Abstract
Aim
To identify subtypes in a large group of children clinically diagnosed with developmental coordination disorder (DCD) based on their pattern of motor, cognitive, and visual‐motor abilities.
Method
Standardized scores for verbal IQ, total IQ, Movement Assessment Battery for Children, Second Edition (MABC‐2) balance, MABC‐2 manual dexterity, MABC‐2 ball skills, and Beery‐Buktenica Developmental Tests of Visual‐Motor Integration (Beery‐VMI), Motor Coordination (Beery‐MC), and Visual Perception (Beery‐VP) were used. The NbClust complete procedure was used to best partition the data on 98 children (84 males, 14 females, mean [SD] age: 8 years [2 years 1 month]) into clusters. Deviation contrasts, multivariate analysis of variance, and post hoc comparisons were used to characterize the clusters.
Results
Four clusters were revealed: two clusters with a broad motor skill problem, one with relatively preserved visual‐motor integration and Beery‐MC skills, and a second with abnormal ball skills, balance, and Beery‐MC skills. A third cluster with more specific gross‐motor problems, and a fourth with relatively preserved ball skills but low Beery‐MC and performance IQ, were identified. Balance scores were ‘at risk’ or ‘abnormal’ in all four clusters.
Interpretation
DCD is a heterogeneous condition. However, subtypes can be discriminated on the basis of more severe difficulties in fine‐motor performance, gross‐motor performance, or both. There was evidence for generalized motor impairments in around half of all children. Importantly, at least borderline level reduced balance was evident in each subtype.
What this paper adds
Four subtypes were identified in a large clinical group of children with developmental coordination disorder (DCD).
Subtypes were based on motor, cognitive, and visual‐motor abilities.
There was evidence of generalized motor impairments in around 50% of children with DCD.
A generalized balance problem is present across all subtypes of DCD.
What this paper adds
Four subtypes were identified in a large clinical group of children with developmental coordination disorder (DCD).
Subtypes were based on motor, cognitive, and visual‐motor abilities.
There was evidence of generalized motor impairments in around 50% of children with DCD.
A generalized balance problem is present across all subtypes of DCD.
This original article is commented on by Green on pages 1316–1317 of this issue.
Abbreviations
- Beery‐MC
Beery‐Buktenica Developmental Test of Motor Coordination
- Beery‐VMI
Beery‐Buktenica Developmental Test of Visual‐Motor Integration
- Beery‐VP
Beery‐Buktenica Developmental Test of Visual Perception
- DCD
Developmental coordination disorder
- MABC‐2
Movement Assessment Battery for Children, Second Edition
Developmental coordination disorder (DCD) is a chronic disorder that affects approximately 5% to 6% of children, but is under‐recognized by health care and educational
professionals. 1 Children diagnosed with DCD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) criteria form a heterogeneous group. 2 While impairment in motor coordination is a defining attribute of this group, heterogeneity exists in the nature and severity of not only the motor problems, but also in the sensory and cognitive problems often associated with DCD. 1 It is increasingly recognized that this heterogeneity hampers the identification and effective treatment of these children, 1 highlighting the need for more comprehensive assessment.
Recent research into the mechanisms of DCD suggests varying levels of motor‐cognitive issues in these children. 1 For example, Wilson et al. 3 showed a persistent deficit in executive function in a subgroup of children with DCD, warranting more elaborate screening for cognition in this group. This profile was more common in children with persistent DCD. This finding, together with previous studies that have examined possible subtypes in DCD, 4 , 5 , 6 , 7 , 8 suggests that further examination of these subtypes in a larger group of children clinically diagnosed with DCD is warranted.
In the present retrospective study, we used a large sample of children that were clinically diagnosed with DCD according to DSM‐5 standards to identify subtypes of DCD, based on their cognitive, visual‐perceptual, and motor abilities (n = 98). The sample was drawn from a large database (the ZOOM‐IN database) that was collected from 2009 onwards by the Sint Maartenskliniek in Nijmegen, the Netherlands. The primary aim of the ZOOM‐IN project was to help children with motor problems who were unable to fully participate in school or community for various reasons. Based on the screening results, professional guidance was given to children and their parents to enhance their engagement at school and participation.
A secondary consequence of the ZOOM‐IN project was the accumulation of a large database of children that were clinically diagnosed with DCD according to the DSM criteria. 2 , 9 We used this database to examine the presence of DCD subtypes via cluster analysis. Specifically, and in line with the recently updated international consensus statement on DCD, 1 we included motor performance, cognition, and visual‐motor integration abilities to determine whether viable clusters are present in the DCD group at large. In our view, identification of these subtypes will advance customized treatment for these children. It can help in deciding where to give treatment, the intensity of any treatment, and follow‐up programme.
