Abstract
Fungicides are used to control pathogenic fungi of crop species, but they have also been shown to alter behavioral, life history and fitness related traits of nontarget insects. Here, we tested the fungicide effects on feeding behavior, survival and physiology of the nontarget pest insect, the Colorado potato beetle (CPB) (Leptinotarsa decemlineata). Feeding behavior was studied by a choice test of adult beetles, which were allowed to choose between a control and a fungicide (fluazinam) treated potato leaf. Larval survival was recorded after 24 and 72 h exposure to control and fungicide‐treated leaves with 2 different concentrations. The adults did not show fungicide avoidance behavior. Similarly, survival of the larvae was not affected by the exposure to fungicides. Finally, to understand the effects of fungicides at the physiological level (gene expression), we tested whether the larval exposure to fungicide alter the expression of 5 metabolic pathway and stress associated genes. Highest concentration and 72‐h exposure caused upregulation of 1 cytochrome P450 (CYP9Z14v2) and 1 insecticide resistance gene (Ldace1), whereas metabolic detoxification gene (Ugt1) was downregulated. At 24‐h exposure, highest concentration caused downregulation of another common detoxification gene (Gs), while both exposure times to lowest concentration caused upregulation of the Hsp70 stress tolerance gene. Despite these overall effects, there was a considerable amount of variation among different families in the gene expression levels. Even though the behavioral effects of the fungicide treatments were minor, the expression level differences of the studied genes indicate changes on the metabolic detoxifications and stress‐related pathways.
Keywords: behavior, fluazinam, gene expression, Leptinotarsa decemlineata, metabolic detoxification, nontarget animal
Introduction
Fungal pathogens of crops are considered one of the most economically significant phyto‐pathogens that can be a severe threat to food security (Pennisi, 2010; National Academy of Sciences, 2011). Agricultural fungicides control fungal diseases of crops and as the severity of fungal diseases has increased over the past few decades (Fisher et al., 2012) so has fungicide usage. In Europe, fungicides are one of the most widely used plant protection chemicals (41.76% in 2015: Eurostat, 2020) and it is likely that fungicide residuals are also present in our environment (air or water) and even in food (Woodrow et al., 1995; Cabras et al., 1997; Caldas et al., 2001; Kreuger et al., 2010; EFSA, 2016) as fungicide exposed animals and plant materials can be found in many food chains (Walorczyk et al., 2013; Mu et al., 2016). Presence of fungicides in food may in turn have negative impacts on the health of consumers. However, the impact of fungicide on animals may vary depending on the active ingredient and its pattern of exposure (Piel et al., 2019). In general, fungicides are designed to target essential cellular or physiological processes in fungi (FRAC, 2021), but they may also target common cellular organelles in other organisms, which are similar in fungi and in eukaryotes (Maltby et al., 2009). Several studies have shown the potential negative impacts of fungicides on many organisms, such as fish, pollinators, arthropods and so forth (Wightwick et al., 2010; Elskus, 2014; Shi et al., 2018). Indeed, excessive use of agricultural fungicides can induce costs to species living in the agro‐environment.
Toxicity information of many fungicide classes is mostly available for fish, rats and mice, although these organisms are unlikely to be regularly exposed to fungicides (Oruc, 2010; Rouabhi, 2010). Nontarget organisms, such as plants, insects, micro‐organisms, aquatic animals and birds that live near to the fungicide application sites, are common nontarget species (Zaller & Brühl, 2019). Moreover, the effects of many commonly used fungicides are still unknown for many likely nontarget organisms such as pollinators or pest insects in the agricultural settings (see Campbell et al., 2016; Clements et al., 2018a). Fungicides can be applied once or multiple times in the field depending on the severity of the fungal diseases (Reilly et al., 2012) and hence species living in the crop fields can be exposed to fungicides for both short and extended periods of time. Effects of the fungicide can also vary depending on the duration of the exposure of the nontarget species to the fungicide (Damalas & Eleftherohorious, 2011). Eco‐toxicological data for fungicides are mostly from short‐term studies focused on the acute toxic effect on mortality, but chronic exposure data and sublethal effects of fungicides on other fitness related traits are also important to understand (Elskus, 2014; Sancho et al., 2016). Fungicide concentrations and the mode of exposure found in agricultural fields may have both acute toxic effects after 1, or chronic effects (on fitness related traits) after multiple exposures (Wightwick et al., 2010; Reilly et al., 2012).
Several studies have reported the effects of fungicides on multiple fitness and life history related traits in nontarget organisms, including fish, amphipods, moths, beneficial and pest insects and so forth (Biggs & Hagley, 1988; Michaud, 2001; Adamski et al., 2011; Vu et al., 2016, 2017). For instance, in Agrotis segetum (turnip moth), fungicide (mancozeb) negatively affected the egg count and development of the larvae, but the larval survival remained unaffected (Adamski et al., 2011). Another fungicide (boscalid) reduced both the survival and reproduction (the number of offspring) in a marine arthropod (Allorchestes compressa) (Vu et al., 2016). Moreover, 2 other fungicides, (propiconazol and chlorothalonil) decreased larval survival and egg hatching success in the Japanese beetle (Popillia japonica) (Obear et al., 2016) and these 2 chemicals also decreased the activity of detoxification related enzymes in the larvae. In the Colorado potato beetle (CPB) (Leptinotarsa decemlineata), chlorothalonil and boscalid altered the activity of glutathione S‐transferase enzyme and multiple genes involved in metabolic detoxification pathways (Clements et al., 2018a). A study with zebra fish (Danio rerio) showed that a commonly used fungicide (fluazinam), can also alter the expression of stress‐related genes (Wang et al., 2018). Therefore, the effects of fungicides on any nontarget species should be studied at multiple levels ranging from behavior to gene expression levels.
Fungicides target fungal cell membrane components or essential cellular functions, like nucleic acid or protein synthesis, cell division, signal transduction or respiration (Yang et al., 2011; FRAC, 2021). Fungicides that have target sites in the mitochondrial respiratory system of the fungi are one of the most popular classes due to their broad‐spectrum anti‐fungal activity and low toxicity to nontarget mammals (EFSA, 2016; FRAC, 2021). Studies with respiratory inhibitor fungicides have shown they do not cause acute toxicity to mammals, birds and bees but are highly toxic to fish, marine and freshwater invertebrates (Tomlin, 2000; Elskus, 2014). Fluazinam, an oxidative phosphorylation (OXPHOS, an essential stage in the mitochondrial adenosine triphosphate [ATP] synthesis reaction) inhibitor fungicide, is commonly used in various crop fields to control a broad range of fungal diseases. These include molds (e.g., Botrytis cinerea), stem and root rot (Sclerotinia sclerotiorum), and potato blights (e.g., Phytophthora infestans and Alternaria solani) (Kalamarakis et al., 2000; Runno‐Paurson et al., 2015; Schepers et al., 2018). In the fungal cell, the mode of action of fluazinam involves the inhibition of ATP synthase enzyme, that is, it blocks ATP production (Vitoratos, 2014). If fluazinam can also affect mitochondria and/or the respiration process in other organisms, this could explain the negative effects of such OXPHOS inhibitor fungicides in nontarget organisms, as complete or partial inhibition of ATP can compromise growth, development, activity and immune function (Campbell et al., 2016; Wang et al., 2018). Earlier studies have indicated that unlike in fungi (where it targets ATP synthase), fluazinam does not have specific target sites in nontarget species, but it impairs to some degree energy production (Guo et al., 1991; Lee et al., 2012; Wang et al., 2018). In addition, fluazinam toxicity may vary between different species due to the difference in metabolism and excretion process of the fungicide in different organisms (Guo et al., 1991). Some animals may also be more susceptible to fungicide than others, due to their physiology or behavior (Oruc, 2010). However, very little information exists on the effects of fungicides on invertebrates that frequently come into contact with these chemicals in agricultural fields.
