell‐mediated immunity to reduce the tumour response. Recently, multiple clinical trials have suggested synergistic effects between radiation therapy and immunotherapy using immune checkpoint inhibitors [41, 42, 43]. However, such benefits have not been confirmed in other clinical studies [44, 45] and the mechanisms of the synergism remain unclear. The results of this study suggest that PD‐L1 expression on monocytes may affect the response to combination therapy and may be useful as a biomarker to determine the optimal neoadjuvant treatment for patients with LARC.
CONFLICT OF INTEREST
All authors declare that there are no conflict of interests.
Supporting information
Figure. S1
ACKNOWLEDGEMENTS
This study was supported by Japan Society for the Promotion of Science (17 K10649, 19 K09225) in animal experiments and adenosine quantification. This study was also supported by Keirin Race Fund from JKA foundation in flowcytometric analysis using LSRFortessa. We greatly thank H. Hayakawa for her excellent technical assistance of flowcytometric analysis. We also thank J. Shinohara, H. Hatakeyama, N. Nishiaki and I. Nieda for technical and clerical works.
Tojo M, Horie H, Koinuma K, Miyato H, Tsukui H, Kaneko Y, Programmed cell death ligand 1 expression on monocytes is inversely correlated with tumour response to preoperative chemoradiotherapy for locally advanced rectal cancer. Colorectal Dis. 2022;24:1140–1149. 10.1111/codi.16167
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Pahlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26:3687–94. [DOI] [PubMed] [Google Scholar]
- 2. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic‐Rundic S, Bensadoun RJ, et al. Fluorouracil‐based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long‐term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–90. [DOI] [PubMed] [Google Scholar]
- 3. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short‐course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 4. Cellini F, Valentini V. Current perspectives on preoperative integrated treatments for locally advanced rectal cancer: a review of agreement and controversies. Oncology (Williston Park). 2012;26:730–5. 41. [PubMed] [Google Scholar]
- 5. Zheng J, Feng X, Hu W, Wang J, Li Y. Systematic review and meta‐analysis of preoperative chemoradiotherapy with or without oxaliplatin in locally advanced rectal cancer. Medicine (Baltimore). 2017;96:e6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chee CG, Kim YH, Lee KH, Lee YJ, Park JH, Lee HS, et al. CT texture analysis in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: a potential imaging biomarker for treatment response and prognosis. PLoS One. 2017;12:e0182883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pyo DH, Choi JY, Lee WY, Yun SH, Kim HC, Huh JW, et al. A nomogram for predicting pathological complete response to neoadjuvant chemoradiotherapy using semiquantitative parameters derived from sequential PET/CT in locally advanced rectal cancer. Front Oncol. 2021;11:742728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryan JE, Warrier SK, Lynch AC, Ramsay RG, Phillips WA, Heriot AG. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis. 2016;18:234–46. [DOI] [PubMed] [Google Scholar]
- 9. Dayde D, Tanaka I, Jain R, Tai MC, Taguchi A. Predictive and prognostic molecular biomarkers for response to neoadjuvant chemoradiation in rectal cancer. Int J Mol Sci. 2017;18:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673–88. [DOI] [PubMed] [Google Scholar]
- 11. Conde‐Muino R, Cuadros M, Zambudio N, Segura‐Jimenez I, Cano C, Palma P. Predictive biomarkers to chemoradiation in locally advanced rectal cancer. Biomed Res Int. 2015;2015:921435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pettit C, Walston S, Wald P, Webb A, Williams TM. Molecular profiling of locally‐advanced rectal adenocarcinoma using microRNA expression (review). Int J Oncol. 2017;51:393–404. [DOI] [PubMed] [Google Scholar]
- 13. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Twyman‐Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature. 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–9. [DOI] [PubMed] [Google Scholar]
- 17. Akiyoshi T, Tanaka N, Kiyotani K, Gotoh O, Yamamoto N, Oba K, et al. Immunogenomic profiles associated with response to neoadjuvant chemoradiotherapy in patients with rectal cancer. Br J Surg. 2019;106:1381–92. [DOI] [PubMed] [Google Scholar]