METHOD
ZOOM‐IN setting and identification of the children with DCD
The ZOOM‐IN project was developed and implemented in a specialized consulting centre, the Sint Maartenskliniek. This centre is a collaboration that combines the expertise of a regional expertise centre on childhood disability, a school for special education, and a rehabilitation centre in which a multidisciplinary team of professionals work together. A prerequisite for admission into ZOOM‐IN was a referral by a physician, initiated by the parents themselves or by recommendation of the schoolteacher. A critical inclusion criterion for ZOOM‐IN was a dedicated request for help regarding motor skill development. Children diagnosed with a major medical or psychiatric condition, with the exception of attention‐deficit/hyperactivity disorder and (after the introduction of DSM‐5) 2 autism spectrum disorders, were excluded. A case manager guided the child and parents during the diagnostic, advisory, and follow‐up process. The child and parents visited the centre three times over the course of 1 week for interviews and assessments by the ZOOM‐IN team which consisted of a paediatric physician, a child psychologist, a paediatric physical therapist, an occupational therapist, a speech therapist, and, upon request or upon indication, a social worker. In the following week, the multidisciplinary team analysed and discussed the results of the assessment and, in the third week, parents were informed about the results in a joint consultation with the paediatric physician, the psychologist, and the case manager. Subsequently, a meeting was arranged at the school of the child, with the support‐teacher of the Rivierenland regional expertise centre, parents, and teacher present, to discuss the results and recommendations for the educational setting.
The presence or absence of DCD was determined according to DSM‐IV 9 and later DSM‐52 criteria operationalized for the Netherlands in the Dutch DCD guideline (https://richtlijnendatabase.nl/richtlijn/developmental_coordination_disorder_dcd/startpagina_‐_developmental_coordination_disorder_dcd.html). To summarize, children are seen and tested by a multidisciplinary team (including a medical doctor who performs the neurological examination of minor neurological dysfunction, and a physical therapist who administers the Movement Assessment Battery for Children, Second Edition [MABC‐2]) after referral. Parents and the teacher complete questionnaires regarding their request for help, developmental history of the child, motor performance, and behavioural issues.
Cohort
Between March 2009 and December 2018, a total of 891 children were registered on the ZOOM‐IN database. Parents/caregivers of these children were informed via email about the database and its planned use for the present study. They were invited by the ZOOM‐IN secretary via e‐mail to give informed consent digitally (ticking a box) for the inclusion of the anonymized data of their child. Responses for 479 (53.8%) children were received. For 82.9% (n = 379), consent was provided, of which 123 (32.5%) (104 males, 19 females, mean [SD] age: 8 years 1 month [2 years 3 months]) were clinically diagnosed as having DCD. Ethical approval for use of the clinical database was granted by the local Ethics Committee of the Faculty of Social Science at Radboud University (reference: ECSW‐2020‐133) and the local ethics committee of the rehabilitation centre (reference: 2018/06/20a/MVo/eb).
Assessments
Motor function
The MABC‐2 (Dutch translation) 10 was used to identify and describe the level of motor functioning on three domains of skill (ball skills, balance, manual dexterity). Component standard scores (raw scores corrected for age [mean = 10; SD = 3], based on Dutch norms) 10 on each domain were used in the analyses since these scores provide the most valid index of motor performance and are recommended for scientific use. 10 Centile scores for each domain and for the total test score were used to characterize the sample and individual clusters. Scores on or below the 5th centile indicate a significant movement difficulty and those between the 6th and 16th centile indicate being ‘at risk’ of a movement difficulty. Scores above the 16th centile indicate ‘unlikely to have a movement difficulty’. 10 Following the recommendations in the Dutch DCD guideline, DSM‐5 criterion A is also met with a subscore on or below the 5th centile.
Visual‐motor integration
Beery‐Buktenica Developmental Test of Visual‐Motor Integration (Beery‐VMI) 11 and its two supplementary tests (Visual Perception [Beery‐VP] and Motor Coordination [Beery‐MC]) were used to measure the ability to integrate or coordinate visual‐perceptual and motor abilities when performing manual actions (visual‐motor integration). 11 The test consists of three parts: visual‐motor integration, visual perception, and motor coordination. Raw scores are converted to standardized scores with a mean of 100 and SD of 15. 11 A standard score of 90 or above is considered normal, 80 to 89 as below average, 70 to 79 as low, and below 70 as very low. 11
Cognition
The Dutch version of Wechsler Intelligence Scale for Children, Third Edition 12 was used to assess IQ as a measure of cognitive functioning. Total IQ, verbal IQ, and performance IQ were determined. Raw scores were converted to standard scores with a mean of 100 and SD of 15. A score below 85 is considered ‘at risk’ and 85 or above as normal. A total IQ cut‐off of 70 was used as an inclusion criterion for ZOOM‐IN.
Procedure
First, scores were standardized (i.e. z‐scores [mean = 0, SD = 1]) for: cognition (performance IQ, verbal IQ, total IQ), motor performance (MABC‐2 balance, MABC‐2 manual dexterity, MABC‐2 ball skills, MABC‐2‐total score), and visual‐motor integration (Beery‐VMI, Beery‐MC, Beery‐VP). Outliers (>3 SD) were recoded as missing values (n = 6). Absolute skewness and kurtosis values of the remaining z‐scores were less than 1.96 (p > 0.05) (min–max 0.02–0.62).