During the growing season, potato fields can be heavily infested with early and late blight (A. solani and P. infestans) which are controlled by fungicides, including fluazinam (FRAC MoA C5) (Reilly et al., 2012; Schepers et al., 2018) and nontarget species like arthropods living in the agricultural field can also be affected. As the most likely nontarget species of crop fields, pest insects are particularly interesting, because they can be repeatedly affected by fungicides. Even though the effects of few classes of fungicides, such as boscalid, chlorothalonil and mancozeb have been studied in some pest insects, information about the effects of many common fungicides on nontarget pest species are still unavailable (Adamski et al., 2011; Patterson & Alyokhin, 2014; Obear et al., 2016; Clements et al., 2018a). Moreover, the effects of the most commonly used class of respiratory inhibitor fungicide, that is, OXPHOS inhibitors, of a specific crop, like potato, are still unknown for its most likely nontarget pest species, CPBs.
The CPB is the most harmful insect pest of potato (Solanum tuberosum) (Alyokhin et al., 2008). As it is an invasive pest species, chemical insecticides are used to control its occurrence worldwide, and hence many CPB populations have developed resistance to various insecticides (Huseth et al., 2014; Mota‐Sanchez & Wise, 2021). Besides the insecticides that are applied frequently in the fields, other chemical inputs may also play a role in the development of insecticide resistance in the species (Clements et al., 2018a). One of the important factors can be the cross‐resistance between insecticides and fungicides that may facilitate the rapid development of resistance in a population. Earlier studies have shown that fungicides can negatively affect larval survival and development, and can also activate metabolic detoxification related pathways similar to those affected by the insecticide treatments in CPB (Patterson & Alyokhin, 2014; Clements et al., 2018a, 2018b). However, the effects of the OXPHOS inhibitor fungicides, like fluazinam, on CPB have not been investigated. Beetles are most likely exposed to fluazinam in the potato field multiple times during either their larval and/or adult stages since the fungicide is applied throughout the growing season (Schepers et al., 2018). If fluazinam affects the respiratory system of the beetle, it may affect the survival or physiology of the larvae (by altering enzyme and gene activity). Exposure to fungicide, as often shown in insecticide exposure, may also induce behavioral responses (avoidance of the fungicide‐treated leaves) in the adult beetles (e.g., feeding behavior). Therefore, our aim of this study was 2 fold: to understand the effects of fluazinam, on (i) the adult and (ii) larval stage of the CPB. We tested the feeding behavior of the CPB adults under 3 field related concentrations of the fluazinam used in Finland and in other European countries (EU Pesticides database, 2021). First, we tested whether the fungicide is aversive to the beetles and whether this aversion is related to the level of fungicide concentrations on the treated potato leaves by measuring feeding behavior. Further, we checked whether there are differences in the feeding behavior between the sexes. Second, we investigated the direct effects of fungicide on larval performance by measuring larval survival under different fungicide treatments (i.e., control, 24 and 72‐h exposure to 0.25 and 0.66 mg/L of fluazinam). Finally, we investigated gene expression level differences between different treatments using 3 metabolic detoxification related genes (CYP9Z14v2, Ugt1, and Gs), 1 insecticide resistance gene (Ldace1), and 1 stress tolerance gene (Hsp70).
Materials and methods
Study species
We used the laboratory population of the CPB originally collected from Vermont, USA (44°43′ N, 73°20′ W) in 2010. To maintain the lab population, we mated the field collected adult beetles in laboratory conditions and new adults of the next generation were overwintered in controlled chambers at 5 °C (detailed rearing conditions are described in Lehmann et al., 2014; Margus, 2018; Margus et al., 2019). We used the 7th generation of the adults for the behavior trials (choice and food consumption experiments, summer 2017) and larvae from the 8th generation for the survival and gene expression experiments (summer 2018).
Fungicide choice experiment on adults
We chose randomly 133 adults (62 females and 71 males) from 24 families of the CPB for the fungicide choice experiment, where we allowed each individual to choose from either control or fungicide‐treated leaves (Table 1). Commercial fungicide product, Shirlan (Syngenta Crop Protection AG, Switzerland) which contains fluazinam (500 g/L) as active ingredient was used for the experiment. We used half of the lowest (0.12 mg/L), lowest (0.25 mg/L) and highest (0.66 mg/L) field recommended concentration of fluazinam for the experiment. Before the experiment the adult beetles were weighed (± 1 mg) (AM100, Mettler, Columbus, OH, USA) and then randomly divided into 3 fungicide concentration groups: 0.12 mg/L (N = 42), 0.25 mg/L (N = 46) and 0.66 mg/L (N = 45). We allowed beetles to choose from 2 potato leaf discs (1.7 cm in diameter), which were either dipped in distilled water (control) or in the different fungicide solutions. The 2 leaf discs were offered to the beetles on a Petri dish (9 cm in diameter). The adult beetle was put on its back in the center of the Petri dish and the beetle's choice was counted when it started eating the leaf discs. The choice the beetles made (control or fungicide) and the time it took to choose the leaf were recorded. Each of the adults was allowed to choose 3 times between a new set of treated and control leaf discs.
Table 1.
Experimental setup for feeding behavior, sample size and descriptive statistics by different concentrations and sexes
| Fungicide concentration | |||
|---|---|---|---|
| 0.12 mg/L | 0.25 mg/L | 0.66 mg/L | |
| Females, n | 19 | 22 | 21 |
| First choice control: fungicide | 10 : 9 | 12 : 10 | 9: 12 |
| Average choice (SEM) | 0.56 (± 0.1) | 0.47 (± 0.1) | 0.49 (± 0.1) |
| Time in first trial (SEM), min | 8.16 (± 1.8) | 18.73 (± 3.9) | 15.57 (± 3.3) |
| Average time (SEM), min | 10.78 (± 1.7) | 12.53 (± 1.6) | 14.17 (± 1.6) |
| Males, n | 23 | 24 | 24 |
| First choice control: fungicide | 14 : 9 | 12 : 12 | 12 : 12 |
| Average choice (SEM) | 0.46 (± 0.1) | 0.51 (± 0.1) | 0.51 (± 0.1) |
| Time in first trial, (SEM) min, by type | control 15.03 (± 2.5): fungicide 22.36 (± 3.2) | ||
| Average time (SEM), min | 14.09 (± 2.1) | 11.17 (± 1.9) | 13.19 (± 2.2) |
First choice refers to which treated leaf the beetles chose first, average choice (± SEM) to how beetles chose on average (random choice = 0.5), the time it took the first leaf for females, by different concentrations, and for different leaf types for males and finally the average time (± SEM) in min it took for beetles to choose the leaves.
Larval survival experiment
To test the direct effect of 2 field related fluazinam concentrations (0.25 mg/L and 0.66 mg/L) and treatment types (24 and 72‐h exposure) on larval survival, we exposed the 2nd instar (i.e., 2–3‐d‐old) larvae (N = 875) from 12 different families either to control (distilled water) or to a fluazinam‐treated leaf disc. We had 5 treatments in total (control, 24‐h exposure of 0.25 and 0.66 mg/L and 72‐h exposure of 0.25 and 0.66 mg/L of fluazinam). We took 15 larvae from each family and randomly divided them into 5 treatment groups where each group had 3 larvae. We repeated each of the families 4–5 times for each treatment group. For the exposure, we dipped a leaf into a fungicide solution and placed them into a randomly chosen well (36 mm in diameter) of a 6‐well falcon plate (127 mm in length). In the 24‐h exposure treatments, we exposed the larvae once to the treated leaf at the beginning of the experiment and subsequently gave beetles fresh leaves after 24 and 48 h. For the 72‐h exposure, we exposed the larvae to the new fungicide‐treated leaf 3 times (at the beginning, after 24 h and after 48 h). We recorded the 72‐h survival and snap froze living larvae with liquid nitrogen and stored them at −80 °C for RNA extractions.