- 18. Xu Y, Lou X, Liang Y, Zhang S, Yang S, Chen Q, et al. Predicting neoadjuvant chemoradiotherapy response in locally advanced rectal cancer using tumor‐infiltrating lymphocytes density. J Inflamm Res. 2021;14:5891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer. 2011;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng YX, Lin JZ, Peng JH, Zhao YJ, Sui QQ, Wu XJ, et al. Lymphocyte‐to‐monocyte ratio before chemoradiotherapy represents a prognostic predictor for locally advanced rectal cancer. Onco Targets Ther. 2017;10:5575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vallard A, Garcia MA, Diao P, Espenel S, de Laroche G, Guy JB, et al. Outcomes prediction in pre‐operative radiotherapy locally advanced rectal cancer: leucocyte assessment as immune biomarker. Oncotarget. 2018;9:22368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh SY, Heo J, Noh OK, Chun M, Cho O, Oh YT. Absolute lymphocyte count in preoperative chemoradiotherapy for rectal cancer: changes over time and prognostic significance. Technol Cancer Res Treat. 2018;17:1533033818780065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen‐specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. [DOI] [PubMed] [Google Scholar]
- 24. Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor‐specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–66. [DOI] [PubMed] [Google Scholar]
- 25. Wichmann MW, Meyer G, Adam M, Hochtlen‐Vollmar W, Angele MK, Schalhorn A, et al. Detrimental immunologic effects of preoperative chemoradiotherapy in advanced rectal cancer. Dis Colon Rectum. 2003;46:875–87. [DOI] [PubMed] [Google Scholar]
- 26. Tada N, Kawai K, Tsuno NH, Ishihara S, Yamaguchi H, Sunami E, et al. Prediction of the preoperative chemoradiotherapy response for rectal cancer by peripheral blood lymphocyte subsets. World J Surg Oncol. 2015;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 28. Auffray C, Fogg D, Garfa M, Elain G, Join‐Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–70. [DOI] [PubMed] [Google Scholar]
- 29. Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubo H, Mensurado S, Goncalves‐Sousa N, Serre K, Silva‐Santos B. Primary tumors limit metastasis formation through induction of IL15‐mediated cross‐talk between patrolling monocytes and NK cells. Cancer Immunol Res. 2017;5:812–20. [DOI] [PubMed] [Google Scholar]
- 31. Narasimhan PB, Eggert T, Zhu YP, Marcovecchio P, Meyer MA, Wu R, et al. Patrolling monocytes control NK cell expression of activating and stimulatory receptors to curtail lung metastases. J Immunol. 2020;204:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, Rahbari NN, et al. Ly6Clo monocytes drive immunosuppression and confer resistance to anti‐VEGFR2 cancer therapy. J Clin Invest. 2017;127:3039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartley G, Regan D, Guth A, Dow S. Regulation of PD‐L1 expression on murine tumor‐associated monocytes and macrophages by locally produced TNF‐alpha. Cancer Immunol Immunother. 2017;66:523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, et al. PD‐L1 on host cells is essential for PD‐L1 blockade‐mediated tumor regression. J Clin Invest. 2018;128:580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, et al. PD‐L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020;11:4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shao R, Fang Y, Yu H, Zhao L, Jiang Z, Li CS. Monocyte programmed death ligand‐1 expression after 3‐4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit Care. 2016;20:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang ZY, Xu P, Li JH, Zeng CH, Song HF, Chen H, et al. Clinical significance of dynamics of programmed death Ligand‐1 expression on circulating CD14(+) monocytes and CD19(+) B cells with the progression of hepatitis B virus infection. Viral Immunol. 2017;30:224–31. [DOI] [PubMed] [Google Scholar]
- 39. Pan T, Zhou T, Li L, Liu Z, Chen Y, Mao E, et al. Monocyte programmed death ligand‐1 expression is an early marker for predicting infectious complications in acute pancreatitis. Crit Care. 2017;21:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pico de Coana Y, Wolodarski M, van der Haar AI, Nakajima T, Rentouli S, Lundqvist A, et al. PD‐1 checkpoint blockade in advanced melanoma patients: NK cells, monocytic subsets and host PD‐L1 expression as predictive biomarker candidates. Onco Targets Ther. 2020;9:1786888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 42. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non‐small‐cell lung cancer: a secondary analysis of the KEYNOTE‐001 phase 1 trial. Lancet Oncol. 2017;18:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Procureur A, Simonaggio A, Bibault JE, Oudard S, Vano YA. Enhance the immune checkpoint inhibitors efficacy with radiotherapy induced immunogenic cell death: A comprehensive review and latest developments. Cancers (Basel). 2021;13:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐043): a multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure. S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