Cross‐correlations (Pearson's r) between total test and factor scores were computed to identify redundant factors (Table S1). As expected, high (≥0.70) correlations were found between verbal IQ and total IQ, and between performance IQ and total IQ. The correlation between performance IQ and verbal IQ was weak. Moderate‐to‐high correlations were found between the MABC‐2 total standard score and MABC‐2 domain scores (manual dexterity, ball skills, and balance). Correlations between domain scores were negligible. For the Beery scales, the correlation was mild between visual‐motor integration and visual perception, moderate between visual‐motor integration and motor coordination, and very low between visual perception and motor coordination. As subscale scores represent different domains that were expected to be important in defining the hypothesized clusters, total test scores were omitted from the analysis. Complete data were available for 98 children (Table 1). MABC‐2 scores of 13 children were not available, Beery‐VMI scores were missing for 10 children, both Beery‐VP and Beery‐MC scores were missing for 11 children. In addition, performance IQ scores were missing for two children and verbal IQ scores for one child.
Table 1.
Descriptive statistics of the unstandardized values of each variable for the total sample and each separate cluster
| Total (n = 98) | Cluster 1 (n = 37) | Cluster 2 (n = 24) | Cluster 3 (n = 18) | Cluster 4 (n = 19) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Min–Max | F(1,96), p, η2a | Mean (SD) | Min–Max | Dev. | Mean (SD) | Min–Max | Dev. | Mean (SD) | Min–Max | Dev. | Mean (SD) | Min–Max | Dev. | ||
| IQ | Performance | 89.6 (12.2) | 66–123 | 12.53, <0.001, 0.29 | 86.7 (9.2) | 66–105 | 0 | 100.8 (13.0) | 80–123 | + | 86.9 (11.3) | 67–109 | 0 | 83.7 (8.0) | 70–102 | − |
| Verbal | 103.4 (12.3) | 80–140 | 16.15, <0.001, 0.34 | 98.6 (7.6) | 80–111 | − | 114.9 (12.5) | 91–140 | + | 96.4 (12.4) | 80–112 | − | 104.7 (8.7) | 96–129 | 0 | |
| MABC‐2 (centile) | Manual dexterity | 10.8 (12.5) | 0.1–63 | 5.40, 0 .002, 0.15 | 8.7 (9.5) | 0.1–37 | 0 | 18.6 (16.3) | 1–63 | + | 8.3 (11.5) | 0.5–50 | 0 | 7.5 (9.4) | 0.1–37 | 0 |
| Ball skills | 18.7 (22.1) | 0.1–91 | 35.30, <0.001, 0.53 | 12.4 (12.6) | 0.1–50 | − | 14.3 (12.9) | 0.5–50 | 0 | 2.3 (2.2) | 0.1–5 | − | 51.9 (23.4) | 16–91 | + | |
| Balance | 9.26 (12.8) | 0.1–75 | 2.80, 0.044, 0.08 | 9.3 (15.5) | 0.1–75 | 0 | 9.4 (10.9) | 0.1–37 | 0 | 4.1 (6.1) | 0.1–25 | − | 14.0 (12.7) | 0.1–37 | + | |
| Beery‐VMI (standard scores) | VMI | 86.7 (10.2) | 58–111 | 17.22, <0.001, 0.36 | 89.0 (7.3) | 75–102 | + | 94.4 (7.0) | 83–111 | + | 80.7 (8.4) | 66–94 | − | 78.4 (11.1) | 58–93 | − |
| VP | 97.3 (13.3) | 63–129 | 8.31, <0.001, 0.21 | 93.6 (11.5) | 70–117 | − | 107.9 (11.9) | 85–129 | + | 93.8 (11.5) | 63–116 | 0 | 94.6 (13.3) | 67–118 | 0 | |
| MC | 79.7 (12.2) | 45–104 | 22.48, <0.001, 0.42 | 83.2 (8.6) | 67–104 | + | 89 (10.3) | 66–104 | + | 67.7 (9.9) | 53–90 | − | 72.8 (9.5) | 45–87 | − | |
Abbreviations: Dev., deviation contrast test result: ‘–’ = lower than the grand average of the clusters, ‘0’ = not different from the grand average, ‘+’ = higher than the grand average, p < 0.006; MABC‐2, Movement Assessment Battery for Children, Second Edition; MANOVA, multivariate analysis of variance; MC, Motor Coordination; VMI, Visual‐Motor Integration; VP, Visual Perception.
MANOVA test results are included and deviation contrast test results are presented for each cluster.
The NbClust R package 13 was used to identify the optimal number of clusters according to the majority rule 13 whereby the number of clusters that is indicated by the majority of the indices is deemed the best fitting. Accordingly, the NbClust complete procedure with Euclidean distance 13 was used to best partition the data into the optimal number of clusters. The resulting clusters were subsequently analysed using SPSS (v. 25, IBM Corp., Armonk, NY, USA). Deviation contrasts were used to characterize the scores within each identified cluster relative to the grand mean. Multivariate analysis of variance was used to test differences between clusters. Shapiro–Wilk's test was used to assess multivariate normality. No significant (corrected alpha of 0.002) deviations from normality were found. Since Box's M test was significant (p = 0.002), Pillai's Trace was reported. Differences between clusters on all metrics (performance IQ, verbal IQ, MABC‐2 manual dexterity, MABC‐2 ball skills, MABC‐2 balance, Beery‐VMI, Beery‐VP, and Beery‐MC) were tested with a corrected alpha of 0.006 to infer statistical significance, followed by post hoc comparisons on specific metrics. Levene's test was used to test homogeneity of variances. Where variances were unequal (performance IQ, verbal IQ, and Beery‐VMI), Games‐Howell correction was reported instead of Bonferroni. Demographic and clinical characteristics of the identified clusters were also described.