Target gene selection
We chose to test the effects of fungicide treatment on the expression levels of 5 candidate genes, which have been previously associated with metabolic detoxification (3 genes), insecticide resistance (1 gene), and stress tolerance (1 gene) in CPB (detailed below).
CPB has shown to have metabolic resistance against carbamate, organophosphate and pyrethroid insecticides (reviewed in Kaplanoglu, 2016). A common enzyme group involved in the metabolic detoxification is cytochrome P450 (CYPs; Li et al., 2007; Feyereisen, 2012). CYP9 genes of this family has been found to be associated with resistance to pyrethroids and organophosphate insecticides in several different insects, like in Bombyx mori (silkworm) and Rhynchophorus ferrugineus (weevil beetle) (Zhao et al., 2011; Antony et al., 2019) as well as in CPB (Clements et al., 2016; Zhu et al., 2016). For this study, we selected detoxification gene CYP9Z14v2, which has been shown to be upregulated in the neonicotinoid resistant individuals of CPB (Zhang et al., 2008; Kaplanoglu et al., 2017). We also chose another metabolic detoxification related gene, Uridine diphosphate glycoronosyltransferase 1 (Ugt1) which is associated with the metabolic detoxification of insecticides in CPB (Kaplanoglu et al., 2017; Clements et al., 2018b). In addition, we used Glutathione synthetase (Gs) gene, which is known to be related to neonicotinoid resistance in CPB (Clements et al., 2016, 2017) and acetylcholine esterase1 gene (Ldace1), with high expression levels associated with resistance against organophosphate and carbamate insecticides in CPB (Revuelta et al., 2011; Margus et al. 2021). Finally, we measured the expression levels of a heat shock protein gene, Hsp70, which is an early marker of stress associated with the respiration process and temperature shock (Lee et al., 2012; Chen et al., 2014; Wang et al., 2018).
Primers for 4 of the above‐mentioned target genes, CYP9Z14v2, Gs, Ldace1 and Hsp70 and for 2 reference genes used in quantitative real‐time polymerase chain reaction (qPCR), ribosomal protein S18 gene (RpS18) and 50S ribosomal protein L13e gene (L13e) were collected from published studies (Table 2). In addition, we designed primers for Ugt1 gene using the annotated transcriptome of the CPB (DDBJ/EMBL/GenBank accession: GEEF00000000, Clements et al., 2016) with programs Net Primer (http://www.premierbiosoft.com/netprimer/) and Primer3 (version 0.4.0, http://bioinfo.ut.ee/primer3‐0.4.0/).
Table 2.
Primer sequences used in the quantitative real‐time polymerase chain reaction analysis
| Target genes | E% | R 2 | Forward and reverse primers (5′–3′) | Product size (bp) | Response (treatment/pesticide) |
|---|---|---|---|---|---|
| CYP9Z14v2 | 100.4 | 0.998 |
F: ACCAATGCGTTTCAATCCCG R: CCAACCCGAATGGCAAATAAG |
82 | Environmental stress and host plant response 1 |
| Ugt1* | 90.3 | 0.996 |
F: CGCTGAAGAGTTTGGGCTGT R: TCAGATCGGGACAGTGAGGAA |
270 | Chlorothalonil and imidacloprid2 |
| Gs | 97.8 | 0.999 |
F: CAGAGCAGGGTATGAACCTAATC R: CCAGCCAAGTGATACTGAATCG |
114 | Chlorothalonil3 |
| Ldace1 | 96.7 | 0.998 |
F: CGCCGAGTTACAAAATACCC R: TAGCGTTTCCATCCAATTCC |
124 | Organophosphate4 |
| Hsp70 | 101.8 | 0.999 |
F: GACGAGAAGCAAAGGCAAAG R: TGAGCGGTCTGTTTGATCTG |
77 | Heat shock5 |
| L13e | 91.3 | 0.999 |
F: TATTCACCAGCCATCCATCA R: GCGTCCTTCACTCTCTTTGC |
138 | Reference gene6 |
| RpS18 | 91.8 | 0.999 |
F: TCCTCGCCAGTACAAAATCC R: ACACGGAGACCCCAGTAGTG |
174 | Reference gene7 |
Primers were either obtained from published studies or designed for the current study. Primer amplification efficiency (E%) and R‐squared (R 2) values are given for each of the primer pairs. Response gives the information about the stress conditions under which the gene was tested in the published study.
Extraction of RNA, cDNA synthesis and gene expression analyses with qPCR
For gene expression analysis we randomly chose 6 unrelated CPB families out of 12 that were exposed to 5 fungicide treatments (control, 24 and 72‐h exposure to 0.25 and 0.66 mg/L of fluazinam). We then randomly took 5 larvae (out of 15 treated) from each of the treatments for each the 6 families for RNA extractions. We had in total 150 larvae from 5 treatment groups (5 larvae for 6 families/treatment).
Total RNA was extracted from single larvae with TRI Reagent (Sigma‐Aldrich, St. Louis, MO, USA) and RNeasy RNA isolation kit (Qiagen, Hilden, Germany). Concentration and the integrity of the RNA were measured with TapeStation (Agilent 2200, Santa Clara, CA, USA). The concentration of the RNA from each of the single larval samples were then normalized to 400 and 20 ng/μL and were used for complementary DNA (cDNA) synthesis using iScript cDNA Synthesis Kit (Bio‐Rad, Laboratories Inc., Hercules, CA, USA) according to the manufacturer's protocol. qPCR reaction was performed with a total volume of 20 μL by using 1 μL of cDNA, 10 μL of 2 × SYBR Green Supermix (Bio‐Rad Laboratories Inc.), 1 μL of the forward (10 μmol/L) and 1 μL of the reverse (10 μmol/L) gene‐specific primers, and 7 μL of nuclease‐free water. qPCR reactions were run on a CFX96 instrument (Bio‐Rad) with the following temperature cycles: initiation at 95 °C for 3 min and 39 cycles of 10 s at 95 °C, 10 s at 56 °C and 30 s at 72 °C. Melting curves of the reactions to check their amplification purity were then measured at 65 °C to 95 °C. For each treatment group and family, we used 5 larvae (biological replicates) with 3 technical replicates and the final threshold value (Cq) was defined as a mean of the technical replicates that produced good quality data. Normalization of the qPCR data was done with ∆∆(Ct) normalization method (Pfaffl, 2001) with RpS18 and L13e as reference genes (these genes had equal expression levels in all the compared samples, data not shown) using Bio‐Rad CFX Maestro 1.1 program. Efficiency of the genes was quantified using 2‐times dilution series with the same software. Statistical significance of the expression level differences between different treatments and family groups was analyzed with REST (http://rest.gene‐quantification.info/) software with 10 000 iterations and using real efficiency values.
Statistical analyses
Choice (i.e., control or fungicide) made by the adult beetles was analyzed with Chi‐square test. To analyze the time it took for the beetles to choose treated and untreated leaves, we used the time (in minutes) taken to make the first choice as a dependent variable in analysis of covariance (ANCOVA) where fungicide treatment, first choice (control/fungicide) and sex were used as fixed factors, and the weight of the beetle as a covariate. We analyzed 72‐h larval survival with the binary logistic regression where the fungicide concentration treatment was used as a categorical factor and family as a random factor. Analyses were carried out using SPSS v.24 (IBM Corp. Armonk, NY, USA).