RESULTS
Among 27 indices, the NbClust procedure identified that most (10 indices) had four as the optimal number of clusters. Figure 1 presents the mean z‐scores of the standard scores per metric and cluster, based on this best partition solution. Next, deviation contrasts were defined to describe each cluster relative to the grand average of the sample (Table 1).
Figure 1.
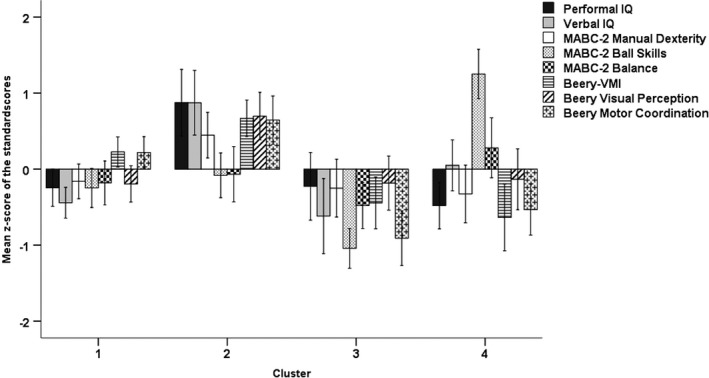
Mean z‐scores of the standard test scores on cognition (performance IQ, verbal IQ), motor function (MABC‐2: manual dexterity, ball skills, balance), and visual‐motor integration (Beery‐VMI: visual perception, visual‐motor integration, motor coordination) (n = 98). Whiskers show the 95% confidence intervals. Cluster 1: n = 37; Cluster 2: n = 24; Cluster 3: n = 18; Cluster 4: n = 19. Abbreviations: Beery‐VMI, Beery‐Buktenica Developmental Test of Visual‐Motor Integration; MABC‐2, Movement Assessment Battery for Children, Second Edition.
There was a statistically significant difference between the clusters (F[24,267] = 11.86, p < 0.001; Pillai's Trace = 1.55, partial η 2 = 0.52). Test of between‐subject (i.e. cluster) effects showed significant differences between the four clusters on all metrics except MABC‐2 balance (Table 1). For each performance metric below, post hoc comparisons are reported to describe differences between individual clusters (Table 2).
Table 2.
Multiple comparisons for each metric in the cluster analysis (n = 98)
| Dependent variable | Cluster (A) | Cluster (B) | Mean difference (A–B) | 95% CI | p | Dependent variable | Cluster (A) | Cluster (B) | Mean difference (A–B) | 95% CI | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Performance IQ | 1 | 2 | −1.12 | −1.77, −0.47 | <0.001 | MABC‐2 Balance | 1 | 2 | 0.11 | −0.69, 0.46 | 1.000 |
| 3 | −0.02 | 0.68, 0.64 | 1.000 | 3 | 0.30 | −0.33, 0.93 | 1.000 | ||||
| 4 | 0.24 | −0.27, 0.74 | 0.600 | 4 | −0.46 | −1.08, 0.16 | 0.286 | ||||
| 2 | 3 | 1.10 | 0.30, 1.90 | 0.004 | 2 | 3 | 0.41 | −0.27, 1.10 | 0.651 | ||
| 4 | 1.36 | 0.67, 2.04 | <0.001 | 4 | −0.35 | −1.02, 0.33 | 1.000 | ||||
| 3 | 4 | 2.25 | −0.44, 0.95 | 0.754 | 3 | 4 | −0.76 | −1.48, −0.04 | 0.034 | ||
| Verbal IQ | 1 | 2 | −1.32 | −1.94, −0.70 | <0.001 | Beery‐VMI | 1 | 2 | −0.44 | −0.85, −0.04 | 0.026 |
| 3 | 0.18 | −0.53, 0.88 | 0.901 | 3 | 0.67 | 0.16, 1.18 | 0.007 | ||||
| 4 | −0.49 | −1.00, 0.02 | 0.062 | 4 | 0.86 | 0.23, 1.50 | 0.005 | ||||
| 2 | 3 | 1.49 | 0.65, 2.33 | <0.001 | 2 | 3 | 1.11 | 0.57, 1.65 | <0.001 | ||
| 4 | 0.82 | 0.13, 1.52 | 0.015 | 4 | 1.31 | 0.65, 1.96 | <0.001 | ||||
| 3 | 4 | −0.67 | −1.44, 0.10 | 0.109 | 3 | 4 | 0.19 | −0.52, 0.91 | 0.886 | ||
| MABC‐2 manual dexterity | 1 | 2 | −0.61 | −1.12, −0.09 | 0.012 | Beery‐VP | 1 | 2 | 0.89 | −1.42, −0.37 | <0.001 |
| 3 | 0.09 | −0.47, 0.65 | 1.000 | 3 | 0.01 | −0.59, 0.57 | 1.000 | ||||
| 4 | 0.17 | −0.39, 0.72 | 1.000 | 4 | −0.06 | −0.63, 0.51 | 1.000 | ||||
| 2 | 3 | 0.70 | 0.09, 1.31 | 0.016 | 2 | 3 | 0.88 | 0.25, 1.51 | 0.002 | ||
| 4 | 0.77 | 0.17, 1.38 | 0.005 | 4 | 0.83 | 0.21, 1.45 | 0.003 | ||||
| 3 | 4 | 0.08 | −0.57, 0.72 | 1.000 | 3 | 4 | 0.05 | −0.71, 0.61 | 1.000 | ||
| MABC‐2 ball skills | 1 | 2 | 0.17 | −0.66, 0.33 | 1.000 | Beery‐MC | 1 | 2 | 0.43 | −0.91, 0.06 | 0.123 |
| 3 | 0.79 | 0.25, 1.33 | 0.001 | 3 | 1.13 | 0.60, 1.66 | <0.001 | ||||
| 4 | −1.50 | −2.03, −0.97 | <0.001 | 4 | 0.75 | 0.23, 1.28 | 0.001 | ||||
| 2 | 3 | 0.96 | 0.37, 1.54 | <0.001 | 2 | 3 | 1.56 | 0.98, 2.13 | <0.001 | ||
| 4 | −1.33 | −1.91, −0.76 | <0.001 | 4 | 1.18 | 0.61, 1.75 | <0.001 | ||||