Results
Effects of the fungicide on feeding behavior
We offered both control and fungicide‐treated leaf discs to CPB adults and measured their feeding and response time behavior. We did not identify any general fungicide avoidance behavior since the beetles chose the fungicide‐treated leaves as likely as control leaves on their first choice (χ 2 = 0.957, n = 133, df = 2, P = 0.620). Moreover, there was no avoidance of fungicide‐treated leaves over the 3 trials (average choice, ANCOVA F 2,129 = 0.053, P = 0.948). However, there was a difference in time it took for the female and male beetles to choose the treated leaves in the different fungicide treatments in the first trial (ANCOVA, F 2,130 = 3.284, P = 0.041) and therefore the responses of the sexes were separately analyzed. There was a tendency that the fungicide treatment affected the time it took for females to take the first leaf disc (ANCOVA, F 2,55 = 2.924, P = 0.066) and females seemed to be more hesitant to choose the leaf discs in the average (0.25 mg/L concentrations). However, for the males it appeared to take longer to take the fungicide‐treated leaf discs although the difference was not statistically significant (ANCOVA, F 1,64 = 3.507, P = 0.067). When the average times for all trials were analyzed, these differences disappeared (females: fungicide treatment ANCOVA F 2,58 = 1.329, P = 0.273; males: fungicide treatment ANCOVA F 2,67 = 0.504, P = 0.606). When data were analyzed by individual choices there were altogether 93 adults that did not show any clear preference to fungicide or to control treated discs (Fig. 1). However, there were 40 beetles, that systematically chose only either the control (n = 22) or the fungicide discs (n = 18). Males (n = 9) that systematically chose fungicide discs were on average 12.9 min slower compared to males (n = 12) that chose the control discs (ANCOVA, F 1,20 = 8.127, P = 0.011, Fig. 1B) whereas no such difference was observed in females (ANCOVA, F 1,18 = 1.143, P = 0.301, Fig. 1A).
Fig. 1.
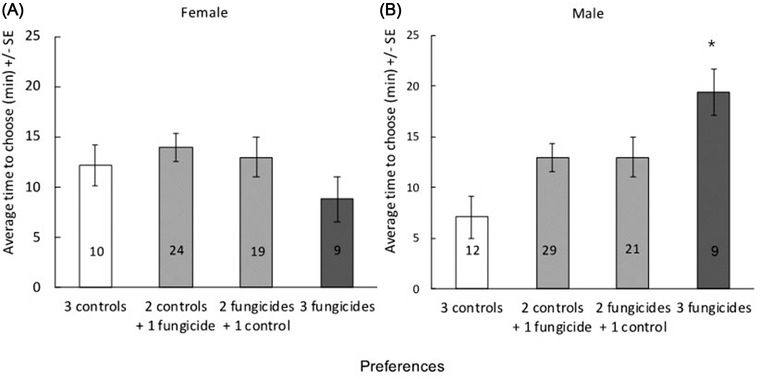
Average time (in min) taken to choose the 3 potato discs for (A) female and (B) male beetles with different preferences when all concentration treatments are combined. White bars indicate the beetles that chose only the control treated leaves, and light gray bars for the choice of both fungicide and control treated leaves after 3 trials. Dark gray bars indicate the beetles that chose only the fungicide‐treated leaf after 3 trials. Numbers in the bars show the number of individuals.
Effects of fungicide treatments on the survival of CPB larvae
The overall 72‐h larval survival (n = 875) was very high (>95% for all groups, only 16 individuals died). The survival under 72‐h exposure to the highest fungicide concentration (0.66 mg/L, survival 95.5%) did not differ significantly from the other groups (control survival 100%; 24‐h exposure to 0.25 mg/L, survival 97.7%; 72‐h exposure to 0.25 mg/L, survival 98.3%; or from 24‐h exposure to 0.66 mg/L, survival 99.4 %) (Wald = 5.444, df = 4, P = 0.245). Finally, survival of the larvae did not differ among different families (Wald = 3.809, df = 11, P = 0.975).
Expression of metabolic detoxification, insecticide resistance and stress tolerance genes under different fungicide concentrations in different beetle families
Long‐term exposure (when the larvae were exposed to fungicide for 72 h) to the highest fungicide concentration (0.66 mg/L) altered the expression of the investigated metabolic detoxification genes CYP9Z14v2, Ugt1 and insecticide resistance gene, Ldace1 (Fig. 2) in CPB larvae. The expression of CYP9Z14v2 and Ldace1 genes overall samples were significantly upregulated while that of Ugt1 was downregulated. Moreover, short‐term (24 h) exposure to the highest fungicide concentration caused downregulation of Gs gene. Finally, both 24 and 72‐h exposure to the lowest field concentration of fungicide (0.25 mg/L) increased the expression of a stress tolerance gene Hsp70 (Fig. 2).
Fig. 2.
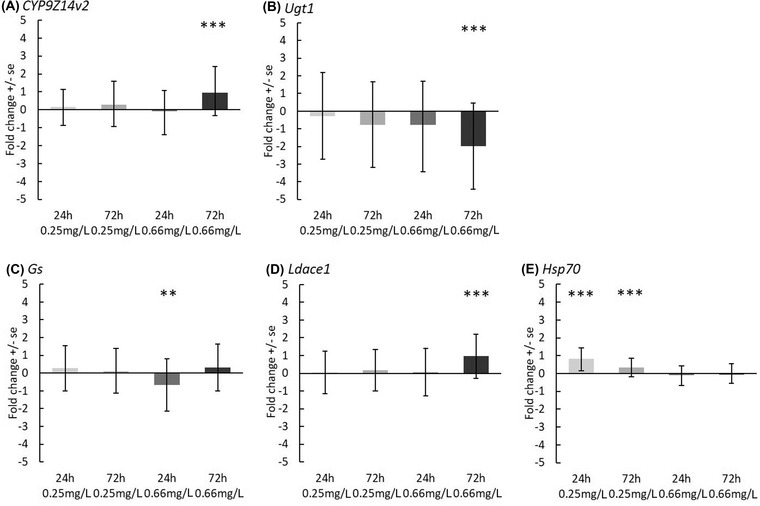
Fold change (log2 transformed ± SE) of the target genes, (A) CYP9Z14v2, (B) Ugt1, (C) Gs, (D) Ldace1, (E) Hsp70, in Colorado potato beetle (CPB) larvae under fungicide treatments (24 h of single and 72 h of repeated [3 times] exposure to 0.25 mg/L and 0.66 mg/L of fluazinam) when compared to control (nontreated) sample overall families. Expression levels of the study genes were normalized using L13e and RpS18 reference genes. Significant differences between the control and the treatment groups are marked with asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P = 0.001).
Due to the large variation in the gene expression levels over all samples, we separately analyzed expression changes in each of the CPB families (Fig. 3). There were no significant differences in the expression levels of CYP9Z14v2 under 24 or 72‐h exposure to the lowest fungicide concentration in any of the families. However, the expression increased significantly in 1 family under 24‐h exposure, and in 2 families under 72‐h exposure to the highest fungicide concentration. The expression of Ugt1 was unaffected in all the families under 24‐h exposure to the lower concentration while it showed significant downregulation in all except 2 families in the other treatments. Interestingly, in 1 family, Ugt1 was significantly upregulated under 24‐h exposure to the higher fungicide concentration. Unfortunately, gene expression results of the third metabolic detoxification gene, Gs, are more difficult to interpret as this gene was significantly upregulated in one of the families under 72‐h exposure to both lower and higher concentrations while 2 other families showed significant downregulation in the 24‐h but not in the 72‐h treatment to the higher concentration. Expression levels of the insecticide resistance gene, Ldace1, were unaffected by both the 24 and 72‐h exposure to the lower concentration, as well as by the 24‐h exposure to the higher concentration. However, this gene was significantly upregulated in all except 1 family under the longer treatment of higher fungicide concentration when compared to control samples. Finally, expression of the stress tolerance gene, Hsp70, increased significantly in 3 families under the 24‐h exposure of the lower concentration. However, surprisingly, under the 72‐h exposure to the higher concentration, expression level of this gene both increased and decreased in different families while it remained unchanged under 72‐h exposure to the lower concentration and under 24‐h exposure to the higher fungicide concentrations in all families.
Fig. 3.
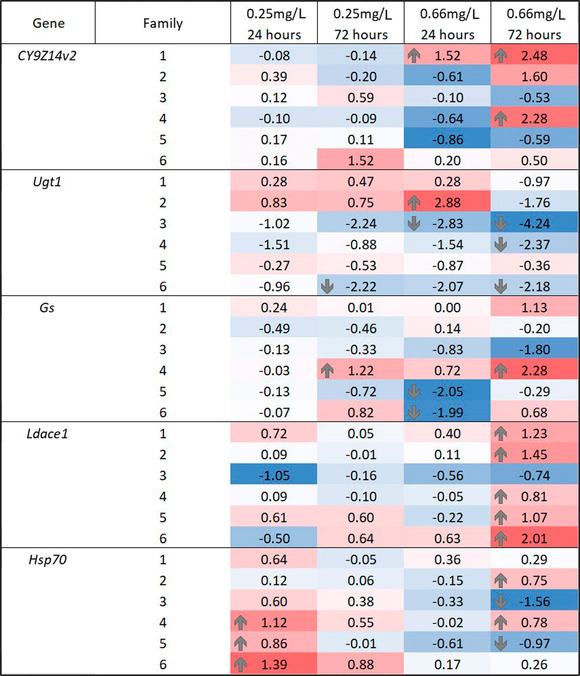
Fold changes (log2 transformed) of the 5 target genes (CYP9Z14v2, Ugt1, Gs, Ldace1, Hsp70) when compared to the control (nontreated) sample (n = 5) under 4 treatments (24‐h and 72‐h exposure to 0.25 mg/L and to 0.66 mg/L of fluazinam) in 6 Colorado potato beetle (CBP) families. Expression levels of the study genes were normalized using L13e and RpS18 reference genes. Red color and positive values denote upregulation and blue and negative values indicate downregulation. Numbers from 1 to 6 indicate the 6 different families used in the study. Intensity of the color indicates the level of expression, and families with significant differences (collected from REST, see text for details) between the control and the treatment groups are marked with upward or downward arrows.
Discussion
In agriculture, fungicides are important pesticides to reduce crop losses due to plant pathogens, but at the same time, their effects even at field related concentrations can extend to nontarget pest species. In this paper, we ran a series of experiments to test the effects of field related concentrations of fluazinam (a common fungicide used against the potato late blight) on the nontarget pest species, CPB. To get a comprehensive picture of the effects, we tested the fluazinam effects on beetles on 3 different levels: (i) at the behavioral (adult feeding behavior); (ii) at the individual (larval) fitness; and (iii) at the gene expression level.
In the adult behavioral tests beetles did not clearly avoid fungicide‐treated leaf discs, as they chose to eat both control and fungicide‐treated leaves at the same rate, and they did not even differentiate between different fungicide concentrations, suggesting that field related concentrations of fluazinam are not aversive to the beetles. Alternatively, it is possible that these concentrations were not high enough to be detected by the beetles. In aquatic ecosystems aversion behavior has been observed in juvenile zebra fish (D. rerio) and frog tadpoles (Leptodactylus latrans), which both avoided fungicide (pyrimethanil; FRAC MoA D1) contaminated water (Araújo et al., 2014a; 2014b). Although the results among different species and environments are not directly comparable (Elskus, 2014; Müller, 2018), the lack of choosing either of the leaves suggests that the CPB adults would be exposed to the fungicides in the potato field. However, there was individual level variation in the choosiness of the individuals (see Fig. 1), which may contribute to the exposure of fungicides in the field.
In addition to the adult behavior, neither of the field related fluazinam concentrations increased short‐term larval mortality in our study. Similar results have been observed before in zebrafish (Wang et al., 2018), although higher fluazinam concentrations disrupted mitochondrial bioenergetics and induced oxidative stress. Moreover, Clements et al., 2018a showed that boscalid fungicide (FRAC MoA C2) did not increase mortality directly, but that it delayed the CPB larval growth rate and larvae gained less mass compared to the control group, which also lead to a smaller size. Therefore, it is possible that delayed fitness effects related to fungicide exposure could also require longer experiments or fungicides used in combination with other stressors (see e.g., Cullen et al., 2019).
While there were no effects on adult behavior or larval mortality, the field related concentrations of fungicides still affected gene expression levels of the beetle larvae (see Shi et al., 2018; Fig. 2). Overall, out of the 5 genes tested, the smallest concentration affected only Hsp70 gene expression, whereas the higher fluazinam concentration affected all the other 4 genes (Fig. 2), although there were big differences between different CBP families (Fig. 3). The fact that the higher concentration and repeated exposure increased gene expression the most, is a very general dose‐dependent finding (Wang et al., 2018) and suggests that even these low field related fluazinam concentrations may have metabolic costs for individuals. These costs may in turn have long‐term fitness consequences which were not observed here.
Out of the tested metabolic genes, CYP9Z14v2 gene was upregulated and Gs and Ugt1 were downregulated after exposure to the higher concentration of fungicide. Upregulation of cytochrome P450 (CYP) gene family could indicate that larvae were metabolizing fungicides like other toxic compounds (Terriere, 1984; Werck‐Reichhart & Feyereisen, 2000 see also Shang et al., 2020) such as insecticides (Clements et al., 2018a, 2018b) to minimize oxidative damages. Previously, upregulation of several CYP genes has been reported in CPB adults fed with fungicide (chlorothalonil, FRAC MoA M05) treated leaves (Clements et al., 2018a). Contrary to our findings, Clements et al. 2018a, 2018b) showed upregulation of Ugt1 and Gs in CPB adults under fungicide treatments, suggesting that these genes are involved in the insect xenobiotic metabolism pathway. It is possible that the difference among the results is due to the exposure time or differences between the fungicides. However, both experiments suggest that these genes are important xenobiotic metabolic genes to study further.
The insecticide resistance associated gene, Ldace1 (Fournier et al., 1992; Zhu & Gao, 1999; Clark et al., 2001), was upregulated under the highest concentration of fluazinam, suggesting that this chemical can act similarly like insecticides as high expression levels of Ldace1 have been previously associated with the resistance against organophosphate and carbamate insecticides in CPB (Revuelta et al., 2011; Margus et al., 2021). On the other hand, if fluazinam slows down the growth of the larvae (see Clements et al., 2018a) it is possible that this difference between treatments is generated by the developmental differences among larvae as Ldace1 expression goes down when the larvae grow (Revuelta et al., 2011). In contrast to our results, glyphosate‐based herbicides inhibited Ldace1 expression in CPB larvae (Modesto & Martinez, 2010; Margus et al., 2019). The fact that both fungicides and herbicides can affect resistance associated genes underlines that even though fungicide effects are minor, as they can affect the same pathways as insecticides, they may also contribute to overall insecticide resistance.
Out of the studied genes, only Hsp70 was upregulated on the lower fungicide concentration. HSPs perform as molecular chaperones that typically stabilize the structure and functions of the proteins in the cells under thermal stress conditions (Sun et al., 2014) or insecticide toxicity (Jing et al., 2013). Previously it has been shown that insecticide (imidacloprid) can increase the expression levels of the Hsp70 gene of CPB under optimal temperature conditions (Chen et al., 2016). It has been also suggested that sublethal insecticide stress could select for higher temperature tolerance through their impact on the HSP pathways (Ge et al., 2013). Whether fungicides could act in a similar manner remains to be tested.
The variation in gene expression levels within each treatment was large in all the studied genes (Fig. 2) and hence we analyzed the overall treatment effect of the fungicide at the family level (Fig. 3). We found that the expression of all the investigated genes among the families show a more complex pattern. For example, whereas the overall expression of Hsp70 showed no difference from the control samples at the higher concentration, there were differences among the families to different directions, which cancels out the overall effect. Similarly, even though the overall expression of CYP9Z14v2 and Ugt1 genes did not change under highest concentration and 24‐h exposure, there were some families which did respond to the fungicide. Similar variation in the expression levels of Ugt and 1 CYP gene was found among CPB adults under fungicide (chlorothalonil) treatment (Clements et al., 2018b), which highlights individual level variation in these detoxifying enzymes. Differences in the gene expression levels in different families under the same treatment group indicates that responses can vary within a beetle population and that there might be individual level genetic differences in responses to fungicides. It remains to be tested what are the underlying mechanisms for these differences.