| 3 | 4 | −2.29 | −2.91, −1.68 | <0.001 | 3 | 4 | −0.38 | −0.99, 0.23 | 0.595 |
Standardized z‐scores are reported. Bonferroni is reported, except for performance IQ, verbal IQ, and Beery VMI. Here, Games‐Howell is reported. Abbreviations: Beery‐MC, Beery‐Buktenica Developmental Test of Motor Coordination; Beery‐VMI, Beery‐Buktenica Developmental Test of Visual‐Motor Integration; Beery‐VP, Beery‐Buktenica Developmental Test of Visual Perception; CI, confidence interval; MABC‐2, Movement Assessment Battery for Children, Second Edition.
Cluster 1
Average IQ, visual‐motor integration, and visual perception skills were within the normal range, whereas performance scores on the MABC‐2 and Beery‐MC subtests were in the at‐risk range (Table 1). Relative to the grand mean of the total sample, scores on verbal IQ, ball skills, and Beery‐VP were significantly lower in cluster 1, while Beery‐VMI and Beery‐MC were higher (Table 1). Multiple comparisons showed that performance IQ, verbal IQ, manual dexterity, Beery‐VMI, and Beery‐VP scores were significantly lower in cluster 1 compared with cluster 2 (Table 2). Ball skills in cluster 1 were better than those in cluster 3, but worse than cluster 4. Beery‐VMI and Beery‐MC scores in cluster 1 were higher than those in clusters 3 and 4. All other differences were not significant.
Cluster 2
Average IQ, manual dexterity, and all Beery scores were within the normal range (Table 1). Average ball and balance skills were in the at‐risk range (Table 1). Scores of children in cluster 2 were near (ball and balance skills) or below the grand mean of the total sample, while Beery‐VMI and both Beery‐MC and Beery‐VP were higher (Table 1). Multiple comparisons (Table 2) showed that scores in cluster 2 were higher than those in all other clusters on all metrics except ball skills and balance. Ball skills were comparable to those in cluster 1, but higher than those in cluster 3 and lower than cluster 4.
Cluster 3
Cluster 3 was characterized by, on average, abnormal ball skills, balance, and Beery‐MC skills. Average manual dexterity and Beery‐VP scores were at risk, whereas IQ and Beery‐VMI were within the normal range (Table 1). Relative to the total sample, children in this cluster scored near (performance IQ, manual dexterity, and visual perception) or below the grand mean on all metrics (Table 1). Multiple comparisons (Table 2) showed that IQ, manual dexterity, and Beery‐VP scores were lower in cluster 3 than in cluster 2. Ball skills was the lowest of all clusters, whereas balance was significantly lower than cluster 4 only. Beery‐VMI and Beery‐MC performance was lower compared with cluster 1 and 2. All other differences were not significant.
Cluster 4
On average, cluster 4 showed well‐developed ball skills relative to other clusters (Table 1). Also, the average verbal IQ and Beery‐VP were within the normal range. Average scores on all other metrics were within the at‐risk range. Relative to the grand mean of the sample (Table 1), ball and balance skills were above average, while verbal IQ, manual dexterity, and visual perception were average. Performance IQ, Beery‐VMI, and Beery‐MC were below average. Multiple comparisons (Table 2) showed that cluster 4 outperformed all other clusters on ball skills. IQ, manual dexterity, and visual perception were lower than in cluster 2. Beery‐VMI and Beery‐MC skills were lower in cluster 4 than both clusters 1 and 2. Finally, balance scores were higher than cluster 3. All other differences were not significant.