Conclusions
The field related concentrations of the fluazinam (0.12, 0.25 and 0.66 mg/L) did not have any significant negative effect on the behavior of a common nontarget pest species. Furthermore, there were no effects on larval survival even when individuals were exposed to fungicides for 3 d. However, we observed that this repeated exposure altered expression of the metabolic detoxification, insecticide resistance and stress tolerance genes. Since the expression levels differed among the families under different fungicide concentrations, this further suggests that there may be genetic differences in the tolerance to fungicides. Together, these results suggest that even though fluazinam only imposes minor effects in the short‐term, long‐term consequences should be also tested to fully understand how these widely used pesticides affect nontarget pest species and whether they will increase cross‐resistance with other pesticides.
Disclosure
Authors declare they have no conflict of interest.
Acknowledgments
This study was supported by the Finish Cultural foundation and the Academy of Finland (Grant NO. 308302 to LL and 322980 to MK). We would also like to thank E. Räsänen and S. Varjola for their assistance in the beetle lab. As the CPB is a quarantine species, the study was done under the license by Evira (4057/0614/2016).
References
- Adamski, Z. , Krawiec, J. , Markiewicz, E. , Bankiet, M. , Rybska, E. , and Ratajczak, M. , et al. (2011) Effect of dithiocarbamate fungicide mancozeb on development, reproduction and ultrastructure of fat body of Agrotis segetum moths. Karaelmas Science and Engineering Journal, 1, 7–16. [Google Scholar]
- Alyokhin, A. , Baker, M. , Mota‐Sanchez, D. , Dively, G. and Grafius, E. (2008) Colorado potato beetle resistance to insecticides. American Journal of Potato Research, 85, 395–413. [Google Scholar]
- Antony, B. , Johny, J. , Abdelazim, M.M. , Jakše, J. , Al‐Saleh, M.A. and Pain, A. (2019) Global transcriptome profiling and functional analysis reveal that tissue‐specific constitutive overexpression of cytochrome P450s confers tolerance to imidacloprid in palm weevils in date palm fields. BMC Genomics, 20, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, C.V.M. , Shinn, C. , Mendes, L.B. , Delello‐Schneider, D. , Sanchez, A.L. and Espíndola, E.L.G. (2014a) Avoidance response of Danio rerio to a fungicide in a linear contamination gradient. Science of the Total Environment, 484, 36–42. [DOI] [PubMed] [Google Scholar]
- Araújo, C.V.M. , Shinn, C. , Vasconcelos, A.M. , Ribeiro, R. and Espíndola, E.L.G. (2014b) Preference and avoidance responses by tadpoles: The fungicide pyrimethanil as a habitat disturber. Ecotoxicology (London, England), 23, 851–860. [DOI] [PubMed] [Google Scholar]
- Biggs, A.R. and Hagley, E.A.C. (1988) Effects of two sterol‐inhibiting fungicides on populations of pest and beneficial arthropods on apple. Agriculture, Ecosystems and Environment, 20, 235–244. [Google Scholar]
- Cabras, P. , Angioni, A. , Garau, V.L. , Melis, M. , Pirisi, F.M. , Minelli, E.V. , et al. (1997) Fate of some new fungicides (Cyprodinil, Fludioxonil, Pyrimethanil, and Tebuconazole) from vine to wine. Journal of Agricultural and Food Chemistry, 45, 2708–2710. [Google Scholar]
- Caldas, E.D. , Conceição, M.H. , Miranda, M.C.C. , de Souza, L.C.K.R. and Lima, J.F. (2001) Determination of dithiocarbamate fungicide residues in food by a spectrophotometric method using a vertical disulfide reaction system. Journal of Agricultural and Food Chemistry, 49, 4521–4525. [DOI] [PubMed] [Google Scholar]
- Campbell, J.B. , Nath, R. , Gadau, J. , Fox, T. , DeGrandi‐Hoffman, G. and Harrison, J.F. (2016) The fungicide Pristine® inhibits mitochondrial function in vitro but not flight metabolic rates in honeybees. Journal of Insect Physiology, 86, 11–16. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Alyokhin, A. , Mota‐Sanchez, D. , Baker, M. and Whalon, M. (2014) Variation in fitness among geographically isolated Colorado potato beetle (Coleoptera: Chrysomelidae) populations. Annals of the Entomological Society of America, 107, 128–135. [Google Scholar]
- Chen, J. , Kitazumi, A. , Alpuerto, J. , Alyokhin, A. and de Los Reyes, B. (2016) Heat‐induced mortality and expression of heat shock proteins in Colorado potato beetles treated with imidacloprid. Insect Science, 23, 548–554. [DOI] [PubMed] [Google Scholar]
- Clark, M.J. , Lee, S.H. , Kim, H.J. , Yoon, K.S. and Zhang, A. (2001) DNA‐based genotyping techniques for the detection of point mutations associated with insecticide resistance in Colorado potato beetle Leptinotarsa decemlineata . Pest Management Science, 57, 968–974. [DOI] [PubMed] [Google Scholar]
- Clements, J. , Schoville, S. , Petersen, N. , Lan, Q. and Groves, R.L. (2016) Characterizing molecular mechanisms of imidacloprid resistance in select populations of Leptinotarsa decemlineata in the Central Sands Region of Wisconsin. PLoS ONE, 11, e0147844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements, J. , Schoville, S. , Clements, N. , Chapman, S. and Groves, R.L. (2017) Temporal patterns of imidacloprid resistance throughout a growing season in Leptinotarsa decemlineata populations. Pest Management Science, 73, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements, J. , Sanchez‐Sedillo, B. , Bradfield, C.A. and Groves, R.L. (2018a) Transcriptomic analysis reveals similarities in genetic activation of detoxification mechanisms resulting from imidacloprid and chlorothalonil exposure. PLoS ONE, 13, e0205881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements, J. , Schoville, S. , Clements, A. , Amezian, D. , Davis, T. , Sanchez‐Sedillo, B. , et al. (2018b) Agricultural fungicides inadvertently influence the fitness of Colorado potato beetles, Leptinotarsa decemlineata, and their susceptibility to insecticides. Scientific Reports, 8, 13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, M.G. ,, Thompson, L.J. , Carolan, J.C. , Stout, J.C. and Stanley, D.A. (2019. ) Fungicides, herbicides and bees: a systematic review of existing research and methods. PLoS ONE, 14, e0225743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damalas, C.A. and Eleftherohorinos, I.G. (2011) Pesticide exposure, safety issues, and risk assessment indicators. International Journal of Environmental Research and Public Health, 8, 1402–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2016) The European Union Report on Pesticide Residues in Food. EFSA Journal, 14, e04611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elskus, A.A. (2014) Toxicity, sublethal effects, and potential modes of action of select fungicides on freshwater fish and invertebrates. U.S. Geological Survey Open‐File Report, (2012–1213) version1.1., 42 p. [Google Scholar]
- EU Pesticides Database (2021) https://ec.europa.eu/food/plants/pesticides/eu‐pesticides‐database_en
- Eurostat (2020) https://ec.europa.eu/eurostat/
- Feyereisen, R. (2012) Insect CYP genes and P450 enzymes. Insect Molecular Biology and Biochemistry (ed. Gilbert L.I.), pp. 236–316. Academic Press. [Google Scholar]
- Fisher, M.C. , Henk, D.A. , Briggs, C.J. , Brownstein, J.S. , Madoff, L.C. , McCraw, S.L. , et al. (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature, 484, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier, D. Bride, J.M. , Hoffmann, F. and Karchi, F. (1992) Acetylcholinesterase, two types of modifications confer resistance to insecticide. The Journal of Biological Chemistry, 267, 14270–14274. [PubMed] [Google Scholar]
- FRAC (2021) FRAC Code List ©2021: Fungal control agents sorted by cross resistance pattern and mode of action (including coding for FRAC Groups on product labels) https://www.frac.info/docs/default‐source/publications/frac‐code‐list/frac‐code‐list‐2021–final.pdf?sfvrsn=f7ec499a_2
- Ge, L.Q. , Huang, L.J. , Yang, G.Q. , Song, Q.S. , Stanley, D. , Gurr, G.M. , et al. (2013) Molecular basis for insecticide‐enhanced thermotolerance in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Molecular Ecology, 22, 5624–5634. [DOI] [PubMed] [Google Scholar]
- Guo, Z. , Miyoshi, H. , Komyoji, T. , Haga, T. and Fujita, T. (1991) Uncoupling activity of a newly developed fungicide, fluazinam [3‐chloro‐N‐(3‐chloro‐2,6‐dinitro‐4‐trifluoromethylphenyl)‐5‐trifluoromethyl‐2‐pyridinamine.]. Bioenergetics, 1056, 89–92. [Google Scholar]
- Huseth, A.S. , Groves, R.L. , Chapman, S.A. , Alyokhin, A. , Kuhar, T.P. , Macrae, I.V. , et al. (2014) Managing Colorado potato beetle insecticide resistance: new tools and strategies for the next decade of pest control in potato. Journal of Integrated Pest Management, 5, 1–8. [Google Scholar]
- Jing, J. , Liu, H. , Chen, H. , Hu, S. , Xiao, K. and Ma, X. (2013) Acute effect of copper and cadmium exposure on the expression of heat shock protein 70 in the Cyprinidae fish Tanichthys albonubes . Chemosphere, 91, 1113–1122. [DOI] [PubMed] [Google Scholar]
- Kalamarakis, A.E. , Petsikos‐Panagiotarou, N. , Mavroidis, B. and Ziogas, B.N. (2000) Activity of fluazinam against strains of Botrytis cinerea resistant to benzimidazoles and/or dicarboximides and to a benzimidazole‐phenylcarbamate mixture. Journal of Phytopathology, 148, 449–455. [Google Scholar]
- Kaplanoglu, E. (2016) Multi‐gene resistance to neonicotinoids in the Colorado potato beetle, Leptinotarsa decemlineata . Electronic Thesis and Dissertation Repository, 3953. https://ir.lib.uwo.ca/etd/3953 [Google Scholar]
- Kaplanoglu, E. , Chapman, P. , Scott, I.M. and Donly, C. (2017) Overexpression of a cytochrome P450 and a UDP‐glycosyltransferase is associated with imidacloprid resistance in the Colorado potato beetle. Leptinotarsa decemlineata. Scientific Reports, 7, 1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger, J. , Graaf, S. , Patring, J. and Adielsson, S. (2010) Pesticides in surface water in areas with open ground and greenhouse horticultural crops in Sweden 2008. Swedish University of Agricultural Science. Technical report. Ekohydrologi, 117, 1–45. [Google Scholar]
- Lee, J.E. , Kang, J.S. , Ki, Y.W. , Park, J.H. , Shin, I.C. and Koh, H.C. (2012) Fluazinam targets mitochondrial complex I to induce reactive oxygen species‐dependent cytotoxicity in SH‐SY5Y cells. Neurochemistry International, 60, 773–781. [DOI] [PubMed] [Google Scholar]
- Lehmann, P. , Piiroinen, S. , Kankare, M. , Lyytinen, A. , Paljakka, M. and Lindström, L. (2014) Photoperiodic effects on diapause‐associated gene expression trajectories in European Leptinotarsa decemlineata populations. Insect Molecular Biology, 23, 566–578. [DOI] [PubMed] [Google Scholar]
- Li, X. , Schuler, M.A. and Berenbaum, M.R. (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annual Review of Entomology, 52, 231–253. [DOI] [PubMed] [Google Scholar]
- Maltby, L. , Brock, T.C.M. and van den Brink, P.J. (2009) Fungicide risk assessment for aquatic ecosystems: importance of interspecific variation, toxic mode of action, and exposure regime. Environmental Science and Technology, 43, 7556–7563. [DOI] [PubMed] [Google Scholar]
- Margus, A. (2018) Adaptation to stressful environments: invasion success of the Colorado potato beetle (Leptinotarsa decemlineata). JYU Dissertations 11. University of Jyväskylä.
- Margus, A. , Piiroinen, S. , Lehmann, P. , Tikka, S. , Karvanen, J. and Lindström, L. (2019) Sublethal pyrethroid insecticide exposure carries positive fitness effects over generations in a pest insect. Scientific Reports, 9, 11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margus, A. , Piiroinen, S. , Lehmann, P. , Grapputo, A. , Gilbert, L. , Chen, Y.H. , Lindström, L. (2021) Sequence variation and regulatory variation in acetylcholinesterase genes contribute to insecticide resistance in different populations of Leptinotarsa decemlineata. Ecology and Evolution, 11, 15995–16005. 10.1002/ece3.8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margus, A. , Rainio, M. , Lindström, L. (2019) Can Indirect Herbicide Exposure Modify the Response of the Colorado Potato Beetle to an Organophosphate Insecticide?. Journal of Economic Entomology, 112, 2316–2323. 10.1093/jee/toz115 [DOI] [PubMed] [Google Scholar]
- Michaud, J.P. (2001) Responses of two ladybeetles to eight fungicides used in Florida citrus: implications for biological control. Journal of Insect Science, 1, 1–6. [PMC free article] [PubMed] [Google Scholar]
- Modesto, K.A. and Martinez, C.B. (2010) Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus . Chemosphere, 78, 294–299. [DOI] [PubMed] [Google Scholar]
- Mota‐Sanchez, D. and Wise, J. (2021) Arthropod pesticide resistance database (APRD). Available in: http://www.pesticideresistance.org/.