Demographic and clinical characteristics of the identified clusters (Table 3) revealed no (significant) differences between the clusters (p > 0.05). Number of MABC‐2 classifications for total and subscale scores per cluster are presented in Table S2.
Table 3.
Demographic and clinical characteristics of the identified clusters (n = 98)
| Cluster 1 (n = 37) | Cluster 2 (n = 24) | Cluster 3 (n = 18) | Cluster 4 (n = 19) | |
|---|---|---|---|---|
| MABC‐2 total score (centile) | ||||
| Mean | 3.05 | 5.27 | 0.93 | 9.47 |
| SD | 4.06 | 4.65 | 1.15 | 12.12 |
| Min–max | 0.1–16 | 0.5–16 | 0.1–5 | 0.5–50 |
| Sex | ||||
| Male (n) | 29 | 22 | 14 | 19 |
| Female (n) | 8 | 2 | 4 | 0 |
| Known comorbidity | ||||
| ADHD (n) | 2 | 1 | 3 | 2 |
| ASD (n) | 1 | 1 | 1 | 1 |
| Dyslexia (n) | 2 | 0 | 0 | 0 |
| Age (months) | ||||
| Mean | 94.32 | 90.92 | 96.78 | 105.53 |
| SD | 27.44 | 23.21 | 22.65 | 23.63 |
| BMI | ||||
| Mean | 16.64 | 16.06 | 16.37 | 17.40 |
| SD | 2.61 | 3.16 | 1.93 | 3.02 |
| Min–max | 12.90–22.28 | 12.44–24.74 | 13.66–19.90 | 13.23–24.36 |
| Gestational age at birth (weeks) | ||||
| Mean | 39.03 | 39.32 | 39.33 | 40.06 |
| SD | 1.99 | 2.48 | 1.85 | 1.64 |
| Preterm (<37 weeks) (n) | 3 | 2 | 2 | 1 |
| Birthweight (g) | ||||
| Mean | 3352 | 3203 | 3632 | 3737 |
| SD | 531.2 | 583.1 | 336.3 | 569.1 |
| Min–max | 2315–4580 | 2185–4290 | 3025–4200 | 3030–4560 |
| <2500 g (n) | 2 | 2 | 0 | 0 |
| APGAR score 1 minute, median (min–max) | 9 (3–10) | 9 (2–10) | 9 (6–10) | 9 (5–10) |
| APGAR score 5 minutes, median (min–max) | 10 (6–10) | 10 (6–10) | 10 (9–10) | 10 (8–10) |
| Education | ||||
| Year repetition (valid %) | 32.4 | 8.7 | 44.4 | 21.1 |
| Mainstream | 37 | 24 | 14 | 16 |
| Special | 0 | 0 | 4 | 3 |
Missing data cluster (n): ADHD: 1 (1). ASD: 1 (1). Dyslexia: 1 (1). BMI: 1 (10), 2 (5), 3 (4), 4 (1). Gestational age: 1 (4), 2 (2), 4 (2). Birthweight: 1 (10), 2 (4), 3 (4), 4 (3). APGAR score 1 minute: 1 (14), 2 (11), 3 (7), 4 (5). APGAR score 5 minute: 1 (16), 2 (10), 3 (7), 4 (5). Year repetition: 1 (3), 2 (1). Abbreviations: ADHD, attention‐deficit/hyperactivity disorder; ASD, autism spectrum disorder; BMI, body mass index; MABC‐2, Movement Assessment Battery for Children, Second Edition.
DISCUSSION
The aim of our study was to identify possible subtypes within a large group of children diagnosed with DCD, based on performance profiles across measures of motor, cognitive, visual‐motor integration, and visual perceptual function. The cluster analysis identified four viable clusters. Clusters 1 and 3 were formed by groups of children that showed below‐average performance on all aspects of motor function and at least one aspect of perception and borderline performance IQ. Cluster 3 was distinguished, however, by much lower levels of gross‐motor skill (below the 5th centile, compared with the 5th to 15th centile range for cluster 1).
For children in cluster 2, difficulties were confined to gross‐motor/balance skills only (scores between the 5th and 15th centile); scores on all other measures were in the normal range. Finally, children in cluster 4 were distinguished by their relatively low performance on tasks requiring fine‐motor skills (MABC‐2 manual dexterity and Beery‐MC), combined with lower visual‐perceptual skill and performance IQ. Ball skills were age‐appropriate in this group, however.
The results of studies that investigate subtypes in DCD are difficult to compare because of differences in samples, measures included in the cluster analysis, and the statistical procedures used. 8 Despite these differences, we identified two clusters (comprising 56% of our total sample) with poor performance on almost all included measures. While this subtype is recognized in earlier studies, 4 , 5 , 6 , 7 , 8 , 14 , 15 the percentage of children that we identified with generalized problems (56%) is in line with the study by Vaivre‐Douret et al. only (44%); 15 other studies report much lower estimates (12–18%). 6 , 7 , 8 In our study, these two clusters were characterized by relatively poor performance on both gross‐ and fine‐motor skills, fine‐motor coordination as measured with the visual‐motor integration, and lower performance IQ scores. We conclude that approximately half of the children with DCD show fairly generalized difficulties across motor and perceptual skills.