- Mu, Z. , Feng, X. , Zhang, Y. and Zhang, H. (2016) Trace analysis of three fungicides in animal origin foods with a modified QuEChERS method and liquid chromatography‐tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 408, 1515–1522. [DOI] [PubMed] [Google Scholar]
- Müller, C. (2018) Impacts of sublethal insecticide exposure on insects–Facts and knowledge gaps. Basic and Applied Ecology, 30, 1–10. [Google Scholar]
- National Academy of Sciences (2011) Fungal Diseases: An Emerging Threat to Human, Animal, and Plant Health: Workshop Summary. Institute of Medicine (US) Forum on Microbial Threats. Washington: (DC: ): National Academies Press; (US; ); 2011. [PubMed] [Google Scholar]
- Obear, G.R. , Williamson, R.C. , Held, D.W. , Liesch, P.J. and Adesanya, A.W. (2016) Fungicides affect Japanese beetle Popillia japonica (Coleoptera: Scarabaeidae) egg hatch, larval survival and detoxification enzymes. Pest Management Science, 72, 966–973. [DOI] [PubMed] [Google Scholar]
- Oruc, H.H. (2010) Fungicides and Their Effects on Animals, in: Fungicides. InTech. 10.5772/12959 [DOI]
- Patterson, M. and Alyokhin, A. (2014) Survival and development of Colorado potato beetles on potatoes treated with phosphite. Crop Protection, 61, 38–42. [Google Scholar]
- Pauchet, Y. , Wilkinson, P. , van Munster, M. , Augustin, S. , Pauron, D. and Ffrench‐Constant, R.H. (2009) Pyrosequencing of the midgut transcriptome of the poplar leaf beetle Chrysomela tremulae reveals new gene families in Coleoptera. Insect Biochemistry and Molecular Biology, 39, 403–413. [DOI] [PubMed] [Google Scholar]
- Pennisi, E. (2010) Technologies for a better future. Science, 327, 803. [Google Scholar]
- Pfaffl, M.W. (2001) A new mathematical model for relative quantification in real‐time RT–PCR. Nucleic Acid Research, 29, e45. (see https://www.ncbi.nlm.nih.gov/pmc/articles/PMC55695/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel, C. , Pouchieu, C. , Carles, C. , Béziat, B. , Boulanger, M. , Bureau, M. et al. (2019) Agricultural exposures to carbamate herbicides and fungicides and central nervous system tumour incidence in the cohort AGRICAN. Environment International, 130, 104875. [DOI] [PubMed] [Google Scholar]
- Reilly, T.J. , Smalling, K.L. , Orlando, J.L. and Kuivila, K.M. (2012) Occurrence of boscalid and other selected fungicides in surface water and groundwater in three targeted use areas in the United States. Chemosphere, 89, 228–234. [DOI] [PubMed] [Google Scholar]
- Revuelta, L. , Ortego, F. , Díaz‐Ruíz, J.R. , Castañera, P. , Tenllado, F. and Hernández‐Crespo, P. (2011) Contribution of ldace1 gene to acetylcholinesterase activity in colorado potato beetle. Insect Biochemistry and Molecular Biology, 41, 795–803. [DOI] [PubMed] [Google Scholar]
- Rouabhi, R. (2010) Introduction and Toxicology of Fungicides. In Tech, 10.13140/RG.2.1.2099.9125 [DOI] [Google Scholar]
- Runno‐Paurson, E. , Loit, K. , Hansen, M. , Tein, B. , Williams, I.H. and Mänd, M. (2015) Early blight destroys potato foliage in the northern Baltic region. Acta Agriculturae Scandinavica, Section B–Soil and Plant Science, 65, 422–432. [Google Scholar]
- Sancho, E. , Villarroel, M.J. and Ferrando, M.D. (2016) Assessment of chronic effects of tebuconazole on survival, reproduction and growth of Daphnia magna after different exposure times. Ecotoxicology and Environmental Safety, 124, 10–17. [DOI] [PubMed] [Google Scholar]
- Schepers, H.T.A.M. , Kessel, G.J.T. , Lucca, F. , Förch, M.G. , van den Bosch, G.B.M. , Topper, C.G. , et al. (2018) Reduced efficacy of fluazinam against Phytophthora infestans in the Netherlands. European Journal of Plant Pathology, 151, 947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, Y. , Wang, Y. , Deng, J. , Liu, X. , Fang, Y. , Rao, Q. , et al. (2020) Comparative transcriptome analysis reveals the mechanism related to fluazinam stress of Panonychus citri (Acarina: Tetranychidae). Insects, 11, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, T. , Burton, S. , Zhu, Y. , Wang, Y. , Xu, S. and Yu, L. (2018) Effects of field‐realistic concentrations of carbendazim on survival and physiology in forager honey bees (Hymenoptera: Apidae). Journal of Insect Science, 18, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Sheng, Y. , Bai, L. , Zhang, Y. , Xiao, Y. , Xiao, L. , et al. (2014) Characterizing heat shock protein 90 gene of Apolygus lucorum (Meyer‐Dür) and its expression in response to different temperature and pesticide stresses. Cell Stress and Chaperones, 19, 725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terriere, L.C. (1984) Induction of detoxication enzymes in insects. Annual Review of Entomology, 29, 71–88. [DOI] [PubMed] [Google Scholar]
- Tomlin, S.C. (2000) The Pesticide Manual. 12th edn, British Crop Protection Council, Surry. [Google Scholar]
- Vitoratos, A.G. (2014) Mode of action and genetic analysis of resistance to fluazinam in Ustilago maydis . Journal of Phytopathology, 162, 737–746. [Google Scholar]
- Vu, H.T. , Keough, M.J. , Long, S.M. and Pettigrove, V.J. (2016) Effects of the boscalid fungicide Filan® on the marine amphipod Allorchestes compressa at environmentally relevant concentrations. Environmental Toxicology and Chemistry, 35, 1130–1137. [DOI] [PubMed] [Google Scholar]
- Vu, H.T. , Keough, M.J. , Long, S.M. and Pettigrove, V.J. (2017) Effects of two commonly used fungicides on the amphipod Austrochiltonia subtenuis . Environmental Toxicology and Chemistry, 36, 720–726. [DOI] [PubMed] [Google Scholar]
- Walorczyk, S. , Drozzyński, D. , Kowalska, J. , Remlein‐Starosta, D. , Ziółkowski, A. , Przewoźniak, M. , et al. (2013) Pesticide residues determination in Polish organic crops in 2007–2010 applying gas chromatography‐tandem quadrupole mass spectrometry. Food Chemistry, 139, 482–487. [DOI] [PubMed] [Google Scholar]
- Wang, X.H. , Zheng, S.S. , Huang, T. , Su, L.M. , Zhao, Y.H. , Souders, C.L. , et al. (2018) Fluazinam impairs oxidative phosphorylation and induces hyper/hypo‐activity in a dose specific manner in zebrafish larvae. Chemosphere, 210, 633–644. [DOI] [PubMed] [Google Scholar]
- Werck‐Reichhart, D. and Feyereisen, R. (2000) Cytochromes P450 a success story. Genome Biology, 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightwick, A. , Allinson, G. , Reichman, S. , Menzies, N. and Walters, R. (2010) Environmental risks of fungicides used in horticultural production systems, fungicides. In Tech, 10.5772/13032 [DOI] [Google Scholar]
- Woodrow, J.E. , Seiber, J.N. and Fitzell, D. (1995) Analytical method for the dithiocarbamate fungicides ziram and mancozeb in air: preliminary field results. Journal of Agricultural and Food Chemistry, 43, 1524–1529. [Google Scholar]
- Yang, C. , Hamel, C. , Vujanovic, V. and Gan, Y. (2011) Fungicide: modes of action and possible impact on nontarget microorganisms. ISRN Ecology, 2011, 130289. [Google Scholar]
- Youcum, G.D. , Rinehart, J.P. , Chrimumamilla‐Chapra, A. and Larson, M.A. (2009) Characterization of gene expression patterns during the initiation and maintenance phases of diapause in the Colorado potato beetle, Leptinotarsa decemlineata . Journal of Insect Physiology, 55, 32–39. [DOI] [PubMed] [Google Scholar]
- Zaller, J.G. and Brühl, C.A. (2019) Editorial: nontarget effects of pesticides on organisms inhabiting agroecosystems. Frontiers in Environmental Science, 7, 75. [Google Scholar]
- Zhang, J.H. , Pelletier, Y. and Goyer, C. (2008) Identification of potential detoxification enzyme genes in Leptinotarsa decemlineata (Say) and study of their expression in insects reared on different plants. Canadian Journal of Plant Science, 88, 621–629. [Google Scholar]
- Zhao, G.D. , Zhao, S.S. , Gao, R.N. , Wang, R.X. , Zhang, T. , Ding, H. , et al. (2011) Transcription profiling of eight cytochrome P450s potentially involved in xenobiotic metabolism in the silkworm, Bombyx mori . Pesticide Biochemistry and Physiology, 100, 251–255. [Google Scholar]
- Zhu, F. , Moural, T.W. , Nelson, D.R. and Palli, S.R. (2016) A specialist herbivore pest adaptation to xenobiotics through up‐regulation of multiple Cytochrome P450s. Scientific Reports, 6, 20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, K.Y. and Gao, J.R. (1999) Increased activity associated with reduced sensitivity of acetylcholinesterase in organophosphate‐resistant greenbug, Schizaphis graminum . Pest Management Science, 55, 11–17. [Google Scholar]