The identification of a cluster (cluster 4) with primarily fine‐motor problems and poor visual perceptual skills is also in line with the results of previous studies.6–8,15 The size of this group (19%) is comparable to two other studies (10–18%), 6 , 7 but smaller than that reported by Macnab et al. (32%) 8 and Vaivre‐Douret et al. (44%). 15 Our cluster 2, that primarily showed gross‐motor problems (both balance and ball skills) on the MABC‐2, is a unique subtype not present in earlier subtyping studies. Wright and Sugden reported a cluster with problems confined mainly to catching, 14 and Macnab et al. to running speed and agility. 8 Notwithstanding these distinctions, the percentage of children in (broadly) gross‐motor clusters is consistent across studies (14–18%). These findings suggest that clinicians need to be aware that children with DCD can present with difficulties that are more confined to gross‐motor skills. These children may not be as readily identified by teachers and parents as having a developmental disorder since the level of impairment is moderate, and there are few other issues of note.
A striking result in our study is that impaired balance was evident across all subtypes (mean balance subtests were all <15th centile), unlike earlier subtyping studies. However, our finding is in line with the results of a very recent systematic review and meta‐analysis on balance problems in DCD. 16 This review showed that children with DCD have particular deficits in anticipatory balance control, leading to greater reliance on slower feedback‐based control processes compared with typically developing children. 16 Such deficits are likely to impede performance of functional motor tasks that rely on good postural control, such as ball skills, especially in an open environment. 17 The implication of this generalized balance problem in DCD for clinical practice is that postural control should be addressed within task‐oriented treatment of DCD.
Although our sample size was sufficient, it was just within the rule of thumb of a minimum of 10 times the number of variables. 18 Consequently, a possible effect of the inclusion and exclusion of children with known comorbidity could not be established. 6 A second limitation of the present retrospective study is that the choice of cognitive, motor, and visual‐perceptual metrics was confined to the ZOOM‐IN protocol. These metrics comply with international guidelines for diagnosing DCD 1 and are representative of data available in other rehabilitation centres. However, we had no influence on the inclusion of variables in the database. A factor like executive functioning could therefore not be included, which may have been worthwhile with regard to implications for intervention. Third, like in other studies, we were limited to parents who gave their informed consent. For the purpose of retrospective database research we advise rehabilitation centres to enable the anonymous use of data by including information and a consent form in their standard procedures. Subtype studies should ideally also provide information about aetiology and presence of comorbidity. 19 The groups in our study did not differ on demographic or clinical variables, such as perinatal characteristics like birthweight and gestational age. Interestingly, it was noted that children in clusters 1, 3, and 4 often had to repeat their school year (21.2–44%). These percentages are much higher than the national figure in the Netherlands (max 13%), 20 and demonstrate the high incidence of comorbid learning difficulties in these children as well as the need to provide these children with the necessary support early in their school career to prevent class repeating.
Our results show viable subtypes of DCD, which have implications for our understanding of the disorder and its clinical management. Our study revealed two clusters with a broad motor skill problem: one with relatively preserved visual‐motor integration and Beery‐MC skills, and another with abnormal ball skills, balance, and Beery‐MC skills. A third cluster with more specific gross‐motor problems, and a fourth with relatively preserved ball skills, but low Beery‐MC and performance IQ, were identified. For clinical practice, our results should alert clinicians to the more common performance profiles across motor, cognitive, and perceptual function. Clinical assessment should cover the full spectrum of motor, cognitive, and perceptual performance to reveal the personal strengths and needs of a child, and to enable individually tailored interventions. Specifically, children in cluster 2 may benefit solely with the help of a primary care physiotherapist, while children in clusters 1 and 3 (having more broad motor problems) are likely to require the help of the multidisciplinary care team in a specialized rehabilitation centre. In addition, assessment and treatment of children in cluster 4 could be optimized by the help of an occupational therapist and more in‐depth assessment of their visual perception skills. Future research is warranted to provide more detailed information on the possible longer‐term differences in outcome for the children in each cluster. Indeed, a comprehensive assessment should be conducted in cases where there is any suspicion of difficulty, if only to then set up task‐oriented interventions that target specific performance issues (i.e. early identification and intervention). To investigate the differential effects of intervention, it is important to identify the type and frequency of use of intervention techniques. For instance, the type and frequency of strategy use during a cognitive orientation to daily occupational performance (CO‐OP) approach intervention has been suggested to be important in relation to individual treatment effects. 6 Finally, to ascertain whether these subtypes are defined by unique neurocognitive profiles/mechanisms as well, neuroimaging studies are recommended in the future to clarify aetiology.
Supporting information
Table S1: Pearson correlation matrix of standardized z‐scores.
Table S2: Frequency of MABC‐2 centile score classifications for total and subscale scores per cluster.
ACKNOWLEDGMENTS
We would like to express our gratitude towards all parents who consented to include their child's data in our database. The authors have no interests that might be perceived as posing a conflict or bias.
Lust JM, Steenbergen B, Diepstraten JEM, Wilson PH, Schoemaker MM, Poelma MJ. The subtypes of developmental coordination disorder. Dev Med Child Neurol. 2022;64(11):1366–1374. 10.1111/dmcn.15260
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Blank R, Barnett AL, Cairney J, Green D, Kirby A, Polatajko H, et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev Med Child Neurol. 2019;61(3):242–85. Available from: 10.1111/dmcn.14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3. Wilson P, Ruddock S, Rahimi‐Golkhandan S, Piek J, Sugden D, Green D, et al. Cognitive and motor function in developmental coordination disorder. Dev Med Child Neurol 2020; 62: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 4. Asonitou K, Koutsouki D. Cognitive process‐based subtypes of developmental coordination disorder (DCD). Hum Mov Sci [Internet]. 2016. Jun [cited 2016 May 9];47:121–34. Available from: http://www.sciencedirect.com/science/article/pii/S0167945716300021 [DOI] [PubMed] [Google Scholar]
- 5. Dewey D, Kaplan BJ. Subtyping of Developmental Motor Deficits. Dev Neuropsychol. 1994. Jan 1;10(3):265–84. [Google Scholar]
- 6. Green D, Chambers ME, Sugden DA. Does subtype of developmental coordination disorder count: Is there a differential effect on outcome following intervention? Hum Mov Sci [Internet]. 2008;27(2):363–82. Available from: http://www.sciencedirect.com/science/article/pii/S0167945708000195 [DOI] [PubMed] [Google Scholar]
- 7. Hoare D. Subtypes of Developmental Coordination Disorder. Vol. 11, ADAPTED PHYSICAL ACTIVITY QUARTERLY. 1994.
- 8. Macnab JJ, Miller LT, Polatajko HJ. The search for subtypes of DCD: is cluster analysis the answer? Hum Mov Sci [Internet]. 2001. Mar [cited 2020 Mar 13];20(1–2):49–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11471397 [DOI] [PubMed] [Google Scholar]
- 9. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10. Smits‐Engelsman BCM. Handleiding Movement ABC‐2‐NL. Amsterdam: Pearson; 2010. [Google Scholar]
- 11. Beery KE, Buktenica NA, Beery NA. The Beery‐Buktenica Developmental Test of Visual‐Motor Intergration: Administration, scoring, and teaching manual (6th ed.). Minneapolis, MN: Pearson; 2010. [Google Scholar]
- 12. Kort W, Schittekatte M, Bosmans M, Compaan E, Dekker P, Vermeir G, et al. WISC‐III‐NL Wechsler Intelligence Scale for Children. Third edition NL. Amsterdam: Harcourt Test Publishers; 2005. [Google Scholar]
- 13. Charrad M, Ghazzali N, Boiteau V, Niknafs A. Nbclust: An R package for determining the relevant number of clusters in a data set. J Stat Softw [Internet]. 2014. Nov 3 [cited 2021 Feb 8];61(6):1–36. Available from: https://www.jstatsoft.org/index.php/jss/article/view/v061i06/v61i06.pdf [Google Scholar]
- 14. Wright HC, Sugden DA. The Nature of Developmental Coordination Disorder: Inter‐ and Intragroup Differences. Adapt Phys Act Q [Internet]. 1996. Oct 1 [cited 2021 Jul 9];13(4):357–71. Available from: http://journals.humankinetics.com/view/journals/apaq/13/4/article‐p357.xml [Google Scholar]
- 15. Vaivre‐Douret L, Lalanne C, Ingster‐Moati I, Boddaert N, Cabrol D, Dufier J‐L, et al. Subtypes of Developmental Coordination Disorder: Research on Their Nature and Etiology. Dev Neuropsychol [Internet]. 2011. Jul [cited 2020 Mar 13];36(5):614–43. Available from: 10.1080/87565641.2011.560696 [DOI] [PubMed] [Google Scholar]
- 16. Verbecque E, Johnson C, Rameckers E, Thijs A, van der Veer I, Meyns P, et al. Balance control in individuals with developmental coordination disorder: A systematic review and meta‐analysis. Gait Posture. 2021. Jan 1;83:268–79. [DOI] [PubMed] [Google Scholar]
- 17. Davids K, Bennett S, Kingsbury D, Jolley L, Brain T. Effects of postural constraints on children's catching behavior. Res Q Exerc Sport [Internet]. 2000. [cited 2021 Jul 9];71(1):69–73. Available from: https://pubmed.ncbi.nlm.nih.gov/10763523/ [DOI] [PubMed] [Google Scholar]
- 18. Qiu W, Joe H. Cluster Generation: Random Cluster Generation (with Specified Degree of Separation). 2009.
- 19. Visser J. Developmental coordination disorder: a review of research on subtypes and comorbidities. Hum Mov Sci [Internet]. 2003. Nov [cited 2014 Aug 26];22(4–5):479–93. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0167945703000678 [DOI] [PubMed] [Google Scholar]
- 20. Inspectie van het Onderwijs. De staat van het onderwijs 2021 [Internet]. Utrecht The Netherlands; 2021. Available from: https://www.onderwijsinspectie.nl/documenten/rapporten/2021/04/14/de‐staat‐van‐het‐onderwijs‐2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Pearson correlation matrix of standardized z‐scores.
Table S2: Frequency of MABC‐2 centile score classifications for total and subscale scores per cluster.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


